Transparent Ion-Exchange Membrane Exhibiting Intense Emission under a Specific pH Condition Based on Polypyridyl Ruthenium(II) Complex with Two Imidazophenanthroline Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures
2.2. Preparation of the Films
2.3. DFT Calculation
3. Results and Discussion
3.1. Determination of the Loaded Molecules into Nafion by UV-Vis Spectroscopy
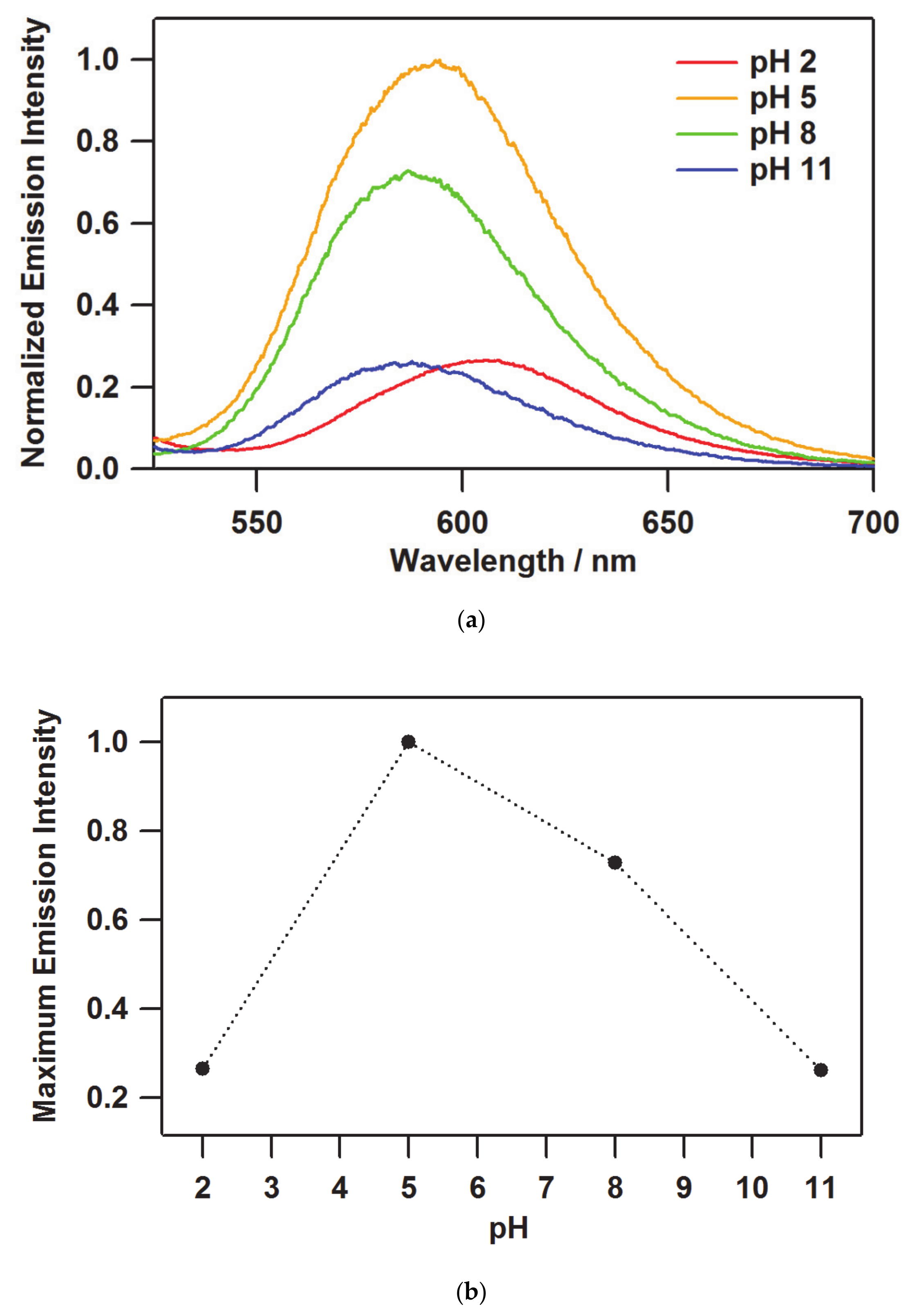
3.2. Emission Spectroscopy and Photophysical Property of the Film
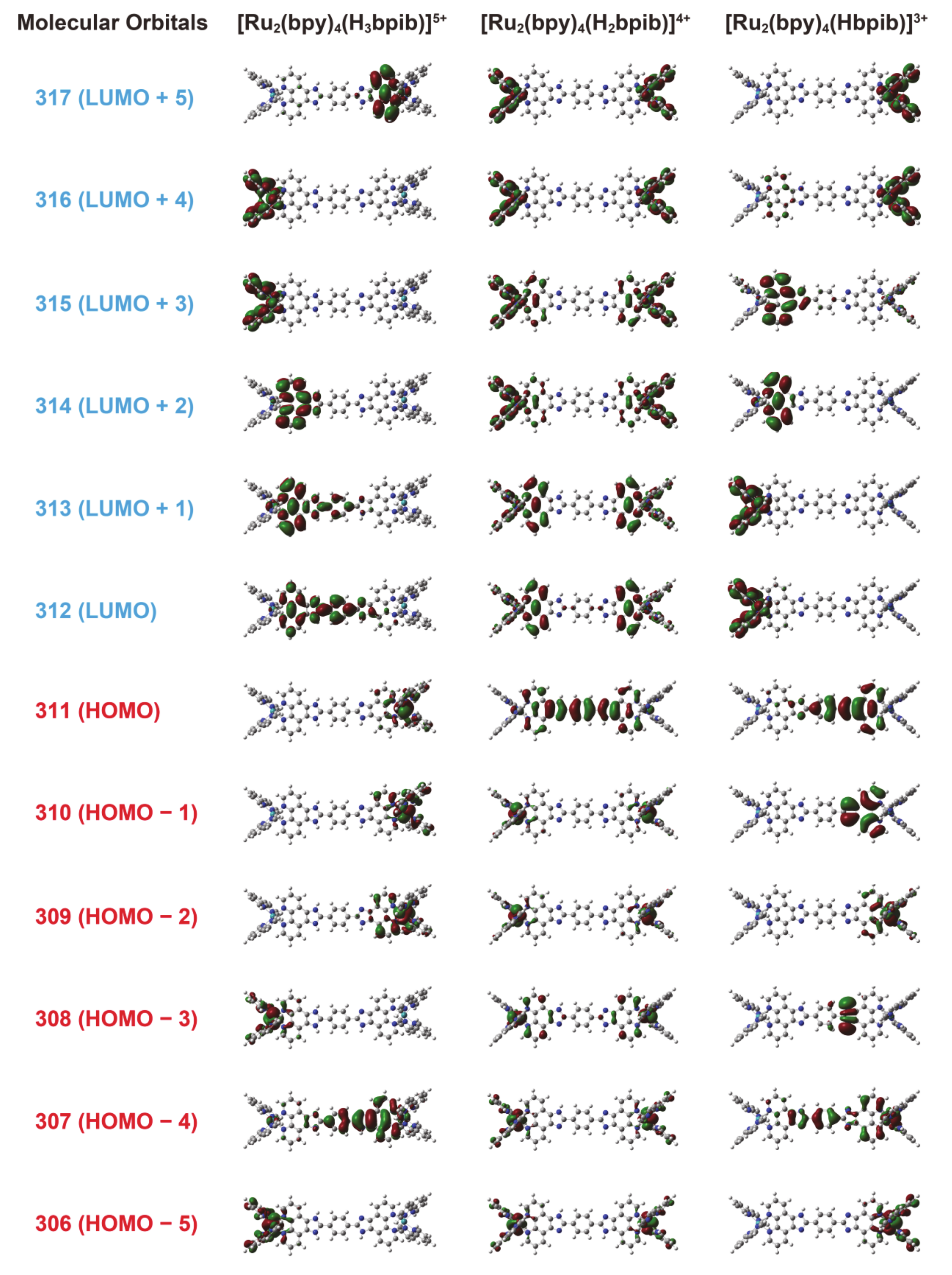
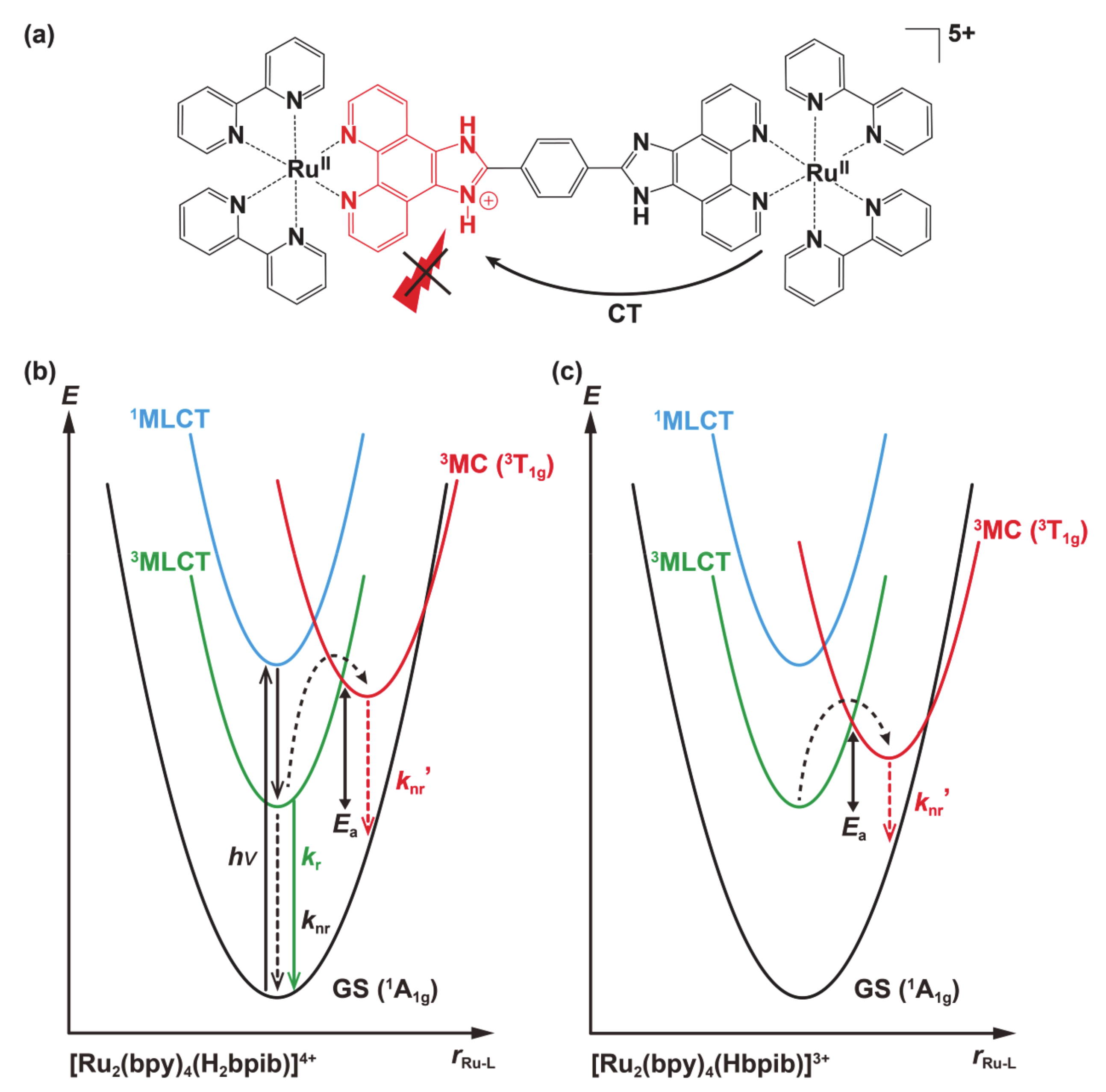
3.3. DFT Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Kitai, A. (Ed.) Luminescent Materials and Applications; Wiley: Chichester, UK, 2008. [Google Scholar]
- Choi, M.K.; Yang, J.; Hyeon, T.; Kim, D.-H. Flexible quantum dot light-emitting diodes for next-generation displays. npj Flex. Electron. 2018, 2, 10. [Google Scholar] [CrossRef]
- Alvarado, S.R.; Guo, Y.; Ruberu, T.P.A.; Tavasoli, E.; Vela, J. Inorganic chemistry solutions to semiconductor nanocrystal problems. Coord. Chem. Rev. 2014, 263–264, 182–196. [Google Scholar] [CrossRef]
- Liu, C.; Qian, B.; Ni, R.; Liu, X.; Qiu, J. 3D printing of multicolor luminescent glass. RSC Adv. 2018, 8, 31564–31567. [Google Scholar] [CrossRef]
- Seshadri, M.; de Carvalho dos Anjos, V.; Bell, M.J.V. Luminescence: An Outlook on the Phenomena and Their Applications; Thitumalai, J., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Karbowiak, M.; Cichos, J.; Buczko, K. Interaction of lanthanide β-diketonate complexes with polyvinylpyrrolidone: Proton-controlled switching of Tb3+ luminescence. J. Phys. Chem. B 2014, 118, 226–239. [Google Scholar] [CrossRef]
- Deschamps, J.; Potdevin, A.; Caperaa, N.; Chadeyron, G.; Therias, S.; Mahiou, R. A promising way to obtain large, luminescent and transparent thick films suitable for optical devices. New J. Chem. 2010, 34, 385–387. [Google Scholar] [CrossRef]
- Kamebuchi, H.; Yoshioka, T.; Tadokoro, M. Development of tuneable green-to-red emitting transparent film based on Nafion with TbIII/EuIII β-diketonate complexes modulated by pH and proton flow. Mater. Adv. 2020, 1, 569–573. [Google Scholar] [CrossRef]
