Abstract
The similar physico-chemical properties of propylene and propane molecules have made the separation process of propylene/propane challenging. Membrane separation techniques show substantial prospects in propylene/propane separation due to their low energy consumption and investment costs, and they have been proposed to replace or to be combined with the conventional cryogenic distillation process. Over the past decade, organosilica membranes have attracted considerable attention due to their significant features, such as their good molecular sieving properties and high hydrothermal stability. In the present review, holistic insight is provided to summarize the recent progress in propylene/propane separation using polymeric, inorganic, and hybrid membranes, and a particular inspection of organosilica membranes is conducted. The importance of the pore subnano-environment of organosilica membranes is highlighted, and future directions and perspectives for propylene/propane separation are also provided.
1. Introduction
Propylene (C3H6) plays a vital role in industrial applications because it is widely used for the production of downstream chemicals that are closely related to daily life [1,2]. For instance, most propylene is utilized to produce polypropylene and for the manufacture of commodities [1]. The annual production of C3H6 was approximately 100 million tons worldwide in 2016 and is expected to grow at a rate of 3.6% by 2025 [3]. To obtain high-purity C3H6, the separation of C3H6 from other components, such as propane (C3H8), is inevitable and is currently conducted by the cryogenic distillation process [1,2,4]. Nevertheless, the cryogenic distillation process consumes substantial energy due to the quite similar physical properties of C3H6 and C3H8 molecules (Table 1). Two huge splitter columns of 180 trays are usually used for the separation of C3H6/C3H8 in industry, which creates high capital and operating costs [5]. The intensive energy required for this process is estimated to equal the annual energy expenditure of Singapore [2]. In general, energy consumption is about 40% of total energy used in petrochemicals, estimated at about 1014 Btu per year. It also has an investment cost of over $50 million [6]. For this reason, even minor optimization of the purification process would have a significant impact on the energy savings for processing C3H6 and C3H8 mixtures [7]. Consequently, it is urgent to exploit new separation techniques with lower energy consumption.

Table 1.
Summary of physical properties of C3H6 and C3H8 molecules.
Alternatively, separation processes based on absorption [8,9], adsorption [10,11], and membranes [12,13] have been extensively studied with the target of energy-efficient C3H6 purification. In comparison to the cryogenic distillation, these energy-saving techniques have shown much prospects in C3H6/C3H8 separation. Gas adsorption is a spontaneous process in which gas molecules adhere to the surface of the adsorbents. Indeed a lot of materials have been developed and displayed tremendous promise for propylene/propane separation, some issues still need to be addressed for further practical separation [7]. For instance, the high cost of the synthesized adsorbents and the regeneration issues after the adsorption. Membrane-based separation processes have attracted considerable attention due to their intrinsic advantages of lower energy use, continuous operation, and low investment costs [12,13,14,15]. In comparison to the energy-intensive cryogenic distillation, membrane based technology would use 90% less energy than distillation process [2]. A membrane with a C3H6 permeability of 1 barrer and C3H6/C3H8 selectivity of 35 was believed to have the high potential to compete with industrial cryogenic distillation [16,17]. However, it should be pointed out that permeance rather than the permeability is the direct indicator of real membrane-based separation performance, since the membrane thickness has no effect. In addition, a hybrid membrane-distillation configuration was proposed to improve the economics of C3H6/C3H8 separation. Koros and Lively clarified the target performance for a membrane to debottleneck the cryogenic distillation in a hybrid membrane-distillation configuration [18]. An economically acceptable process would require a membrane with a minimum C3H6 permeability of 10 barrer and C3H6/C3H8 selectivity of 20. Consequently, a variety of membrane materials have been developed, such as the polymeric membranes [19,20,21,22,23,24,25], mixed matrix membranes (MMMs) [26,27,28,29,30], carbon molecular sieve (CMS) membranes [31,32,33], zeolite membranes [34,35], and zeolitic imidazolate framework (ZIF) membranes, as illustrated in Figure 1a. However, the existence of intrinsic drawbacks still hampers the real application of these membranes for separating C3H6 and C3H8 mixtures. For instance, the large scale production of these membranes for C3H6/C3H8 separation is still difficult to be achieved. Organosilica membranes, an important part of the membrane family, have shown great applicability on various separation processes, including pervaporation, vapor permeation, reverse osmosis, and gas separations [36,37,38,39,40].
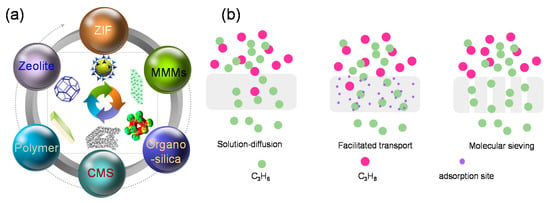
Figure 1.
(a) Overview of the various membrane materials for C3H6/C3H8 separation. (b) General permeation mechanisms for C3H6/C3H8 separation through membranes.
Generally, the mechanisms of C3H6/C3H8 separation through membranes can be classified into three categories, as presented in Figure 1b [13,41]. Polymeric membranes are dominated by a solution-diffusion model, where the adsorbed C3H6 and C3H8 molecules dissolve into the membrane materials and diffuse to the permeate side, driven by a concentration, pressure, or temperature gradient [42]. Another separation mechanism was attributed to the affinity between the membrane and the C3H6 molecules [43]. The specific affinity (formation of σ or π bonds) between the incorporated metal ions in the facilitated transport membranes and C3H6 molecules accelerated the preferential permeation of C3H6. Additionally, the permeation of C3H6 molecules through (organo)silica membranes can also be enhanced by the generation of hydrogen bonds between the silanol (Si-OH) groups and the π bonds in C3H6 [44,45]. The third separation mechanism is accepted as molecular sieving, which discriminates between C3H6 and C3H8 based on their molecular sizes or shapes. This mechanism is common in the CMS, ZIF-8, and organosilica membranes. Based on the discussion above, both the membrane’s molecular sieving properties and the affinities between C3H6 and the membrane matrix play decisive roles in the promotion of C3H6/C3H8 separation with an elevated efficiency.
In the past several decades, a wide variety of membranes have been extensively exploited, and many reviews have been presented that discuss C3H6/C3H8 separation. However, most of these reviews focused only on the polymeric membranes and ZIF-8 membranes. To the best of our knowledge, however, few specific papers have reviewed the development and the current status of C3H6/C3H8 separation through inorganic membranes, especially for the organosilica membranes. The fast development and the increased interest on the organosilica materials and membranes prompted us to summarize the current progress and consider the applications of C3H6/C3H8 separation more deeply. Holistic insight is first provided to summarize the propylene/propane separation using organosilica membranes. A brief summary of polymeric membranes and other types of inorganic membranes is also provided. Furthermore, the importance of the pore subnano-environment of organosilica membranes is highlighted, and future directions and perspectives for C3H6/C3H8 separation using organosilica membranes are presented.
2. Current Membrane Materials for C3H6/C3H8 Separation
2.1. Polymeric Membranes
The low costs and easy processability have widened the applications of polymeric membranes. Polyimide membranes, obtaining high mechanical properties, good thermal stability, and high chemical tolerance, are usually prepared from the polymerization reaction of dianhydride and diamine precursors [46]. However, the low permeability and plasticization effect at high pressure hamper the long-term operations [19,47]. Facilitated transport membranes were fabricated from the incorporation of metals with the target of overcoming the drawbacks of traditional polymeric membranes, such as low permeability and selectivity [5,48]. In comparison with the polymeric membranes, facilitated transport membranes display much potential for C3H6/C3H8 separation. Nevertheless, the further applications of these membranes were still restricted by the instability of the carriers.
2.1.1. Polyimide Membranes
Polyimide (PI) membranes prepared from the polymerization reaction between di-anhydride and diamine precursors exhibit high chemical and mechanical stabilities. Thus, they have attracted considerable attention and are applied in C3H6/C3H8 separation [46]. A solution casting method was generally used for the fabrication of PI membranes. PI solutions were obtained by the polycondensation reaction using dianhydrides and diamines. Subsequently, the PI solution was casted onto the support and then vacuum drying process was further conducted. Finally, the supported PI membranes were fabricated. In Table 2, the C3H6/C3H8 separation performances of some representative polymeric membranes are listed. Nevertheless, the swelling and plasticization problems still perplex researchers and restrict further industrial scale-up. Koros’s group studied the permeation of C3H6/C3H8 mixtures through 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA)-derived PI membranes [20]. The experimental results indicated that the separation performance is highly related to the types of precursors used during the membrane synthesis process. The fabricated PI membranes showed more permeability of C3H6 than C3H8, and the selectivity of C3H6/C3H8 ranged from 10–16. Nevertheless, the selectivity of C3H6/C3H8 mixtures was almost 50% lower than the ideal gas selectivity, even at low feed pressures. To solve the problem of plasticization in PI membranes, Das et al. proposed an approach to suppress the plasticization effect via a finely controlled annealing procedure for membrane fabrication [19]. No plasticization phenomenon was evident when the pressure was increased to 483 kPa at 70 °C. However, the low permeabilities of these polyimide membranes must still be improved.

Table 2.
Separation performance of C3H6/C3H8 for polyimide membranes.
Polymers of intrinsic microporosity (PIMs) comprising relatively inflexible macromolecular architectures with contortion sites have been shown to simultaneously boost the permeability and selectivity for membrane separation. Triptycene (KAUST-PI-1)- and spirobisindane (PIM-PI-1)-based PI membranes were systematically investigated for C3H6/C3H8 separation. The KAUST-PI-1 membrane displayed an ideal C3H6 permeability of 817 barrer with a C3H6/C3H8 selectivity of 16, allowing it to outperform the reported PI membranes [47]. The C3H6/C3H8 selectivity in the binary separation system, however, significantly dropped to 5 at a C3H6 partial pressure of 200 kPa because of the effect of plasticization and competitive sorption.
2.1.2. Facilitated Transport Membranes
C3H6/C3H8 separation through polyimide membranes is dominated by the solution-diffusion mechanism. However, this mechanism is intrinsically not sufficient for distinguishing C3H6 and C3H8 molecules due to the similar physical properties of C3H6 and C3H8 molecules, as presented in Table 1 [13]. The small difference of the solubility between C3H6 and C3H8 molecules cannot result in effective discrimination. To promote the separation performance of C3H6/C3H8 mixtures, facilitated transport membranes have been exploited. The specific affinity between C3H6 molecules and metal ions cause the metal ions to act as the carriers, which facilitates the preferential sorption and diffusion of C3H6 through the membranes. As a result, both the C3H6 permeance and C3H6/C3H8 selectivity were obviously enhanced. Table 3 illustrates the C3H6/C3H8 separation performance of some facilitated transport membranes. The π-complexation intensity between the metals and the C3H6 molecules affect the carrier abilities of metals, which is determined by the electronegativity of the metal ion. Table 4 illustrates the electronegativities of some transition metals [5,48]. Generally, the higher electronegativity of a metal is, the stronger the intensity of the π-complexation becomes. Nevertheless, the excessive electronegativities of metals restrict the reversible reactivity and makes the desorption process of C3H6 more difficult. In contrast, metals with low electronegativities cannot provide a sufficiently high affinity for C3H6 molecules. The suitable electronegativity is reportedly in the range of 1.6–2.3, as studied by Kang et al. [48].

Table 3.
Separation performance of C3H6/C3H8 for facilitated transport membranes.

Table 4.
Electronegativity of the transition metals [5].
Liao et al. explored C3H6/C3H8 separation behaviors by incorporating metal ions such as Zn2+, Mg2+, and Ag+ into PIMs [22]. Zn2+ increased the membrane permeabilities by enlarging the free volume of the matrix, while the affinity between the membranes and C3H6 molecules was enhanced after modification with Mg2+. The Ag+-functionalized PIMs resulted in a facilitated transport mechanism for the permeation of C3H6. In short, metal-ion-incorporated PIM membranes showed higher C3H6/C3H8 selectivities than those of pristine PIM membranes. The specific data for pristine and metal-modified PIM membranes for C3H6/C3H8 separation are shown in Figure 2. Kasahara et al. also reported facilitated transport in an ion-gel membrane with a high permeability of C3H6, which was attributed to the addition of silver ions [23]. However, the stability of facilitated transport membranes in C3H6/C3H8 separation requires further improvement. Jose et al. found that the C3H6/C3H8 selectivity of Ag-doped polymer membranes continuously decreased with the operation time due to the reduction of Ag+ during the reaction [24]. The agglomeration of Ag may lead to the appearance of defects, which is detrimental to the membrane separation performance.
