Effect of Acid Flow Rate, Membrane Surface Area, and Capture Solution on the Effectiveness of Suspended GPM Systems to Recover Ammonia
Abstract
:1. Introduction
2. Materials and Methods
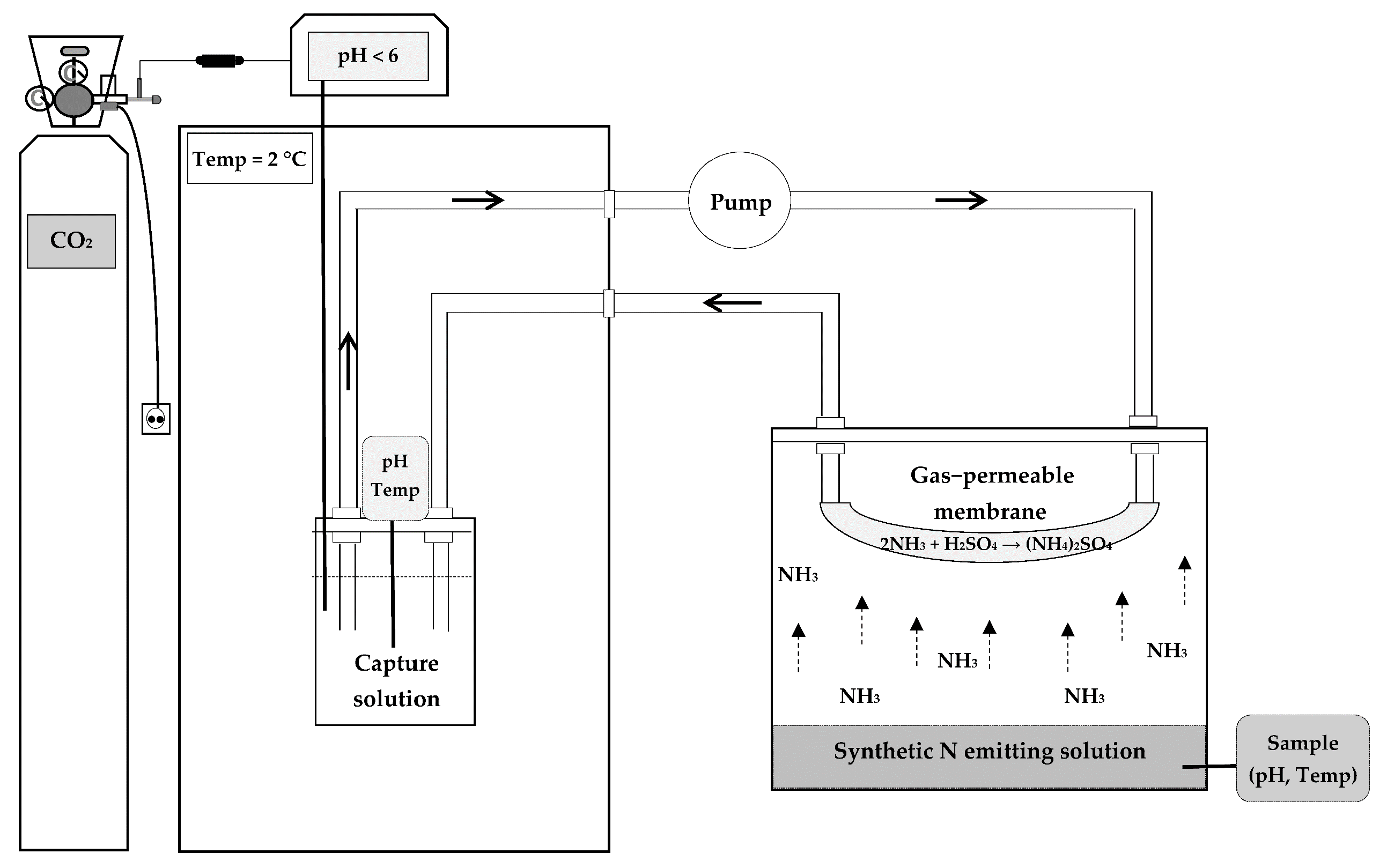
2.1. Experimental Design
2.2. Analysis Methodology
2.3. Data Calculations
2.4. Statistical Analyses
3. Results and Discussion
3.1. Effect of Membrane Surface Area
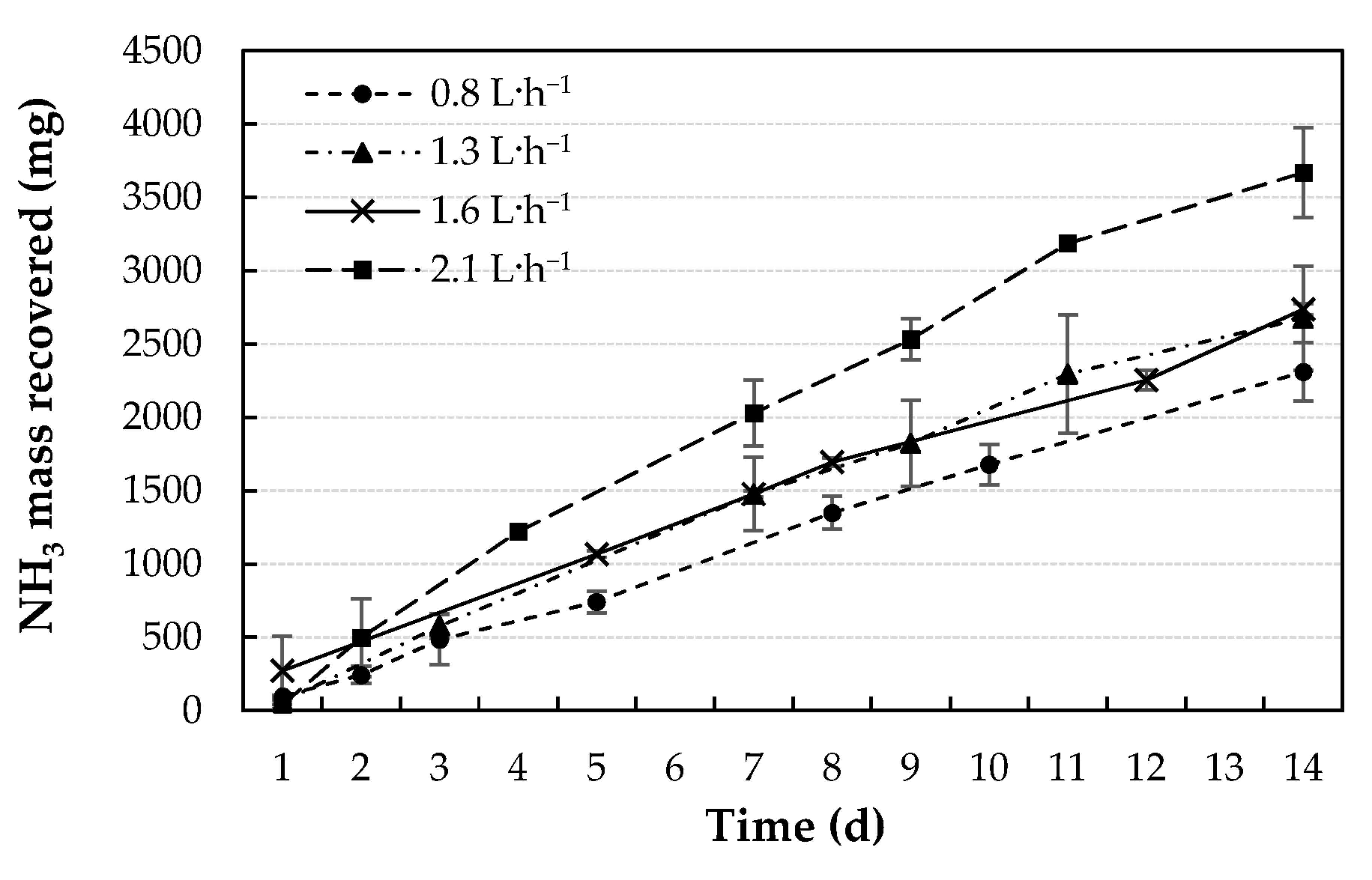
3.2. Effect of Acid Flow Rate
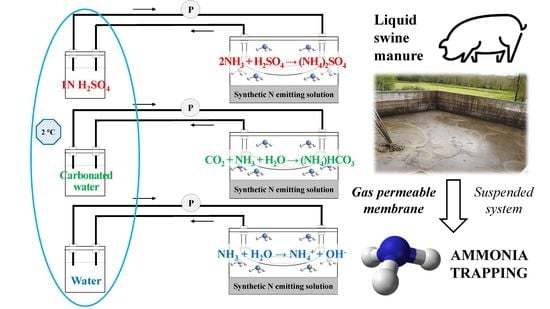
3.3. Effect of Ammonia Capture Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Follett, R.; Hatfield, J. Nitrogen in the Environment: Sources, Problems, and Management. Sci. World J. 2001, 1, 920–926. [Google Scholar] [CrossRef]
- Philippe, F.-X.; Cabaraux, J.-F.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Krupa, S. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Ni, J. Mechanistic Models of Ammonia Release from Liquid Manure: A Review. J. Agric. Eng. Res. 1999, 72, 1–17. [Google Scholar] [CrossRef]
- Olesen, J.; Sommer, S. Modelling effects of wind speed and surface cover on ammonia volatilization from stored pig slurry. Atmos. Environ. Part A Gen. Top. 1993, 27, 2567–2574. [Google Scholar] [CrossRef]
- Sürmeli, R.Ö.; Bayrakdar, A.; Çalli, B. Ammonia recovery from chicken manure digestate using polydimethylsiloxane membrane contactor. J. Clean. Prod. 2018, 191, 99–104. [Google Scholar] [CrossRef]
- Sommer, S. Ammonia volatilization from farm tanks containing anaerobically digested animal slurry. Atmos. Environ. 1997, 31, 863–868. [Google Scholar] [CrossRef]
- Teye, F.K.; Hautala, M. Adaptation of an ammonia volatilization model for a naturally ventilated dairy building. Atmos. Environ. 2008, 42, 4345–4354. [Google Scholar] [CrossRef]
- Tabase, R.K.; Millet, S.; Brusselman, E.; Ampe, B.; De Cuyper, C.; Sonck, B.; Demeyer, P. Effect of ventilation control settings on ammonia and odour emissions from a pig rearing building. Biosyst. Eng. 2020, 192, 215–231. [Google Scholar] [CrossRef]
- Poteko, J.; Zähner, M.; Schrade, S. Effects of housing system, floor type and temperature on ammonia and methane emissions from dairy farming: A meta-analysis. Biosyst. Eng. 2019, 182, 16–28. [Google Scholar] [CrossRef]
- Koerkamp, P.G.; Metz, J.; Uenk, G.; Phillips, V.; Holden, M.; Sneath, R.; Short, J.; White, R.; Hartung, J.; Seedorf, J.; et al. Concentrations and Emissions of Ammonia in Livestock Buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 79–95. [Google Scholar] [CrossRef]
