1. Introduction
Ultrafiltration is an established pressure-driven membrane filtration process. These membranes are capable of retaining species in the size range 2000 to 500,000 Da. Rejected species include proteins, enzymes, DNA, virus particles, polymers and colloidal particles [
1]. Though ultrafiltration is considered a size-based separation process, numerous studies have indicated the importance of membrane–solute interactions as well as solution conditions [
2]. In fact, by tailoring the membrane pore surface properties, one can tune membrane selectivity [
3,
4].
More recently, there has been growing interest in developing membranes whose performance can be modulated by environmental conditions. These stimuli-responsive membranes respond to an external stimulus [
5,
6,
7]. One could include the responsive groups in the bulk membrane polymer or incorporate the responsive groups after membrane formation [
8]. It is this latter approach that is used here. Numerous external stimuli have been investigated, such as pH, ionic strength, temperature, light and electric fields. Here, our focus is on grafting magnetically responsive brushes from the surface of a base ultrafiltration membrane. Magnetically responsive polymer brushes can induce both localized heating and movement of the grafted polymer chains.
A few investigators have considered the development of magnetically responsive ultrafiltration membranes by modifying the base membrane after formation. Superparamagnetic and paramagnetic nanoparticles are attached to the surface of ultrafiltration membranes. In an oscillating magnetic field, these particles can induce movement due to magnetophoretic actuation and alignment [
9,
10,
11,
12,
13]. In addition, in an oscillating magnetic field, the magnetic nanoparticle can induce heating. Several investigators have included a grafted nanostructure consisting of a thermo-responsive polymer which exhibits a lower critical solution temperature (LCST), such as poly(N-isopropylacrylamide) (PNIPAm). Heating by the nanoparticles can lead to the local temperature exceeding the LCST, which is 32 °C in deionized (DI) water. This causes the grafted nanostructure to dehydrate and collapse, which will lead to changes in the membrane barrier properties [
14,
15].
In recent years, significant efforts have been directed towards grafting PNIPAm to the surface of a base membrane structure. Many investigators have focused on developing ‘smart’ membranes whose properties can be modified by changing the conformation of the grafted nanostructure. As the temperature is increased above the LCST of the grafted nanostructure, the nanostructure dehydrates and collapses, while below its LCST, it is hydrated and swells [
16,
17,
18,
19,
20,
21,
22]. Others have considered the antifouling properties of PNIPAm grafted membrane surfaces. These investigators have considered the ability to detach adsorbed foulants by switching the feed temperature above and below the LCST of the grafted polymer nanostructure [
22,
23,
24,
25,
26,
27,
28,
29].
Our approach to develop magnetically responsive ultrafiltration membranes is different. We have tethered a superparamagnetic nanoparticle to the end of the polymer chains that are grafted from the surface of the base membrane. In our earlier work, we used atom transfer radical polymerization (ATRP) to graft polymer chains from the surface of nanofiltration and microfiltration membranes, followed by attachment of a superparamagnetic nanoparticle to the chain end. In a magnetic field, the particles experience a force and torque. Further, in an oscillating magnetic field, the particles can induce heating. By using PNIPAm as the polymer chain, heating induced in an oscillating magnetic field can cause the grafted PNIPAm nanostructure to collapse at temperatures above its LCST [
30,
31,
32,
33,
34,
35,
36].
In our earlier work, we highlighted the importance of controlling the location of initiator immobilization prior to ATRP [
37]. Often, if the initiator attaches to the pore surface, subsequent polymerization can lead to pore plugging. In this work, regenerated cellulose ultrafiltration membranes with a nominal molecular weight cut-off (NMWCO) of 300 kDa have been modified using ATRP. Three different strategies for initiator immobilization have been considered. In the first, the initiator is immobilized throughout the membrane inner pore surface and outer surface. The second strategy involves blocking the membrane pore surface by filling the pores with glycerol during initiator immobilization. In this way, grafting from the pore surface is suppressed. Finally, we attempt to immobilize the initiator only to the inner pore surface and not the outer membrane surface. After polymerization, a superparamagnetic nanoparticle is attached to the chain end and membrane performance is evaluated in an oscillating magnetic field. The frequency of the oscillating magnetic field is chosen to either maximize movement of the PNIPAm chains or induce heating of the PNIPAm chains, and hence collapse of the grafted nanostructure.
2. Materials and Methods
2.1. Materials
All reagents used were American Chemical Society (ACS) reagent grade or higher unless otherwise specified. Methanol, glycerol and acetonitrile were purchased from MilliporeSigma (Billerica, MA, USA). Triethylamine (TEA), 4-dimethylaminopyridine (DMAP), N,N,N′,N″,N″-pentamethyl diethylenetriamine (PMDETA, 99%), sodium phosphate monobasic (99%), sodium phosphate dibasic (99%), copper (I) bromide and copper (II) bromide (CuBr and CuBr2, 99.999% trace metal basis) were purchased from Sigma-Aldrich (Munich, Germany). N-isopropylacrylamide (NIPAm, >98%, stabilized with mequinol) was procured from TCI America (Boston, MA, USA). α-bromoisobutyryl bromide (BIB, 98%) was purchased from Alfa-Aesar (Ward Hill, MA, USA). 1,2-Epoxy-5-hexene was purchased from Acros Organic (98%, Pittsburgh, PA, USA). Bovine serum albumin (BSA, biotechnology grade) and L-(+)-ascorbic acid (AA) were purchased from Amresco (Solon, OH, USA). 2,2′-bipyridine (Bpy) was purchased from BeanTown Chemical (Hudson, NH, USA). Iron oxide superparamagnetic nanoparticles with a 15 nm core diameter and a 5 nm coating layer functionalized with amine groups were purchased from Ocean Nanotech (San Diego, CA, USA). The deionized (DI) water used in the present investigation was obtained from a Thermo Fisher 18 MΩ Barnstead Smart2Pure system (Schwerte, Germany). Commercially available regenerated cellulose membrane discs (44.5 mm diameter) with a nominal molecular weight cut-off of 300 kDa were obtained from MilliporeSigma.