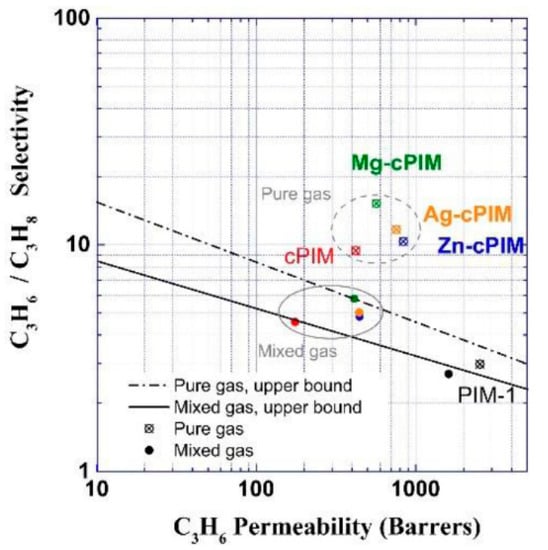
Figure 2.
Trade-off of C3H6/C3H8 separation performance of pristine and metal-modified polymer of intrinsic microporosity (PIM) membranes [22].
2.2. Inorganic Membranes
In comparison to polymeric membranes, inorganic membranes composed of molecular sieves, such as CMSs, zeolites, and zeolite imidazolate frameworks (ZIFs), are important candidates for C3H6/C3H8 separation because of their high separation performances and excellent chemical and thermal stabilities. Table 5 briefly summarizes the characteristics and intrinsic drawbacks of these membranes.

Table 5.
Characteristics and intrinsic drawbacks of inorganic membranes used for C3H6/C3H8 separation.
2.2.1. Carbon Molecular Sieve Membranes
CMS membranes fabricated from the pyrolysis treatment of polymers with intrinsic porosity yield an amorphous network structure and relatively broader distribution of the pore size [49]. The high processability also makes CMS membranes prepared via the pyrolysis of polymeric precursors suitable for C3H6/C3H8 separation. Steel and Koros examined the effect of the precursors and pyrolysis temperature on the microstructure and gas separation properties of CMS membranes [31]. The 6FDA/BPDA-DAM-derived membrane exhibited C3H6 permeability of 200 barrer and C3H6/C3H8 selectivity of 100 than Matrimid®-derived CMSs (permeability of 0.1 barrer and selectivity of 10, respectively) because of the larger fractional free-volume. In addition, the 6FDA-BPDA-DAM membrane pyrolyzed at 550 °C also exhibited much higher separation performances (permeability of 200 barrer and selectivity of 100) than that pyrolyzed at 800 °C (permeability of 1.3 barrer and selectivity of 7.9). A hypothetical model was proposed to explain this phenomenon, as shown in Figure 3. Both C3H6 and C3H8 could only permeate through the CMS membranes via the pores, as shown at the tail end of the hypothetical pore size distribution curve. However, the number of effective pores drastically decreased when the pyrolysis temperatures increased from 550 to 800 °C, and as a result, undesirable losses in permeability and selectivity were observed. Subsequently, Xu et al. found that the permeance decreased for the Matrimid®-derived CMS fiber membranes because of the increased thickness attributed to the structure collapse [32]. To suppress the collapse of the morphological structure for the CMS membrane, pre-pyrolysis treatment via a sol–gel crosslinking reaction was conducted [51]. A significant reduction in membrane thickness of up to 5–6 times (from ~16–17 to ~3–4 μm) was achieved, resulting in an efficient elevation in the membrane permeance.
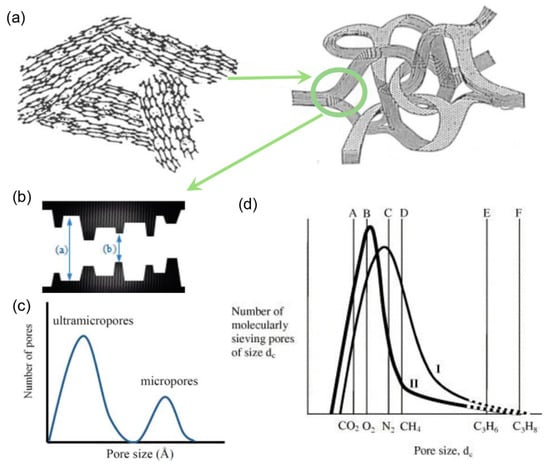
Figure 3.
(a) Structure of pyrolytic carbon material [51]. (b) Schematic image of the “slit-like” structure and (c) the bimodal pore size distribution [49]. (d) Hypothetical semi-quantitative ultramicropore size distribution of CMS membrane [31].
The thickness can also affect the microstructure and separation properties of the CMS membrane [33]. The 6FDA-derived CMS membranes with thicknesses of 520 nm have exhibited C3H6/C3H8 permeance ratios of ~31 and C3H6 permeances of ~1.0 × 10−8 mol m−2 s−1 Pa−1 (~30 GPU, 1GPU = 3.348 × 10−10 mol m−2 s−1 Pa−1). As the thickness decreased to 300 nm, the permeance ratio of He/N2 increased, while that of C3H6/C3H8 decreased. The hypothetical qualitative model of the pore size distribution for CMS membranes proposed by Steel and Koros [31] was suitable for explaining this experimental phenomenon. The micropore size distribution was transformed to a region characterized by a smaller pore size as the membrane thickness decreased, which resulted in a significant reduction in the effective number of micropores available for C3H6 permeation. The scale-up of C3H6/C3H8 separation using CMS membranes, however, was difficult to achieve due to the low oxygen resistance and fragility. The specific performances of some CMS membranes are provided in Table 6.

Table 6.
Separation performance of C3H6/C3H8 for CMS membranes.
2.2.2. Zeolite Membranes
Zeolite membranes are drawing enormous interest due to their controllable pore sizes and strong adsorption properties. Different types of zeolite membranes have been extensively implemented for gas separations [34,35]. The faujasite (FAU)-type zeolite membranes with low Si/Al ratios were fabricated and applied for hydrocarbon separations for the first time by Nikolakis et al. [54]. The resultant membrane exhibited unsaturated hydrocarbon selectivity properties for benzene/cyclohexane, benzene/n-hexane, C3H6/C3H8, toluene/n-heptane, and ethylene/methane pairs. The size exclusion and the strong affinity between unsaturated hydrocarbons and zeolite cations are responsible for this. Giannakopoulos et al. also synthesized FAU membranes with thicknesses of 20 μm and examined the effect of operating temperatures and feed compositions for C3H6/C3H8 separation in detail [55]. A separation factor of 13.7 ± 1 was attained at 100 °C. Interestingly, the propane permeation was enhanced by the existence of propylene molecules in binary mixtures when the differences between the temperature-dependent single and binary C3H6/C3H8 separations were carefully analyzed.
Inspired by the facilitated transport mechanism, Sakai et al. reported an Ag-exchanged zeolite membrane that exhibited a high C3H6/C3H8 selectivity of 55 with a C3H6 permeance of 4.1 × 10−8 mol m−2 s−1 Pa−1 (122.5 GPU) at 80 °C, as shown in Figure 4 [56]. The C3H6/C3H8 selectivity was improved after the exchange of Na to Ag cations. The stability of Ag-exchanged zeolite membranes for C3H6/C3H8 separation was evidenced by the temperature-cycle measurements of binary gas separations. More importantly, no distinct decrease in membrane performance was measured after discontinuous use for more than one month. The authors claimed that the strong affinity between C3H6 and Ag cations was significant for the high separation performance. The adsorption properties of C3H6 and C3H8 on Ag-exchanged zeolite membranes confirmed the contribution of adsorption selectivity to the C3H6/C3H8 separation performance [57]. In view of the good consistency between the calculated C3H6 purity in the adsorbed phase and the C3H6 purity downstream of the Ag-exchanged zeolite membrane, the permeation of this membrane was estimated to be governed by the adsorption selectivity. However, non-selective defects are always formed in the zeolite membranes during the hydrothermal synthesis and high-temperature calcination process. The presence of these defects largely deteriorated the membrane separation performance. Consequently, the patching of defects in zeolite membranes has attracted much attention. Many strategies have been proposed to repair the defects, such as surface coating, chemical vapor deposition, coke deposition, rapid thermal processing, and hydrothermal treatment [58].
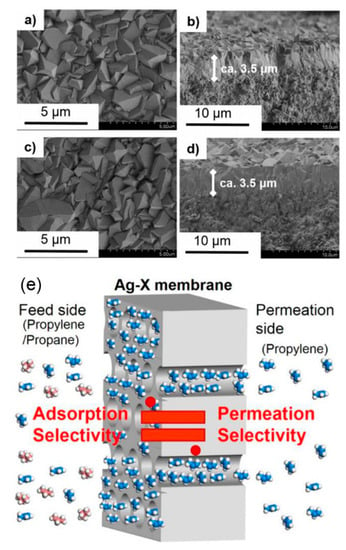
Figure 4.
Typical field-emission scanning electron microscopy (FE-SEM) images of (a,b) Na-X and (c,d) Ag-X membrane ion exchanged with 10 mM AgNO3 solution [56]. (e) Schematic illustration of the relationship between the adsorption selectivity and permeation selectivity of the Ag-exchanged zeolite membrane [57].
2.2.3. Graphene Membranes
Graphene, a 2D material consisting of sp2-hybridized carbon atoms, have been utilized for membrane-based gas separations after creating effective pores, since pristine graphene is inherently impermeable for any gases [59,60]. As one of the most promising materials, graphene, has attracted considerable attention. However, few practical graphene membranes have been reported for applications in C3H6/C3H8 separation. Nonetheless, some researchers have tried to predict the C3H6/C3H8 separation properties by utilizing molecular simulation approaches [61,62]. Jiang et al. conducted molecular dynamics and first-principles density functional theory to examine the separation behaviors of porous graphene membranes for C3H6 and C3H8 mixtures [61]. Molecular models of porous graphene membranes with different numbers of pores were attained by drilling carbon atoms on the original graphene, as shown in Figure 5a. In comparison with other membranes, the pore-13 membrane retained a good balance between the C3H6 permeability and C3H6/C3H8 selectivity. Nevertheless, the C3H6/C3H8 selectivity was still unsatisfactory. To further promote the separation performance, the decoration of the pore-13 membrane was implemented with N and H atoms, as shown in Figure 5b. The N-H-decorated membrane exhibited the best separation performance when compared to the N-modified and H-modified membranes. The N-H-decorated membrane possessed an attractive potential energy well for C3H6 and an energy barrier for C3H8, which enabled the easier permeation of C3H6 through the pores. Although the current experimental study of the utilization of practical porous graphene membranes for C3H6/C3H8 separation has progressed slowly, future work is still anticipated to be accelerated by the continuous development of advanced techniques.
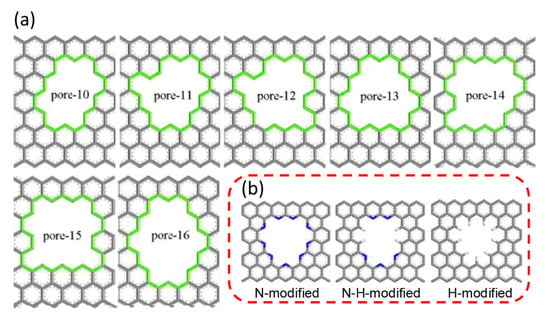
Figure 5.
(a) Structure of porous graphene models. (b) Structure of the modified pore-13 [61].
2.2.4. Zeolitic Imidazolate Framework (ZIF) Membranes
Metal organic framework (MOF) membranes have received a great deal of attention over the years due to their potential for use in high-efficiency gas and liquid purification [15,63,64]. Among the large family of MOF membranes, ZIF membranes, consisting of zinc ions bridged with the imidazolate ligands show great potential for hydrocarbon separations [63,65,66,67,68,69,70]. In this section, we mainly focus on the development of ZIF-8 and ZIF-67 membranes for the C3H6/C3H8 separation based on many reported studies. The specific separation performances of ZIF-8 and ZIF-67 membranes are listed in Table 7. Bux et al. first studied the gas permeation performances of ZIF-8 membranes in 2009, which exhibited outstanding H2/N2 and H2/CH4 separation properties [71]. The first report of C3H6/C3H8 separations utilizing ZIF-8 membranes was conducted by Pan et al. [65]. A defect-free ZIF-8 membrane fabricated by a hydrothermal seeded growth method showed superior performance for C3H6 and C3H8 mixtures. At the measurement temperature of −15 °C, a C3H6 permeance of 3 × 10−8 mol m−2 s−1 Pa−1 (90 GPU) and a C3H6/C3H8 selectivity of around 50 were surprisingly achieved. The first successful trial of C3H6/C3H8 separation by ZIF-8 membranes promoted subsequent extensive research work around the world.