- Dai, X.; Blanes-Vidal, V. Emissions of ammonia, carbon dioxide, and hydrogen sulfide from swine wastewater during and after acidification treatment: Effect of pH, mixing and aeration. J. Environ. Manag. 2013, 115, 147–154. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.; Christensen, M.; Sommer, S.G. Solid–Liquid Separation of Animal Slurry in Theory and Practice. In Sustainable Agriculture Volume 2; Springer: Dordrecht, The Netherlands, 2011; pp. 953–986. [Google Scholar]
- Zilio, M.; Orzi, V.; Chiodini, M.; Riva, C.; Acutis, M.; Boccasile, G.; Adani, F. Evaluation of ammonia and odour emissions from animal slurry and digestate storage in the Po Valley (Italy). Waste Manag. 2020, 103, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Masse, L.; Massé, D.; Pellerin, Y.; Dubreuil, J. Osmotic pressure and substrate resistance during the concentration of manure nutrients by reverse osmosis membranes. J. Membr. Sci. 2010, 348, 28–33. [Google Scholar] [CrossRef]
- Bonmati, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Milan, Z.; Sánchez, E.; Weiland, P.; Pozas, C.D.L.; Borja, R.; Mayari, R.; Rovirosa, N. Ammonia removal from anaerobically treated piggery manure by ion exchange in columns packed with homoionic zeolite. Chem. Eng. J. 1997, 66, 65–71. [Google Scholar] [CrossRef]
- Demirer, S.U.; Demirer, G.; Chen, S. Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process. Biochem. 2005, 40, 3667–3674. [Google Scholar] [CrossRef]
- Garcia-González, M.; Vanotti, M. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Manag. 2015, 38, 455–461. [Google Scholar] [CrossRef]
- Bellahsen, N.; Varga, G.; Halyag, N.; Kertész, S.; Tombácz, E.; Hodúr, C. Pomegranate peel as a new low-cost adsorbent for ammonium removal. Int. J. Environ. Sci. Technol. 2021, 18, 711–722. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.; Li, T.; Teasdale, P. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef]
- Zarebska, A.; Nieto, D.R.; Christensen, K.; Søtoft, L.F.; Norddahl, B. Ammonium Fertilizers Production from Manure: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 45, 1469–1521. [Google Scholar] [CrossRef]
