Polyunsaturated Fatty Acids Mediated Regulation of Membrane Biochemistry and Tumor Cell Membrane Integrity
Abstract
1. Introduction
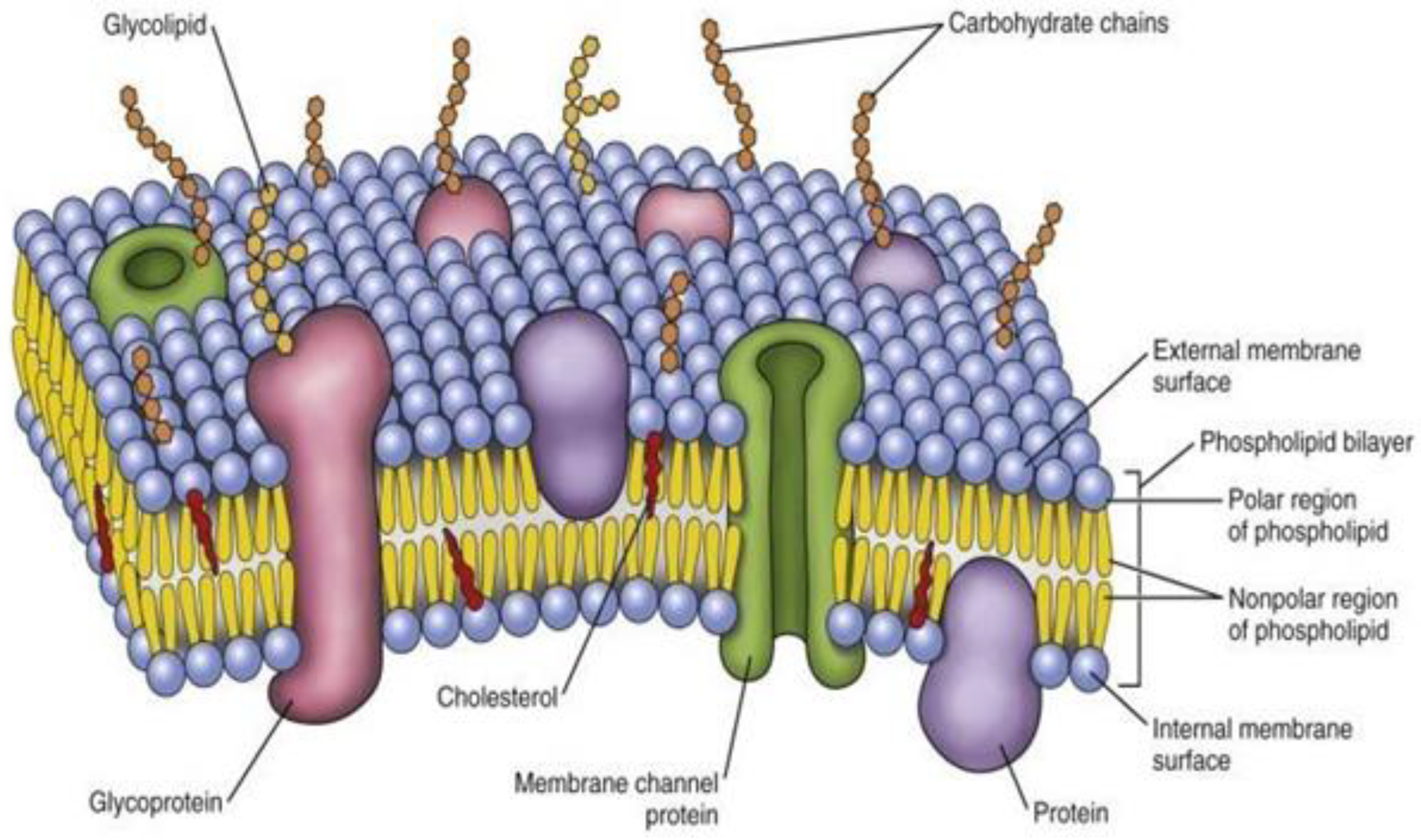
2. Biophysical Architecture of Cellular Membrane
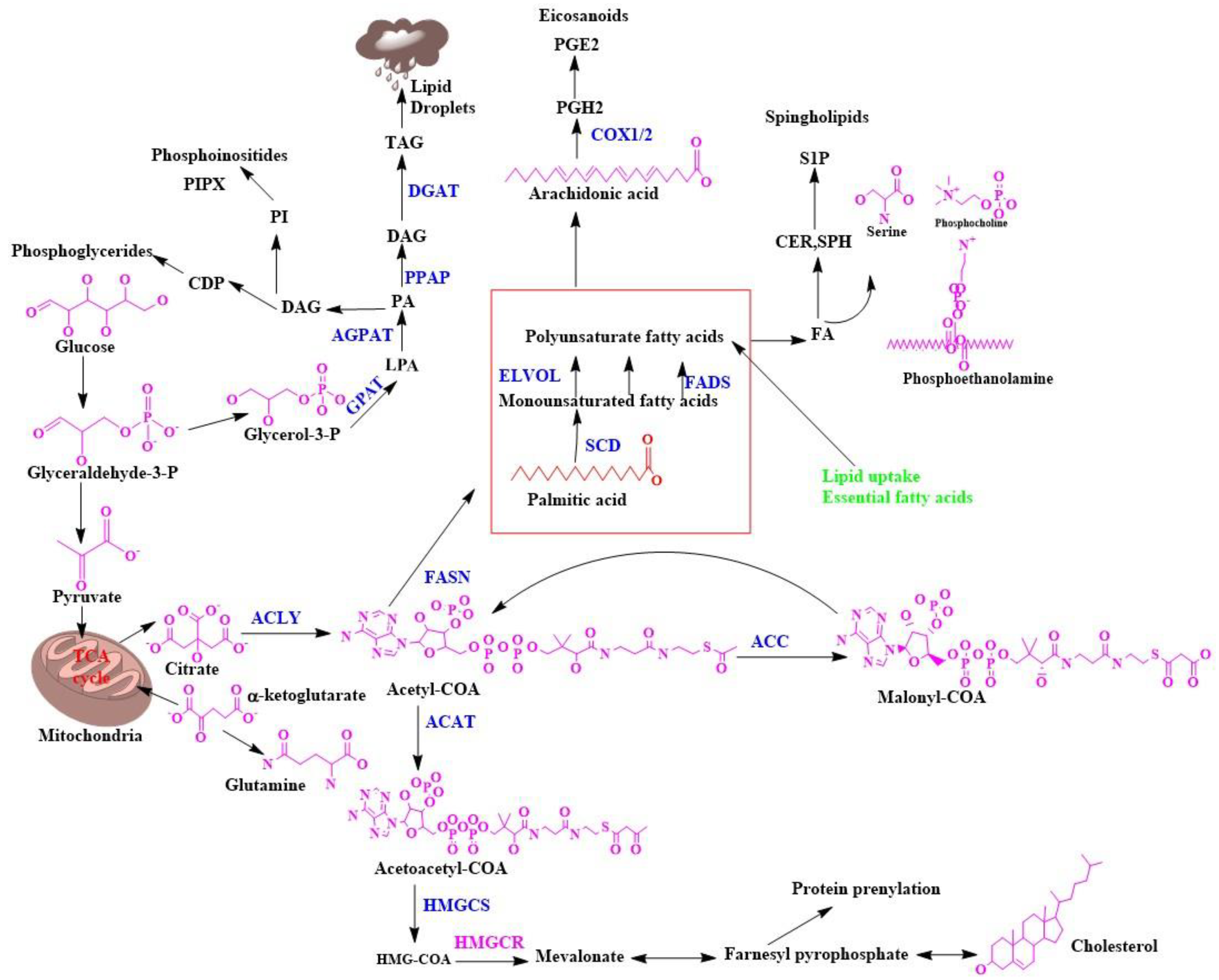
Biosynthesis of Fatty Acids in the Cell Membrane
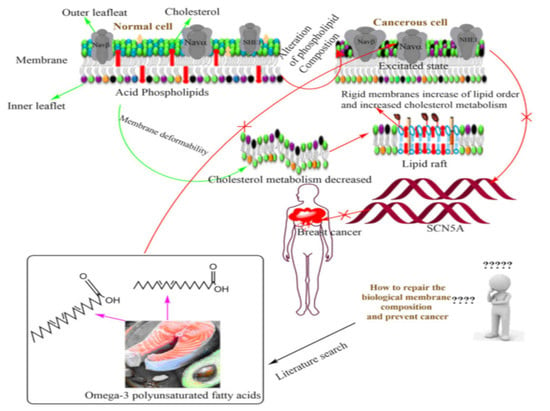
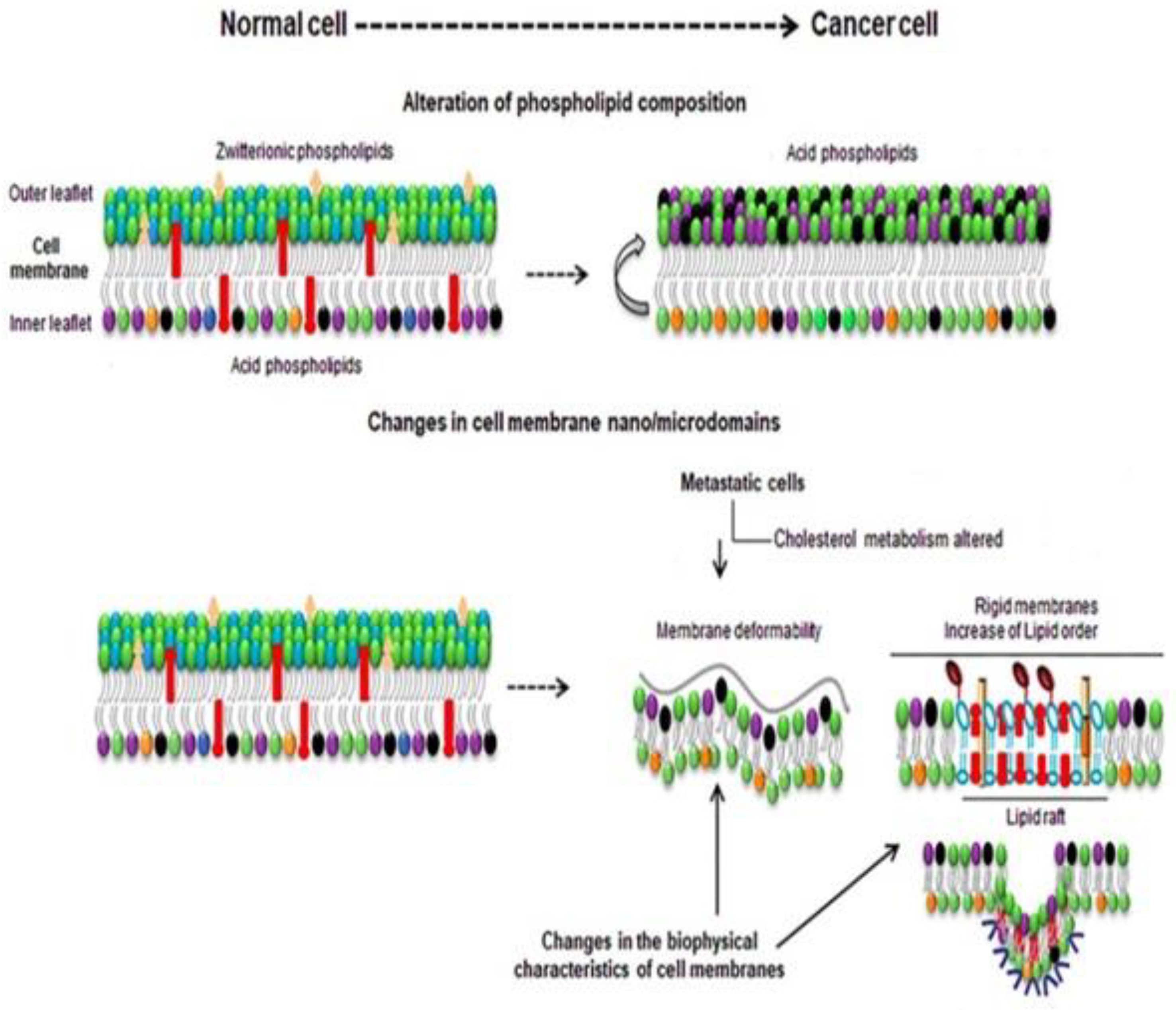
3. Membrane Biophysical Difference between a Normal Cell and Cancer Cell
4. Pivotal Role of Membrane Lipid and Cholesterol for Reprogramming of Breast Cancer
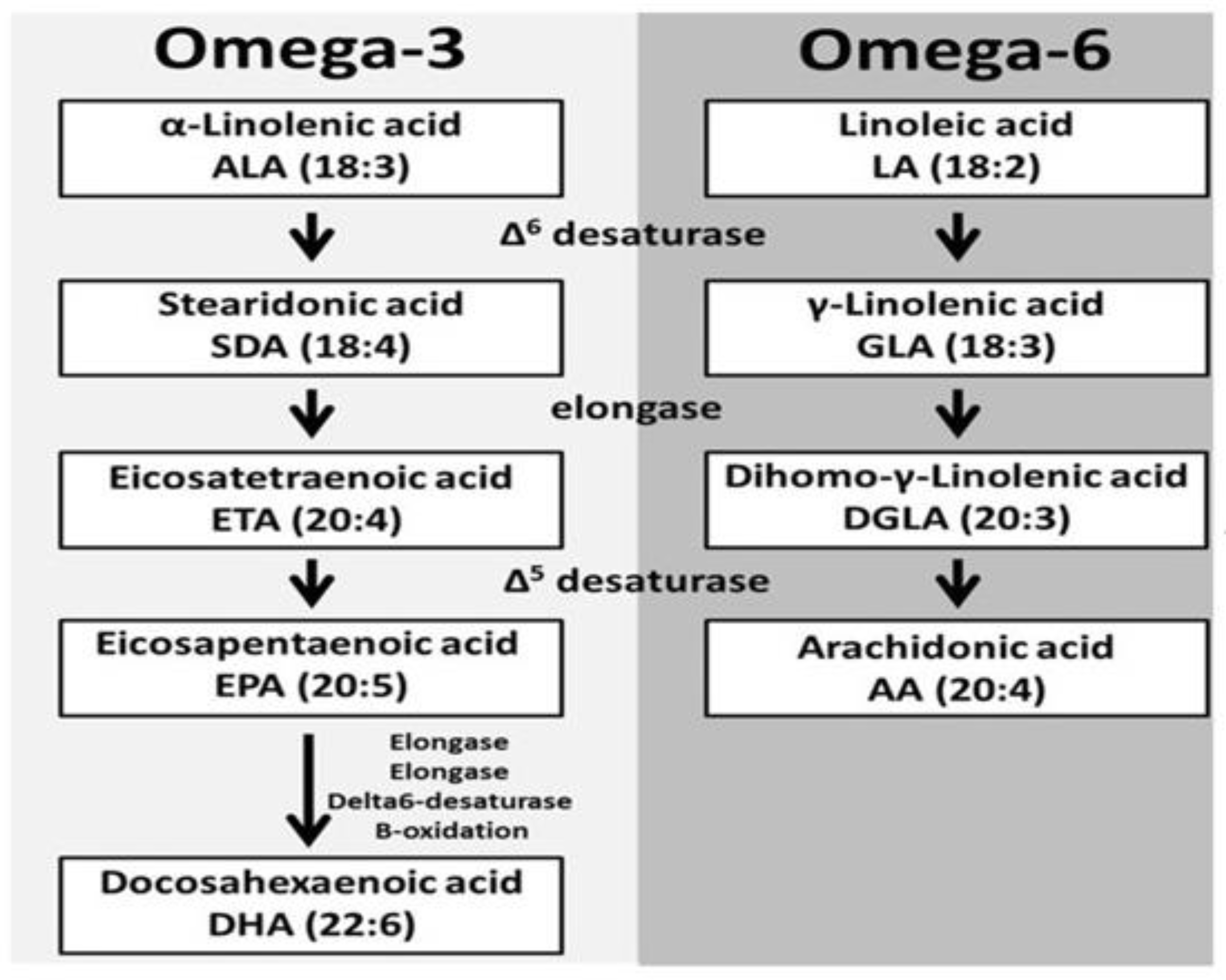
5. Introductory Concept of Omega-3 PUFAs
6. Intermediate Cross-Talk of ALA and Gamma-Linolenic Acid (GLA)
7. Mechanism of GLA for Breast Cancer Prevention
8. Role of Omega-3 PUFAs in the Regulation of Membrane Channel Activity
9. Translational Impact of PUFAs
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howe, L.R.; Subbaramaiah, K.; Hudis, C.A.; Dannenberg, A.J. Molecular pathways: Adipose inflammation as a mediator of obesity-associated cancer. Clin. Cancer Res. 2013, 19, 6074–6083. [Google Scholar] [CrossRef]
- Baumgarten, S.C.; Frasor, J. Minireview: Inflammation: An instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol. Endocrinol. 2012, 26, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.R.; Hassan, H.I. Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: Preliminary observations. J. Clin. Pathol. 2006, 59, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W. Macrophages define the invasive microenvironment in breast cancer. J. Leukoc. Biol. 2008, 84, 623–630. [Google Scholar] [CrossRef]
- McDermott, R.S.; Beuvon, F.; Pauly, M.; Pallud, C.; Vincent-Salomon, A.; Mosseri, V.; Pouillart, P.; Scholl, S.M. Tumor antigens and antigen-presenting capacity in breast cancer. Pathobiology 2002, 70, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef]
- Weylandt, K.H.; Chiu, C.Y.; Gomolka, B.; Waechter, S.F.; Wiedenmann, B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins Other Lipid Mediat. 2012, 97, 73–82. [Google Scholar] [CrossRef]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins. Leukot. Essent. Fat. Acids 2013, 88, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Ravacci, G.R.; Brentani, M.M.; Tortelli, T.; Torrinhas, R.S.M.; Saldanha, T.; Torres, E.A.F.; Waitzberg, D.L. Lipid raft disruption by docosahexaenoic acid induces apoptosis in transformed human mammary luminal epithelial cells harboring HER-2 overexpression. J. Nutr. Biochem. 2013, 24, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Manral, C.; Roy, S.; Singh, M.; Gautam, S.; Yadav, R.K.; Rawat, J.K.; Devi, U.; Ansari, M.N.; Saeedan, A.S.; Kaithwas, G. Effect of β-sitosterol against methyl nitrosourea-induced mammary gland carcinoma in albino rats. BMC Complementary Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Rani, A.; Roy, S.; Singh, M.; Devi, U.; Yadav, R.K.; Gautam, S.; Rawat, J.K.; Ansari, M.N.; Saeedan, A.S.; Prakash, A.; et al. α-Chymotrypsin regulates free fatty acids and UCHL-1 to ameliorate N-methyl nitrosourea induced mammary gland carcinoma in albino wistar rats. Inflammopharmacology 2016, 24, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Yun, U.J.; Koo, K.H.; Sung, J.Y.; Shim, J.; Ye, S.K.; Hong, K.M.; Kim, Y.N. Down-regulation of lipid raft-associated onco-proteins via cholesterol-dependent lipid raft internalization in docosahexaenoic acid-induced apoptosis. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 190–203. [Google Scholar] [CrossRef]