Table 7.
Separation performances of C3H6/C3H8 for zeolitic imidazolate framework (ZIF) membranes.
In comparison with the hydrothermal seeded growth method, many other strategies have been exploited to fabricate ZIF-8 membranes for C3H6/C3H8 separation [15,63]. ZIF-8 membranes synthesized by a secondary growth method in aqueous solutions were evaluated based on the gas permeation, diffusion, and adsorption properties of C3H6 and C3H8 [72]. The secondary-growth-method-derived ZIF-8 membranes showed an over 30 times higher diffusivity for C3H6 (1.25 × 10−8 cm2 s−1) than for C3H8 (3.99 × 10−10 cm2 s−1). For C3H6 and C3H8 equimolar mixtures, ZIF-8 membranes exhibited stable separation performances (C3H6 permeance: 1.1 × 10−8 mol m−2 s−1 Pa−1, 33 GPU, C3H6/C3H8 selectivity: 30) during a discontinuous operation process for 40 days. A counter-diffusion concept was proposed by Kwon and Jeong for the in-situ synthesis of ZIF-8 membranes, as shown in Figure 6a [17]. The α-Al2O3 support was immersed in a metal ion solution and then transferred to a solvothermal growth environment. A reaction zone was formed by the diffusion of metal ions and ligands. Subsequently, a nucleation reaction occurred homogeneously in the vicinity of the support, resulting in the generation of high-quality ZIF-8 membranes. The resultant membrane showed a promising C3H6/C3H8 selectivity of 55.
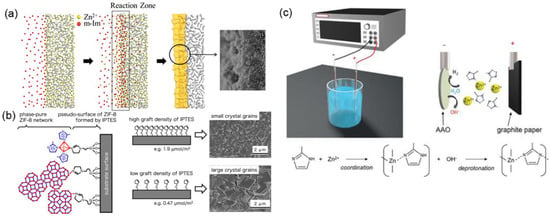
Figure 6.
(a) Schematic illustration of membrane synthesis using the counter-diffusion method [17]. (b) Schematic image of the heterogeneous nucleation and crystal growth on the 3-(2-imidazolin-1-yl)propyltriethoxysilane (IPTES)-modified surface [73]. (c) Illustration of the experimental apparatus of atomic layer deposition (ALD) for the fabrication of ZIF-8 membranes [69].
Tanaka et al. reported a seeding-free aqueous synthesis strategy for the preparation of ZIF-8 membranes with controlled grain sizes [73]. The 3-(2-imidazolin-1-yl)propyltriethoxysilane (IPTES), acting as an imidazolate linker, was used to modify the surface of the membrane support. It was found that the grain sizes of the ZIF-8 membranes could be well controlled by fine-tuning of the grafting density of IPTES, as revealed in Figure 6b. The evaluation of single gas permeation measurement clearly indicated that the ideal C3H6/C3H8 selectivity increased with the IPTES density and approached 36, with a C3H6 permeance of 8.5 × 10−8 mol m−2 s−1 Pa−1 (254 GPU). Recently, an aqueously cathodic (ACD) deposition strategy was developed for the preparation of ZIF-8 membranes by Wei et al., as shown in Figure 6c [69]. No electrolyte, modulator, or organic solvent was added during the synthesis procedure, and thus, this is an eco-friendly and energy-saving approach. The resultant ZIF-8 membranes exhibited high C3H6/C3H8 separation performances (6.1 × 10−8 mol m−2 s−1 Pa−1 C3H6 permeance (182 GPU) and 142 C3H6/C3H8 selectivity), demonstrating great potential for the scalable application of ZIF-8 membranes.
It is well known that the pressure-resistance properties and the defect issues are challenging for ZIF-8 membranes in C3H6/C3H8 separation [66]. Yu et al. reported the dramatic deterioration of the C3H6/C3H8 selectivity (from 61 to 14) as the transmembrane pressure drop increased (from 0 to 300 kPa), which may be attributed to the network flexibility rather than the permanent formation of defects because the separation performance could be easily recovered when the transmembrane pressure drop decreased. To solve this problem, Sheng et al. proposed a coating strategy using polydimethylsiloxane (PDMS) to simultaneously hinder the flexibility and patch the defects of ZIF-8 membranes, as shown in Figure 7a,b [74]. ZIF-8 membranes with PDMS coatings showed significantly enhanced C3H6/C3H8 selectivities and improved pressure resistance properties. The C3H6/C3H8 selectivity of hybrid PDMS-ZIF-8 membranes showed a slight increase from 93 to 105 when the transmembrane pressure increased from 0 to 600 kPa and could easily return to the initial level as the pressure reaches 0. In a subsequent study, Li et al. adopt a similar coating strategy to synthesize PDMS-ZIF-8 membranes on the porous tubular α-Al2O3 support for C3H6/C3H8 separation [75]. The effect of the operating pressure, temperature, and permeation conditions (with or without a sweep gas and a vacuum on the permeation side, as shown in Figure 7c–e, respectively) were carefully assessed. The permeation/separation behaviors of C3H6/C3H8 were found to be highly dependent on the permeation conditions. The conditions with a sweep gas and a vacuum pump were beneficial for the C3H6 permeation and the improved C3H6/C3H8 selectivity due to the quick removal of permeated C3H6. However, the operation with a vacuum pump was more desirable in practical applications than that using sweep gas, where the further separation of C3H6 and sweep gas is necessary.
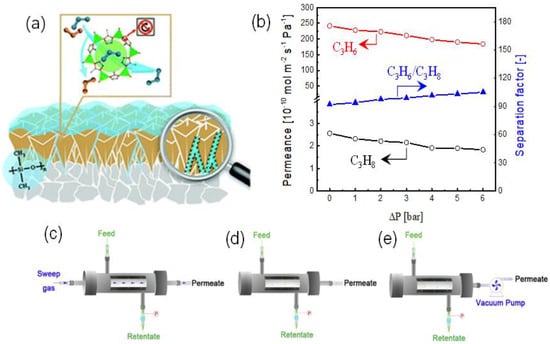
Figure 7.
(a) Schematic illustration of the PDMS-coated ZIF-8 membrane [74]. (b) Separation properties of C3H6/C3H8 as a function of the transmembrane pressure [74]. Permeation conditions for C3H6/C3H8 separation (c) with sweep gas, (d) without sweep gas, and (e) with vacuum pump [75].
In addition to the extensively studied ZIF-8 membranes, ZIF-67 membranes were also confirmed to be alternative candidates for C3H6/C3H8 separation [15,68,76]. Jeong et al. fabricated well inter-grown ZIF-67 membranes via heteroepitaxially growing ZIF-67 using ZIF-8 as the seed layers (Figure 8a), which exhibited an ultrahigh C3H6/C3H8 selectivity of approximately 200 along with a C3H6 permeance of 3.7 × 10−8 mol m−2 s−1 Pa−1 (106 GPU) [76]. In comparison with classical ZIF-8 membranes, the network structures of the ZIF-67 membranes were more rigid because the shorter Co-N bonds substituted the Zn-N bonds in the ZIF-8. However, few papers focused on using ZIF-67 membranes for C3H6/C3H8 separation with high selectivity. The low separation performance was possibly attributed to the poor grain boundary structure derived from the ultrafast crystallization reaction. In a recently published paper, Hou et al. proposed a crystal engineering strategy to balance the grain boundary structure and the flexibility within ZIF-67 membranes [68]. The Zn2+ was incorporated into the framework of ZIF-67 membranes to fabricate a series of bimetallic ZIFs membranes with different Co2+/Zn2+ ratios, as revealed in Figure 8b. A high C3H6/C3H8 separation performance could not be obtained in ZIFs membranes with Co2+/Zn2+ ratios that were too high or too low. The optimal balance between the grain boundary structure and the flexibility was attained by the membrane with a Co2+/Zn2+ ratio of 18/82, which exhibited a C3H6/C3H8 selectivity of around 175 with a C3H6 permeance of 2 × 10−8 mol m−2 s−1 Pa−1 (60 GPU).
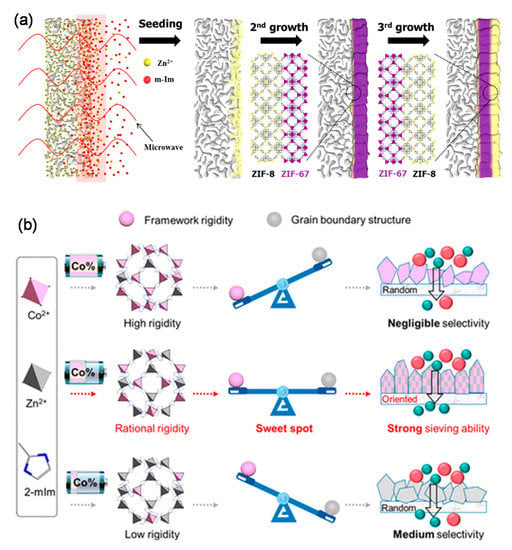
Figure 8.
(a) Schematic illustration of the membrane synthesis via heteroepitaxial growth [76]. (b) Schematic illustration of the design of bimetallic Zn(100-x)CoxZIF membranes [68].
2.3. Mixed Matrix Membranes (MMMs)
To combine the advantages of a superior porosity, high permeance of inorganic membranes, easy processability, and low cost of polymeric membranes, MMMs were intensively implemented for the target molecular separation [26]. Liu et al. reported the “conformation-controlled molecular sieving effect” on the C3H6/C3H8 separation of MMMs [27]. The incorporated Zr-fum-fcu-MOF in the 6FDA-DAM matrix, which had a contracted triangular pore-aperture, efficiently improved the C3H6/C3H8 separation performance and overcame the problem of the plasticization effect. The separation properties (C3H6 permeance: 20–30 barrer, C3H6/C3H8 selectivity: 15–20) far exceeded the upper bound of polymer membranes. Zhang et al. fabricated ZIF-8/6FDA-DAM membranes on the hollow fiber supports for possible scale-up applications [28]. The as-synthesized MMMs with a 30 wt% ZIF-8 loading exhibited a high C3H6/C3H8 selectivity of around 27.5. However, the C3H6 permeance was much lower than those of pure ZIF-8 membranes. Furthermore, the maximum loading of inorganic fillers of the aforementioned MMMs was limited to 30 wt% due to compatibility issues. Ma et al. reported that a ZIF-8 incorporated PIM-6FDA-OH membrane with a high loading ratio up to 65 wt% demonstrated a satisfying C3H6/C3H8 selectivity of 28.7 together a C3H6 permeability of 30 barrer and good plasticization resistance at a feed pressure of 700 kPa [29]. The improved compatibility was attributed to the (N···O-H)-induced hydrogen bonding between the ZIF-8 filler and the PIM-6FDA-OH matrix, as confirmed by Fourier-transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) characterization.
The reported MMMs exhibited higher C3H6 permeances and C3H6/C3H8 selectivities than those of pure polymer membranes, yet the performances were inherently restrained by the bulk polymer matrix. To exceed this limitation, Rashidi et al. first proposed the concept of “all-nanoporous hybrid membranes” based on ZIF-8 and MFI, in which both the dispersed and continuous phases were nanoporous materials [30]. The separation performance of the all-nanoporous ZIF-8/MFI membrane exceeded those of the individual ZIF-8 and MFI membranes, as well as those of traditional MMMs. The high-level and the proposed concept of C3H6/C3H8 separation redefined the upper bound and initiated a new generation of membranes for molecular separation applications. The characteristics and the intrinsic disadvantages of general MMMs and the nanoporous hybrid membranes are summarized in Table 8. The detailed separation properties of MMMs were summarized in Table 9.

Table 8.
Characteristics of mixed matrix membranes (MMMs) used for C3H6/C3H8 separation.

Table 9.
Separation performance of C3H6/C3H8 for MMMs.