- Daguerre-Martini, S.; Vanotti, M.; Rodriguez-Pastor, M.; Rosal, A.; Moral, R. Nitrogen recovery from wastewater using gas-permeable membranes: Impact of inorganic carbon content and natural organic matter. Water Res. 2018, 137, 201–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanotti, M.B.; Szögi, A.A. Use of Gas-Permeable Membranes for the Removal and Recovery of Ammonia from High Strength Livestock Wastewater. Proc. Water Environ. Fed. 2011, 2011, 659–667. [Google Scholar] [CrossRef]
- Vanotti, M.; Dube, P.; Szogi, A.; González, M.C.G. Recovery of ammonia and phosphate minerals from swine wastewater using gas-permeable membranes. Water Res. 2017, 112, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of Ammonia from Poultry Litter Using Gas-Permeable Membranes. Trans. ASABE 2010, 53, 1267–1275. [Google Scholar] [CrossRef]
- Rothrock, M.; Szögi, A.; Vanotti, M. Recovery of ammonia from poultry litter using flat gas permeable membranes. Waste Manag. 2013, 33, 1531–1538. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J. Separation of ammonia from radioactive wastewater by hydrophobic membrane contactor. Prog. Nucl. Energy 2016, 86, 97–102. [Google Scholar] [CrossRef]
- Ashrafizadeh, S.; Khorasani, Z. Ammonia removal from aqueous solutions using hollow-fiber membrane contactors. Chem. Eng. J. 2010, 162, 242–249. [Google Scholar] [CrossRef]
- Imai, M.; Furusaki, S.; Miyauchi, T. Separation of volatile materials by gas membranes. Ind. Eng. Chem. Process. Des. Dev. 2002, 21, 421–426. [Google Scholar] [CrossRef]
- Schneider, M.; Marison, I.; Von Stockar, U. Principles of an efficient new method for the removal of ammonia from animal cell cultures using hydrophobic membranes. Enzym. Microb. Technol. 1994, 16, 957–963. [Google Scholar] [CrossRef]
- Shin, H.S.; Hwang, Y.H.; Ahn, Y.T. Application of PTFE membrane for ammonia removal in a membrane contactor. Water Sci. Technol. 2011, 63, 2944–2948. [Google Scholar] [CrossRef]
- Majd, A.M.S.; Mukhtar, S. Ammonia Recovery Enhancement Using a Tubular Gas-Permeable Membrane System in Laboratory and Field-Scale Studies. Trans. ASABE 2013, 56, 1951–1958. [Google Scholar] [CrossRef]
- Darestani, M.; Haigh, V.; Couperthwaite, S.; Millar, G.; Nghiem, L. Hollow fibre membrane contactors for ammonia recovery: Current status and future developments. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.-L.; Chen, S.-H.; Liou, R.-M. Removing aqueous ammonia by membrane contactor process. Desalination Water Treat. 2013, 51, 5307–5310. [Google Scholar] [CrossRef]