2.2. Membrane Modification
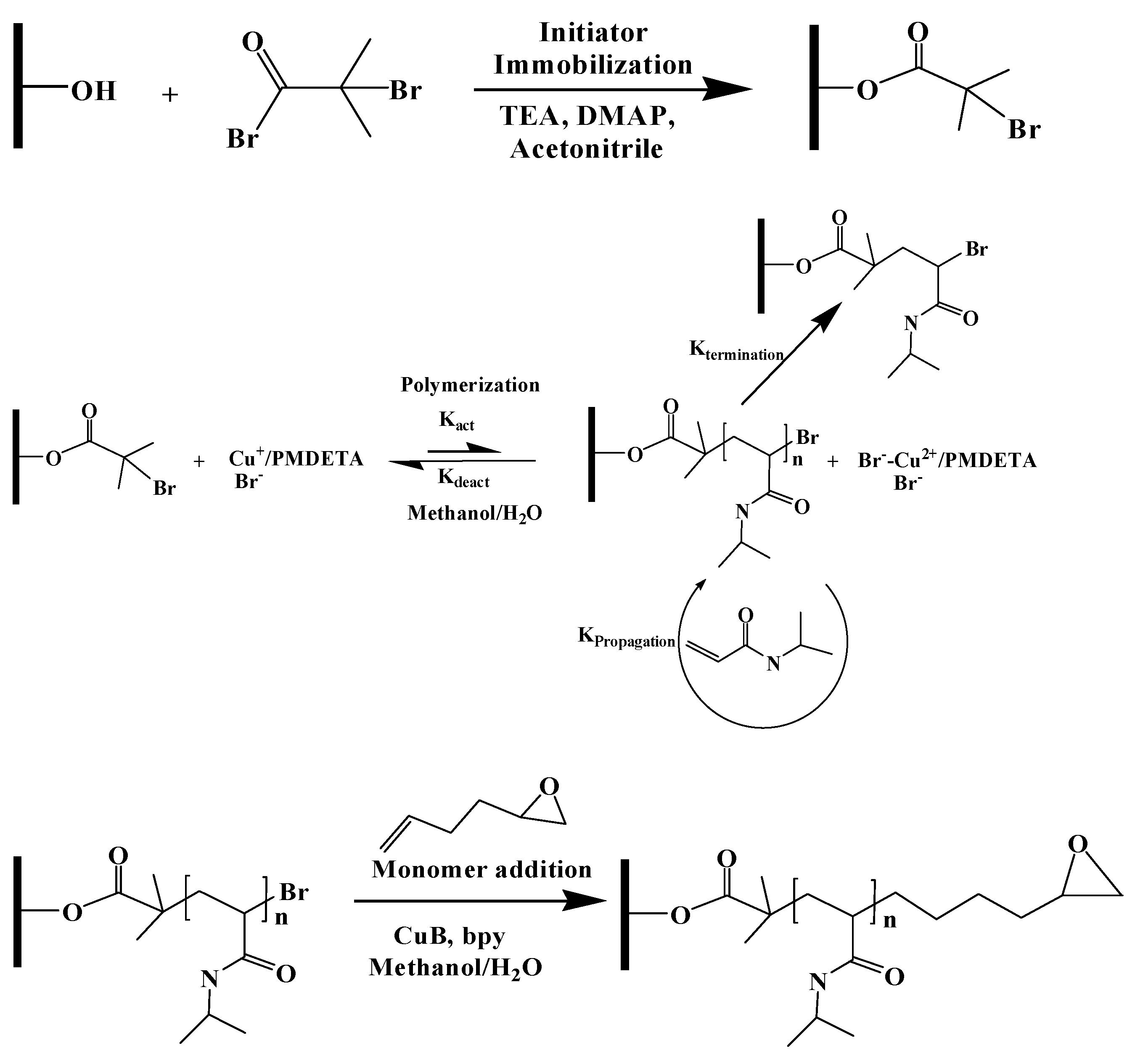
Scheme 1 provides the membrane modification protocol. The first step was initiator immobilization. Three strategies were used: immobilization on the outer membrane surface and inner pore surface, immobilization only on the outer membrane surface and immobilization on the inner pore surface only. The second step was atom transfer radical polymerization, whereby PNIPAm chains were grown from the membrane surface. Next, monomer addition (atom transfer radical addition) was conducted to add 1-2 epoxy-5-hexane to the PNIPAm chain ends. Finally, the amine functionalized superparamagnetic nanoparticles were attached via epoxide ring opening.
2.2.1. Initiator Immobilization
The membranes discs were washed three times in 25 mL of methanol for 20 min and then washed for 30 min in DI water. Acetonitrile was dried over activated molecular sieves prior to use. A washed membrane disc was rinsed 3 times with dry acetonitrile (20 mL) for 20 min. The reactive initiator immobilization solution was prepared by adding 100 mM BIB, 100 mM TEA and 5 mM DMAP to 20 mL of acetonitrile.
The membrane was transferred to the immobilization solution, then quickly sealed in the reaction vessel and the reaction was allowed to proceed at room temperature.