- Yadav, S.; Tiwari, V.; Singh, M.; Yadav, R.K.; Roy, S.; Devi, U.; Gautam, S.; Rawat, J.K.; Ansari, M.N.; Saeedan, A.S.; et al. Comparative efficacy of alpha-linolenic acid and gamma-linolenic acid to attenuate valproic acid-induced autism-like features. J. Physiol. Biochem. 2017, 73, 187–198. [Google Scholar] [CrossRef]
- Rogers, K.R.; Kikawa, K.D.; Mouradian, M.; Hernandez, K.; McKinnon, K.M.; Ahwah, S.M.; Pardini, R.S. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis 2010, 31, 1523–1530. [Google Scholar] [CrossRef]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668, S50–S58. [Google Scholar] [CrossRef]
- Wen, Z.H.; Su, Y.C.; Lai, P.L.; Zhang, Y.; Xu, Y.F.; Zhao, A.; Yao, G.Y.; Jia, C.H.; Lin, J.; Xu, S.; et al. Critical role of arachidonic acid-activated mTOR signaling in breast carcinogenesis and angiogenesis. Oncogene 2013, 32, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.M.; Calder, P.C.; Rainger, G.E. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Friborg, J.T.; Melbye, M. Cancer patterns in Inuit populations. Lancet Oncol. 2008, 9, 892–900. [Google Scholar] [CrossRef]
- Abel, S.; Riedel, S.; Gelderblom, W.C. Dietary PUFA and cancer. Proc. Nutr. Soc. 2014, 73, 361–367. [Google Scholar] [CrossRef]
- Abramczyk, H.; Surmacki, J.; Kopeć, M.; Olejnik, A.K.; Lubecka-Pietruszewska, K.; Fabianowska-Majewska, K. The role of lipid droplets and adipocytes in cancer. Raman imaging of cell cultures: MCF10A, MCF7, and MDA-MB-231 compared to adipocytes in cancerous human breast tissue. Analyst 2015, 140, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Accioly, M.T.; Pacheco, P.; Maya-Monteiro, C.M.; Carrossini, N.; Robbs, B.K.; Oliveira, S.S.; Kaufmann, C.; Morgado-Diaz, J.A.; Bozza, P.T.; Viola, J.P. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 2008, 168, 1732–1740. [Google Scholar] [CrossRef]
- Adachi, S.; Nagao, T.; Ingolfsson, H.I.; Maxfield, F.R.; Andersen, O.S.; Kopelovich, L.; Weinstein, I.B. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007, 67, 6493–6501. [Google Scholar] [CrossRef]
- Agnihotri, N.; Sharma, G.; Rani, I.; Bhatnagar, A. Fish oil prevents colon cancer by modulation of structure and function of mitochondria. Biomed. Pharmacother. 2016, 82, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, J.D.; Bieberich, A.A.; Terry, C.; Harvey, K.A.; VanHorn, J.F.; Xu, Z.; Davisson, V.J.; Siddiqui, R.A. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: Unique signaling not explained by the effects of either compound alone. BMC Cancer 2011, 11, 1–6. [Google Scholar] [CrossRef]
- Ariotti, N.; Fernández-Rojo, M.A.; Zhou, Y.; Hill, M.M.; Rodkey, T.L.; Inder, K.L.; Tanner, L.B.; Wenk, M.R.; Hancock, J.F.; Parton, R.G. Caveolae regulate the nanoscale organization of the plasma membrane to remotely control Ras signaling. J. Cell Biol. 2014, 204, 777–792. [Google Scholar] [CrossRef]
- Arora, A.; Singh, S.; Bhatt, A.N.; Pandey, S.; Sandhir, R.; Dwarakanath, B.S. Interplay between metabolism and oncogenic process: Role of microRNAs. Transl. Oncogenom. 2015, 7, 11. [Google Scholar]