Most of the reported MMMs are synthesized by mixing two individual phases (a dispersed phase and a continuous phase). However, the critical conditions must be controlled to suppress the possible generation of interfacial voids. Recently, the concept of in-situ growth of MOFs in a polymer matrix was proposed for the fabrication of MMMs for gas separation [78,79,80]. As revealed in Figure 9, four steps are required: polymer hydrolysis, ion exchange, ligand treatment, and imidization for the in situ formation of MMMs [77]. The in situ growth method potentially eliminates the formation of interfacial voids and enhances the dispersity of inorganic fillers in the polymer matrix. In addition, the resultant novel MMMs showed much higher C3H6/C3H8 selectivities than that of the conventional MMMs. The authors also demonstrated the possible scalability of the MMMs derived by the in situ growth method using commercial hollow fiber membranes, which also exhibited acceptable C3H6/C3H8 separation properties (C3H6 permeance: 2.17 GPU, C3H6/C3H8 selectivity: ~20).
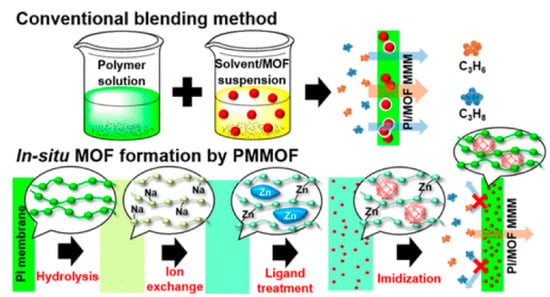
Figure 9.
Schematic image of the in situ formation of metal organic framework (MOF)-doped MMMs [77].
3. Organosilica Membranes
Compared to pure silica membranes, organosilica membranes exhibit enhanced hydrothermal stability, lower permeation resistance, and wider applicability due to the incorporation of organic groups [81,82,83,84]. Since the first round of the successful application of organosilica membranes derived from bis(triethoxysilyl)ethane (BTESE) in 2008 [85], the research interest on silica membranes has changed and transitioned to the exploitation of novel organosilica membranes. BTESE membranes obtained from the conventional sol–gel process demonstrated excellent dehydration properties in separating n-butanol/water (95:5) mixtures [85]. The water concentration on the permeation side still remained at 98 wt% during on-stream operation for 1.5 years, showing great promise for practical applications. Our group proposed the concept of the “spacer technique” by the incorporation of bridged organics, which was confirmed to be effective for tailoring the network sizes of conventional silica membranes [36,86]. Subsequently, the number of studies on the gas separation [87,88,89,90,91,92,93], pervaporation [39,90,94], vapor permeation [40,95], and reverse osmosis [37,38,96] using organosilica membranes rapidly increased. Some of the organosilica membranes have also shown great prospects for C3H6/C3H8 separation, and considerable detailed work has been performed [45,89,93,97,98,99,100,101,102]. In this section, we will discuss the progress of C3H6/C3H8 separations using organosilica membranes. The synthesis approaches and the pore subnano-environment engineering of organosilica membranes are summarized in detail.
3.1. Synthesis of Organosilica Membranes
Generally, porous α-Al2O3 ceramics were used as the supports to fabricate organosilica membranes. Prior to the preparation of the top layer, the formation of particle layer and intermediate layer are necessary to reduce the pore size, as demonstrated in Figure 10a. Once the pore size of the intermediate layer is confirmed to be suitable for the deposition of the top layer, the sol–gel method [36,38,39,45,87,88,89,91,92,93,96,97,98,99,100,101,103] (Figure 10b) or the chemical vapor deposition (CVD) technique [104,105,106,107] (Figure 10c) can be conducted for the fabrication of an organosilica selective layer. In a typical sol–gel process, the polymeric and colloidal sol routes are divided based on the different reaction rates. The relatively lower reaction rate in the polymeric sol route allows partial hydrolysis. As a result, the formed organosilica layer is always a linear type with a micropore size lower than 1 nm. This process is commonly controlled using an acid as the catalyst or dropping a small amount of water for the hydrolysis reaction. In contrast, the utilization of a base as the catalyst and the dropping of excessive amount of water accelerate the reaction rate, generating a colloidal sol with abundant silanol (Si-OH) groups. Consequently, the molecules always permeate the interparticle pores within the membranes derived by the colloidal sol route. The overall time for the fabrication of organosilica membranes via sol-gel strategy is estimated to be around 4 h.
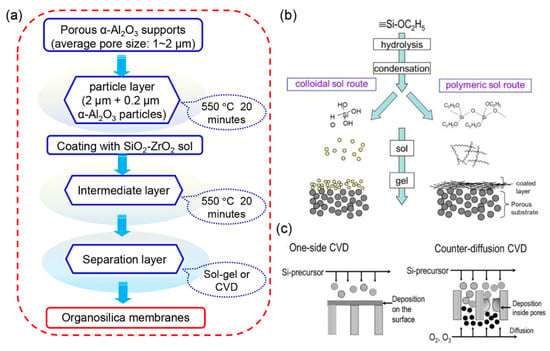
Figure 10.
(a) Schematic illustration of the detailed fabrication process of organosilica membranes. (b) Sol–gel process for the formation of a separation layer including polymeric and colloidal sol routes [81]. (c) One-sided chemical vapor deposition (CVD) and counter-diffusion CVD methods for the deposition of an organosilica layer [81].
Another popular approach for the fabrication of organosilica membranes is the CVD technique, as presented in Figure 10c. The organosilica precursors flow to the surface of the support in a vapor phase, and subsequently, condensation reactions occur at temperature arounds 400–600 °C [107]. Compared to the membranes derived by the sol–gel method with higher permeances and moderate selectivities, the membranes fabricated from the CVD technique often exhibit dense structures with lower permeances but higher selectivities. Even though high-quality membranes can be obtained via the CVD technique, the organic groups have difficulty surviving at high temperatures. Hence, the plasma-enhanced CVD (PECVD) technique was extensively studied to replace the conventional CVD method, which allowed membrane fabrication at much lower temperatures [106,107,108,109]. Nagasawa et al. fabricated a series of organosilica membranes with different O/Si ratios via the PECVD technique [106]. The deposition time of the PECVD method was discussed, which revealed that stable gas permeation properties could be achieved within only 5 min. The PECVD technique effectively reduced the reaction temperature and the time consumption, which contributed to the easy fabrication of organosilica membranes. Nevertheless, no report of the scalable preparation of organosilica membranes used the PECVD strategy. The published papers related to PECVD-derived organosilica membranes are still limited in the lab-scale. In addition, the hollow fibers can be considered to act as the support instead of the ceramics to decrease the fabrication cost in the further studies.
Most of the reported organosilica membranes, however, are fabricated on the expensive inorganic supports, which highly hampers the commercialization process. Therefore, the deposition of organosilica membranes on polymeric support is anticipated to be beneficial for achieving this target. Some successful cases have been reported by Gong et al., where the organosilica sols were spin-coated onto the surface of a porous polymeric support [40,110]. The resultant membranes showed stable dehydration performance for the water/isopropanol mixtures. Nevertheless, the spin-coating process is still not suitable for the industrial application, since it is difficult to be implemented on a large-area polymer membrane. To solve this problem, Gong et al. proposed an approach named “flow-induced deposition” for scalable fabrication of a continuous and uniform organosilica membrane on the porous polymeric support as shown in Figure 11 [111]. The “flow-induced deposition” derived organsilica membranes demonstrate high NaCl rejection in a reverse osmosis desalination process. Interestingly, this membrane still showed high stability and flexibility even rolled into a curvature radius of 11 mm. It is fact that the fabrication of organosilica membranes on polymeric support is feasible, however, the gas molecular sieving properties need to be significantly improved and the mechanical stability of polymeric support should also be carefully considered.
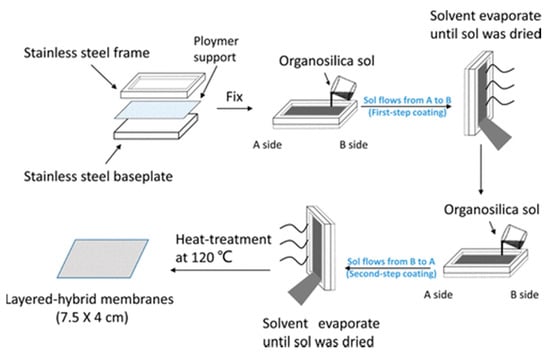
Figure 11.
Schematic diagram for fabrication of organosilica/polymeric support membrane via the “flow-induced deposition” approach [111].
3.2. Pore Subnano-Environment Engineering of Organosilica Membranes
Generally, the pore subnano-environment engineering of organosilica membranes consists of pore size control and affinity control. Pore size control strategy includes spacer technique, incorporation of metal ions, and co-condensation reaction, respectively. In addition, affinity control method includes control of calcination parameters and post treatment.
3.2.1. Pore Size Control
Spacer Technique
The “spacer technique” proposed by our group is effective for the tuning of the membrane pore size, which is beneficial for various types of organosilica precursors [36,86,87,89,93,112]. In comparison with the pure silica membrane derived from tetraethoxysilane (TEOS), the existence of Si-R-Si units (R represents the organic bridge) in the organosilica networks changed the minimum constructive unit, and as a result, the network size can be efficiently adjusted. TEOS, bis(triethoxysilyl)methane (BTESM), and BTESE were selected as the precursors for the preparation of organosilica membranes, as shown in Figure 12a [89]. The single gas permeation properties clearly illustrated the effect of the precursors on the formation of amorphous structures. Based on the modified gas translation model, the average pore size of the BTESM membrane was determined to be between those of the TEOS and BTESE membranes. More importantly, BTESM membranes with bridges of the methane groups showed prospects for C3H6/C3H8 separation (C3H6 permeance of 6.32 × 10−8 mol m−2 s−1 Pa−1, C3H6/C3H8 selectivity around 8.8) due to the controlled network size. However, the C3H6/C3H8 separation performance of BTESM membranes still must be improved, including both the C3H6 permeance and C3H6/C3H8 selectivity.
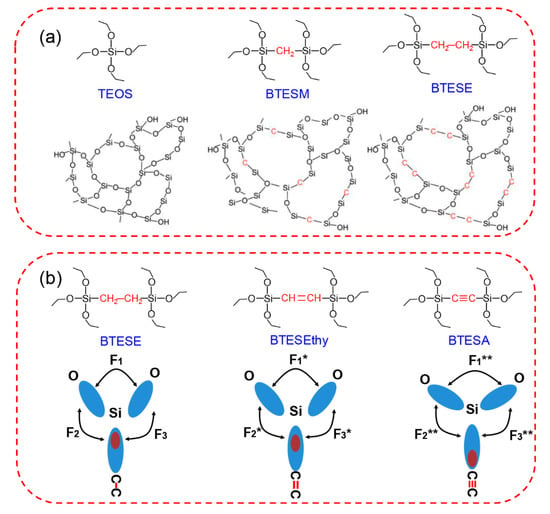
Figure 12.
(a) Schematic illustration of amorphous network structures derived from tetraethoxysilane (TEOS), bis(triethoxysilyl)methane (BTESM), and bis(triethoxysilyl)ethane (BTESE) [89]. (b) Bonding structure model of BTESE-, BTESEthy-, and BTESA-derived networks [93].
Recently, we proposed a novel strategy to tailor the microstructures and permeation properties of organosilica membranes via control of the bond angles, as shown in Figure 12b [93]. Sol–gel derived organosilica membranes with different linking groups (ethane, ethylene, and acetylene) were fabricated using BTESE, bis(triethoxysilyl)ethylene (BTESEthy), and bis(triethoxysilyl)acetylene (BTESA) precursors. The microstructure and gas permeation properties were successfully tailored by controlling the Si-O-Si and Si-CC bond angles. The changes of the Si-CC bond angles were easily confirmed by the different conformations of the bridged groups of BTESE, BTESEthy, and BTESA, which were attributed to the different unsaturated degrees of the bridges. A nearly linear shape of the Si-C≡C bonds indicated the rigid structures of the BTESA membranes. In the case of the Si-O-Si bonds, the bonding structure model was utilized to interpret this interesting evolution trend. The locations of the electrons in the Si-C≡C bonds tended to be much further from Si atoms than those in Si-C-C and Si-C=C bonds, which enhanced the electron pair repulsion (F1** > F1* > F1). The increases in the Si-O-Si and Si-CC bond angles contributed to the construction of a loose and uniform structure, which was also evidenced by the FT-IR spectra. BTESA membranes featured a more open and accessible pore structure, which was found to be suitable for the separation of C3H6/C3H8. BTESA membranes with acetylene-bridged groups showed a superior C3H6 permeance of 1.0–2.0 × 10−7 mol m−2 s−1 Pa−1 (299–597 GPU) with an ideal C3H6/C3H8 selectivity of 11–14 in single gas permeation measurements, demonstrating great potential for membrane-based hydrocarbon separations.