The membrane disc was placed in a stirred cell (8050, MilliporeSigma) and the feed reservoir filled with glycerol. The feed pressure was increased to 1.5 bar and filtration continued until 5 mL of permeate was obtained. The membrane was then removed from the stirred cell and incubated in glycerol overnight. Next, the membrane surface was patted dry with a Kimwipe and then with a roller wiper in order to remove glycerol from the surface. The membrane was then placed on a glass plate, and an ethylenepropylenediene monomer (EPDM) gasket with a circular cutout 35 mm in diameter was centered on the sample. A piece of high-density polyethylene (HDPE) with dimensions matching the gasket was placed on top of the setup and the assembly was secured with binder clips. The initiator immobilization solution was added on top of the membrane and the reaction was allowed to proceed at room temperature.
The outer surface of the barrier layer of the membrane was coated with glycerol. The membrane was then placed in a stirred cell (8050) with the functional layer facedown, as shown in
Figure S1. The reactive monomer solution was added to the stirred cell and the reaction was allowed to proceed at room temperature.
For all three strategies, a reaction time of 1 min was investigated based on our earlier work [
37]. Next, the reaction was quenched with water. The membrane was washed in a 1:1 (
v/v) methanol/water mixture 3 times for 1 h. The modified region was cut out with a 25 mm punch and then incubated overnight in water.
2.2.2. Activator Generated by Electron Transfer (AGET) ATRP
Surface-initiated activator-generated electron transfer (AGET) ATRP was used to graft N-isopropylacrylamide (NIPAm), from the initiator sites on the membrane surface [
37]. The reaction solution was prepared by adding (NIPAm) (0.08 M), CuBr
2 (0.8 mM) and PMDETA (2.4 mM) to a 1:9 (
v/v) methanol/water mixture. Next, ascorbic acid at a concentration of 0.8 mM was added. The color of solution changed from blue to light purple. The reaction solution was introduced to the reaction vessel and the initiator functionalized membrane was added to the solution. The reaction vessel was placed on a shaker table and the reaction was allowed to proceed for 1, 2, 3 or 4 h for membranes with initiator immobilized on the inner pore and outer membrane surface, as well as the outer membrane surface only. For initiator immobilization on the inner pore surface only, 1- and 2-h polymerization times were investigated. After the specified reaction time had elapsed, the polymerization was terminated by adding the membrane into a quenching solution consisting of CuBr
2 (0.22 M) and PMDETA (0.06 M) in a 1:1 (
v/v) methanol/water mixture. The membranes were removed and transferred to the wash solution consisting of a 1:1 (
v/v) methanol/water mixture for 2 h. The membranes were again washed and then stored in DI water for 1 h.
2.2.3. Monomer Addition
The PNIPAm grafted membrane was placed in a Schlenk flask equipped with rubber stoppers. The flask was sealed with parafilm, evacuated and backfilled with argon three times. 1-2 epoxy-5-hexane (17.7 mM) and bpy (28.8 mM) were dissolved in a 1:1 (v/v) methanol/water mixture and purged with nitrogen for 30 min. Next, copper (I) bromide (5.58 mM) was added to the solution with rapid stirring under argon for 15 min. Finally, 30 mL of the reaction solution was added into the Schlenck flask that contained the modified membrane and kept at 50 °C for 24 h. After the reaction, the membrane was washed with a 1:1 (v/v) mixture of methanol/water 3 times for 1 h. The membrane was then immersed in water overnight.
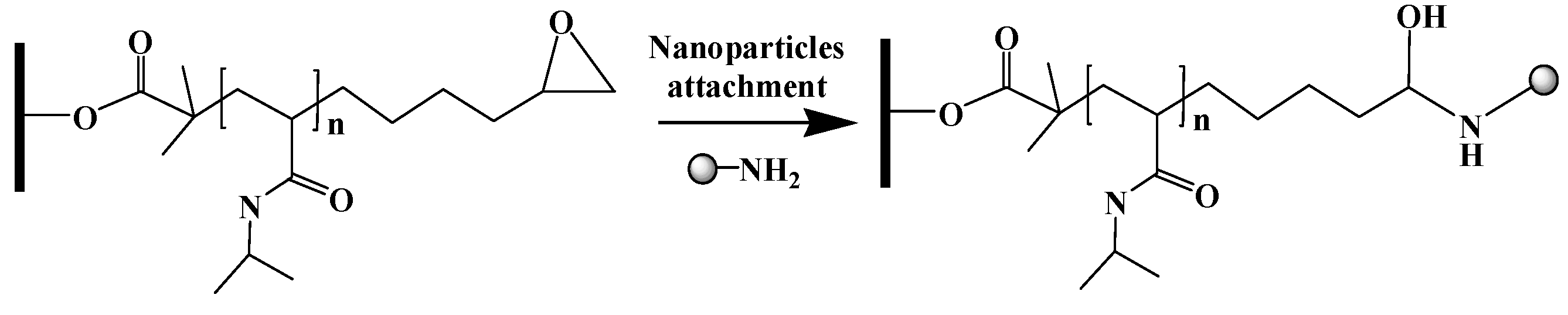
2.2.4. Nanoparticle Attachment
The membrane was rinsed and incubated in 20 mM phosphate buffer at pH 12 for 30 min. The reaction solution was prepared using 15 μL of the nanoparticle feedstock in 20 mL of 20 mM phosphate buffer at pH 12. The membrane was incubated in the reaction solution for 48 h on a shaker under gentle shaking at room temperature. Finally, the membrane was rinsed 3 times with water. The membrane was stored in water prior to use.