- Fuentes, N.R.; Kim, E.; Fan, Y.Y.; Chapkin, R.S. Omega-3 fatty acids, membrane remodeling and cancer prevention. Mol. Asp. Med. 2018, 64, 79–91. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, L.K.; Lupton, J.R.; Davidson, L.A.; Taddeo, S.S.; Murphy, M.E.; Carroll, R.J.; Chapkin, R.S. Dietary fish oil reduces oxidative DNA damage in rat colonocytes. Free Radic. Biol. Med. 2003, 135, 149–159. [Google Scholar] [CrossRef]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van Den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Barker, N.; Ridgway, R.A.; Van Es, J.H.; Van De Wetering, M.; Begthel, H.; Van Den Born, M.; Danenberg, E.; Clarke, A.R.; Sansom, O.J.; Clevers, H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009, 457, 608–611. [Google Scholar] [CrossRef]
- Barman, S.; Nayak, D.P. Lipid raft disruption by cholesterol depletion enhances influenza A virus budding from MDCK cells. J. Virol. 2007, 181, 12169–12178. [Google Scholar] [CrossRef] [PubMed]
- Alagumuthu, M.; Dahiya, D.; Nigam, P.S. Phospholipid—the dynamic structure between living and non-living world; a much obligatory supramolecule for present and future. AIMS Mol. Sci. 2019, 26, 1–9. [Google Scholar]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef]
- Barrera, N.P.; Zhou, M.; Robinson, C.V. The role of lipids in defining membrane protein interactions: Insights from mass spectrometry. Trends Cell Biol. 2013, 23, 1–8. [Google Scholar] [CrossRef]
- Bayram, I.; Erbey, F.; Celik, N.; Nelson, J.L.; Tanyeli, A. The use of a protein and energy dense eicosapentaenoic acid containing supplement for malignancy-related weight loss in children. Pediatric Blood Cancer 2009, 52, 571–574. [Google Scholar] [CrossRef]
- Bene, L.; Bodnár, A.; Damjanovich, S.; Vámosi, G.; Bacsó, Z.; Aradi, J.; Berta, A.; Damjanovich, J. Membrane topography of HLA I, HLA II, and ICAM-1 is affected by IFN-γ in lipid rafts of uveal melanomas. Biochem. Biophys. Res. Commun. 2004, 1322, 678–683. [Google Scholar] [CrossRef]
- Beyaz, S.; Mana, M.D.; Roper, J.; Kedrin, D.; Saadatpour, A.; Hong, S.J.; Bauer-Rowe, K.E.; Xifaras, M.E.; Akkad, A.; Arias, E.; et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 2016, 531, 53–58. [Google Scholar] [CrossRef]
- Bloch, K.E. Sterol, structure and membrane function. Crit. Rev. Biochem. 1983, 14, 47–92. [Google Scholar] [CrossRef]
- Bohdanowicz, M.; Grinstein, S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol. Rev. 2013, 93, 69–106. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Decoux-Poullot, A.G.; Tanti, J.F.; Clavel, S. Energy disruptors: Rising stars in anticancer therapy? Oncogenesis 2016, 5, e188. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, D.M.; Gardner, J.S.; Malone, K.E.; Heckbert, S.R.; Blough, D.K.; Daling, J.R. The association between 3-hydroxy-3-methylglutaryl conenzyme A inhibitor use and breast carcinoma risk among postmenopausal women. Cancer 2004, 100, 2308–2316. [Google Scholar] [CrossRef]
- Bougnoux, P.; Hajjaji, N.; Ferrasson, M.N.; Giraudeau, B.; Couet, C.; Le Floch, O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: A phase II trial. Br. J. Cancer 2009, 101, 1978–1985. [Google Scholar] [CrossRef] [PubMed]
- Bozza, P.T.; Viola, J.P. Lipid droplets in inflammation and cancer. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2010, 82, 243–250. [Google Scholar] [CrossRef]
- Broitman, S.A.; Cerda, S.; Wilkinson, J. Cholesterol metabolism and colon cancer. Prog. Food Nutr. Sci. 1993, 17, 1–40. [Google Scholar]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Calviello, G.; Di Nicuolo, F.; Serini, S.; Piccioni, E.; Boninsegna, A.; Maggiano, N.; Ranelletti, F.O.; Palozza, P. Docosahexaenoic acid enhances the susceptibility of human colorectal cancer cells to 5-fluorouracil. Cancer Chemother. Pharmacol. 2005, 55, 12–20. [Google Scholar] [CrossRef]