- Szogi, A.A.; Vanotti, M.B.; Rothrock, M.J. Gaseous Ammonia Removal System. USA Patent 8,906,332 B2, 9 December 2014. [Google Scholar]
- Dube, P.; Vanotti, M.; Szogi, A.; González, M.C.G. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Bavarella, S.; Brookes, A.; Moore, A.; Vale, P.; Di Profio, G.; Curcio, E.; Hart, P.; Pidou, M.; McAdam, E.J. Chemically reactive membrane crystallisation reactor for CO2–NH3 absorption and ammonium bicarbonate crystallisation: Kinetics of heterogeneous crystal growth. J. Membr. Sci. 2020, 599, 117682. [Google Scholar] [CrossRef]
- Cámara, A. Captura y almacenamiento de CO2. In Proceedings of the X Congreso Nacional del Medio Ambiente (CONAMA 10), Madrid, Spain, 22–26 November 2010. [Google Scholar]
- Damtie, M.M.; Volpin, F.; Yao, M.; Tijing, L.D.; Hailemariam, R.H.; Bao, T.; Park, K.-D.; Shon, H.K.; Choi, J.-S. Ammonia recovery from human urine as liquid fertilizers in hollow fiber membrane contactor: Effects of permeate chemistry. Environ. Eng. Res. 2020, 26. [Google Scholar] [CrossRef]
- Beltrán-Rocha, J.C.; Guajardo-Barbosa, C.; Barceló-Quintal, I.D.; López-Chuken, U.J. Biotratamiento de efluentes secundarios municipales utilizando microalgas: Efecto del pH, nutrientes (C, N y P) y enriquecimiento con CO2. Rev. Biol. Mar. Oceanogr. 2017, 52, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington DC, USA, 2017. [Google Scholar]
- Blet, V.; Pons, M.-N.; Greffe, J. Separation of ammonia with a gas-permeable tubular membrane. Anal. Chim. Acta 1989, 219, 309–311. [Google Scholar] [CrossRef]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Fillingham, M.; VanderZaag, A.; Singh, J.; Burtt, S.; Crolla, A.; Kinsley, C.; MacDonald, J.D. Characterizing the Performance of Gas-Permeable Membranes as an Ammonia Recovery Strategy from Anaerobically Digested Dairy Manure. Membranes 2017, 7, 59. [Google Scholar] [CrossRef] [Green Version]
- Becares, E.; Álvarez, J.A.; León-Cófreces, C.; García-González, M.C. Caracterización de purines de cerdo y eficacias de tratamiento en España Central. Innovación Tecnol. Agroaliment. 2008, 4, 64–75. [Google Scholar]