2.3. Membrane Characterization
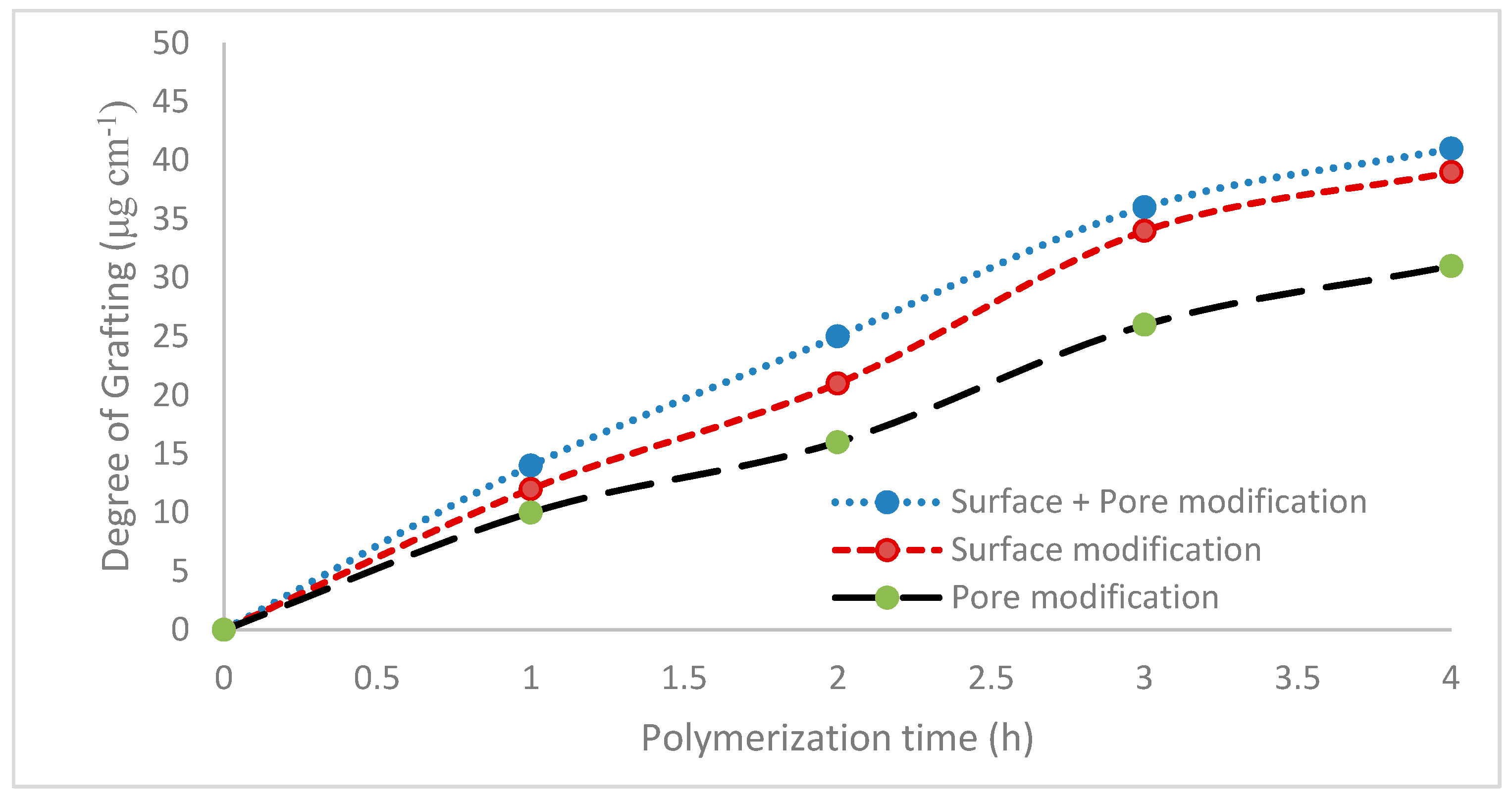
2.3.1. Degree of Grafting
Base membrane coupons were rinsed and dried overnight in a vacuum oven at 40 °C. The dried weight of the membrane was recorded. After modification, the membrane coupon was washed in DI water and then dried overnight in a vacuum oven at 40 °C. Three membrane coupons were modified at each condition. The membrane coupon was then weighed again. The degree of grafting was calculated from the following expression:
where, w
0 and w
1 are the masses of the unmodified and modified membrane respectively, while A
m stands for the area of the membrane used in the present investigation. Each value of the degree of grafting represents the average of three readings obtained for the three membranes modified at each condition. In all cases, the three readings were within 10% of each other. Since regenerated cellulose membranes are hygroscopic, it is critical to standardize the time between drying and weighing the membrane. Consequently, all membranes were weighed 30 min after being taken out of the oven.
