Pilot Demonstration of Reclaiming Municipal Wastewater for Irrigation Using Electrodialysis Reversal: Effect of Operational Parameters on Water Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Water Quality and Analysis
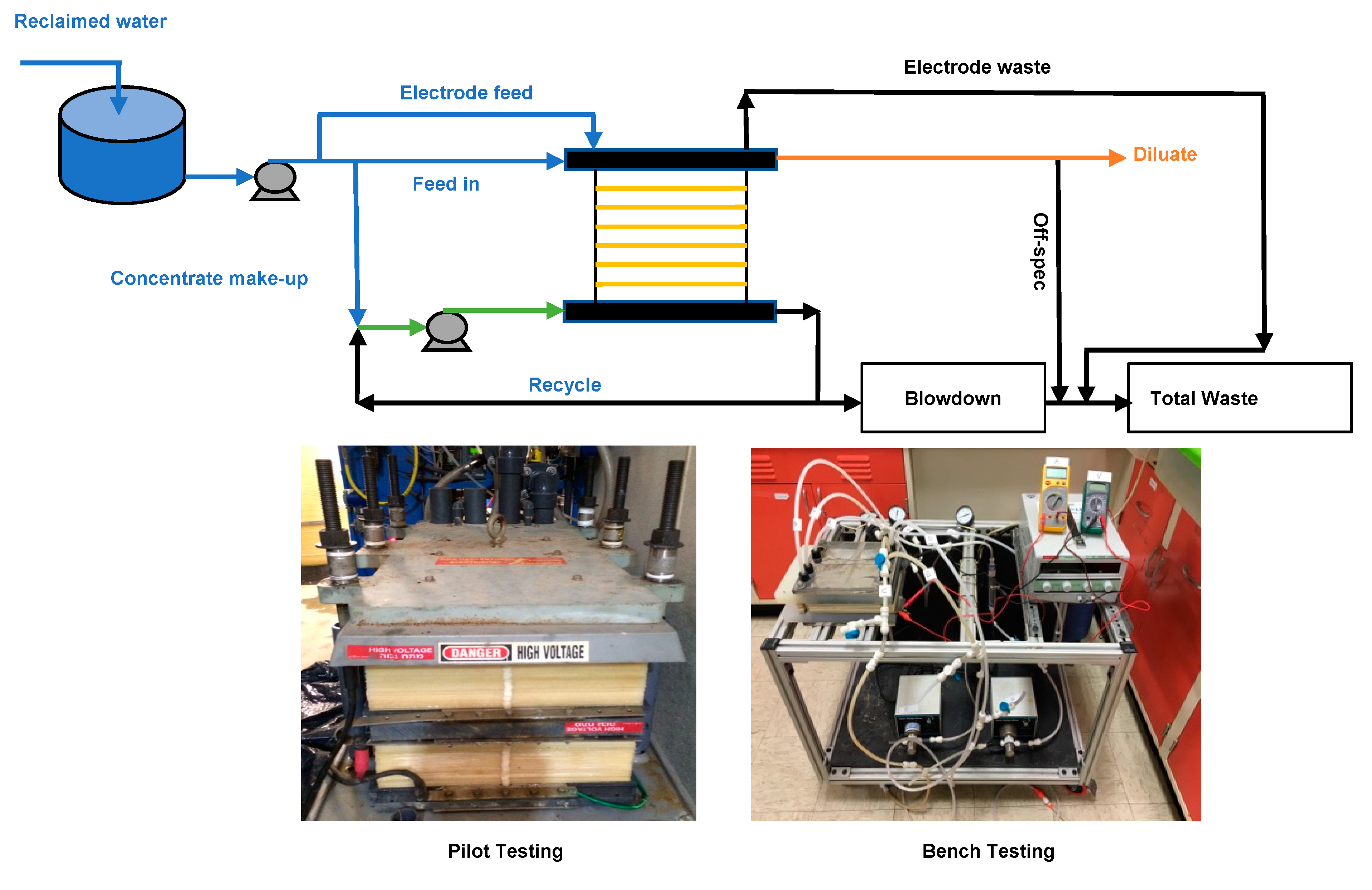
2.2. Bench- and Pilot-Scale Electrodialysis Systems
3. Results and Discussion
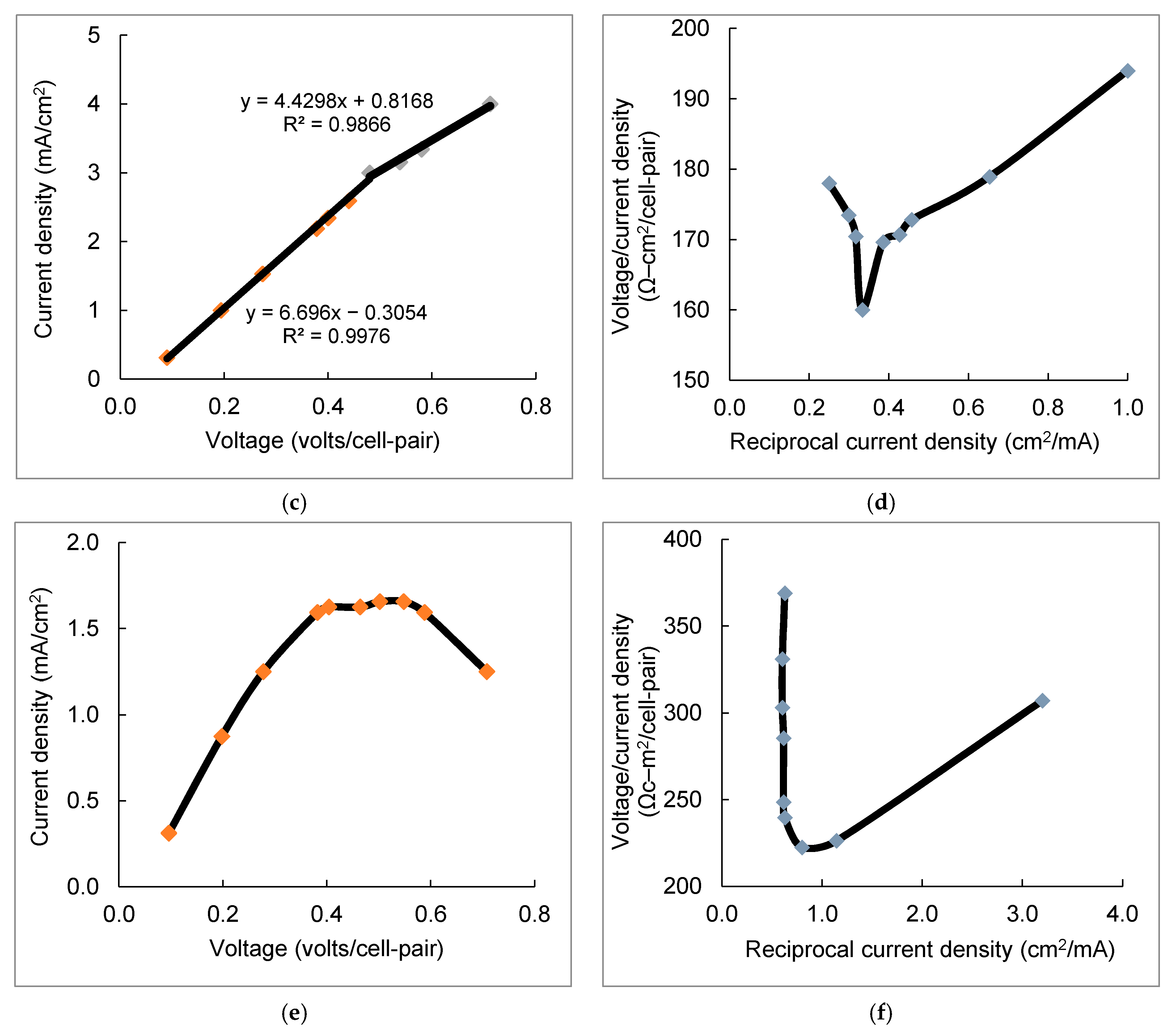
3.1. LCD Measurement
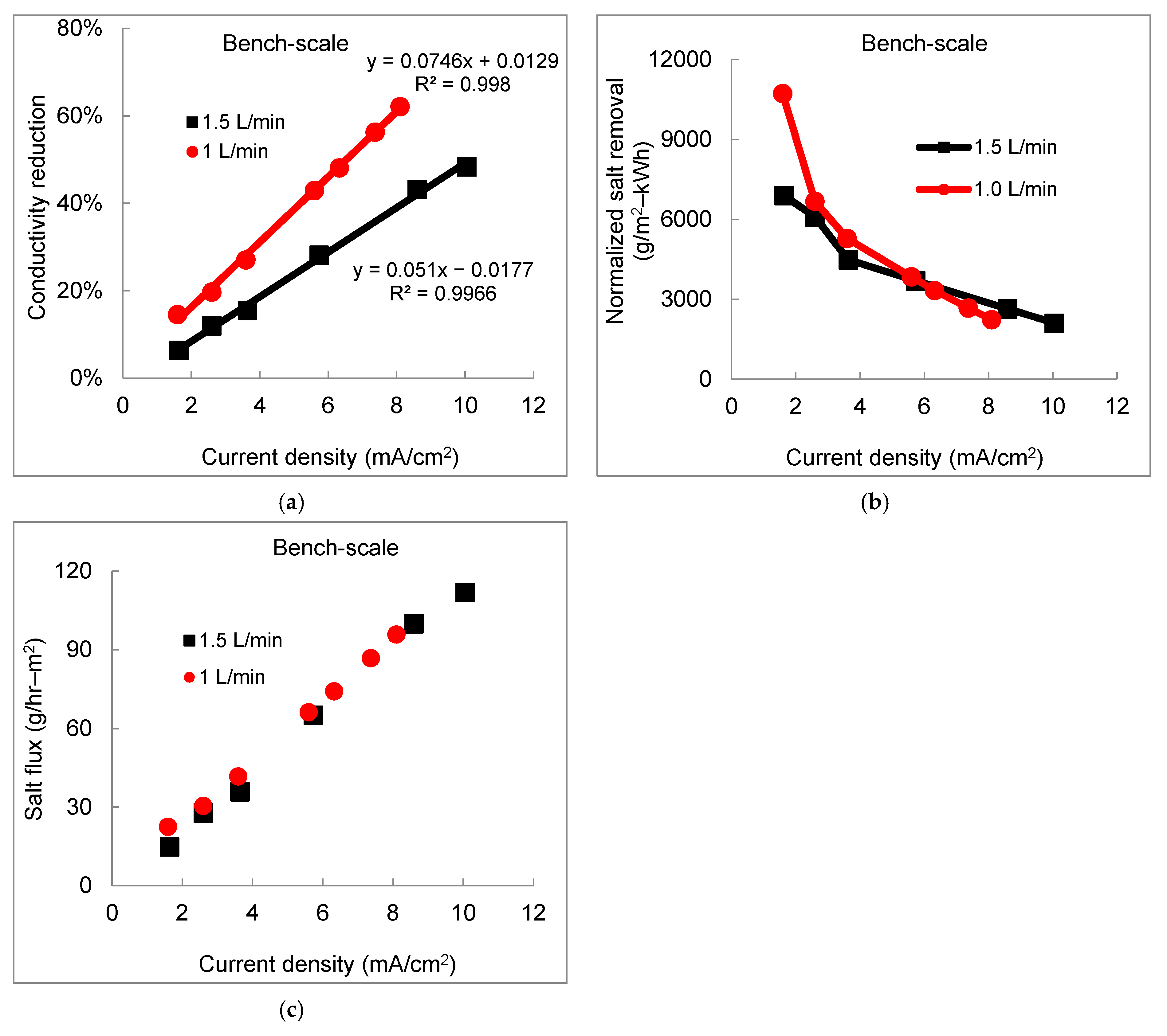
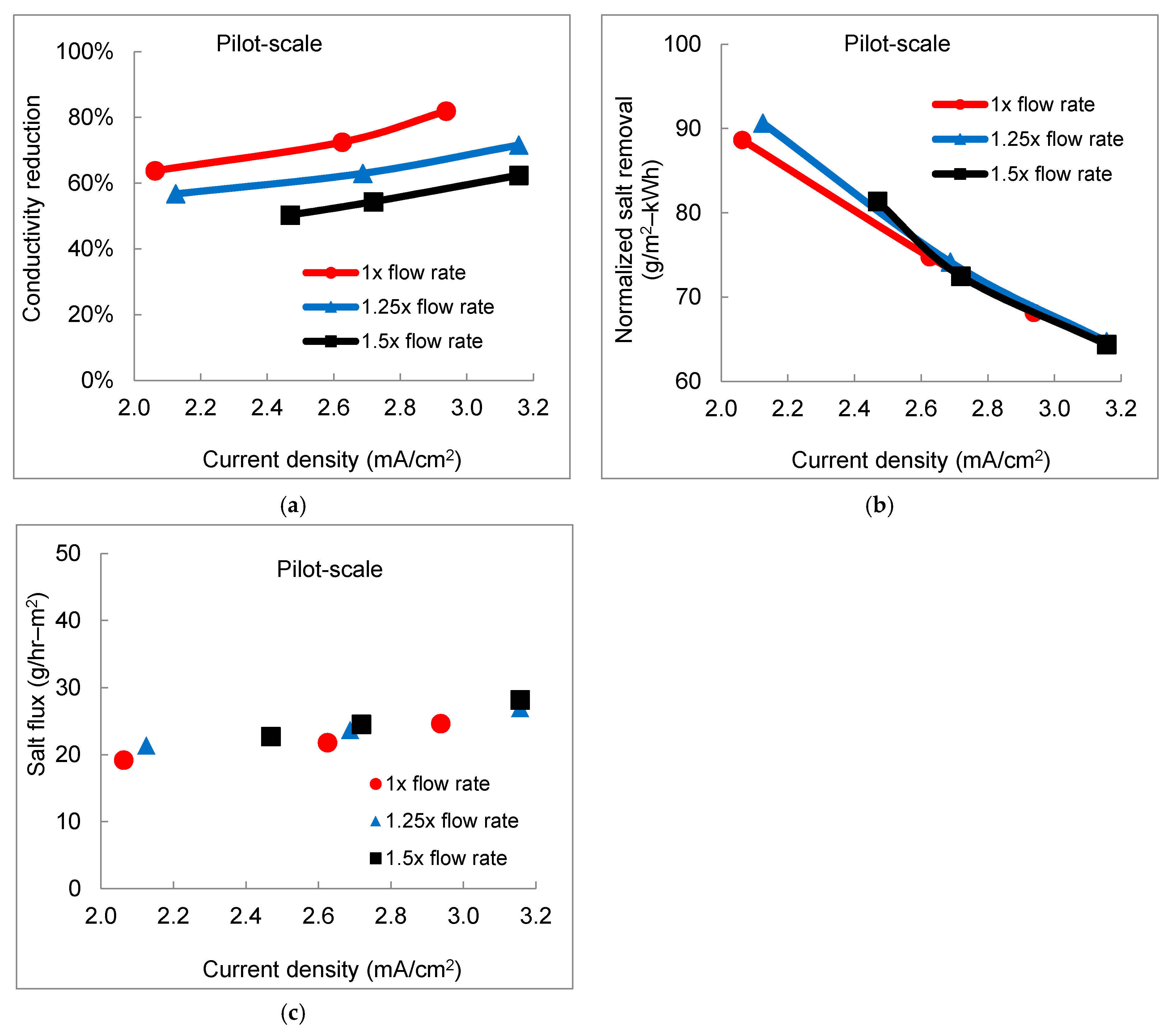
3.2. Effect of Flow Rate on Desalination Performance
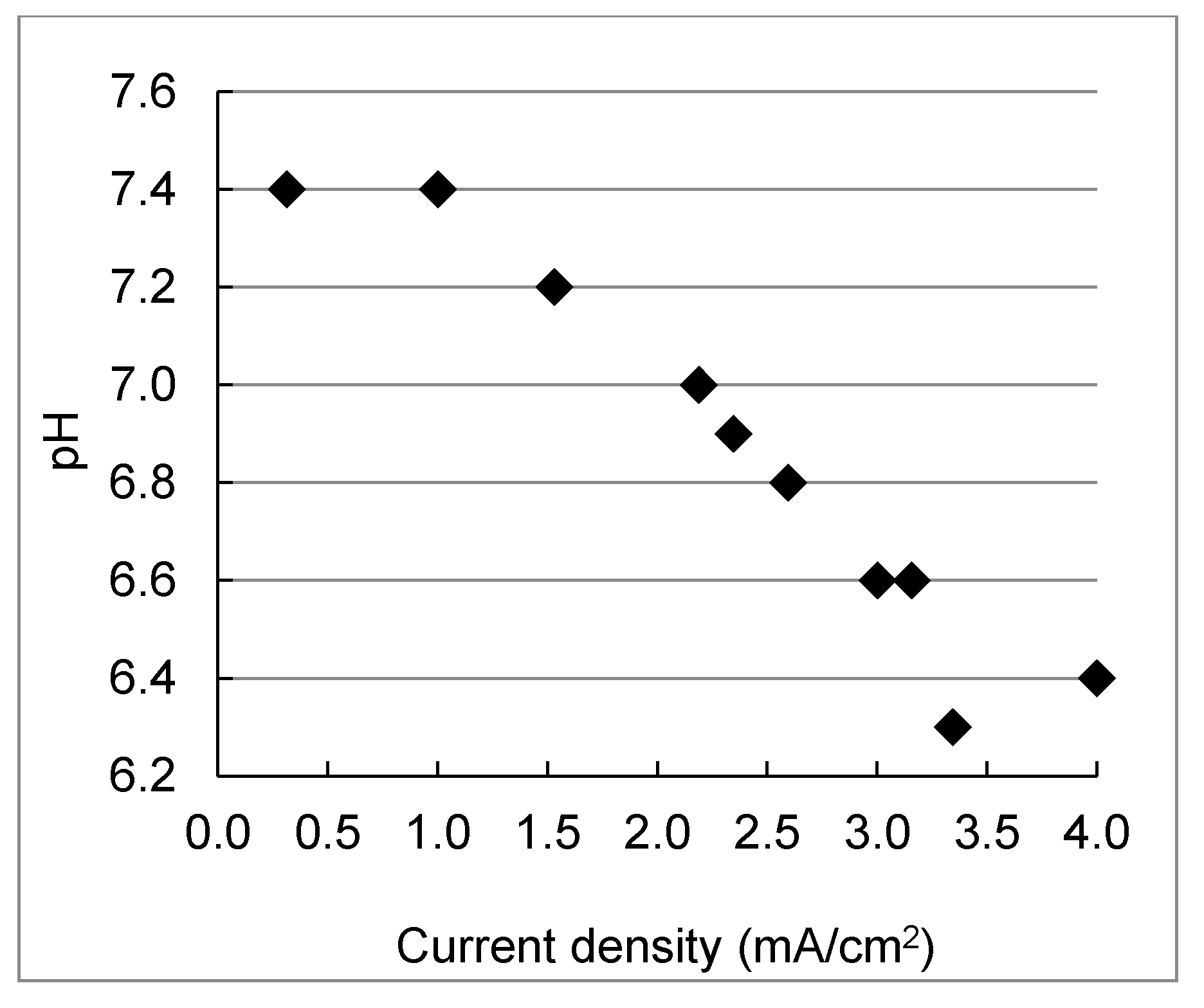
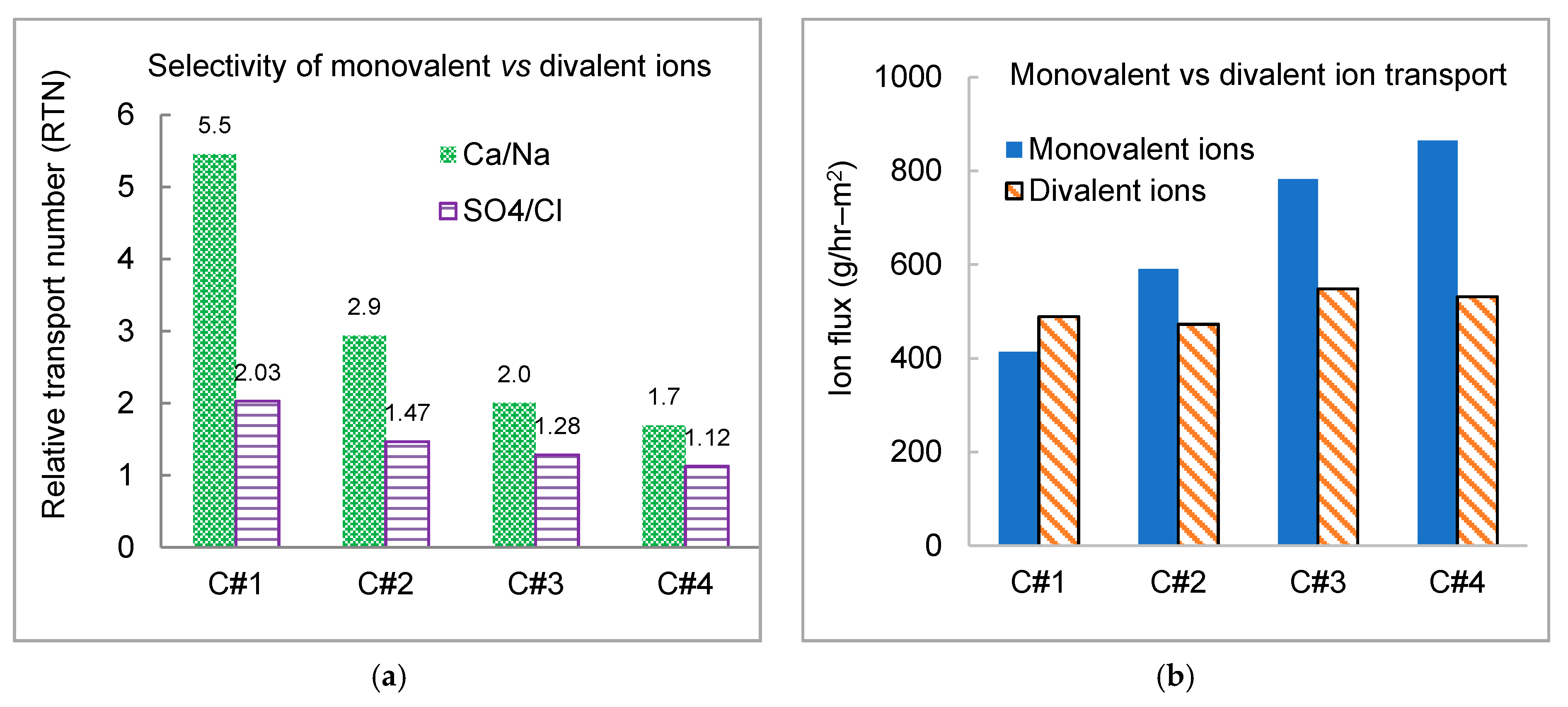
3.3. Impact of Applied Current Density on Desalination and Ion Separation
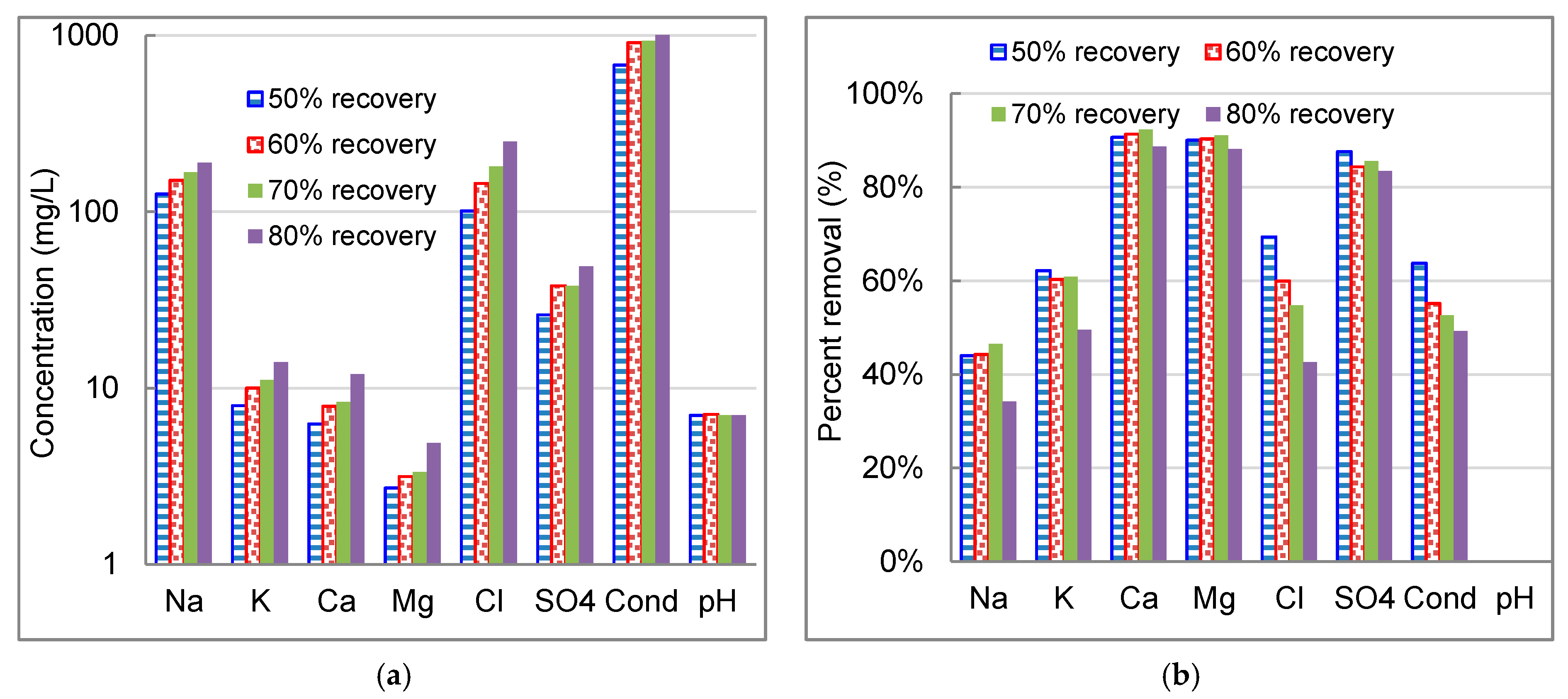
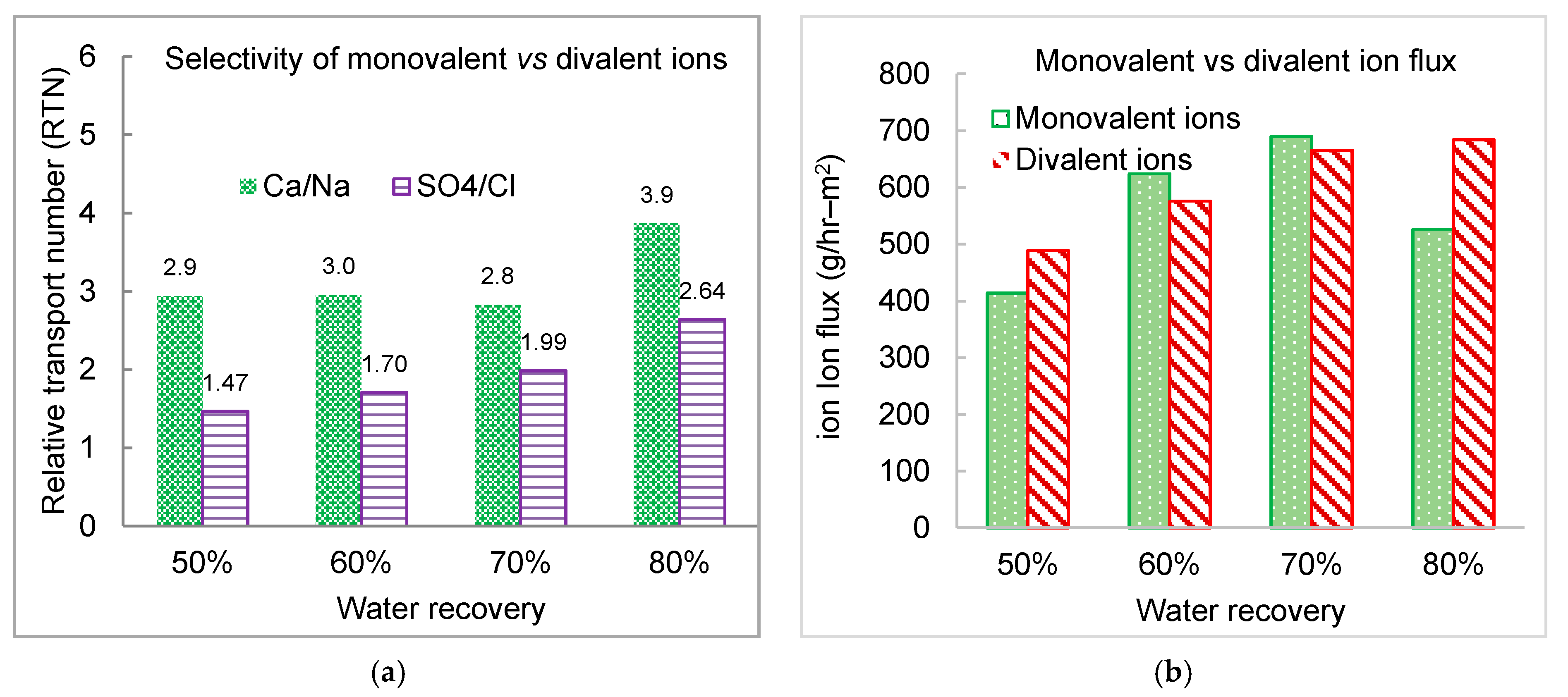
3.4. Effect of Water Recovery
3.5. Techno-Economic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greve, P.; Kahil, T.; Mochizuki, J.; Schinko, T.; Satoh, Y.; Burek, P.; Fischer, G.; Tramberend, S.; Burtscher, R.; Langan, S.; et al. Global assessment of water challenges under uncertainty in water scarcity projections. Nat. Sustain. 2018, 1, 486–494. [Google Scholar] [CrossRef]
- Nobre, R.L.G.; Caliman, A.; Cabral, C.R.; de Carvalho Araújo, F.; Guerin, J.; Dantas, F.D.C.C.; Quesado, L.B.; Venticinque, E.M.; Guariento, R.D.; Amado, A.M.; et al. Precipitation, landscape properties and land use interactively affect water quality of tropical freshwaters. Sci. Total Environ. 2020, 716, 137044. [Google Scholar] [CrossRef]
- MacDonald, G.M. Water, climate change, and sustainability in the southwest. Proc. Natl. Acad. Sci. USA 2010, 107, 21256–21262. [Google Scholar] [CrossRef]
- Pettygrove, G.S. Irrigation with Reclaimed Municipal Wastewater—A Guidance Manual; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Zhang, Y.; Shen, Y. Wastewater irrigation: Past, present, and future. Wiley Interdiscip. Rev. Water 2019, 6, e1234. [Google Scholar] [CrossRef]