- Hasanoğlu, A.; Romero, J.; Pérez, B.; Plaza, A. Ammonia removal from wastewater streams through membrane contactors: Experimental and theoretical analysis of operation parameters and configuration. Chem. Eng. J. 2010, 160, 530–537. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Conde-Cid, D.; Vanotti, M.B. Effect of the Type of Gas-Permeable Membrane in Ammonia Recovery from Air. Environments 2019, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Brennan, B.; Briciu-Burghina, C.; Hickey, S.; Abadie, T.; Awali, S.M.A.M.; Delaure, Y.; Durkan, J.; Holland, L.; Quilty, B.; Tajparast, M.; et al. Pilot Scale Study: First Demonstration of Hydrophobic Membranes for the Removal of Ammonia Molecules from Rendering Condensate Wastewater. Int. J. Mol. Sci. 2020, 21, 3914. [Google Scholar] [CrossRef]
- Jamaludin, Z.; Rollings-Scattergood, S.; Lutes, K.; Vaneeckhaute, C. Evaluation of sustainable scrubbing agents for ammonia recovery from anaerobic digestate. Bioresour. Technol. 2018, 270, 596–602. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Waite, T.D. The impact of absorbents on ammonia recovery in a capacitive membrane stripping system. Chem. Eng. J. 2020, 382, 122851. [Google Scholar] [CrossRef]
- Karami, M.; Keshavarz, P.; Khorram, M.; Mehdipour, M. Analysis of ammonia separation from purge gases in microporous hollow fiber membrane contactors. J. Hazard. Mater. 2013, 260, 576–584. [Google Scholar] [CrossRef]
- Budzianowski, W.; Koziol, A. Stripping of Ammonia from Aqueous Solutions in the Presence of Carbon Dioxide. Chem. Eng. Res. Des. 2005, 83, 196–204. [Google Scholar] [CrossRef]
- Palakodeti, A.; Azman, S.; Rossi, B.; Dewil, R.; Appels, L. A critical review of ammonia recovery from anaerobic digestate of organic wastes via stripping. Renew. Sustain. Energy Rev. 2021, 143, 110903. [Google Scholar] [CrossRef]
- Huang, J.-C.; Shang, C. Air stripping. In Advanced Physicochemical Treatment Processes; Wang, L.K., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 47–79. [Google Scholar] [CrossRef]
- Hamouda, A.S.; Eldien, M.S.; Abadir, M.F. Carbon dioxide capture by ammonium hydroxide solution and its possible application in cement industry. Ain Shams Eng. J. 2020, 11, 1061–1067. [Google Scholar] [CrossRef]
- Heffer, P.; Prud’homme, M. Global nitrogen fertilizer demand and supply: Trend, current level and outlook. In Proceedings of the 2016 International Nitrogen Initiative Conference, Melbourne, Australia, 4–8 December 2016; pp. 4–8. [Google Scholar]

| Membrane Surface Area (cm2) | Theoretical NH3-N Concentration (mg·L−1) | Initial NH3-N (mg N) | Removed NH3-N (mg N) | Recovered NH3-N (mg N) | N Flux (mg·cm−2·d−1) |
|---|---|---|---|---|---|
| 81.7 | 3000 | 2743 ± 68 | 840 ± 103 | 663 ± 93 e | 0.6 ± 0.1 d |