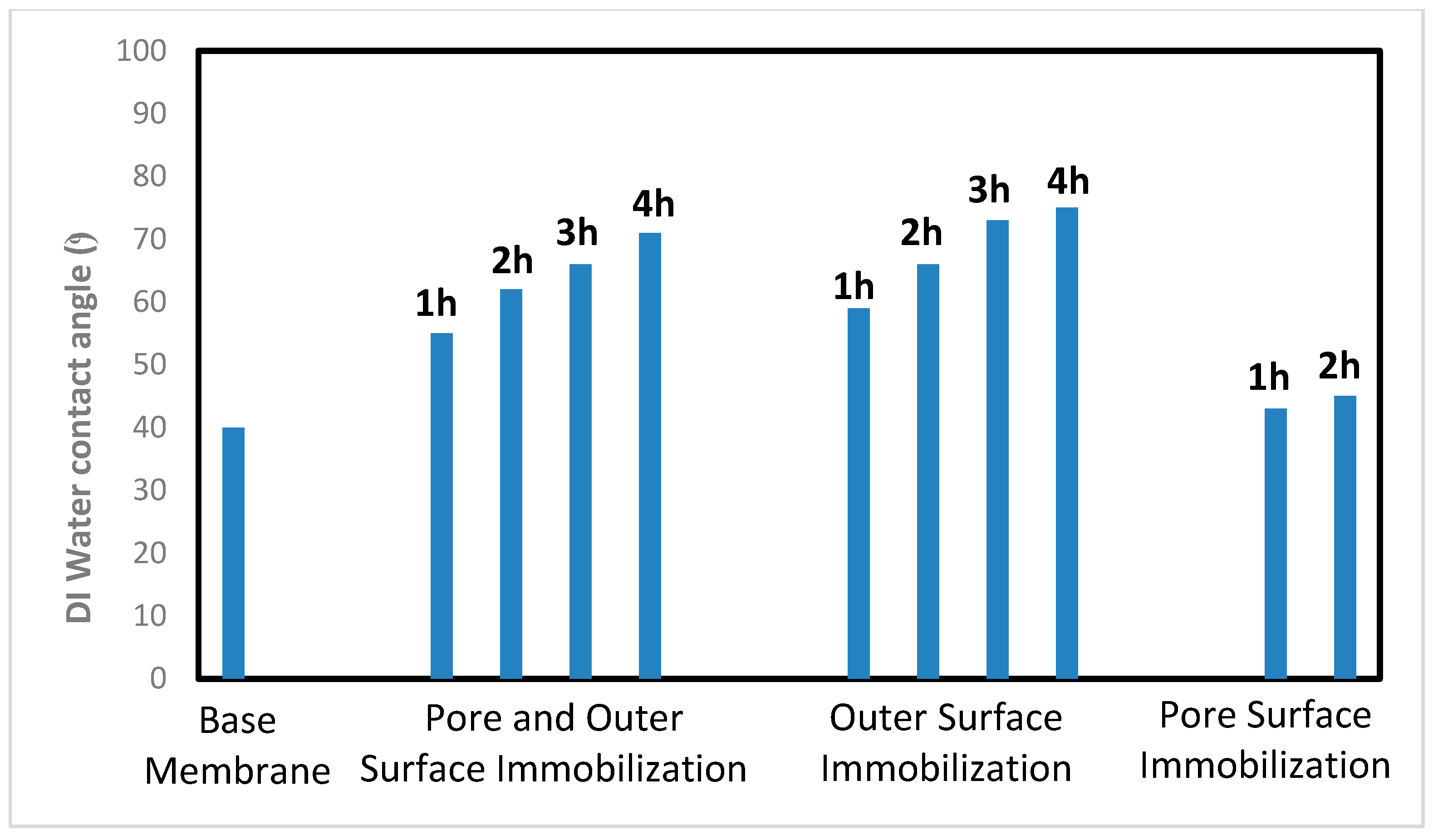
2.3.2. Contact Angle Measurement
The membrane coupon was dried in a vacuum oven at 40 °C overnight. Contact angle measurements were conducted at room temperature with DI water using a Future Digital Scientific, model OCA15EC (Garden City, NY, USA) contact angle goniometer. The droplet size was 2.0 µL. The droplet was dispensed at a speed of 0.5 µL/s. The contact angle was determined using the circle fitting method. Each value is the average of 5 repeat measurements.
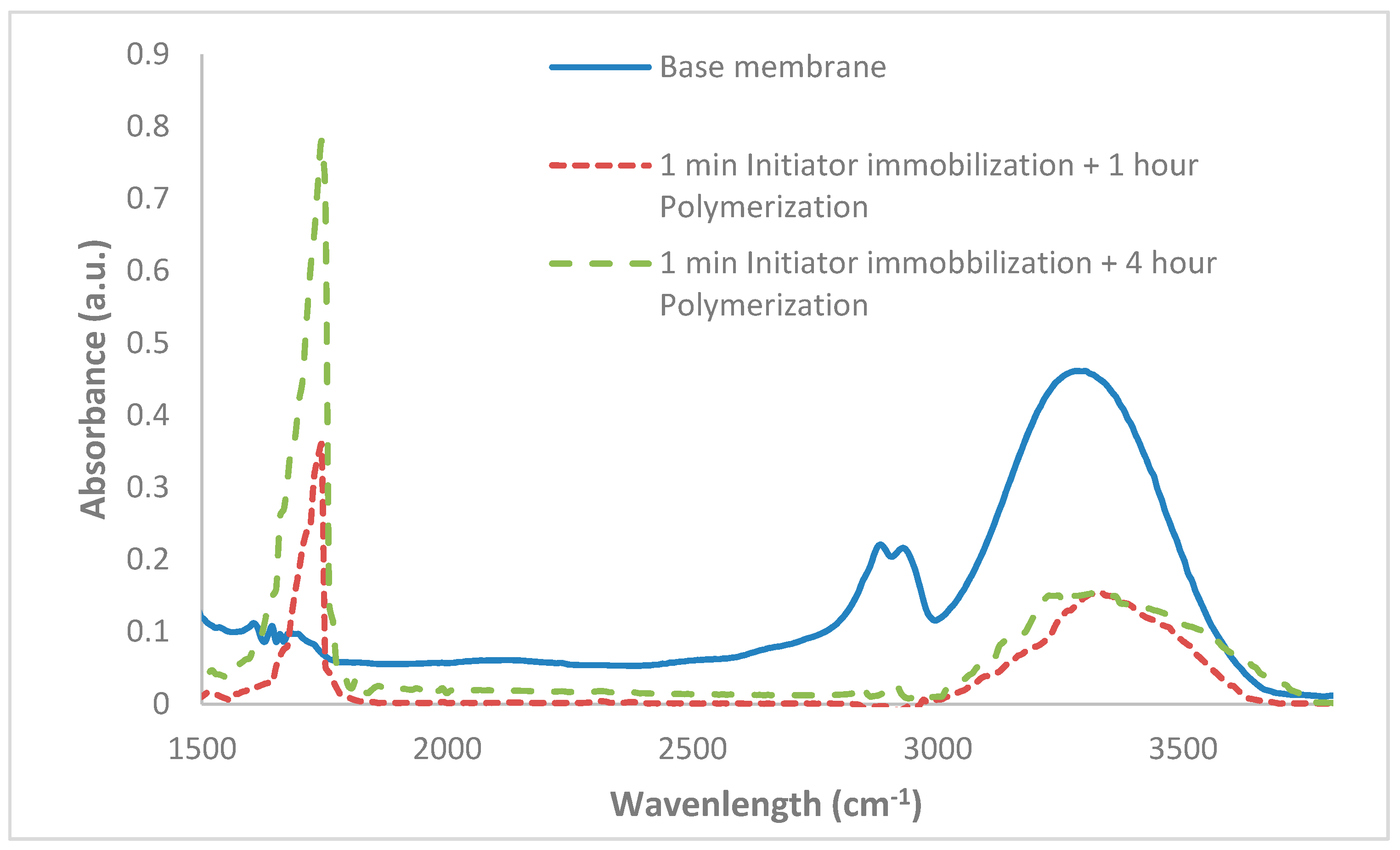
2.3.3. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
ATR-FTIR spectroscopy provides qualitative information about functional groups at the top, approximately 2 µm of the membrane. Data were collected using an IR Affinity instrument (Shimadzu, Columbia, MD, USA) with a horizontal ZnSe accessary. ATR-FTIR spectra were averaged over 100 scans covering a range of 1150–3650 cm−1. Prior to analysis, the membranes were dried overnight in a vacuum oven at 40 °C.