- Cardwell, C.; Hicks, B.M.; Hughes, C.; Murray, L.J. Statin Use After Colorectal Cancer Diagnosis and Survival: A Population-Based Cohort Study. J. Clin. Oncol. 2014, 32, 3177–3183. [Google Scholar] [CrossRef]
- Cauley, J.A.; Zmuda, J.M.; Lui, L.Y.; Hillier, T.A.; Ness, R.B.; Stone, K.L.; Cummings, S.R.; Bauer, D.C. Lipid-lowering drug use and breast cancer in older women: A prospective study. J. Women’s Health 2003, 12, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Devi, U.; Roy, S.; Gupta, P.S.; Saraf, S.A.; Kaithwas, G. Prolyl hydroxylase mediated inhibition of fatty acid synthase to combat tumor growth in mammary gland carcinoma. Breast Cancer 2016, 23, 820–829. [Google Scholar] [CrossRef]
- Singh, M.; Devi, U.; Roy, S.; Gupta, P.S.; Kaithwas, G. Chemical activation of prolyl hydroxylase-2 by BBAP-1 down regulates hypoxia inducible factor-1α and fatty acid synthase for mammary gland chemoprevention. RSC Adv. 2018, 8, 12848–12860. [Google Scholar] [CrossRef]
- Devi, U.; Singh, M.; Roy, S.; Tripathi, A.C.; Gupta, P.S.; Saraf, S.K.; Ansari, N.; Saeedan, A.S.; Kaithwas, G. PHD-2 activation: A novel strategy to control HIF-1α and mitochondrial stress to modulate mammary gland pathophysiology in ER+ subtype. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1239–1256. [Google Scholar] [CrossRef]
- Cerchietti, L.C.; Navigante, A.H.; Castro, M.A. Effects of eicosapentaenoic and docosahexaenoic n-3 fatty acids from fish oil and preferential Cox-2 inhibition on systemic syndromes in patients with advanced lung cancer. Nutr. Cancer 2007, 159, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Singh, M.; Rawat, A.; Devi, U.; Gautam, S.; Yadav, R.K.; Rawat, J.K.; Ansari, M.N.; Saeedan, A.S.; Kumar, D.; et al. GLA supplementation regulates PHD2 mediated hypoxia and mitochondrial apoptosis in DMBA induced mammary gland carcinoma. Int. J. Biochem. Cell Biol. 2018, 96, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Tang, D.G. Detection of apoptosis in cell-free systems. In Apoptosis; Humana Press: Totowa, NJ, USA, 2009; pp. 65–75. [Google Scholar]
- Chapkin, R.S. Fatty acids in foods and their health implications. In Reappraisal of the Essential Fatty Acids, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 557–568. [Google Scholar]
- Chapkin, R.S.; Akoh, C.C.; Miller, C.C. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptidoleukotriene synthesis. J. Lipid Res. 1991, 32, 1205–1213. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Hong, M.Y.; Fan, Y.Y.; Davidson, L.A.; Sanders, L.M.; Henderson, C.E.; Barhoumi, R.; Burghardt, R.C.; Turner, N.D.; Lupton, J.R. Dietary n− 3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids 2002, 37, 193–199. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Wang, N.; Fan, Y.Y.; Lupton, J.R.; Prior, I.A. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2008, 1778, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.; DeClercq, V.; Kim, E.; Fuentes, N.R.; Fan, Y.Y. Mechanisms by which pleiotropic amphiphilic n−3 PUFA reduce colon cancer risk. Curr. Colorectal Cancer Rep. 2014, 10, 442–452. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, H.; Turner, N.D.; Mann, J.C.; Wei, J.; Taddeo, S.S.; Davidson, L.A.; Wang, N.; Vannucci, M.; Carroll, R.J.; et al. A chemoprotective fish oil-and pectin-containing diet temporally alters gene expression profiles in exfoliated rat colonocytes throughout oncogenesis. J. Nutr. 2011, 141, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Turner, N.D.; Davidson, L.A.; Chapkin, R.S.; Carroll, R.J.; Lupton, J.R. A chemoprotective fish oil/pectin diet enhances apoptosis via Bcl-2 promoter methylation in rat azoxymethane-induced carcinomas. Exp. Biol. Med. 2012, 237, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Turner, N.D.; Davidson, L.A.; Chapkin, R.S.; Carroll, R.J.; Lupton, J.R. Colon cancer cell apoptosis is induced by combined exposure to the n-3 fatty acid docosahexaenoic acid and butyrate through promoter methylation. Exp. Biol. Med. 2014, 239, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rawat, A.K.; Sammi, S.R.; Devi, U.; Singh, M.; Gautam, S.; Yadav, R.K.; Rawat, J.K.; Singh, L.; Ansari, M.N.; et al. Alpha-linolenic acid stabilizes HIF-1 α and downregulates FASN to promote mitochondrial apoptosis for mammary gland chemoprevention. Oncotarget 2017, 18, 70049. [Google Scholar] [CrossRef]
- Yadav, R.K.; Singh, M.; Roy, S.; Ansari, M.N.; Saeedan, A.S.; Kaithwas, G. Modulation of oxidative stress response by flaxseed oil: Role of lipid peroxidation and underlying mechanisms. Prostaglandins Lipid Mediat. 2018, 135, 21–26. [Google Scholar] [CrossRef]
- Erlejman, A.G.; Verstraeten, S.V.; Fraga, C.G.; Oteiza, P.I. The interaction of flavonoids with membranes: Potential determinant of flavonoid antioxidant effects. Free Radic. Res. 2004, 38, 1311–1320. [Google Scholar] [CrossRef]
- Fabian, C.J.; Kimler, B.F.; Phillips, T.A.; Nydegger, J.L.; Kreutzjans, A.L.; Carlson, S.E.; Hidaka, B.; Metheny, T.; Zalles, C.M.; Mills, G.B.; et al. Modulation of Breast Cancer Risk Biomarkers by High-Dose Omega-3 Fatty Acids: Phase II Pilot Study in Postmenopausal Women. Cancer Prev. Res. 2015, 8, 922–931. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Hardman, W.E. Omega 3 fatty acids increase the chemo-sensitivity of B-CLL-derived cell lines EHEB and MEC-2 and of B-PLL-derived cell line JVM-2 to anti-cancer drugs doxorubicin, vincristine and fludarabine. Lipids Health Dis. 2013, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Roy, S.; Ansari, M.N.; Saeedan, A.S.; Saraf, S.A.; Kaithwas, G. DuCLOX-2/5 inhibition: A promising target for cancer chemoprevention. Breast Cancer 2016, 24, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Ran, Q.; Toyokuni, S.; Okazaki, Y.; Callaway, E.S.; Lupton, J.R.; Chapkin, R.S. Dietary fish oil promotes colonic apoptosis and mitochondrial proton leak in oxidatively stressed mice. Cancer Prev. Res. 2011, 4, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Zhan, Y.; Aukema, H.M.; Davidson, L.A.; Zhou, L.; Callaway, E.; Tian, Y.; Weeks, B.R.; Lupton, J.R.; Toyokuni, S.; et al. Proapoptotic effects of dietary (n-3) fatty acids are enhanced in colonocytes of manganese-dependent superoxide dismutase knockout mice. J. Nutr. 2009, 139, 1328–1332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yadav, R.K.; Singh, M.; Roy, S.; Gautam, S.; Rawat, J.K.; Singh, L.; Ansari, M.N.; Saeedan, A.S.; Kaithwas, G. Short communication: Evaluation of α-linolenic acid–based intramammary nanosuspension for treatment of subclinical mastitis. J. Dairy Sci. 2020, 103, 2701–2706. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Vaz, F.M.; Chapkin, R.S. Dietary fat and fiber interactively modulate apoptosis and mitochondrial bioenergetic profiles in mouse colon in a site-specific manner. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2017, 26, 301. [Google Scholar] [CrossRef]