The “spacer technique” has been extensively confirmed to be effective for the adjustment of the membrane network structure by varying the bridged groups. Various kinds of precursors have been utilized from the large organosilica family for the fabrication of membranes. Many exciting achievements have been made, and some of these membranes were successfully meet current industrial demands and expectations [113]. For the specific molecular separation process, however, an objective screening of the organosilica precursors should be carefully conducted.
Incorporation of Metal Ions
The effectiveness of metal incorporation on the adjustment of the membrane pore structures has also been evidenced for organosilica membranes (Figure 13) [100,114]. As reported by Kanezashi et al., the sol–gel technique was applied to prepare Al-BTESM membranes for improved C3H6/C3H8 separation [100]. The 29Si and 27Al magic-angle spinning nuclear magnetic resonance (MAS NMR) spectra shown in Figure 13b,c confirmed the existence of Al in the composite network structures. A satisfyingly high C3H6/C3H8 selectivity of around 40 with a C3H6 permeance of 6.3 ✕ 10−9 mol m−2 s−1 Pa−1 (18.8 GPU) was achieved, which was attributed to the fine adjustment of the membrane pore size by the incorporation of Al in the BTESM-derived networks and/or through coordination with silanol (Si-OH) groups. Subsequently, the effect of the Al content on the network size of composite membranes was investigated [97]. The permeance of each gas molecule decreased, while the selectivity of H2/CH4 and H2/C3H8 increased as the Al content increased. The densified structure was also indicated by the increased activation energies of He, H2, N2, and CH4. However, although the C3H6/C3H8 selectivity was clearly enhanced by the introduction of Al, the significant loss of the C3H6 permeance cannot be ignored. For instance, the C3H6/C3H8 selectivity enhanced from 20 to 30 with an approximately one-order-of-magnitude decrease in the C3H6 permeance (from 6.5 ✕ 10−8 to 6.3 ✕ 10−9 mol m−2 s−1 Pa−1, 194 to 18.8 GPU) at 50 °C when BTESM and Al-BTESM (Si-Al 9:1) membranes were compared.
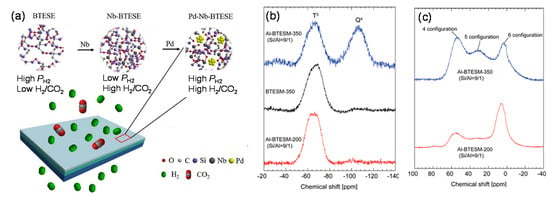
Figure 13.
(a) Schematic image of the possible structures of Pd–Nb–BTESE networks for H2/CO2 separation [114]. (b) 29Si and (c) 27Al magic-angle spinning nuclear magnetic resonance (MAS NMR) spectra for BTESM and Al-BTESM gel powders [100].
Co-Condensation Reaction
Organosilica membranes have exhibited potential for molecule separation. However, sometimes the individual organosilica-precursor-derived membranes cannot simultaneously achieve excellent molecular sieving properties, high hydrothermal stability, and specific interactions with the permeated molecules. Consequently, a co-condensation strategy was proposed to fabricate composite organosilica membranes by mixing different precursors. The membrane structure and the resultant separation performance can be finely controlled by varying the mixing ratios.
A BTESE-TEOS composite membrane with a controlled pore size was prepared via the co-hydrolysis and condensation method [115]. The single gas permeation properties and the estimated pore size determined by the normalized Knudsen-based permeance indicated that the network size of a composite membrane was well tuned between the individual BTESE- and TEOS-derived membranes. Additionally, the composite BTESE-TEOS membrane exhibited a superior O2 permeance which was higher than 10−8 mol m−2 s−1 Pa−1 with an O2/SO2 selectivity of 7.3, revealing the prospects for O2/SO2 separation of this composite membrane. Yu et al. tailored the microporosity properties of organosilica membranes by the co-condensation reaction of 4,6-bis(3-(triethoxysilyl)-1-propoxy)-1,3-pyrimidine with BTESE and/or TEOS [92]. A significantly enhanced CO2 permeance (>2000 GPU) with a CO2/N2 selectivity of ~20 was recorded for the composite organosilica membrane, showing potential for the highly permeable CO2 capture process.
Assisted by the rigid acetylene bridges, BTESA membranes featured a more open and accessible pore structure, showing prospects in C3H6/C3H8 separation [93]. To further advance the membrane separation performance, we fabricated a composite organosilica membrane using BTESA and bis(triethoxysilyl)benzene (BTESB) precursors [101]. The evolution of membrane networks revealed that the steric hindrance of phenyl groups originating from the BTESB played a key role in fine-tuning the membrane pore size. The BTESAB 9:1 (molar ratio of BTESA to BTESB was 9:1) membrane exhibited a high C3H6 permeance of 4.5 × 10−8 mol m−2 s−1 Pa−1 (134 GPU) together with a C3H6/C3H8 selectivity of 33 at 50 °C for an equimolar mixture of C3H6 and C3H8. More importantly, the relationships between the membrane pore size and C3H6/C3H8 separation performance for organosilica membranes in single and binary separation systems were clarified and summarized in detail. As depicted in Figure 14, we investigated the relationship between the membrane pore size (as determined by the modified gas translation model) and the membrane separation performance including the C3H6 permeance and C3H6/C3H8 selectivity. The adopted data were measured at 200 °C to minimize the adsorption effect between the hydrocarbon molecules and membrane materials. In other words, the molecular sieving effect dominated the separation process at this high temperature.
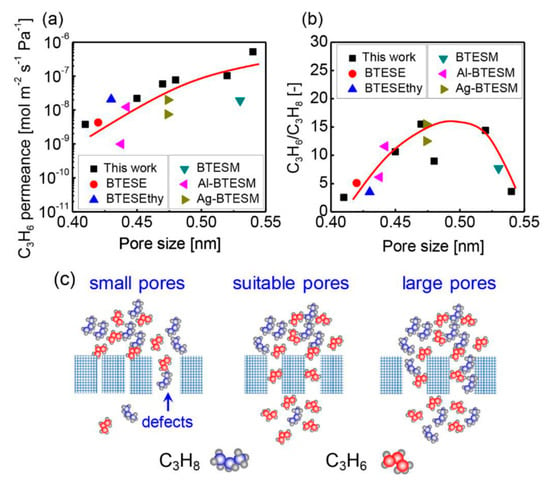
Figure 14.
Relationships between pore size and single gas permeation properties: (a) C3H6 permeance and (b) C3H6/C3H8 permeance ratio at 200 °C for a variety of (composite) organosilica membranes. (c) Possible schematic image for C3H6/C3H8 separation utilizing organosilica membranes with different pore sizes [101].
Membranes with pores that are too small cannot provide enough channels for gas permeation. As a result, C3H6 and C3H8 only permeate through the defects with low selectivity properties. In contrast, membranes with sufficiently large pores can drastically increase the gas permeance. However, these large pores allow both C3H6 and C3H8 molecules to easily permeate without any major resistance or effective molecular sieving properties. In contrast to membranes with pores that are too small or too large, membranes with suitable pore sizes feature much higher selectivities due to the good molecular sieving properties. The suitable pore size determined by modified a gas translation (GT) model ranged from 0.45 to 0.52 nm, which fell approximately between the reported molecular size of C3H6 (0.468 nm) and C3H8 (0.506 nm), providing the possibility to discriminate and separate C3H6 and C3H8 molecules [101]. This explanation can quantify the relationships between the membrane pore sizes and C3H6/C3H8 separation properties and explain why membranes with pores that are too large or too small pores are not suitable for high-efficiency separations.
3.2.2. Affinity Control
Calcination Parameters
Calcination treatment is necessary to facilitate the formation of organosilica networks in a typical sol–gel process. Hence, the control of calcination parameters, such as the temperature, heating/cooling rate, and calcination atmosphere, is particularly important for manufacturing high-quality membranes [39,45,98,116,117].
BTESM membranes calcined at 200 °C, 350 °C, and 600 °C were reported by Kanezashi et al. to illustrate the different gas permeation behaviors [98]. The ratio of the C3H6/C3H8 selectivities obtained from a binary separation system and a single gas permeation system for the BTESM membrane calcined at 200 °C (BTESM-200) was higher than that of BTESM membrane calcined at 350 °C (BTESM-350). The permeation of C3H6 molecules might have shown higher efficiency in blocking the permeation of C3H8 molecules for BTESM-200 than BTESM-350, because of the higher density of Si-OH groups in BTESM-200 can interact with the C3H6 molecules. In addition, it should be noted that the combustion of bridged -CH2- bonds and the construction of Si-O-Si groups simultaneously occurred when a BTESM membrane was calcined at 600 °C, leading to the formation of a silica-like network with a densified structure.
Recently, the C3H6/C3H8 separation performances of BTESA membranes fabricated at different temperatures were also extensively studied [45]. FT-IR, surface energy, and hydrocarbon sorption isotherm characterization revealed that low-temperature calcined BTESA materials with more Si-OH groups exhibited enhanced affinities for C3H6 molecules. A molecular simulation (Figure 15a) also indicated that the hydrogen bonding between the Si-OH and C3H6 π-bonds enhanced the C3H6 affinity of the membrane. BTESA membranes calcined at 150 °C featured a high C3H6/C3H8 selectivity of 52 and C3H6 permeance of 1.7 × 10−8 mol m−2 s−1 Pa−1 (51 GPU) at 50 °C. Figure 15b presents the plausible C3H6/C3H8 separation mechanisms of BTESA membranes with different chemical properties. In contrast to an OH-scarce membrane, an OH-rich membrane accelerated the permeation of C3H6 molecules via stronger interactions and enhanced the C3H6/C3H8 separation properties simultaneously. Interestingly, the network sizes of BTESA membranes calcined at higher temperatures tended to be enlarged, which was opposite to the phenomenon found in BTESM membranes. The decomposition of rigid acetylene bridges resulted in a greater porosity rather than densifying the membrane structures.
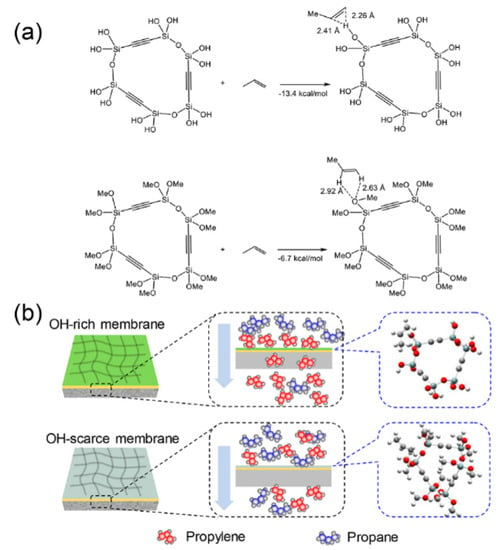
Figure 15.
(a) Model reactions cyclic trimers and optimized geometries of propylene complexes. (b) Plausible C3H6/C3H8 separation mechanisms through BTESA membranes with different chemical properties [45].
Post Treatment
The Si-OH groups within the membrane network structures can accelerate the preferential permeation of C3H6 molecules and enhance the C3H6/C3H8 separation performance, which has been evidenced by BTESM- and BTESA-derived membranes [45,98]. Consequently, determining how to effectively increase the Si-OH density is significant for advancing the C3H6/C3H8 separation performance. Kanezashi et al. prepared a microporous silica membrane utilizing triethoxyfluorosilane (TEFS) as the Si source [118]. It should be emphasized that TEFS is not an organosilica precursor in the strict sense, since no organic groups can be observed. Nevertheless, the similar amorphous structure and the gas permeation mechanism of TEFS and organosilica membranes may still encourage the exploitation of membranes with high permselectivities.