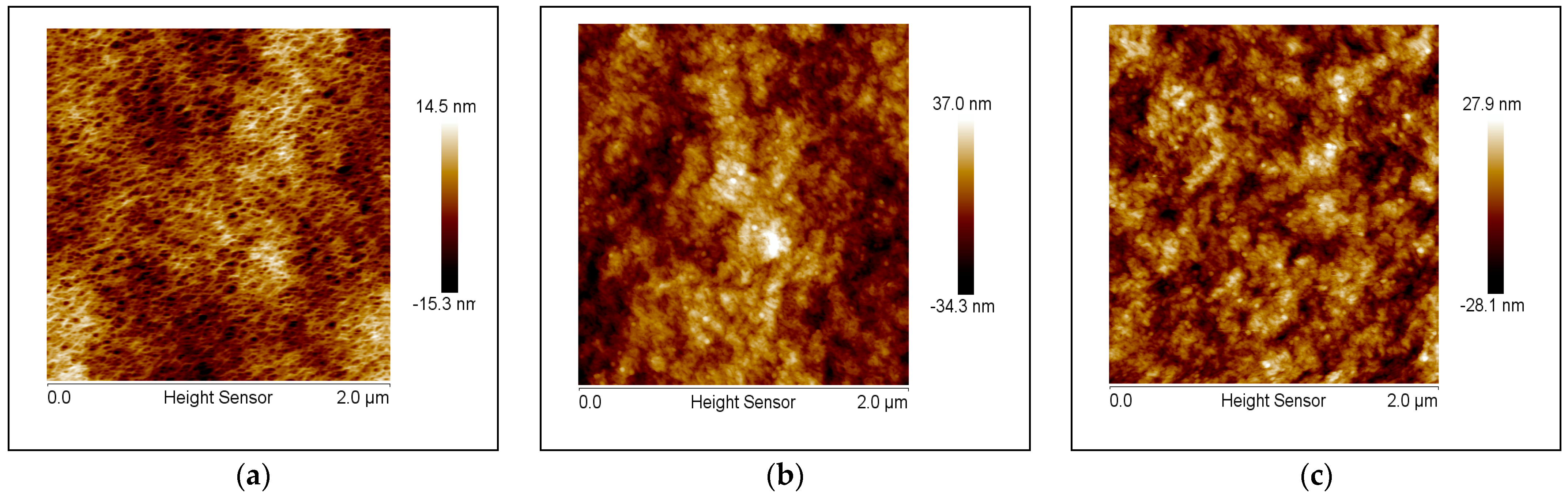
2.3.4. Atomic Force Microscopy (AFM)
The surface topography of the membrane at room temperature was measured using a Dimension Icon AFM (Bruker, Santa Barbara, CA, USA). The NanoScope V815R3sr1 program was used to run the AFM and the NanoScope Analysis program was used to analyze the results. The ScanAsyst mode was used to image the membranes in air using an etched silicon tip on a nitride lever, which was coated with a 100 nm aluminum layer. Membrane coupons were dried overnight at 40 °C prior to analysis. The nominal spring constant of the cantilever used was 0.4 N/m and 70 kHz, respectively. A standard scan rate of 1 Hz with 512 samples per line was used. The measured heights of the images were then flattened in order to obtain the final images.
2.4. Membrane Performance
All testing was conducted at room temperature in dead-end filtration mode using a stirred cell (8010, MilliporeSigma). Prior to flux measurement, the membranes were soaked in methanol for 15 min, then DI water for 15 min. Pressurized nitrogen was used to drive the feed through the membrane. The feed pressure was 10.3 kPa (1.5 psi). The DI water flux was determined after 15 min of operation. Our results indicated that by this time, the flux had reached its steady state value. Feed streams consisting of BSA (1 mg mL
−1) were also tested. The BSA was suspended in 100 mM Tris-HCl, pH 7.5 (Millipore-Sigma). All tests were conducted without stirring in the presence and absence of an oscillating magnetic field. The feed pressure was 10.3 kPa. Flux and rejection data were collected after 15 min of operation. During this period, a pseudo steady-state flux was obtained, where the flux did not vary much with time. BSA rejection was calculated as follows:
where R is the percentage of rejection and C
p and C
f correspond to the permeate and feed concentrations.