- Kamebuchi, H.; Fujimura, Y.; Yoshioka, T.; Okazawa, A.; Tadokoro, M.; Kojima, N. Multicolor emission and photophysical properties of proton-responsive cyclometallated iridium(III) complex in transparent cation-exchange membrane. Crystals 2020, 10, 653. [Google Scholar] [CrossRef]
- Sainz-Gonzalo, F.J.; Popovici, C.; Casimiro, M.; Raya-Barón, A.; López-Ortiz, F.; Fernández, I.; Fernández-Sánchez, J.F.; Fernández-Gutiérrez, A. A novel tridentate bis(phosphinic acid)phosphine oxide based europium(III)-selective Nafion membrane luminescent sensor. Analyst 2013, 138, 6134–6143. [Google Scholar] [CrossRef]
- Shilov, S.M.; Garvronskaya, K.A.; Borisov, A.N.; Pak, V.N. Sensitization of Tb3+ luminescence with polypyridyl ligands in a Nafion membrane. Russ. J. Gen. Chem. 2008, 78, 1775–1779. [Google Scholar] [CrossRef]
- Petushkov, A.A.; Shilov, S.M.; Puzyk, M.V.; Pak, V.N. Luminescence of europium(III) β-diketonate complexes in a Nafion membrane. Russ. J. Phys. Chem. A 2007, 81, 612–616. [Google Scholar] [CrossRef]
- Watanabe, C.N.; Gehlen, M.H. Luminescence quenching of uranyl ion adsorbed in nafion membrane by alcohols and vinyl monomers. J. Photochem. Photobiol. A Chem. 2003, 156, 65–68. [Google Scholar] [CrossRef]
- Chan, C.-M.; Lo, W.; Wong, K.-Y. Application of a luminescence-based pH optrode to monitoring of fermentation by Klebsiella pneumoniae. Biosens. Bioelectron. 2000, 15, 7–11. [Google Scholar] [CrossRef]
- Chan, C.-M.; Fung, C.-S.; Wong, K.-Y.; Lo, W. Evaluation of a luminescent ruthenium complex immobilized inside Nafion as optical pH sensor. Analyst 1998, 123, 1843–1847. [Google Scholar] [CrossRef]
- Lopez, M.; Birch, D.J.S. Enhanced non-radiative energy transfer from excited uranyl to metal ion acceptors in dried Nafion. J. Lumin. 1997, 71, 221–228. [Google Scholar] [CrossRef]
- Blatt, E.; Launikonis, A.; Mau, A.W.-H.; Sasse, W.H.F. Luminescence probe studies of pyrene and two charged derivatives in Nafion. Aust. J. Chem. 1987, 40, 1–12. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K.; Chen, Q. Parallel cylindrical water nanochannels in Nafion fuel-cell membranes. Nat. Mater. 2008, 7, 75–83. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Heitner-Wirguin, C. Recent advances in perfluorinated ionomer membranes: Structure, properties and applications. J. Membr. Sci. 1996, 120, 1–33. [Google Scholar] [CrossRef]