A steam treatment strategy was proposed to increase the Si-OH densities of TEFS membranes via the reaction between steam and Si-F bonds. As revealed in Figure 16, the gas sorption isotherms demonstrated that the adsorbed amount of C3H6 was significantly increased for TEFS powders with steam treatment, while that of C3H8 was almost the same as that of the pristine TEFS powder. In a single gas permeation system, the TEFS membrane with steam treatment featured a superior C3H6 permeance of 2.2 ✕ 10−7 mol m−2 s−1 Pa−1 (657 GPU) with a C3H6/C3H8 selectivity of 42. However, the binary separation properties were not determined for this membrane. Inspired by this, the strategy of steam treatment may be applied for BTESM and BTESA membranes. Additionally, H2O/N2 plasma modification technology can also be implemented to achieve a high grafting efficiency of OH groups while causing no damage to the surface morphology and bulk chemistry [119]. The specific performances of the reported (organo)silica membranes are provided in Table 10.
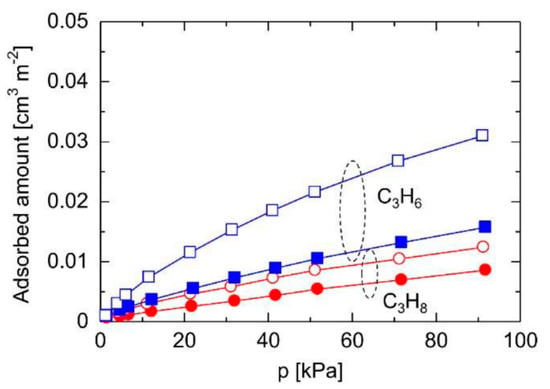
Figure 16.
C3H6 and C3H8 adsorption isotherms at 25 °C for the triethoxyfluorosilane (TEFS) powders calcined at 350 °C before/after steam treatment (closed symbols: before steam treatment, open symbols: after steam treatment) [118].

Table 10.
C3H6/C3H8 separation performances of (organo)silica membranes.
4. Conclusions and Outlook
In this article, we surveyed the progress of membrane-based separation processes of C3H6/C3H8. In Figure 17, the trade-off for the C3H6/C3H8 separation of several kinds of membranes is provided. The specific values of each membranes are summarized in Table S1. The polymeric membranes and MMMs are located at the left-bottom of the trade-off, indicating relatively lower C3H6 permeance and C3H6/C3H8 selectivity values. In comparison, the inorganic membranes, including CMS, ZIF-8, and organosilica, are located at the opposite position. The inorganic membranes exhibited much higher permeance and selectivity values. However, the cost of the membrane materials should not be ignored.
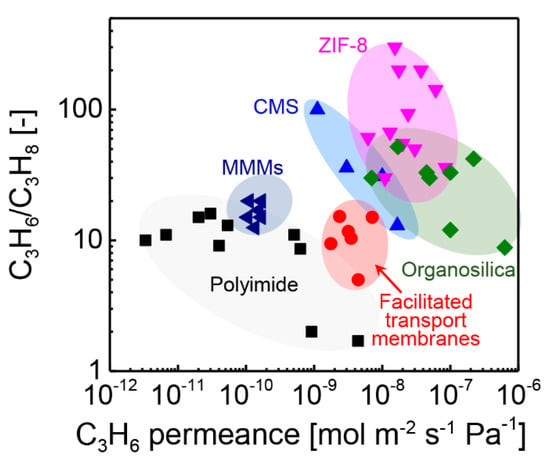
Figure 17.
Trade–off of C3H6/C3H8 for polyimide [20,46], MMMs [27,120], facilitated transport membranes [22,23], CMS [31,33,52,53], ZIF–8 [17,65,66,67,68,69,72,73,74,75,76], and organosilica [45,89,93,97,98,99,101,118] membranes.
Separation mechanisms, including solution-diffusion, facilitated transport, and molecular sieving, dominate the discrimination of C3H6 and C3H8 molecules through membranes. The separation performances of organic, inorganic, and hybrid membranes were highlighted, although they are still hampered by some inherent drawbacks. In addition, we summarized the recent developments of organosilica membranes in detail for the application of C3H6/C3H8 separation. The sol–gel method and the CVD strategy are usually used to fabricate the organosilica membranes. To advance the C3H6/C3H8 separation properties, pore subnano-environment engineering consisting of pore size control (spacer technique, incorporation of metal, and co-condensation strategy) and affinity control (calcination parameters and post treatment) are conducted on silica or organosilica membranes. BTESM and BTESA membrane materials have been shown to be suitable and exhibit great potential for C3H6/C3H8 separation, which is challenging because of the similar physico-chemical properties of C3H6 and C3H8 molecules.
However, some aspects must be examined in future studies of C3H6/C3H8 separation using organosilica membranes. First, no successful industrial application of organosilica membranes for C3H6/C3H8 separation has been achieved. New manufacturing strategies for scalable organosilica membranes must be further exploited for the practical separation of C3H6 and C3H8 mixtures. Low-cost supports such as hollow fibers can also be applied.
Second, the amorphous structures of organosilica membranes always generate a network with a pore size distribution rather than a network with a uniform pore size. As a result, the utilization efficiency of useful pores for C3H6/C3H8 separation is limited to a relatively low level. The template method using a surfactant contributes to the formation of uniform pores of organosilica materials, which is commonly known as periodic mesoporous organosilica (PMO). However, the pore sizes of these material are intrinsically too large for C3H6/C3H8 separation. Hence, the effective decrease in the pore size derived from template methods to the molecular scale is the key to obtaining novel organosilica membranes with superior C3H6 permeances and C3H6/C3H8 selectivities simultaneously. Grafting modification using a pendant-type organosilica precursor with long chains may be an alternative candidate.
Finally, the assessment of the affinity between C3H6 or C3H8 molecules and organosilica membranes is currently conducted through the qualitatively measurement of the single-component adsorption isotherms, in which conditions deviate significantly from the real conditions of binary separations of C3H6/C3H8 mixtures. The binary gas sorption properties or a measurement of the breakthrough may provide more useful information to better understand the separation mechanism of C3H6/C3H8 through organosilica membranes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/membranes11050310/s1, Table S1: The detailed separation performance of various kinds of membranes.
Author Contributions
Writing—Original draft preparation, M.G.; supervision, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eldridge, R.B. Olefin/paraffin separation technology: A review. Ind. Eng. Chem. Res. 1993, 32, 2208–2212. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nat. Cell Biol. 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Nakayama, N. Global Supply and Demand of Petrochemical Products Relied on LPG as Feedstock; International LP Gas Seminar: Tokyo, Japan, 2017. [Google Scholar]
- Ren, T.; Patel, M.; Blok, K. Olefins from conventional and heavy feedstocks: Energy use in steam cracking and alternative processes. Energy 2006, 31, 425–451. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 2012, 73, 261–284. [Google Scholar] [CrossRef]
- Amedi, H.R.; Aghajani, M. Economic Estimation of Various Membranes and Distillation for Propylene and Propane Separation. Ind. Eng. Chem. Res. 2018, 57, 4366–4376. [Google Scholar] [CrossRef]
- Wang, Y.; Peh, S.B.; Zhao, D. Alternatives to Cryogenic Distillation: Advanced Porous Materials in Adsorptive Light Olefin/Paraffin Separations. Small 2019, 15, e1900058. [Google Scholar] [CrossRef] [PubMed]
- Safarik, D.J.; Eldridge, R.B. Olefin/Paraffin Separations by Reactive Absorption: A Review. Ind. Eng. Chem. Res. 1998, 37, 2571–2581. [Google Scholar] [CrossRef]
- Ortiz, A.; Galán, L.M.; Gorri, D.; De Haan, A.B.; Ortiz, I. Reactive Ionic Liquid Media for the Separation of Propylene/Propane Gaseous Mixtures. Ind. Eng. Chem. Res. 2010, 49, 7227–7233. [Google Scholar] [CrossRef]
- Padin, J.; Yang, R.T. New sorbents for olefin/paraffin separations by adsorption via π-complexation: Synthesis and effects of substrates. Chem. Eng. Sci. 2000, 55, 2607–2616. [Google Scholar] [CrossRef]
- And, A.J.; Yang, R.T.; Munson, C.L.; Chinn, D. Deactivation of π-Complexation Adsorbents by Hydrogen and Rejuvenation by Oxidation. Ind. Eng. Chem. Res. 2001, 40, 4370–4376. [Google Scholar] [CrossRef]
- Burns, R.L.; Koros, W.J. Defining the challenges for C3H6/C3H8 separation using polymeric membranes. J. Membr. Sci. 2003, 211, 299–309. [Google Scholar] [CrossRef]
- Hou, J.; Liu, P.; Jiang, M.; Yu, L.; Li, L.; Tang, Z. Olefin/paraffin separation through membranes: From mechanisms to critical materials. J. Mater. Chem. A 2019, 7, 23489–23511. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Ma, X.; Liu, D. Zeolitic Imidazolate Framework Membranes for Light Olefin/Paraffin Separation. Crystals 2018, 9, 14. [Google Scholar] [CrossRef]
- Colling, C.W.; Huff, G.A., Jr.; Bartels, J.V. Processes Using Solid Perm-Selective Membranes in Multiple Groups for Simultaneous Recovery of Specified Products from a Fluid Mixture. U.S. Patent No. 6,830,691, 14 December 2004. [Google Scholar]
- Kwon, H.T.; Jeong, H.-K. In Situ Synthesis of Thin Zeolitic–Imidazolate Framework ZIF-8 Membranes Exhibiting Exceptionally High Propylene/Propane Separation. J. Am. Chem. Soc. 2013, 135, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Koros, W.J.; Lively, R.P. Water and beyond: Expanding the spectrum of large-scale energy efficient separation processes. AIChE J. 2012, 58, 2624–2633. [Google Scholar] [CrossRef]
- Das, M.; Koros, W.J. Performance of 6FDA–6FpDA polyimide for propylene/propane separations. J. Membr. Sci. 2010, 365, 399–408. [Google Scholar] [CrossRef]
- Staudt-Bickel, C.; Koros, W.J. Olefin/paraffin gas separations with 6FDA-based polyimide membranes. J. Membr. Sci. 2000, 170, 205–214. [Google Scholar] [CrossRef]
- Pinnau, I.; Toy, L.G. Solid polymer electrolyte composite membranes for olefin/paraffin separation. J. Membr. Sci. 2001, 184, 39–48. [Google Scholar] [CrossRef]
- Liao, K.-S.; Lai, J.-Y.; Chung, T.-S. Metal ion modified PIM-1 and its application for propylene/propane separation. J. Membr. Sci. 2016, 515, 36–44. [Google Scholar] [CrossRef]
- Kasahara, S.; Kamio, E.; Minami, R.; Matsuyama, H. A facilitated transport ion-gel membrane for propylene/propane separation using silver ion as a carrier. J. Membr. Sci. 2013, 431, 121–130. [Google Scholar] [CrossRef]
- Jose, B.; Ryu, J.H.; Kim, Y.J.; Kim, H.; Kang, Y.S.; Lee, S.D.; Kim, H.S. Effect of plasticizers on the formation of silver nanoparticles in polymer electrolyte membranes for olefin/paraffin separation. Chem. Mater. 2002, 14, 2134–2139. [Google Scholar] [CrossRef]
- Hong, S.U.; Jin, J.H.; Won, J.; Kang, Y.S. Polymer–salt complexes containing silver ions and their application to facilitated olefin transport membranes. Adv. Mater. 2000, 12, 968–971. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Liu, G.; Belmabkhout, Y.; Adil, K.; Eddaoudi, M.; Koros, W. Conformation-Controlled Molecular Sieving Effects for Membrane-Based Propylene/Propane Separation. Adv. Mater. 2019, 31, e1807513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, K.; Xu, L.; LaBreche, Y.; Kraftschik, B.; Koros, W.J. Highly scalable ZIF-based mixed-matrix hollow fiber membranes for advanced hydrocarbon separations. AIChE J. 2014, 60, 2625–2635. [Google Scholar] [CrossRef]
- Ma, X.; Swaidan, R.J.; Wang, Y.; Hsiung, C.-E.; Han, Y.; Pinnau, I. Highly Compatible Hydroxyl-Functionalized Microporous Polyimide-ZIF-8 Mixed Matrix Membranes for Energy Efficient Propylene/Propane Separation. ACS Appl. Nano Mater. 2018, 1, 3541–3547. [Google Scholar] [CrossRef]