- Fournier, E.D.; Keller, A.A.; Geyer, R.; Frew, J. Investigating the energy-water usage efficiency of the reuse of treated municipal wastewater for artificial groundwater recharge. Environ. Sci. Technol. 2016, 50, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Zuurbier, K.; Smeets, P.; Roest, K.; van Vierssen, W. Use of Wastewater in Managed Aquifer Recharge for Agricultural and Drinking Purposes: The Dutch Experience. In Safe Use of Wastewater in Agriculture; Springer: New York, NY, USA, 2018; pp. 159–175. [Google Scholar]
- Wei, X.; Binger, Z.M.; Achilli, A.; Sanders, K.T.; Childress, A.E. A modeling framework to evaluate blending of seawater and treated wastewater streams for synergistic desalination and potable reuse. Water Res. 2020, 170, 115282. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.E.; Theregowda, R.B.; Safari, I.; Abbasian, J.; Arastoopour, H.; Dzombak, D.A.; Hsieh, M.K.; Miller, D.C. Utilization of municipal wastewater for cooling in thermoelectric power plants: Evaluation of the combined cost of makeup water treatment and increased condenser fouling. Energy 2013, 60, 139–147. [Google Scholar] [CrossRef]
- Council, N.R. Water Reuse: Potential for Expanding the Nation’s Water Supply through Reuse of Municipal Wastewater; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Evanylo, G.; Ervin, E.; Zhang, X. Reclaimed water for turfgrass irrigation. Water 2010, 2, 685–701. [Google Scholar] [CrossRef]
- Phogat, V.; Mallants, D.; Cox, J.W.; Šimůnek, J.; Oliver, D.P.; Pitt, T.; Petrie, P.R. Impact of long-term recycled water irrigation on crop yield and soil chemical properties. Agric. Water Manag. 2020, 237, 106167. [Google Scholar] [CrossRef]
- Qian, Y.; Mecham, B. Long-term effects of recycled wastewater irrigation on soil chemical properties on golf course fairways. Agron. J. 2005, 97, 717–721. [Google Scholar] [CrossRef]
- Suarez, D.L.; Wood, J.D.; Lesch, S.M. Infiltration into cropped soils: Effect of rain and sodium adsorption ratio–impacted irrigation water. J. Environ. Qual. 2008, 37 (Suppl. S5), S-169–S-179. [Google Scholar] [CrossRef]
- Goodfellow, W.L.; Ausley, L.W.; Burton, D.T.; Denton, D.L.; Dorn, P.B.; Grothe, D.R.; Heber, M.A.; Norberg-King, T.J.; Rodgers, J.H., Jr. Major ion toxicity in effluents: A review with permitting recommendations. Environ. Toxicol. Chem. Int. J. 2000, 19, 175–182. [Google Scholar] [CrossRef]