- Zawodzinski, T.A., Jr.; Neeman, M.; Sillerud, L.O.; Gottesfeld, S. Determination of water diffusion coefficients in perfluorosulfonate ionomeric membranes. J. Phys. Chem. 1991, 95, 6040–6044. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Gierke, T.D. Ion transport and clustering in nafion perfluorinated membranes. J. Membr. Sci. 1983, 13, 307–326. [Google Scholar] [CrossRef]
- Ogata, Y.; Kawaguchi, D.; Yamada, N.L.; Tanaka, K. Multistep thickening of Nafion thin films in water. ACS Macro Lett. 2013, 2, 856–859. [Google Scholar] [CrossRef]
- Kamebuchi, H.; Enomoto, M.; Kojima, N. Nafion: Properties, Structure and Applications; Sutton, A., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 119–140. [Google Scholar]
- Funasako, Y.; Takaki, A.; Inokuchi, M.; Mochida, T. Photo-, thermo-, and piezochromic Nafion film incorporating cationic spiropyran. Chem. Lett. 2016, 45, 1397–1399. [Google Scholar] [CrossRef]
- Hosokawa, H.; Funasako, Y.; Mochida, T. Colorimetric solvent indicators based on Nafion membranes incorporating nickel(II)-chelate complexes. Chem. Eur. J. 2014, 20, 15014–15020. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, A.; Ono, Y.; Kojima, N.; Matsumura, D.; Yokoyama, T. Spin crossover complex film, [FeII(H-trz)3]-Nafion, with a spin transition around room temperature. Chem. Lett. 2003, 32, 336–337. [Google Scholar] [CrossRef]
- Nakamoto, A.; Kamebuchi, H.; Enomoto, M.; Kojima, N. Study on the spin crossover transition and glass transition for Fe(II) complex film, [Fe(II)(H-triazole)3]@Nafion, by means of Mössbauer spectroscopy. Hyperfine Interact. 2012, 205, 41–45. [Google Scholar] [CrossRef]
- Kamebuchi, H.; Jo, T.; Shimizu, H.; Okazawa, A.; Enomoto, M.; Kojima, N. Development of pH-sensitive spin-crossover iron(II) complex films, [FeII(diAMsar)]-Nafion: Manipulation of the spin state by proton concentration. Chem. Lett. 2011, 40, 888–889. [Google Scholar] [CrossRef]
- Tozawa, M.; Ohkoshi, S.; Kojima, N.; Hashimoto, K. Ion-exchange synthesis and magneto-optical spectra of colored magnetic thin films composed of metal(II) hexacyanochromate(III). Chem. Commun. 2003, 1204–1205. [Google Scholar] [CrossRef]