An oscillating magnetic field was generated using a custom-built system [
30,
31]. The stirred cell was placed between two solenoids. A computer-controlled programmable logic controller (PLC, Click Koya Automation Direct, Cumming, GA, USA) controlled the two solenoids by alternatively activating two solid-state relays. This enabled us to set the frequency of the alternating magnetic field. The solenoids were powered by an Agilent Technologies (Santa Clara, CA, USA) 20 V/25 A power supply. The solenoids were placed on opposite sides of the filtration cell so that the magnetic field was parallel to the barrier layer of the membrane.
The oscillation frequency of the magnetic field was set at 20 or 1000 Hz, where the current was set 2 A and 8 V. Our previous studies indicated that 20 Hz maximizes movement of the superparamagnetic nanoparticles used here [
30,
31]. As higher frequencies induce heating, 1000 Hz was also tested [
36]. Insulated foam was placed between the solenoids and filtration cell in order to prevent heat transfer to the cell from the solenoid.
As we have shown in earlier studies [
36], this is essential to ensure that an increase in the bulk water temperature is suppressed and will not lead to modified fluxes due to changes in viscosity. Further, the frequency we choose, 1000 Hz, is relatively low for applications focused on magnetically induced heating. This is important in order to ensure that heating effects are localized to the grafted polymer chains and do not lead to an increase in the bulk water temperature.
A Mettler Toledo PL 602~S (Columbus, OH, USA) balance connected to a computer was used to measure the weight of the permeate, which in turn was used to calculate the permeate flux. The experimental set-up is shown in
Supplementary Figure S2.
4. Discussion
In this work, we have, for the first time, attached a superparamagnetic nanoparticle to the end of a PNIPAm chain that is grown from the ultrafiltration membrane surface. By controlling the location of grafting, we can probe the effect of changes in the conformation of the PNIPAm chains due to an external stimulus for polymer chains grafted from different membrane surfaces. Here, we have investigated the effect of an external oscillating magnetic field.
Superparamagnetic nanoparticles are unique in that they are single domain nanoparticles. Consequently, they behave like a paramagnet with a giant magnetic moment in the presence of a magnetic field [
38]. For superparamagnetic nanoparticles, the exchange coupling of the atomic magnetic moments is strong, resulting in alignment of the moments with each other. Further, creation of domain walls is energetically unfavorable, resulting in a single magnetic domain. At room temperature, in the absence of a magnetic field, the magnetic anisotropy energy is less than the thermal energy, leading to a random orientation of the giant moment in space. However, the giant moments will readily align in the direction of an external magnetic field. When an external field is removed, superparamagnetic nanoparticles display no remanence. Brownian and Néel relaxation result in the dispersion of the magnetic dipoles [
39,
40].
Brownian relaxation (μ
B) describes the physical rotation of the particles as the magnetic moment within each particle randomizes with respect to neighboring particles. Néel relaxation (μ
N) on the other hand describes the rotation of the magnetic moment within each particle due to this randomization process. Importantly, energy dissipation due to relaxation will lead to heating. Since the particles are tethered to PNIPAm chains, the heat induced can lead to an increase in temperature of the PNIPAM chains above their LCST, which will lead to collapse of the grafted nanostructure. The two relaxation times are determined from the following expressions:
where V
H and V
M are the hydrodynamic and magnetic volumes of the nanoparticles,
is the viscosity, K is the effective anisotropy constant, k
B is the Boltzmann constant, T is the absolute temperature and
is constant. As can be seen, the two relaxation times will be different. For the nanoparticles used here (15 nm magnetic core, 5 nm coating), the calculated Néel relaxation time is much larger than 1 s and the Brownian relaxation time is in the order of 10
−3 s.
In an external magnetic field, the superparamagnetic nanoparticle will experience a torque and a force, as given by Equations (5) and (6):
where
and F are the torque and force experienced by the nanoparticles and
and
are the magnetic moment of the nanoparticles and the external magnetic field. The torque results in rotation of the particles through Brownian and Néel relaxation, as described above. The force on the particles will lead to translational motion, and hence, mixing. As shown in our earlier work [
30], translational motion is maximized at frequencies around 20 Hz. Higher frequencies will lead to greater heat generation. Thus, we have investigated the effect of a 20 and 1000 Hz oscillating magnetic field on membrane performance.
For initiator immobilized on the inner pore and outer membrane surface,
Table 1 indicates that a large decrease in flux is observed, indicating significant pore blocking by the grafted nanostructure. When movement of the grafted polymer chains is maximized at 20 Hz, little change in permeate flux is observed. However, when induced heating is maximized at 1000 Hz, an increase in flux is observed. Here, we insulated the stirred cell to minimize heat transfer from the solenoids. Further, our previous work indicated that insulation in the stirred cell results in little increase in the bulk water temperature [
36]. An increase in the water temperature will lead to a decreased viscosity and can also explain the increased permeate flux.
Collapse of the grafted PNIPAm chains in the membrane pores at temperatures above their LCST will also lead to an increase in membrane pore size and observed flux. This assertion is supported by our earlier studies that indicate an increase in the minimum size of particles found in the retentate of track-etched microfiltration membranes also grafted with PNIPAm chains [
36]. In addition, the data in
Table 3;
Table 5 for initiator immobilized only on the membrane pore surface are consistent with this assertion. BSA rejection data in
Table 5 indicate a decrease in BSA rejection at 1000 Hz for initiator immobilized on the inner pore surface, consistent with our earlier microfiltration studies [
36].
Table 2 indicates that as the thickness of the PNIPAm nanostructure grafted from the outer surface of the membrane increases, the water flux decreases. It is likely that polymer chains grafted at the pore mouth will bridge across the pore entrance, leading to a narrowing of the pore mouth and hence a decrease in permeate flux. The effect will increase as the chain length increases with longer grafting times. The results for BSA filtration shown in
Table 5 are in agreement with the water flux data.