- Pype, M.-L.; Lawrence, M.G.; Keller, J.; Gernjak, W. Reverse osmosis integrity monitoring in water reuse: The challenge to verify virus removal—A review. Water Res. 2016, 98, 384–395. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Xu, P.; Cath, T.Y.; Robertson, A.P.; Reinhard, M.; Leckie, J.O.; Drewes, J.E. Critical Review of Desalination Concentrate Management, Treatment and Beneficial Use. Environ. Eng. Sci. 2013, 30, 502–514. [Google Scholar] [CrossRef]
- Llanos, J.; Cotillas, S.; Cañizares, P.; Rodrigo, M.A. Novel electrodialysis–electrochlorination integrated process for the reclamation of treated wastewaters. Sep. Purif. Technol. 2014, 132, 362–369. [Google Scholar] [CrossRef]
- Xu, X.; Lin, L.; Ma, G.; Wang, H.; Jiang, W.; He, Q.; Nirmalakhandan, N.; Xu, P. Study of Polyethyleneimine Coating on Membrane Permselectivity and Desalination Performance during Pilot-scale Electrodialysis of Reverse Osmosis Concentrate. Sep. Purif. Technol. 2018, 207, 396–405. [Google Scholar]
- Kwon, K.; Han, J.; Park, B.H.; Shin, Y.; Kim, D. Brine recovery using reverse electrodialysis in membrane-based desalination processes. Desalination 2015, 362, 1–10. [Google Scholar] [CrossRef]
- American Water Works Association. Electrodialysis and Electrodialysis Reversal (M38); American Water Works Association: Denver, CO, USA, 1995; Volume 38. [Google Scholar]
- Hansima, M.; Makehelwala, M.; Jinadasa, K.; Wei, Y.; Nanayakkara, K.; Herath, A.; Weerasooriya, R. Fouling of ion exchange membranes used in the electrodialysis reversal advanced water treatment: A review. Chemosphere 2020, 127951. [Google Scholar]
- McGovern, R.K.; Weiner, A.M.; Sun, L.; Chambers, C.G.; Zubair, S.M. On the cost of electrodialysis for the desalination of high salinity feeds. Appl. Energy. 2014, 136, 649–661. [Google Scholar] [CrossRef]
- McGovern, R.K.; Zubair, S.M.; Lienhard, V.J.H. The cost effectiveness of electrodialysis for diverse salinity applications. Desalination 2014, 348, 57–65. [Google Scholar] [CrossRef]