- Kosaka, W.; Tozawa, M.; Hashimoto, K.; Ohkoshi, S. Synthesis and superparamagnetic property of a Co-Cr Prussian blue analogue nanoparticles inside Nafion membrane. Inorg. Chem. Commun. 2006, 9, 920–922. [Google Scholar] [CrossRef]
- Hauser, A.; Adler, J.; Gütlich, P. Light-induced excited spin state trapping (LIESST) in [Fe(2-mephen)3]2+ embedded in polymer matrices. Chem. Phys. Lett. 1988, 152, 468–472. [Google Scholar] [CrossRef]
- Elvin, C.M.; Brownlee, A.G.; Huson, M.G.; Tebb, T.A.; Kim, M.; Lyons, R.E.; Vuocolo, T.; Liyou, N.E.; Hughes, T.C.; Ramshaw, J.A.M.; et al. The development of photochemically crosslinked native fibrinogen as a rapidly formed and mechanically strong surgical tissue sealant. Biomaterials 2009, 30, 2059–2065. [Google Scholar] [CrossRef]
- Funato, Y.; Yoshida, A.; Hirata, Y.; Hashizume, O.; Yamazaki, D.; Miki, H. The oncogenic PRL protein causes acid addiction of cells by stimulating lysosomal exocytosis. Dev. Cell 2020, 55, 387–397. [Google Scholar] [CrossRef]
- Booth, I.R. Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 1985, 49, 359–378. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Rosen, B.P.; Alger, J.R.; Macnab, R.M. pH homeostasis in Escherichia coli: Measurement by 31P nuclear magnetic resonance of methylphosphonate and phosphate. Proc. Natl. Acad. Sci. USA 1981, 78, 6271–6275. [Google Scholar] [CrossRef]
- Padan, E.; Zilberstein, D.; Schuldiner, S. pH homeostasis in bacteria. Biochim. Biophys. Acta 1981, 650, 151–166. [Google Scholar] [CrossRef]
- Padan, E.; Zilberstein, D.; Rottenberg, H. The proton electrochemical gradient in Escherichia coli cells. Eur. J. Biochem. 1976, 63, 533–541. [Google Scholar] [CrossRef]
- Chao, H.; Ye, B.-H.; Zhang, Q.-L.; Ji, L.-N. A luminescent pH sensor based on a diruthenium(II) complex: ‘off–on–off’ switching via the protonation/deprotonation of an imidazole-containing ligand. Inorg. Chem. Commun. 1999, 2, 338–340. [Google Scholar] [CrossRef]