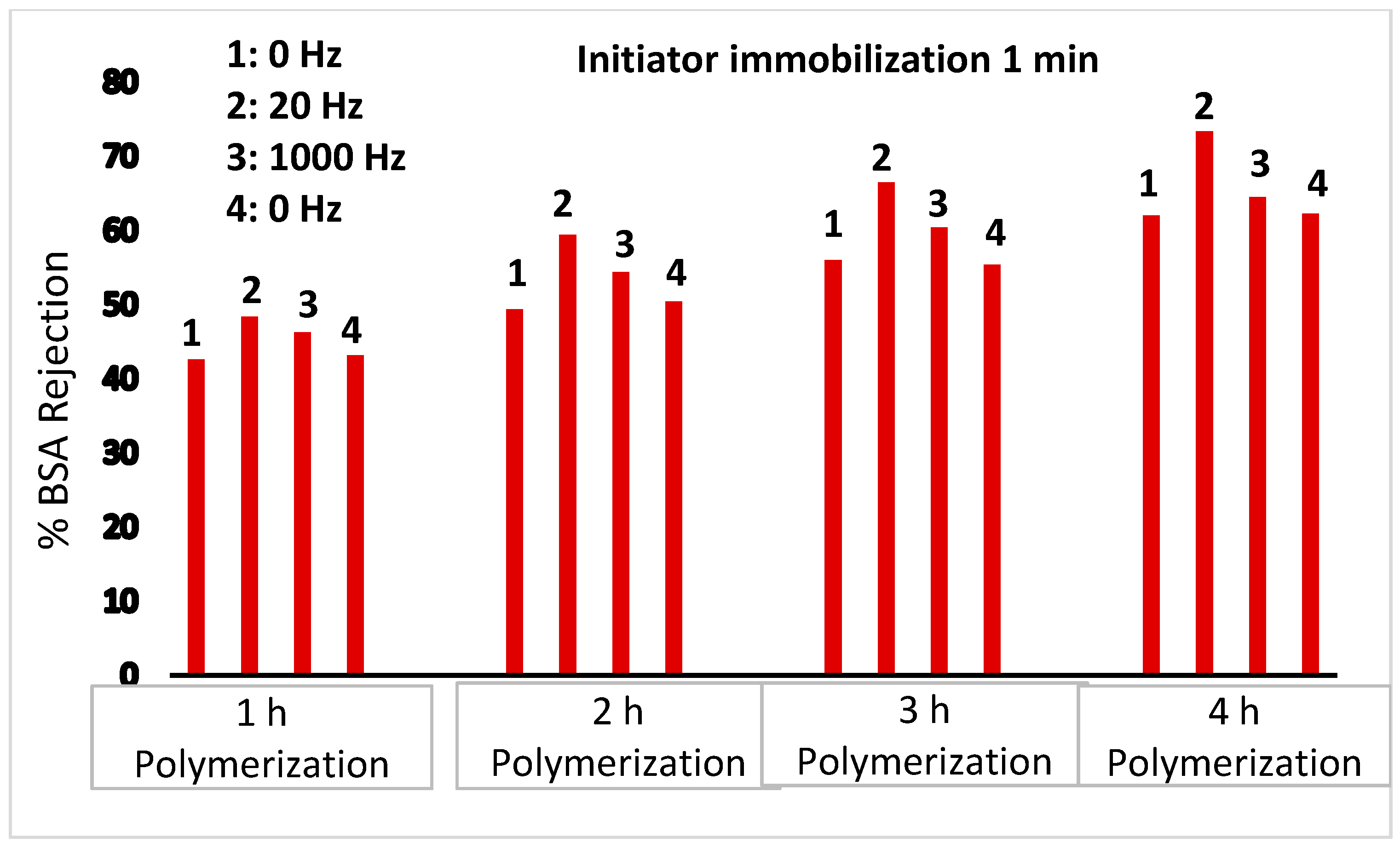
BSA rejection data shown in
Figure 5, however, suggest that for initiator immobilized on the outer membrane surface, the presence of a 20 Hz oscillating magnetic field leads to an increase in the observed rejection. However, no change in the rejection is observed for a 1000 Hz oscillating magnetic field. In a 20 Hz oscillating magnetic field, movement of the grafted PNIPAm chains will be the dominant effect. In our earlier work, we have shown that his movement can lead to break up of the concentration polarization boundary layer [
30,
31]. For nanofiltration membranes, this led to an increase in the observed rejection of partially rejected salts. The result observed here for ultrafiltration membranes is analogous. On the other hand, collapse of the grafted PNIPAm chains due to induced heating is expected to have little effect on BSA rejection for PNIPAm chains grafted from the outer membrane surface.
Taken together, the data indicate for that for ultrafiltration membranes, selectively grafting magnetically and thermally responsive polymer chains on the outer or inner pore surface of the membrane can lead to remote modulation of membrane performance. However, grafting PNIPAm polymer chains from the entire membrane surface limits the degree to which one can tune membrane performance.
In our earlier work, we grafted the same PNIPAm chains from the surface of nanofiltration and track-etched microfiltration membranes [
36]. No attempt was made for surface-specific initiator immobilization. In the case of nanofiltration membranes, the very small pore size present results in little grafting on the pore surface. Diffusional and steric hindrance effects limit monomer and nanoparticle transport into the pores. For microfiltration membranes, given their large pore size (0.4 μm), PNIPAm grafted from the outer membrane surface had little effect on performance. Ultrafiltration membranes lie in between microfiltration and nanofiltration membranes. In this case, careful surface-selective grafting can lead to a high degree of performance modulation.