- Xu, P.; Capito, M.; Cath, T.Y. Selective removal of arsenic and monovalent ions from brackish water reverse osmosis concentrate. J. Hazard. Mater. 2013, 260, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, V.; Afonso, M.D. Limiting current density in the electrodialysis of multi-ionic solutions. J. Membr. Sci. 2010, 360, 499–508. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Razmi, A.; Mohammadi, T. Separation of different ions from wastewater at various operating conditions using electrodialysis. Sep. Purif. Technol. 2007, 54, 147–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Desmidt, E.; Van Looveren, A.; Pinoy, L.; Meesschaert, B.; Van der Bruggen, B. Phosphate separation and recovery from wastewater by novel electrodialysis. Environ. Sci. Technol. 2013, 47, 5888–5895. [Google Scholar] [CrossRef]
- Abou-Shady, A. Recycling of polluted wastewater for agriculture purpose using electrodialysis: Perspective for large scale application. Chem. Eng. J. 2017, 323, 1–18. [Google Scholar] [CrossRef]
- Arola, K.; Ward, A.; Mänttäri, M.; Kallioinen, M.; Batstone, D. Transport of pharmaceuticals during electrodialysis treatment of wastewater. Water Res. 2019, 161, 496–504. [Google Scholar] [CrossRef]
- Mohammadi, R.; Ramasamy, D.L.; Sillanpää, M. Enhancement of nitrate removal and recovery from municipal wastewater through single-and multi-batch electrodialysis: Process optimisation and energy consumption. Desalination 2020, 498, 114726. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis applications in wastewater treatment for environmental protection and resources recovery: A systematic review on progress and perspectives. Membr 2020, 10, 146. [Google Scholar] [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis—Part II: Sensitivity to voltage application and membranes. Desalination 2014, 345, 128–135. [Google Scholar] [CrossRef]
- Lee, H.J.; Sarfert, F.; Strathmann, H.; Moon, S.H. Designing of an electrodialysis desalination plant. Desalination 2002, 142, 267–286. [Google Scholar] [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis—Part I: Sensitivity to superficial velocity. Desalination 2014, 344, 152–162. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis—Part III: Sensitivity to composition and hydraulic recovery. Desalination 2014, 347, 158–164. [Google Scholar] [CrossRef]