- Chao, H.; Li, R.-H.; Jiang, C.-W.; Li, H.; Ji, L.-N.; Li, X.-Y. Mono-, di- and tetra-nuclear ruthenium(II) complexes containing 2,2’-p-phenylenebis(imidazo[4,5-f]phenanthroline): Synthesis, characterization and third-order non-linear optical properties. J. Chem. Soc. Dalton Trans. 2001, 12, 1920–1926. [Google Scholar] [CrossRef]
- Seth, S.K.; Purkayastha, P. Unusually large singlet oxygen (1O2) production by very weakly emissive pyrene-functionalized iridium(III) complex: Interplay between excited 3ILCT/3IL and 3MLCT states. Eur. J. Inorg. Chem. 2020, 2020, 2990–2997. [Google Scholar] [CrossRef]
- Begantsova, Y.E.; Bochkarev, L.N. Cyclometallated ionic iridium(III) binuclear complexes with a bisphenanthroline bridging ligand: Synthesis and photophysical properties. Russ. J. Gen. Chem. 2017, 87, 1198–1203. [Google Scholar] [CrossRef]
- Das, S.; Seth, S.K.; Gupta, P.; Purkayastha, P. Intriguing interaction of a cyclometalated dinuclear Ir(III) complex bridged by imidazolyl phenanthroline with anionic and cationic lipid vesicles. J. Lumin. 2017, 192, 1196–1202. [Google Scholar] [CrossRef]
- Seth, S.K.; Mandal, S.; Srikanth, K.; Purkayastha, P.; Gupta, P. Electronic description of the photophysics of homo- and heterodinuclear cyclometallated iridium and rhodium complexes. Eur. J. Inorg. Chem. 2017, 2017, 873–880. [Google Scholar] [CrossRef]
- Seth, S.K.; Gupta, P.; Purkayastha, P. Efficiency of photoinduced electron transfer in mono- and di-nuclear iridium complexes: A comparative study. New J. Chem. 2017, 41, 6540–6545. [Google Scholar] [CrossRef]
- Yin, H.-J.; Liu, Y.-J.; Gao, J.; Wang, K.-Z. A highly sensitive and selective visible-light excitable luminescent probe for singlet oxygen based on a dinuclear ruthenium complex. Dalton Trans. 2017, 46, 3325–3331. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.K.; Mandal, S.; Purkayastha, P.; Gupta, P. Cyclometalated mono and dinuclear rhodium(III) and iridium(III) complexes with imidazolyl phenanthrolines: Synthesis and, photophysical and electrochemical characterization. Polyhedron 2015, 95, 14–23. [Google Scholar] [CrossRef]
- Mondal, S.; Seth, S.K.; Gupta, P.; Purkayastha, P. Ultrafast photoinduced electron transfer between carbon nanoparticles and cyclometalated rhodium and iridium complexes. J. Phys. Chem. C 2015, 119, 25122–25128. [Google Scholar] [CrossRef]