| 6000 | 5929 ± 454 | 1748 ± 366 | 1545± 62 d | 1.4 ± 0.1 c | |
| 12,000 | 13,153 ± 93 | 5713 ± 499 | 3925 ± 101 b | 3.4 ± 0.1 a | |
| 163.4 | 3000 | 3102 ± 74 | 1655 ± 104 | 1609 ± 10 d | 0.7 ± 0.0 d |
| 6000 | 6167 ± 689 | 3106 ± 352 | 2993 ± 54 c | 1.3 ± 0.0 c | |
| 12,000 | 11,744 ± 85 | 4954 ± 1015 | 4366 ± 14 a | 1.9 ± 0.0 b |
| Flow Rate (L∙h−1) | Initial N-NH3 (mg N) | Removed N-NH3 (mg N) | Recovered N-NH3 (mg N) | N Flux (mg N∙cm−2∙d−1) |
|---|---|---|---|---|
| 0.8 | 6240 ± 107 | 2583 ± 324 | 2311 ± 200 c | 1.35 ± 0.12 c |
| 1.3 | 6039 ± 9 | 2935 ± 431 | 2676 ± 356 bc | 1.56 ± 0.21 bc |
| 1.6 | 5985 ± 250 | 2974 ± 86 | 2737 ± 40 b | 1.60 ± 0.02 b |
| 2.1 | 6108 ± 517 | 4128 ± 470 | 3669 ± 305 a | 2.14 ± 0.18 a |
| Stripping Solution | Initial pH | Final pH | Initial N-NH3 (mg N) | Removed N-NH3 (mg N) | Recovered N-NH3 (mg N) | N Flux (mg N∙cm−2∙d−1) |
|---|---|---|---|---|---|---|
| 1N H2SO4 | 0.3 ± 0.1 | 0.5 ± 0.2 | 6230 ± 90 | 1777 ± 166 | 1602 ± 73 a | 1.4 ± 0.1 a |
| Carbonated water | 4.4 ± 0.1 | 6.3 ± 0.1 | 6285 ± 122 | 828 ± 135 | 414 ± 36 b | 0.4 ± 0.0 b |
| Water | 6.5 ± 0.4 | 8.1 ± 0.1 | 6380 ± 335 | 2128 ± 216 | 1760 ± 134 a | 1.5 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Vanotti, M.B.; Martín-Ramos, P. Effect of Acid Flow Rate, Membrane Surface Area, and Capture Solution on the Effectiveness of Suspended GPM Systems to Recover Ammonia. Membranes 2021, 11, 538. https://doi.org/10.3390/membranes11070538
Soto-Herranz M, Sánchez-Báscones M, Antolín-Rodríguez JM, Vanotti MB, Martín-Ramos P. Effect of Acid Flow Rate, Membrane Surface Area, and Capture Solution on the Effectiveness of Suspended GPM Systems to Recover Ammonia. Membranes. 2021; 11(7):538. https://doi.org/10.3390/membranes11070538
Chicago/Turabian StyleSoto-Herranz, María, Mercedes Sánchez-Báscones, Juan Manuel Antolín-Rodríguez, Matías B. Vanotti, and Pablo Martín-Ramos. 2021. "Effect of Acid Flow Rate, Membrane Surface Area, and Capture Solution on the Effectiveness of Suspended GPM Systems to Recover Ammonia" Membranes 11, no. 7: 538. https://doi.org/10.3390/membranes11070538
APA StyleSoto-Herranz, M., Sánchez-Báscones, M., Antolín-Rodríguez, J. M., Vanotti, M. B., & Martín-Ramos, P. (2021). Effect of Acid Flow Rate, Membrane Surface Area, and Capture Solution on the Effectiveness of Suspended GPM Systems to Recover Ammonia. Membranes, 11(7), 538. https://doi.org/10.3390/membranes11070538