- Myint, M.T. Saving energy consumption and CO2 emission from sustainable efficient operating zones in inland electrodialysis reversal desalination. Water Resour. Ind. 2014, 5, 36–48. [Google Scholar] [CrossRef][Green Version]
- Krol, J.; Wessling, M.; Strathmann, H. Concentration polarization with monopolar ion exchange membranes: Current–voltage curves and water dissociation. J. Membr. Sci. 1999, 162, 145–154. [Google Scholar] [CrossRef]
- Rubinstein, I.; Warshawsky, A.; Schechtman, L.; Kedem, O. Elimination of acid-base generation (‘water-splitting’) in electrodialysis. Desalination 1984, 51, 55–60. [Google Scholar] [CrossRef]
- Lee, H.-J.; Strathmann, H.; Moon, S.-H. Determination of the limiting current density in electrodialysis desalination as an empirical function of linear velocity. Desalination 2006, 190, 43–50. [Google Scholar] [CrossRef]
- Cowan, D.A.; Brown, J.H. Effect of turbulence on limiting current in electrodialysis cells. Ind. Eng. Chem. 1959, 51, 1445–1448. [Google Scholar] [CrossRef]
- Tanaka, Y. Current density distribution and limiting current density in ion-exchange membrane electrodialysis. J. Membr. Sci. 2000, 173, 179–190. [Google Scholar] [CrossRef]
- Xu, X.; He, Q.; Ma, G.; Wang, H.; Nirmalakhandan, N.; Xu, P. Selective separation of mono-and di-valent cations in electrodialysis during brackish water desalination: Bench and pilot-scale studies. Desalination 2018, 428, 146–160. [Google Scholar] [CrossRef]
- Todeschini, S.; Perreault, V.; Goulet, C.; Bouchard, M.; Dubé, P.; Boutin, Y.; Bazinet, L. Assessment of the Performance of Electrodialysis in the Removal of the Most Potent Odor-Active Compounds of Herring Milt Hydrolysate: Focus on Ion-Exchange Membrane Fouling and Water Dissociation as Limiting Process Conditions. Membranes 2020, 10, 127. [Google Scholar] [CrossRef]
- Tanaka, Y. Concentration polarization in ion-exchange membrane electrodialysis—The events arising in a flowing solution in a desalting cell. J. Membr. Sci. 2003, 216, 149–164. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Razmi, A.; Mohammadi, T. Separation of monovalent, divalent and trivalent ions from wastewater at various operating conditions using electrodialysis. Desalination 2007, 205, 53–61. [Google Scholar] [CrossRef]