- Singaravadivel, S.; Velayudham, M.; Babu, E.; Mareeswaran, P.M.; Lu, K.-L.; Rajagopal, S. Sensitized near-infrared luminescence from NdIII, YbIII and ErIII complexes by energy-transfer from ruthenium 1,3-bis([1,10]phenanthroline-[5,6-d]-imidazol-2 –yl)benzene. J. Fluoresc. 2013, 23, 1167–1172. [Google Scholar] [CrossRef]
- Svensson, F.R.; Anderson, J.; Åmand, H.L.; Lincoln, P. Effects of chirality on the intracellular localization of binuclear ruthenium(II) polypyridyl complexes. J. Biol. Inorg. Chem. 2012, 17, 565–571. [Google Scholar] [CrossRef]
- Saha, D.; Das, S.; Mardanya, S.; Baitalik, S. Structural characterization and spectroelectrochemical, anion sensing and solvent dependence photophysical studies of a bimetallic Ru(II) complex derived from 1,3-di(1H-imidazo[4,5-f][1,10]phenanthroline-2-yl)benzene. Dalton Trans. 2012, 41, 8886–8898. [Google Scholar] [CrossRef]
- Srinivasan, P.; Mason, R.H.; MacNeil, J.R.G.; MacLean, B.J. Metal-metal communication in diruthenium complexes of the bridging ligand bis(imidazo[4,5-f][1,10]phenanthroline). Inorg. Chim. Acta 2011, 366, 116–121. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.; Zhou, F.; Weng, L.-P.; Guo, L.-T.; Chen, M.; Chao, H.; Ji, L.-N. pH responsive luminescent switches of ruthenium(II) complexes containing two imidazole groups: Synthesis, spectroscopy, electrochemistry and theoretical calculations. Inorg. Chim. Acta 2009, 362, 4960–4966. [Google Scholar] [CrossRef]
- Han, M.-J.; Gao, L.-H.; Lü, Y.-Y.; Wang, K.-Z. Ruthenium(II) complex of Hbopip: Characterization, pH-induced luminescence “off-on-off” switch, and avid binding to DNA. J. Phys. Chem. B 2006, 110, 2364–2371. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Robinson, R.A. Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Fuentealba, P.; Preuss, H.; Stoll, H.; Von Szentpály, L. A proper account of core-polarization with pseudopotentials: Single valence-electron alkali compounds. Chem. Phys. Lett. 1982, 89, 418–422. [Google Scholar] [CrossRef]
- Naughton, E.M.; Zhang, M.; Troya, D.; Brewer, K.J.; Moore, R.B. Size dependent ion-exchange of large mixed-metal complexes into Nafion® membranes. Polym. Chem. 2015, 6, 6870–6879. [Google Scholar] [CrossRef]