- Fearon, K.C.; Barber, M.D.; Moses, A.G.; Ahmedzai, S.H.; Taylor, G.S.; Tisdale, M.J.; Murray, G.D. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J. Clin. Oncol. 2006, 224, 3401–3407. [Google Scholar] [CrossRef]
- Fedida-Metula, S.; Feldman, B.; Koshelev, V.; Levin-Gromiko, U.; Voronov, E.; Fishman, D. Lipid rafts couple store-operated Ca 2+ entry to constitutive activation of PKB/Akt in a Ca 2+ /calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinogenesis 2012, 33, 740–750. [Google Scholar] [CrossRef]
- Feng, Y.; Schouteden, S.; Geenens, R.; Van Duppen, V.; Herijgers, P.; Holvoet, P.; Van Veldhoven, P.P.; Verfaillie, C.M. Hematopoietic stem/progenitor cell proliferation and differentiation is differentially regulated by high-density and low-density lipoproteins in mice. PLoS ONE 2012, 7, e47286. [Google Scholar] [CrossRef]
- Roy, S.; Singh, M.; Sammi, S.R.; Pandey, R.; Kaithwas, G. ALA-mediated biphasic downregulation of α-7nAchR/HIF-1α along with mitochondrial stress modulation strategy in mammary gland chemoprevention. J. Cell. Physiol. 2019, 234, 4015–4029. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, C.; Segre, O.; Fadda, M.; Monge, T.; Scigliano, M.; Schena, M.; Tinivella, M.; Tiozzo, E.; Catalano, M.G.; Pugliese, M.; et al. Effect of n-3 fatty acids on patients with advanced lung cancer: A double-blind, placebo-controlled study. Br. J. Nutr. 2012, 108, 327–333. [Google Scholar] [CrossRef]
- Forones, N.M.; Falcao, J.B.; Mattos, D.; Barone, B. Cholesterolemia in colorectal cancer. Hepato-Gastroenterol. 1998, 45, 1531–1534. [Google Scholar] [PubMed]
- Frisz, J.F.; Lou, K.; Klitzing, H.A.; Hanafin, W.P.; Lizunov, V.; Wilson, R.L.; Carpenter, K.J.; Kim, R.; Hutcheon, I.D.; Zimmerberg, J.; et al. Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proc. Natl. Acad. Sci. USA 2013, 1110, E613–E622. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.R.; Salinas, M.L.; Kim, E.; Chapkin, R.S. Emerging role of chemoprotective agents in the dynamic shaping of plasma membrane organization. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2017, 1859, 1668–1678. [Google Scholar] [CrossRef]
- Roy, S.; Singh, M.; Rawat, A.; Kumar, D.; Kaithwas, G. Mitochondrial apoptosis and curtailment of hypoxia-inducible factor-1α/fatty acid synthase: A dual edge perspective of gamma linolenic acid in ER+ mammary gland cancer. Cell Biochem. Funct. 2020, 38, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef]
- Gabitova, L.; Restifo, D.; Gorin, A.; Manocha, K.; Handorf, E.; Yang, D.H.; Cai, K.Q.; Klein-Szanto, A.J.; Cunningham, D.; Kratz, L.E.; et al. Endogenous sterol metabolites regulate growth of EGFR/KRAS-dependent tumors via LXR. Cell Rep. 2015, 212, 1927–1938. [Google Scholar] [CrossRef]
- Garcia-Parajo, M.F.; Cambi, A.; Torreno-Pina, J.A.; Thompson, N.; Jacobson, K. Nanoclustering as a dominant feature of plasma membrane organization. J. Cell Sci. 2014, 127, 4995–5005. [Google Scholar] [CrossRef]
- Garwood, E.R.; Kumar, A.S.; Baehner, F.L.; Moore, D.H.; Au, A.; Hylton, N.; Flowers, C.I.; Garber, J.; Lesnikoski, B.A.; Hwang, E.S.; et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res. Treat. 2010, 119, 137–144. [Google Scholar] [CrossRef]
- Adachi, K.; Toyota, M.; Sasaki, Y.; Yamashita, T.; Ishida, S.; Ohe-Toyota, M.; Maruyama, R.; Hinoda, Y.; Saito, T.; Imai, K.; et al. Identification of SCN3B as a novel p53-inducible proapoptotic gene. Oncogene 2004, 23, 7791–7798. [Google Scholar] [CrossRef]