- Rashidi, F.; Leisen, J.; Kim, S.J.; Rownaghi, A.A.; Jones, C.W.; Nair, S. All-Nanoporous Hybrid Membranes: Redefining Upper Limits on Molecular Separation Properties. Angew. Chem. 2019, 131, 242–245. [Google Scholar] [CrossRef]
- Steel, K.M.; Koros, W.J. An investigation of the effects of pyrolysis parameters on gas separation properties of carbon materials. Carbon 2005, 43, 1843–1856. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Koros, W.J. Matrimid® derived carbon molecular sieve hollow fiber membranes for ethylene/ethane separation. J. Membr. Sci. 2011, 380, 138–147. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.S.; Wei, X.; Kniep, J. Ultrathin carbon molecular sieve membrane for propylene/propane separation. AIChE J. 2015, 62, 491–499. [Google Scholar] [CrossRef]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Gascon, J.; Kapteijn, F.; Zornoza, B.; Sebastian, V.; Casado, C.; Coronas, J. Practical approach to zeolitic membranes and coatings: State of the art, opportunities, barriers, and future perspectives. Chem. Mater. 2012, 24, 2829–2844. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Design of Silica Networks for Development of Highly Permeable Hydrogen Separation Membranes with Hydrothermal Stability. J. Am. Chem. Soc. 2009, 131, 414–415. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Development of Robust Organosilica Membranes for Reverse Osmosis. Langmuir 2011, 27, 13996–13999. [Google Scholar] [CrossRef]
- Dong, G.; Nagasawa, H.; Yu, L.; Guo, M.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Energy-efficient separation of organic liquids using organosilica membranes via a reverse osmosis route. J. Membr. Sci. 2020, 597, 117758. [Google Scholar] [CrossRef]
- Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Effect of calcination temperature on the PV dehydration performance of alcohol aqueous solutions through BTESE-derived silica membranes. J. Membr. Sci. 2012, 415–416, 810–815. [Google Scholar] [CrossRef]
- Gong, G.; Wang, J.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Synthesis and characterization of a layered-hybrid membrane consisting of an organosilica separation layer on a polymeric nanofiltration membrane. J. Membr. Sci. 2014, 472, 19–28. [Google Scholar] [CrossRef]
- De Miranda, D.M.V.; Dutra, L.D.S.; Way, D.; Amaral, N.; Wegenast, F.; Scaldaferri, M.C.; Jesus, N.; Pinto, J.C. A Bibliometric Survey of Paraffin/Olefin Separation Using Membranes. Membranes 2019, 9, 157. [Google Scholar] [CrossRef]
- Koros, W.; Fleming, G.; Jordan, S.; Kim, T.; Hoehn, H. Polymeric membrane materials for solution-diffusion based permeation separations. Prog. Polym. Sci. 1988, 13, 339–401. [Google Scholar] [CrossRef]
- Campos, A.C.C.; Dos Reis, R.A.; Ortiz, A.; Gorri, D.; Ortiz, I. A Perspective of Solutions for Membrane Instabilities in Olefin/Paraffin Separations: A Review. Ind. Eng. Chem. Res. 2018, 57, 10071–10085. [Google Scholar] [CrossRef]
- Bandosz, T.J. Analysis of Silica Surface Heterogeneity Using Butane and Butene Adsorption Data. J. Colloid Interface Sci. 1997, 193, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Kanezashi, M.; Nagasawa, H.; Yu, L.; Yamamoto, K.; Gunji, T.; Ohshita, J.; Tsuru, T. Pore subnano-environment engineering of organosilica membranes for highly selective propylene/propane separation. J. Membr. Sci. 2020, 603, 117999. [Google Scholar] [CrossRef]
- Tanaka, K.; Taguchi, A.; Hao, J.; Kita, H.; Okamoto, K. Permeation and separation properties of polyimide membranes to olefins and paraffins. J. Membr. Sci. 1996, 121, 197–207. [Google Scholar] [CrossRef]
- Swaidan, R.J.; Ghanem, B.; Swaidan, R.; Litwiller, E.; Pinnau, I. Pure- and mixed-gas propylene/propane permeation properties of spiro- and triptycene-based microporous polyimides. J. Membr. Sci. 2015, 492, 116–122. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Park, H.-C.; Won, J.-O.; Hong, S.-U.; Kwon, T.-M. Facilitaed Transport Separation Membranes Using Solid State Polymer Electrolytes. U.S. Patents US6468331B2, 15 August 2001. [Google Scholar]
- Fu, S.; Sanders, E.S.; Kulkarni, S.; Chu, Y.-H.; Wenz, G.B.; Koros, W.J. The significance of entropic selectivity in carbon molecular sieve membranes derived from 6FDA/DETDA:DABA(3:2) polyimide. J. Membr. Sci. 2017, 539, 329–343. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Bhuwania, N.; LaBreche, Y.; Achoundong, C.S.; Baltazar, J.; Burgess, S.K.; Karwa, S.; Xu, L.; Henderson, C.L.; Williams, P.J.; Koros, W.J. Engineering substructure morphology of asymmetric carbon molecular sieve hollow fiber membranes. Carbon 2014, 76, 417–434. [Google Scholar] [CrossRef]
- Ma, X.; Lin, B.K.; Wei, X.; Kniep, J.; Lin, Y.S. Gamma-Alumina Supported Carbon Molecular Sieve Membrane for Propylene/Propane Separation. Ind. Eng. Chem. Res. 2013, 52, 4297–4305. [Google Scholar] [CrossRef]
- Okamoto, K.-I.; Kawamura, S.; Yoshino, M.; Kita, H.; Hirayama, Y.; Tanihara, N.; Kusuki, Y. Olefin/Paraffin Separation through Carbonized Membranes Derived from an Asymmetric Polyimide Hollow Fiber Membrane. Ind. Eng. Chem. Res. 1999, 38, 4424–4432. [Google Scholar] [CrossRef]
- Nikolakis, V.; Xomeritakis, G.; Abibi, A.; Dickson, M.; Tsapatsis, M.; Vlachos, D.G. Growth of a faujasite-type zeolite membrane and its application in the separation of saturated/unsaturated hydrocarbon mixtures. J. Membr. Sci. 2001, 184, 209–219. [Google Scholar] [CrossRef]
- Giannakopoulos, I.G.; Nikolakis, V. Separation of propylene/propane mixtures using faujasite-type zeolite membranes. Ind. Eng. Chem. Res. 2005, 44, 226–230. [Google Scholar] [CrossRef]
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin Selective Ag-Exchanged X-Type Zeolite Membrane for Propylene/Propane and Ethylene/Ethane Separation. ACS Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar] [CrossRef]
- Sakai, M.; Fujimaki, N.; Sasaki, Y.; Yasuda, N.; Seshimo, M.; Matsukata, M. Preferential Adsorption of Propylene over Propane on a Ag-Exchanged X-Type Zeolite Membrane. ACS Appl. Mater. Interfaces 2020, 12, 24086–24092. [Google Scholar] [CrossRef]
- Maghsoudi, H. Defects of Zeolite Membranes: Characterization, Modification and Post-treatment Techniques. Sep. Purif. Rev. 2015, 45, 169–192. [Google Scholar] [CrossRef]
- He, G.; Huang, S.; Villalobos, L.F.; Zhao, J.; Mensi, M.; Oveisi, E.; Rezaei, M.; Agrawal, K.V. High-permeance polymer-functionalized single-layer graphene membranes that surpass the postcombustion carbon capture target. Energy Environ. Sci. 2019, 12, 3305–3312. [Google Scholar] [CrossRef]
- Guirguis, A.; Maina, J.W.; Zhang, X.; Henderson, L.C.; Kong, L.; Shon, H.; Dumée, L.F. Applications of nano-porous graphene materials—Critical review on performance and challenges. Mater. Horiz. 2020, 7, 1218–1245. [Google Scholar] [CrossRef]
- Jiang, C.; Hou, Y.; Wang, N.; Li, L.; Lin, L.; Niu, Q.J. Propylene/propane separation by porous graphene membrane: Molecular dynamic simulation and first-principle calculation. J. Taiwan Inst. Chem. Eng. 2017, 78, 477–484. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, J.; Yang, C. Separation of diverse alkenes from C2-C4 alkanes through nanoporous graphene membranes via local size sieving. J. Membr. Sci. 2019, 584, 227–235. [Google Scholar] [CrossRef]
- Lai, Z. Development of ZIF-8 membranes: Opportunities and challenges for commercial applications. Curr. Opin. Chem. Eng. 2018, 20, 78–85. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Liu, G.; Jin, W. Metal-organic framework adsorbents and membranes for separation applications. Curr. Opin. Chem. Eng. 2018, 20, 122–131. [Google Scholar] [CrossRef]
- Pan, Y.; Li, T.; Lestari, G.; Lai, Z. Effective separation of propylene/propane binary mixtures by ZIF-8 membranes. J. Membr. Sci. 2012, 390-391, 93–98. [Google Scholar] [CrossRef]
- Yu, J.; Pan, Y.; Wang, C.; Lai, Z. ZIF-8 membranes with improved reproducibility fabricated from sputter-coated ZnO/alumina supports. Chem. Eng. Sci. 2016, 141, 119–124. [Google Scholar] [CrossRef]
- Zhou, S.; Wei, Y.; Li, L.; Duan, Y.; Hou, Q.; Zhang, L.; Ding, L.-X.; Xue, J.; Wang, H.; Caro, J. Paralyzed membrane: Current-driven synthesis of a metal-organic framework with sharpened propene/propane separation. Sci. Adv. 2018, 4, eaau1393. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Zhou, S.; Wei, Y.; Caro, J.; Wang, H. Balancing the Grain Boundary Structure and the Framework Flexibility through Bimetallic MOF Membranes for Gas Separation. J. Am. Chem. Soc. 2020, 142, 9582–9586. [Google Scholar] [PubMed]
- Wei, R.; Chi, H.; Li, X.; Lu, D.; Wan, Y.; Yang, C.; Lai, Z. Aqueously Cathodic Deposition of ZIF-8 Membranes for Superior Propylene/Propane Separation. Adv. Funct. Mater. 2019, 30, 1907089. [Google Scholar] [CrossRef]
- Li, W.; Wu, W.; Li, Z.; Shi, J.; Xia, Y. Sol–gel asynchronous crystallization of ultra-selective metal–organic framework membranes for gas separation. J. Mater. Chem. A 2018, 6, 16333–16340. [Google Scholar] [CrossRef]
- Bux, H.; Liang, F.; Li, Y.; Cravillon, J.; Wiebcke, M.; Caro, J. Zeolitic Imidazolate Framework Membrane with Molecular Sieving Properties by Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2009, 131, 16000–16001. [Google Scholar] [CrossRef]
- Liu, D.; Ma, X.; Xi, H.; Lin, Y. Gas transport properties and propylene/propane separation characteristics of ZIF-8 membranes. J. Membr. Sci. 2014, 451, 85–93. [Google Scholar] [CrossRef]
- Tanaka, S.; Okubo, K.; Kida, K.; Sugita, M.; Takewaki, T. Grain size control of ZIF-8 membranes by seeding-free aqueous synthesis and their performances in propylene/propane separation. J. Membr. Sci. 2017, 544, 306–311. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, C.; Yang, F.; Xiang, L.; Huang, X.; Yu, J.; Zhang, L.; Pan, Y.; Li, Y. Enhanced C3H6/C3H8 separation performance on MOF membranes through blocking defects and hindering framework flexibility by silicone rubber coating. Chem. Commun. 2017, 53, 7760–7763. [Google Scholar] [CrossRef]
- Li, J.; Lian, H.; Wei, K.; Song, E.; Pan, Y.; Xing, W. Synthesis of tubular ZIF-8 membranes for propylene/propane separation under high-pressure. J. Membr. Sci. 2020, 595, 117503. [Google Scholar] [CrossRef]
- Kwon, H.T.; Jeong, H.-K.; Lee, A.S.; An, H.S.; Lee, J.S. Heteroepitaxially Grown Zeolitic Imidazolate Framework Membranes with Unprecedented Propylene/Propane Separation Performances. J. Am. Chem. Soc. 2015, 137, 12304–12311. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hamid, M.R.A.; Jeong, H.-K. Highly Propylene-Selective Mixed-Matrix Membranes by in Situ Metal–Organic Framework Formation Using a Polymer-Modification Strategy. ACS Appl. Mater. Interfaces 2019, 11, 25949–25957. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, K.; Shin, J.-W.; Park, Y.-K. Analysis of Multistage Membrane and Distillation Hybrid Processes for Propylene/Propane Separation. Chem. Eng. Trans. 2019, 74, 871–876. [Google Scholar]