- Gholamkhass, B.; Koike, K.; Negishi, N.; Hori, H.; Sano, T.; Takeuchi, K. Adjacent- versus remote-site electron injection in TiO2 surfaces modified with binuclear ruthenium complexes. Inorg. Chem. 2003, 42, 2919–2932. [Google Scholar] [CrossRef]
- Durham, B.; Caspar, J.V.; Nagle, J.K.; Meyer, T.J. Photochemistry of Ru(bpy)32+. J. Am. Chem. Soc. 1982, 104, 4803–4810. [Google Scholar] [CrossRef]
- Kaneko, M.; Hayakawa, S. Application of polymer-embeded tris(2,2’bipyridine-ruthenium(II) to photodetection of oxygen. J. Macromol. Sci. Chem. 1988, 25, 1255–1261. [Google Scholar] [CrossRef]
- Reisfeld, R. Spectroscopy and applications of molecules in glasses. J. Non-Cryst. Solids 1990, 121, 254–266. [Google Scholar] [CrossRef]
- Thompson, D.W.; Fleming, C.N.; Myron, B.D.; Meyer, T.J. Rigid medium stabilization of metal-to-ligand charge transfer excited states. J. Phys. Chem. B 2007, 111, 6930–6941. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Knight, T.E.; Stewart, D.J.; Brennaman, M.K.; Meyer, T.J. Rigid medium effects on photophysical properties of MLCT excited states of polypyridyl Os(II) complexes in polymerized poly(ethylene glycol)dimethacrylate monoliths. J. Phys. Chem. A 2014, 118, 10326–10332. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Fang, Z.; Brennaman, M.K.; Meyer, T.J. Long-range photoinduced electron transfer dynamics in rigid media. Phys. Chem. Chem. Phys. 2014, 16, 4880–4891. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Stewart, D.J.; Knight, T.E.; Fang, Z.; Brennaman, M.K.; Meyer, T.J. Excited-state dynamics in rigid media: Evidence for long-range energy transfer. J. Phys. Chem. B 2013, 117, 3428–3438. [Google Scholar] [CrossRef]
- Knight, T.E.; Goldstein, A.P.; Brennaman, M.K.; Cardolaccia, T.; Pandya, A.; DeSimone, J.M.; Meyer, T.J. Influence of the fluid-to-film transition on photophysical properties of MLCT excited states in a polymerizable dimethacrylate fluid. J. Phys. Chem. B 2011, 115, 64–70. [Google Scholar] [CrossRef]
- Grigg, R.; Norbert, W.D.J.A. Luminescent pH sensors based on di(2,2’-bipyridyl) (5,5’-diaminomethyl-2,2’-bipyridyl)-ruthenium(II) complexes. J. Chem. Soc. Chem. Commun. 1992, 18, 1300–1302. [Google Scholar] [CrossRef]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; von Zelewsky, A. Ru(II) polypyridyne complexes: Photophysics, photochemistry, electrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar] [CrossRef]