- Agwa, A.J.; Peigneur, S.; Chow, C.Y.; Lawrence, N.; Craik, D.J.; Tytgat, J.; King, G.F.; Henriques, S.T.; Schroeder, C.I. Gating modifier toxins isolated from spider venom: Modulation of voltage-gated sodium channels and the role of lipid membranes. J. Biol. Chem. 2018, 293, 9041–9052. [Google Scholar] [CrossRef]
- Amara, S.; Ivy, M.T.; Myles, E.L.; Tiriveedhi, V. Sodium channel γENaC mediates IL-17 synergized high salt induced inflammatory stress in breast cancer cells. Cell. Immunol. 2016, 302, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Hon, D.; Robertson, F.; Robertson, G.; Owen, S.; Rogers, G.; Lydon, E.; Lee, N.; Hales, T. Potent inhibition by ropivacaine of metastatic colon cancer SW620 cell invasion and Na V 1.5 channel function. Br. J. Anaesth. 2014, 113, i39–i48. [Google Scholar] [CrossRef]
- Batcioglu, K.; Uyumlu, A.B.; Satilmis, B.; Yildirim, B.; Yucel, N.; Demirtas, H.; Onkal, R.; Guzel, R.M.; Djamgoz, M.B. Oxidative Stress in the in vivo DMBA Rat Model of Breast Cancer: Suppression by a Voltage-gated Sodium Channel Inhibitor (RS 100642). Basic Clin. Pharmacol. Toxicol. 2012, 111, 137–141. [Google Scholar] [CrossRef]
- 97. Bergareche, A.; Bednarz, M.; Sánchez, E.; Krebs, C.E.; Ruiz-Martinez, J.; De La Riva, P.; Makarov, V.; Gorostidi, A.; Jurkat-Rott, K.; Marti-Masso, J.F.; et al. SCN4A pore mutation pathogenetically contributes to autosomal dominant essential tremor and may increase susceptibility to epilepsy. Hum. Mol. Genet. 2015, 24, 7111–7120. [Google Scholar] [CrossRef]
- Biswas, A.K.; Acharyya, S. Understanding cachexia in the context of metastatic progression. Nat. Rev. Cancer 2020, 20, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Mollinedo, F.; Gajate, C.; Martín-Santamaría, S.; Gago, F. ET-18-OCH3 (edelfosine): A selective antitumour lipid targeting apoptosis through intracellular activation of Fas/CD95 death receptor. Curr. Med. Chem. 2004, 11, 3163–3184. [Google Scholar] [CrossRef]
- Delmas, D.; Aires, V.; Colin, D.J.; Limagne, E.; Scagliarini, A.; Cotte, A.K.; Ghiringhelli, F. Importance of lipid microdomains, rafts, in absorption, delivery, and biological effects of resveratrol. Ann. N. Y. Acad. Sci. 2013, 1290, 90–97. [Google Scholar] [CrossRef]
- Veldman, R.J.; Zerp, S.; Van Blitterswijk, W.J.; Verheij, M. N-hexanoyl-sphingomyelin potentiates in vitro doxorubicin cytotoxicity by enhancing its cellular influx. Br. J. Cancer 2004, 90, 917–925. [Google Scholar] [CrossRef]
- Van Hell, A.J.; Melo, M.N.; Van Blitterswijk, W.J.; Gueth, D.M.; Braumuller, T.M.; Pedrosa, L.R.; Song, J.Y.; Marrink, S.J.; Koning, G.A.; Jonkers, J.; et al. Defined lipid analogues induce transient channels to facilitate drug-membrane traversal and circumvent cancer therapy resistance. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Novotný, J.; Janůsová, B.; Novotný, M.; Hrabálek, A.; Vavrova, K. Short-Chain Ceramides Decrease Skin Barrier Properties. Ski. Pharmacol. Physiol. 2009, 22, 22–30. [Google Scholar] [CrossRef]
- Grösch, S.; Schiffmann, S.; Geisslinger, G. Chain length-specific properties of ceramides. Prog. Lipid Res. 2012, 51, 50–62. [Google Scholar] [CrossRef]
- Chiantia, S.; Kahya, N.; Schwille, P. Raft domain reorganization driven by short-and long-chain ceramide: A combined AFM and FCS study. Langmuir 2007, 23, 7659–7665. [Google Scholar] [CrossRef] [PubMed]