- Lee, H.-J.; Song, J.-H.; Moon, S.-H. Comparison of electrodialysis reversal (EDR) and electrodeionization reversal (EDIR) for water softening. Desalination 2013, 314, 43–49. [Google Scholar] [CrossRef]
- Han, L.; Galier, S.; Roux-de Balmann, H. Ion hydration number and electro-osmosis during electrodialysis of mixed salt solution. Desalination 2015, 373, 38–46. [Google Scholar] [CrossRef]
- Ma, G.; Xu, X.; Tesfai, M.; Wang, H.; Xu, P. Developing anti-biofouling and energy-efficient cation-exchange membranes using conductive polymers and nanomaterials. J. Membr. Sci. 2020, 603, 118034. [Google Scholar] [CrossRef]
- Cooke, B. Concentration polarization in electrodialysis—I. The electrometric measurement of interfacial concentration. Electrochim. Acta. 1961, 3, 307–317. [Google Scholar] [CrossRef]
- Valero, F.; Arbós, R. Desalination of brackish river water using Electrodialysis Reversal (EDR): Control of the THMs formation in the Barcelona (NE Spain) area. Desalination 2010, 253, 170–174. [Google Scholar] [CrossRef]
- Sata, T.; Teshima, K.; Yamaguchi, T. Permselectivity between two anions in anion exchange membranes crosslinked with various diamines in electrodialysis. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 1475–1482. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghyselbrecht, K.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. Electrodialysis on RO concentrate to improve water recovery in wastewater reclamation. J. Membr. Sci. 2011, 378, 101–110. [Google Scholar] [CrossRef]
- Kwon, B.; Lee, S.; Cho, J.; Ahn, H.; Lee, D.; Shin, H.S. Biodegradability, DBP formation, and membrane fouling potential of natural organic matter: Characterization and controllability. Environ. Sci. Technol. 2005, 39, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.; Dysert, L.R.; Bates, J.; Burton, D.; Creese, R.C.; Hollmann, J. Cost Estimate Classification System—As Applied in Engineering, Procurement, and Construction for the Process Industries; AACE, Inc: Morgantown, WV, USA, 2011. [Google Scholar]



| Parameters | Case#1(C#1) | Case#2(C#2) | Case#3(C#3) | Case#4(C#4) | ||||
|---|---|---|---|---|---|---|---|---|
| Electrical Stage | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd |
| Voltage, (V) | 14 | 13 | 19 | 19 | 23 | 21 | 26 | 24 |
| Current, (A) | 4.7 | 3.6 | 6.7 | 4.8 | 8.3 | 4.8 | 9.5 | 4.9 |
| Current Density, (mA/cm)2 | 1.5 | 1.1 | 2.1 | 1.5 | 2.6 | 1.5 | 3.0 | 1.5 |
| Salt Removal, (%) | 29.6% | 22.3% | 37.3% | 42.2% | 46.9% | 48.1% | 55.5% | 59.4% |
| Salt Removal Rate, (g/hr) | 571 | 302 | 719 | 510 | 904 | 492 | 1070 | 509 |
| Energy Consumption, (kWh) | 65.8 | 46.8 | 127.3 | 91.2 | 193.2 | 98.7 | 244.4 | 117.6 |
| Energy Consumption, (kWh/kg salt) | 0.12 | 0.15 | 0.18 | 0.18 | 0.21 | 0.20 | 0.23 | 0.23 |
| Na+, (mg/L) | 244 | 194 | 204 | 126 | 191 | 97 | 169 | 75 |
| Cl−, (mg/L) | 279 | 205 | 204 | 101 | 171 | 72 | 147 | 50 |
| Parameters | Baseline (UF/RO) | 2-Stage EDR | 4-Stage EDR | ||
|---|---|---|---|---|---|
| EDR (WATSYS) | EDR (Testing) | EDR (WATSYS) | EDR (Testing) | ||
| Feed Water Flow (mgd) | 1 | 1 | 1 | 1 | 1 |
| Feed Water Na (mg/L) | 235 | 235 | 235 | 235 | 235 |
| % Flow Treated | 60.5% | 69.0% | 100.0% | 69.0% | 78.0% |
| Overall Recovery | 88% | 93% | 92% | 93% | 92% |
| Unit Recovery | 85% | 90% | 90% | 90% | 90% |
| Blended Water Flow (mgd) | 0.88 | 0.93 | 0.92 | 0.93 | 0.92 |
| Product Water Na (mg/L) | 110 | 110 | 129 | 110 | 110 |
| Product TDS (mg/L) | 530 | 522 | 489 | 522 | 433 |
| Concentrate Flow (gpm) | 60 | 48 | 69 | 48 | 54 |
| Concentrate TDS (mg/L) | 7530 | 9662 | 7130 | 9662 | 9662 |
| Concentrate Na (mg/L) | 1524 | 2136 | 1715 | 1927 | 1715 |
| Number of Product Line | - | 7 | 8 | 7 | 6 |
| Number of Stages | - | 2 | 2 | 4 | 4 |
| Parameters | Baseline (UF/RO) | 2-Stage EDR | 4-Stage EDR | ||
|---|---|---|---|---|---|
| EDR (WATSYS) | EDR (Testing) | EDR (WATSYS) | EDR (Testing) | ||
| UF | $599,000 | $- | $- | $- | $- |
| Residuals Handling | $765,000 | $- | $- | $- | $- |
| RO | $889,000 | $- | $- | $- | $- |
| EDR | $ - | $1,820,000 | $2,080,000 | $2,520,000 | $2,940,000 |
| Building | $652,000 | $435,000 | $435,000 | $435,000 | $435,000 |
| Civil Site Works (5%) | $146,000 | $113,000 | $126,000 | $148,000 | $169,000 |
| Electrical and I&C (25%) | $726,000 | $564,000 | $629,000 | $739,000 | $844,000 |
| Contingency (30%) | $1,189,000 | $924,000 | $1,030,000 | $1,210,000 | $1,382,000 |