- Park, S.; Jeong, H.-K. In-situ linker doping as an effective means to tune zeolitic-imidazolate framework-8 (ZIF-8) fillers in mixed-matrix membranes for propylene/propane separation. J. Membr. Sci. 2020, 596, 117689. [Google Scholar] [CrossRef]
- Marti, A.M.; Venna, S.R.; Roth, E.A.; Culp, J.T.; Hopkinson, D.P. Simple Fabrication Method for Mixed Matrix Membranes with in Situ MOF Growth for Gas Separation. ACS Appl. Mater. Interfaces 2018, 10, 24784–24790. [Google Scholar] [CrossRef]
- Tsuru, T. Silica-Based Membranes with Molecular-Net-Sieving Properties: Development and Applications. J. Chem. Eng. Jpn. 2018, 51, 713–725. [Google Scholar] [CrossRef]
- Ren, X.; Tsuru, T. Organosilica-Based Membranes in Gas and Liquid-Phase Separation. Membranes 2019, 9, 107. [Google Scholar] [CrossRef]
- Agirre, I.; Arias, P.L.; Castricum, H.L.; Creatore, M.; Elshof, J.E.T.; Paradis, G.G.; Ngamou, P.H.; van Veen, H.M.; Vente, J.F. Hybrid organosilica membranes and processes: Status and outlook. Sep. Purif. Technol. 2014, 121, 2–12. [Google Scholar] [CrossRef]
- Elshof, J.E.T.; Dral, A.P. Structure–property tuning in hydrothermally stable sol–gel-processed hybrid organosilica molecular sieving membranes. J. Sol-Gel Sci. Technol. 2016, 79, 279–294. [Google Scholar] [CrossRef]
- Castricum, H.L.; Sah, A.; Kreiter, R.; Blank, D.H.A.; Vente, J.F.; Elshof, J.E.T. Hybrid ceramic nanosieves: Stabilizing nanopores with organic links. Chem. Commun. 2008, 9, 1103–1105. [Google Scholar] [CrossRef]
- Chang, K.-S.; Yoshioka, T.; Kanezashi, M.; Tsuru, T.; Tung, K.-L. A molecular dynamics simulation of a homogeneous organic–inorganic hybrid silica membrane. Chem. Commun. 2010, 46, 9140–9142. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yada, K.; Yoshioka, T.; Tsuru, T. Organic–inorganic hybrid silica membranes with controlled silica network size: Preparation and gas permeation characteristics. J. Membr. Sci. 2010, 348, 310–318. [Google Scholar] [CrossRef]
- Niimi, T.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Ito, K.; Tsuru, T. Preparation of BTESE-derived organosilica membranes for catalytic membrane reactors of methylcyclohexane dehydrogenation. J. Membr. Sci. 2014, 455, 375–383. [Google Scholar] [CrossRef]
- Kanezashi, M.; Kawano, M.; Yoshioka, T.; Tsuru, T. Organic–Inorganic Hybrid Silica Membranes with Controlled Silica Network Size for Propylene/Propane Separation. Ind. Eng. Chem. Res. 2011, 51, 944–953. [Google Scholar] [CrossRef]
- Castricum, H.L.; Paradis, G.G.; Mittelmeijer-Hazeleger, M.C.; Kreiter, R.; Vente, J.F.; Ten Elshof, J.E. Tailoring the separation behavior of hybrid organosilica membranes by adjusting the structure of the organic bridging group. Adv. Funct. Mater. 2011, 21, 2319–2329. [Google Scholar] [CrossRef]
- Ren, X.; Nishimoto, K.; Kanezashi, M.; Nagasawa, H.; Yoshioka, T.; Tsuru, T. CO2 Permeation through Hybrid Organosilica Membranes in the Presence of Water Vapor. Ind. Eng. Chem. Res. 2014, 53, 6113–6120. [Google Scholar] [CrossRef]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Guo, M.; Moriyama, N.; Ito, K.; Tsuru, T. Tailoring Ultramicroporosity To Maximize CO2 Transport within Pyrimidine-Bridged Organosilica Membranes. ACS Appl. Mater. Interfaces 2019, 11, 7164–7173. [Google Scholar] [CrossRef]
- Guo, M.; Kanezashi, M.; Nagasawa, H.; Yu, L.; Yamamoto, K.; Gunji, T.; Ohshita, J.; Tsuru, T. Tailoring the microstructure and permeation properties of bridged organosilica membranes via control of the bond angles. J. Membr. Sci. 2019, 584, 56–65. [Google Scholar] [CrossRef]
- Moriyama, N.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Pervaporation dehydration of aqueous solutions of various types of molecules via organosilica membranes: Effect of membrane pore sizes and molecular sizes. Sep. Purif. Technol. 2018, 207, 108–115. [Google Scholar] [CrossRef]
- Gong, G.; Mamoru, M.; Nagasawa, H.; Kanezashi, M.; Hu, Y.; Tsuru, T. Vapor-permeation dehydration of isopropanol using a flexible and thin organosilica membrane with high permeance. J. Membr. Sci. 2019, 588, 117226. [Google Scholar] [CrossRef]
- Xu, R.; Ibrahim, S.M.; Kanezashi, M.; Yoshioka, T.; Ito, K.; Ohshita, J.; Tsuru, T. New Insights into the Microstructure-Separation Properties of Organosilica Membranes with Ethane, Ethylene, and Acetylene Bridges. ACS Appl. Mater. Interfaces 2014, 6, 9357–9364. [Google Scholar] [CrossRef]
- Kanezashi, M.; Miyauchi, S.; Nagasawa, H.; Yoshioka, T.; Tsuru, T. Gas permeation properties through Al-doped organosilica membranes with controlled network size. J. Membr. Sci. 2014, 466, 246–252. [Google Scholar] [CrossRef]
- Kanezashi, M.; Shazwani, W.; Yoshioka, T.; Tsuru, T. Separation of propylene/propane binary mixtures by bis(triethoxysilyl) methane (BTESM)-derived silica membranes fabricated at different calcination temperatures. J. Membr. Sci. 2012, 415-416, 478–485. [Google Scholar] [CrossRef]
- Kanezashi, M.; Miyauchi, S.; Hayakawa, S.; Nagasawa, H.; Yoshioka, T.; Tsuru, T. Propylene/propane Permeation Properties of Metal-doped Organosilica Membranes with Controlled Network Sizes and Adsorptive Properties. J. Jpn. Pet. Inst. 2016, 59, 140–148. [Google Scholar] [CrossRef][Green Version]
- Kanezashi, M.; Miyauchi, S.; Nagasawa, H.; Yoshioka, T.; Tsuru, T. Pore size control of Al-doping into bis (triethoxysilyl) methane (BTESM)-derived membranes for improved gas permeation properties. RSC Adv. 2013, 3, 12080–12083. [Google Scholar] [CrossRef]
- Guo, M.; Kanezashi, M.; Nagasawa, H.; Yu, L.; Yamamoto, K.; Gunji, T.; Tsuru, T. Fine-tuned, molecular-composite, organosilica membranes for highly efficient propylene/propane separation via suitable pore size. AIChE J. 2020, 66, 16850. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Xu, R.; Nagasawa, H.; Naka, A.; Ohshita, J.; Yoshioka, T.; Kanezashi, M.; Tsuru, T. A closer look at the development and performance of organic–inorganic membranes using 2,4,6-tris[3(triethoxysilyl)-1-propoxyl]-1,3,5-triazine (TTESPT). RSC Adv. 2014, 4, 12404–12407. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Reverse osmosis performance of organosilica membranes and comparison with the pervaporation and gas permeation properties. AIChE J. 2012, 59, 1298–1307. [Google Scholar] [CrossRef]
- Lee, D.; Zhang, L.; Oyama, S.; Niu, S.; Saraf, R. Synthesis, characterization, and gas permeation properties of a hydrogen permeable silica membrane supported on porous alumina. J. Membr. Sci. 2004, 231, 117–126. [Google Scholar] [CrossRef]
- Ohta, Y.; Akamatsu, K.; Sugawara, T.; Nakao, A.; Miyoshi, A.; Nakao, S.-I. Development of pore size-controlled silica membranes for gas separation by chemical vapor deposition. J. Membr. Sci. 2008, 315, 93–99. [Google Scholar] [CrossRef]
- Nagasawa, H.; Minamizawa, T.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Microporous organosilica membranes for gas separation prepared via PECVD using different O/Si ratio precursors. J. Membr. Sci. 2015, 489, 11–19. [Google Scholar] [CrossRef]
- Tsuru, T.; Shigemoto, H.; Kanezashi, M.; Yoshioka, T. 2-Step plasma-enhanced CVD for low-temperature fabrication of silica membranes with high gas-separation performance. Chem. Commun. 2011, 47, 8070–8072. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Minamizawa, T.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. High-temperature stability of PECVD-derived organosilica membranes deposited on TiO2 and SiO2–ZrO2 intermediate layers using HMDSO/Ar plasma. Sep. Purif. Technol. 2014, 121, 13–19. [Google Scholar] [CrossRef][Green Version]
- Nagasawa, H.; Shigemoto, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Characterization and gas permeation properties of amorphous silica membranes prepared via plasma enhanced chemical vapor deposition. J. Membr. Sci. 2013, 441, 45–53. [Google Scholar] [CrossRef]
- Gong, G.; Wang, J.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Fabrication of a layered hybrid membrane using an organosilica separation layer on a porous polysulfone support, and the application to vapor permeation. J. Membr. Sci. 2014, 464, 140–148. [Google Scholar] [CrossRef]
- Gong, G.; Nagasawa, H.; Kanezashi, M.; Tsuru, T.; Genghao, G. Facile and Scalable Flow-Induced Deposition of Organosilica on Porous Polymer Supports for Reverse Osmosis Desalination. ACS Appl. Mater. Interfaces 2018, 10, 14070–14078. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yoneda, Y.; Nagasawa, H.; Tsuru, T.; Yamamoto, K.; Ohshita, J. Gas permeation properties for organosilica membranes with different Si/C ratios and evaluation of microporous structures. AIChE J. 2017, 63, 4491–4498. [Google Scholar] [CrossRef]
- Van Veen, H.M.; Rietkerk, M.D.; Shanahan, D.P.; Van Tuel, M.M.; Kreiter, R.; Castricum, H.L.; Elshof, J.E.T.; Vente, J.F. Pushing membrane stability boundaries with HybSi® pervaporation membranes. J. Membr. Sci. 2011, 380, 124–131. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Y.; Qi, H. Palladium-niobium bimetallic doped organosilica membranes for H2/CO2 separation. Microporous Mesoporous Mater. 2020, 305, 110279. [Google Scholar] [CrossRef]
- Meng, L.; Kanezashi, M.; Wang, J.; Tsuru, T. Permeation properties of BTESE–TEOS organosilica membranes and application to O2/SO2 gas separation. J. Membr. Sci. 2015, 496, 211–218. [Google Scholar] [CrossRef]
- Song, H.; Wei, Y.; Qi, H. Tailoring pore structures to improve the permselectivity of organosilica membranes by tuning calcination parameters. J. Mater. Chem. A 2017, 5, 24657–24666. [Google Scholar] [CrossRef]
- Yang, J.; Fan, W.; Bell, C.-M. Effect of calcination atmosphere on microstructure and H2/CO2 separation of palladium-doped silica membranes. Sep. Purif. Technol. 2019, 210, 659–669. [Google Scholar] [CrossRef]
- Kanezashi, M.; Matsutani, T.; Nagasawa, H.; Tsuru, T. Fluorine-induced microporous silica membranes: Dramatic improvement in hydrothermal stability and pore size controllability for highly permeable propylene/propane separation. J. Membr. Sci. 2018, 549, 111–119. [Google Scholar] [CrossRef]
- Ren, X.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Plasma treatment of hydrophobic sub-layers to prepare uniform multi-layered films and high-performance gas separation membranes. Appl. Surf. Sci. 2015, 349, 415–419. [Google Scholar] [CrossRef]
- Yu, J.; Wang, C.; Xiang, L.; Xu, Y.; Pan, Y. Enhanced C3H6/C3H8 separation performance in poly(vinyl acetate) membrane blended with ZIF-8 nanocrystals. Chem. Eng. Sci. 2018, 179, 1–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).