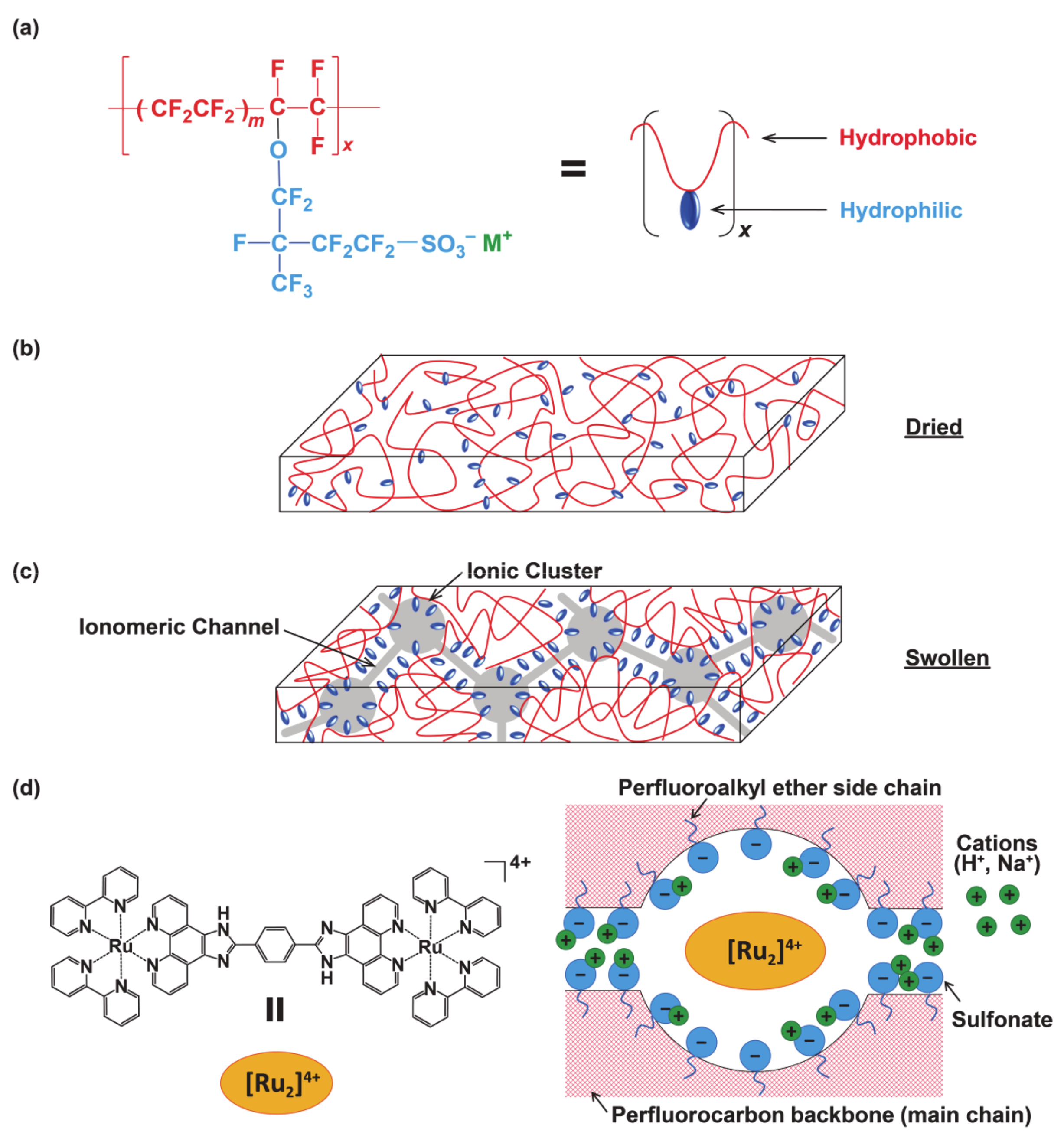
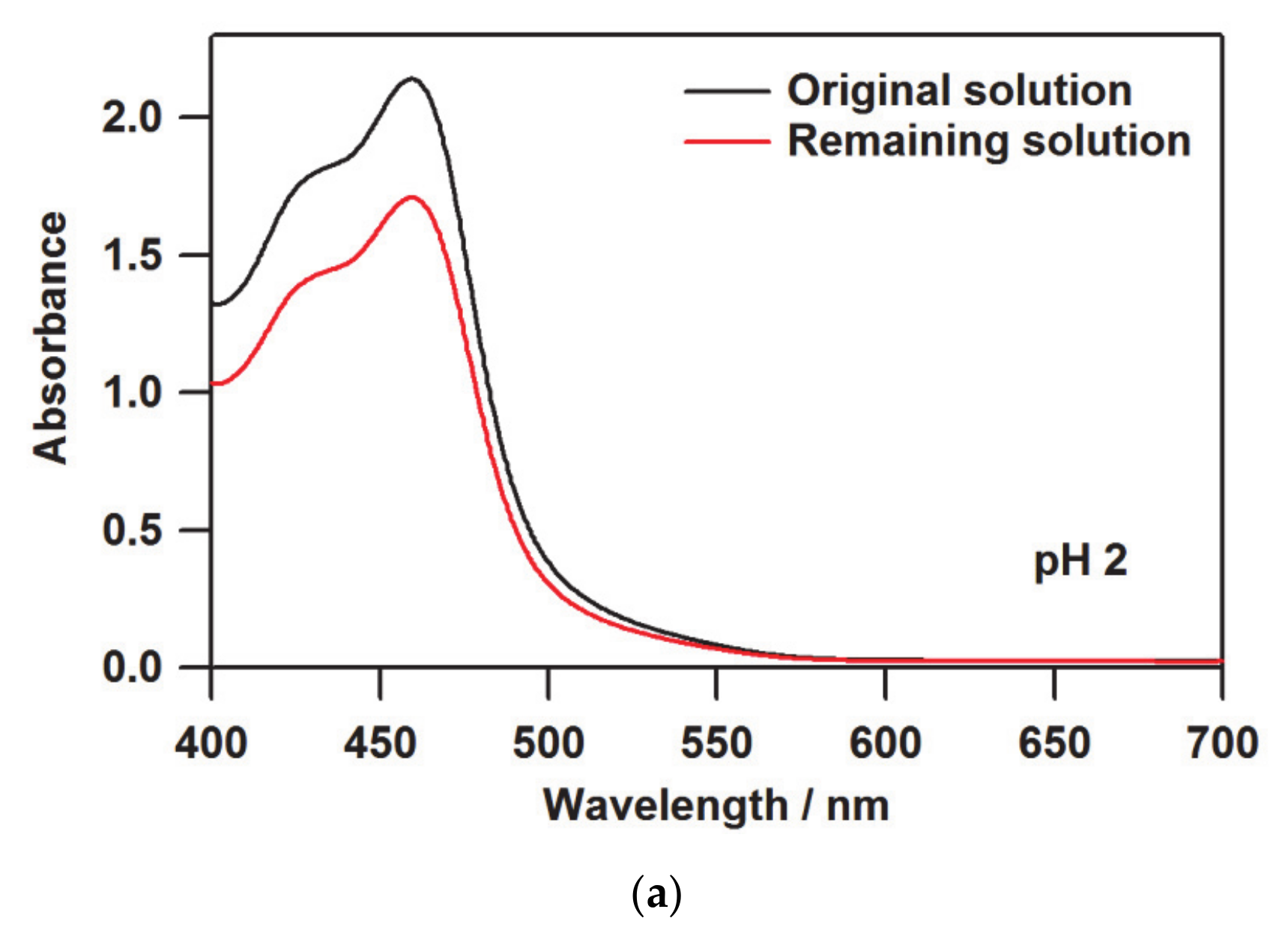
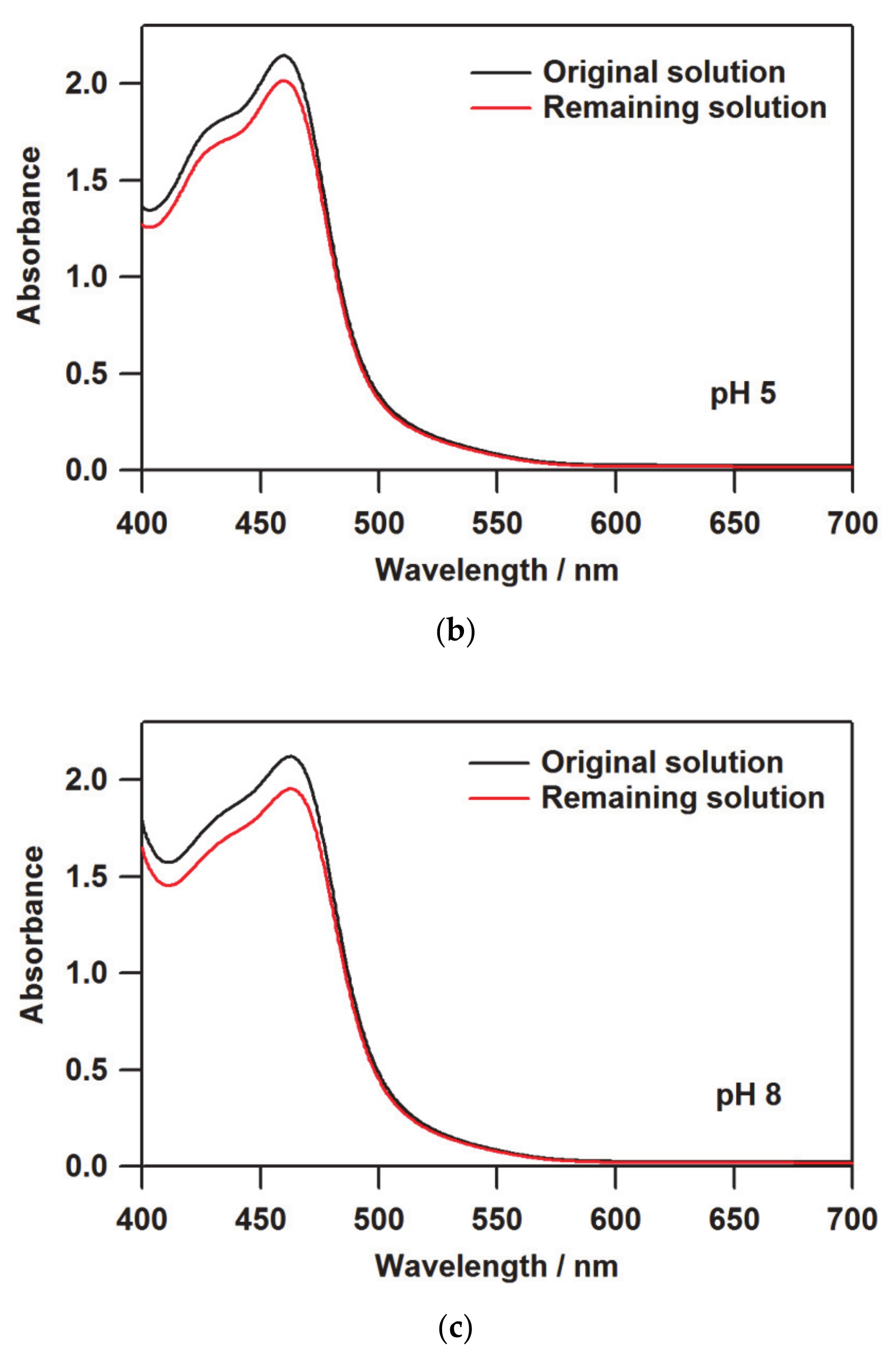

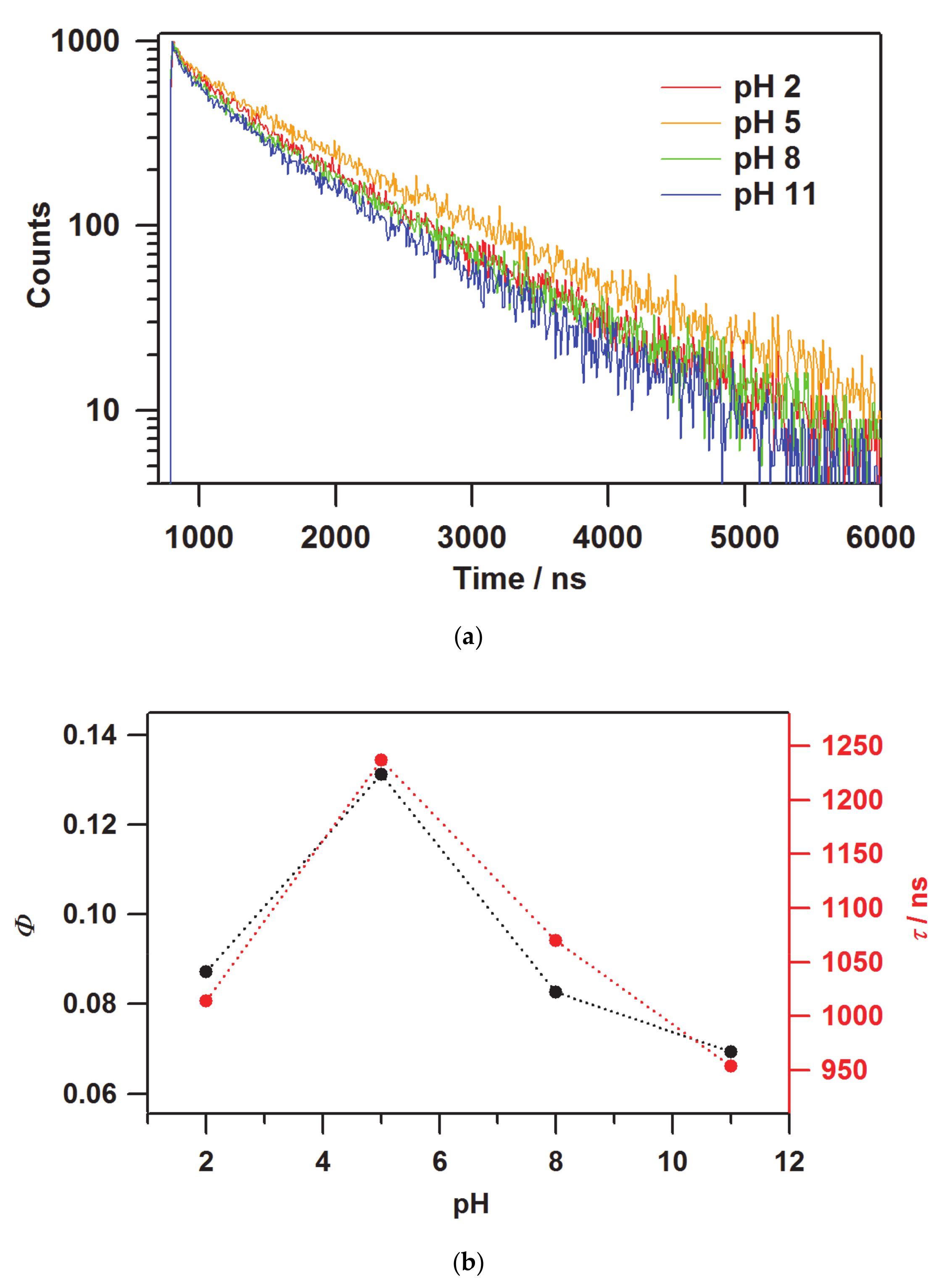
| pH | A/A0 a | Loaded Quantity/10−7 mol g−1 b | Ratio/% c | I(380) d |
|---|---|---|---|---|
| 2 | 0.798 | 4.429 | 0.049 | 0.755 |
| 5 | 0.939 | 1.328 | 0.015 | 0.223 |
| 8 | 0.921 | 1.719 | 0.019 | 0.262 |
| 11 | - | - | - | 0.246 |
| pH | λmaxem/nm | Max. Intensity | Φ | τ/ns | kr a/105 s−1 | knr b/105 s−1 |
|---|---|---|---|---|---|---|
| 2 | 608.2 | 0.265 | 0.087 | 2,521,014 | 0.86 | 9.00 |
| 5 | 594.4 | 1 | 0.131 | 2,681,237 | 1.06 | 7.03 |
| 8 | 586.4 | 0.729 | 0.083 | 1,991,070 | 0.77 | 8.57 |
| 11 | 587.6 | 0.263 | 0.069 | 208,954 | 0.73 | 9.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamebuchi, H.; Tamaki, S.; Okazawa, A.; Kojima, N. Transparent Ion-Exchange Membrane Exhibiting Intense Emission under a Specific pH Condition Based on Polypyridyl Ruthenium(II) Complex with Two Imidazophenanthroline Groups. Membranes 2021, 11, 400. https://doi.org/10.3390/membranes11060400
Kamebuchi H, Tamaki S, Okazawa A, Kojima N. Transparent Ion-Exchange Membrane Exhibiting Intense Emission under a Specific pH Condition Based on Polypyridyl Ruthenium(II) Complex with Two Imidazophenanthroline Groups. Membranes. 2021; 11(6):400. https://doi.org/10.3390/membranes11060400
Chicago/Turabian StyleKamebuchi, Hajime, Satoshi Tamaki, Atsushi Okazawa, and Norimichi Kojima. 2021. "Transparent Ion-Exchange Membrane Exhibiting Intense Emission under a Specific pH Condition Based on Polypyridyl Ruthenium(II) Complex with Two Imidazophenanthroline Groups" Membranes 11, no. 6: 400. https://doi.org/10.3390/membranes11060400
APA StyleKamebuchi, H., Tamaki, S., Okazawa, A., & Kojima, N. (2021). Transparent Ion-Exchange Membrane Exhibiting Intense Emission under a Specific pH Condition Based on Polypyridyl Ruthenium(II) Complex with Two Imidazophenanthroline Groups. Membranes, 11(6), 400. https://doi.org/10.3390/membranes11060400