| General Conditions: Mobilization & Demobilization (5%) | $189,000 | $147,000 | $164,000 | $193,000 | $220,000 |
| Engineering, Administration, and Legal (18%) | $928,000 | $721,000 | $804,000 | $944,000 | $1,078,000 |
| Total Capital Costs ($) | 6,081,000 | 4,721,000 | 5,266,000 | 6,187,000 | 7,066,000 |
| Unit Capital Costs ($/gpd) | 6.88 | 5.05 | 5.72 | 6.64 | 7.68 |
| Total Power Cost ($/year) | 84,000 | 28,000 | 20,000 | 44,000 | 28,000 |
| Total Chemical Cost ($/year) | 69,000 | 67,000 | 67,000 | 67,000 | 67,000 |
| Total Labor Cost ($/year) | 126,000 | 126,000 | 126,000 | 126,000 | 126,000 |
| Total Replacement Cost ($/year) | 17,000 | 14,000 | 13,000 | 14,000 | 13,000 |
| Contingency (20%) | 59,000 | 47,000 | 45,000 | 50,000 | 47,000 |
| Total O&M Costs | 353,000 | 280,000 | 269,000 | 299,000 | 278,000 |
| Unit O&M Costs ($/kgal) | 1.09 | 0.82 | 0.80 | 0.88 | 0.83 |
| Total Life Cycle Costs ($) | 12,800,000 | 10,100,000 | 10,400,000 | 11,900,000 | 12,400,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; He, Q.; Ma, G.; Wang, H.; Nirmalakhandan, N.; Xu, P. Pilot Demonstration of Reclaiming Municipal Wastewater for Irrigation Using Electrodialysis Reversal: Effect of Operational Parameters on Water Quality. Membranes 2021, 11, 333. https://doi.org/10.3390/membranes11050333
Xu X, He Q, Ma G, Wang H, Nirmalakhandan N, Xu P. Pilot Demonstration of Reclaiming Municipal Wastewater for Irrigation Using Electrodialysis Reversal: Effect of Operational Parameters on Water Quality. Membranes. 2021; 11(5):333. https://doi.org/10.3390/membranes11050333
Chicago/Turabian StyleXu, Xuesong, Qun He, Guanyu Ma, Huiyao Wang, Nagamany Nirmalakhandan, and Pei Xu. 2021. "Pilot Demonstration of Reclaiming Municipal Wastewater for Irrigation Using Electrodialysis Reversal: Effect of Operational Parameters on Water Quality" Membranes 11, no. 5: 333. https://doi.org/10.3390/membranes11050333
APA StyleXu, X., He, Q., Ma, G., Wang, H., Nirmalakhandan, N., & Xu, P. (2021). Pilot Demonstration of Reclaiming Municipal Wastewater for Irrigation Using Electrodialysis Reversal: Effect of Operational Parameters on Water Quality. Membranes, 11(5), 333. https://doi.org/10.3390/membranes11050333