- van Lummel, M.; van Blitterswijk, W.J.; Vink, S.R.; Veldman, R.J.; van der Valk, M.A.; Schipper, D.; Dicheva, B.M.; Eggermont, A.M.; Hagen, T.L.; Verheij, M.; et al. Enriching lipid nanovesicles with short-chain glucosylceramide improves doxorubicin delivery and efficacy in solid tumors. FASEB J. 2011, 25, 280–289. [Google Scholar] [CrossRef] [PubMed]
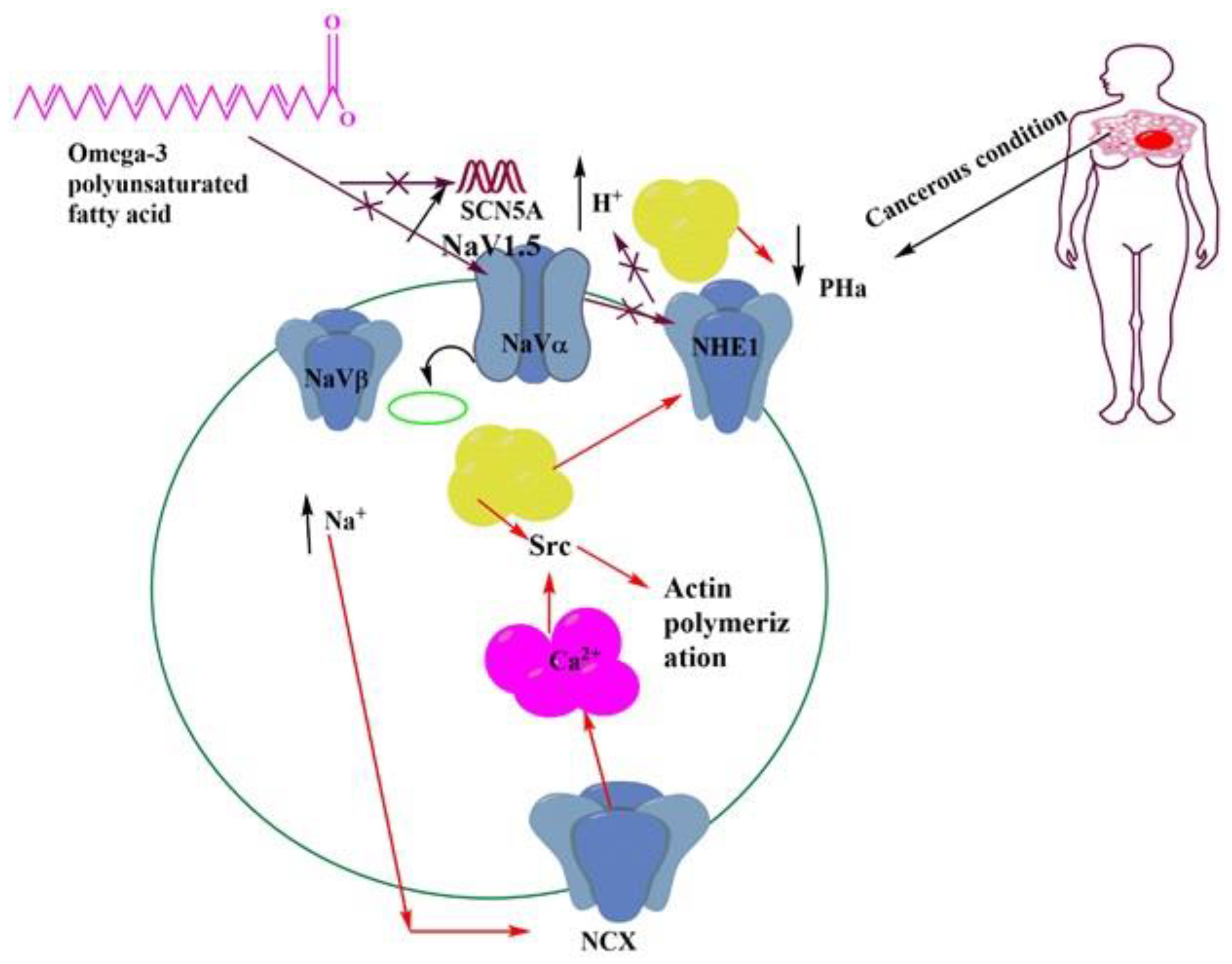
| Name of Sodium Channel | Type of Cancers | Expression | Mechanism |
|---|---|---|---|
| Nav1.5 | Breast | Up-regulated mRNA, and protein | Increase invasion by increasing Src activity and allosteric activation of NHE-1 |
| Nav1.5 | Colorectal | Up-regulated mRNA, and protein | Increase invasion by the regulation of transcriptional pathway Pka/Erk |
| Nav1.5 | Ovarian | Up-regulated mRNA, and protein | Increase migration, invasion, and proliferation by increasing the window currently |
| Nav1.6 | Cervix | Up-regulated mRNA, and protein | Increased invasion and boosted activity of MMP2 and NHE-1 |
| Nav1.7 | Prostate | Up-regulated mRNA, and protein | Cell motility increased via galvanotaxis |
| Nav1.7 | Lung | Up-regulated mRNA, and protein | Increased invasion and dysregulation of sodium homeostasis, an increase in sodium ion, and depolarization of cell membrane |
| Navβ1 | Breast | Down-regulated mRNA, protein | Increased invasion by decreasing cell adhesion and facilitating cell migration |
| Navβ1 | Lung | Down-regulated mRNA, protein | Increased invasion by decreasing cell adhesion and facilitating cell migration |
| Navβ2 | Prostate | Up-regulated mRNA, and protein | Increased invasion by promotion of bipolar cell morphology enhanced cell adhesion |
| Navβ3 | Bone | Up-regulated mRNA, and protein | Increased apoptosis by increasing the p53 dependent apoptotic pathway |
| Navβ4 | Breast | Down-regulated mRNA, protein | Increased invasion by enhancing RhoA activity |
| Name of PUFA | Type of Cancer | Cell Line and Animal Study | Effective Dose |
|---|---|---|---|
| Eicosapentaenoic acid | B lymphocyte (lymphoblast) | U 266 | 50 μM |
| Docosahexaenoic acid | B lymphocyte (lymphoblast) | U 266 | 100 μM |
| Eicosapentaenoic acid | Plasma cell leukemia | L363 | 50 μM |
| Docosahexaenoic acid | Plasma cell leukemia | L363 | 100 μM |
| Arachidonic acid | Prostate cancer | PC3 | 50 μM |
| Eicosapentaenoic acid | Prostate cancer | PC3 | 1 μM |
| Docosahexaenoic acid | Breast cancer | MDA-MB-231 | 20 μM |
| Docosahexaenoic acid | Non-small cell lung cancer | A459 | 25 μM |
| Eicosapentaenoic acid | Colon cancer | CR HT-29 | 20 μmol/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukerjee, S.; Saeedan, A.S.; Ansari, M.N.; Singh, M. Polyunsaturated Fatty Acids Mediated Regulation of Membrane Biochemistry and Tumor Cell Membrane Integrity. Membranes 2021, 11, 479. https://doi.org/10.3390/membranes11070479
Mukerjee S, Saeedan AS, Ansari MN, Singh M. Polyunsaturated Fatty Acids Mediated Regulation of Membrane Biochemistry and Tumor Cell Membrane Integrity. Membranes. 2021; 11(7):479. https://doi.org/10.3390/membranes11070479
Chicago/Turabian StyleMukerjee, Souvik, Abdulaziz S. Saeedan, Mohd. Nazam Ansari, and Manjari Singh. 2021. "Polyunsaturated Fatty Acids Mediated Regulation of Membrane Biochemistry and Tumor Cell Membrane Integrity" Membranes 11, no. 7: 479. https://doi.org/10.3390/membranes11070479
APA StyleMukerjee, S., Saeedan, A. S., Ansari, M. N., & Singh, M. (2021). Polyunsaturated Fatty Acids Mediated Regulation of Membrane Biochemistry and Tumor Cell Membrane Integrity. Membranes, 11(7), 479. https://doi.org/10.3390/membranes11070479