The Bacteriostatic Activity of 2-Phenylethanol Derivatives Correlates with Membrane Binding Affinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Lipids and Chemicals
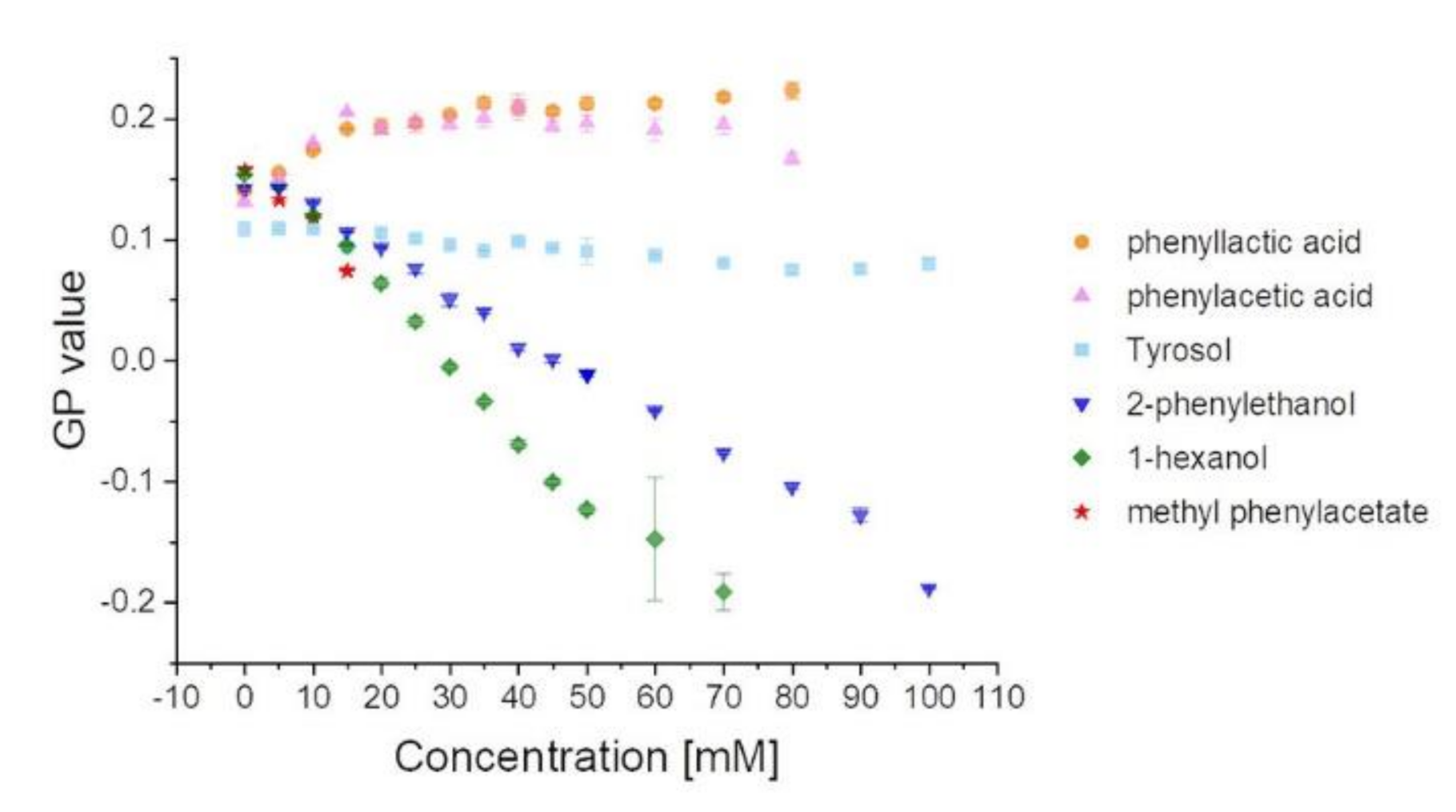
2.2. Laurdan Fluorescence Spectroscopy and Generalized Polarization (GP) Values
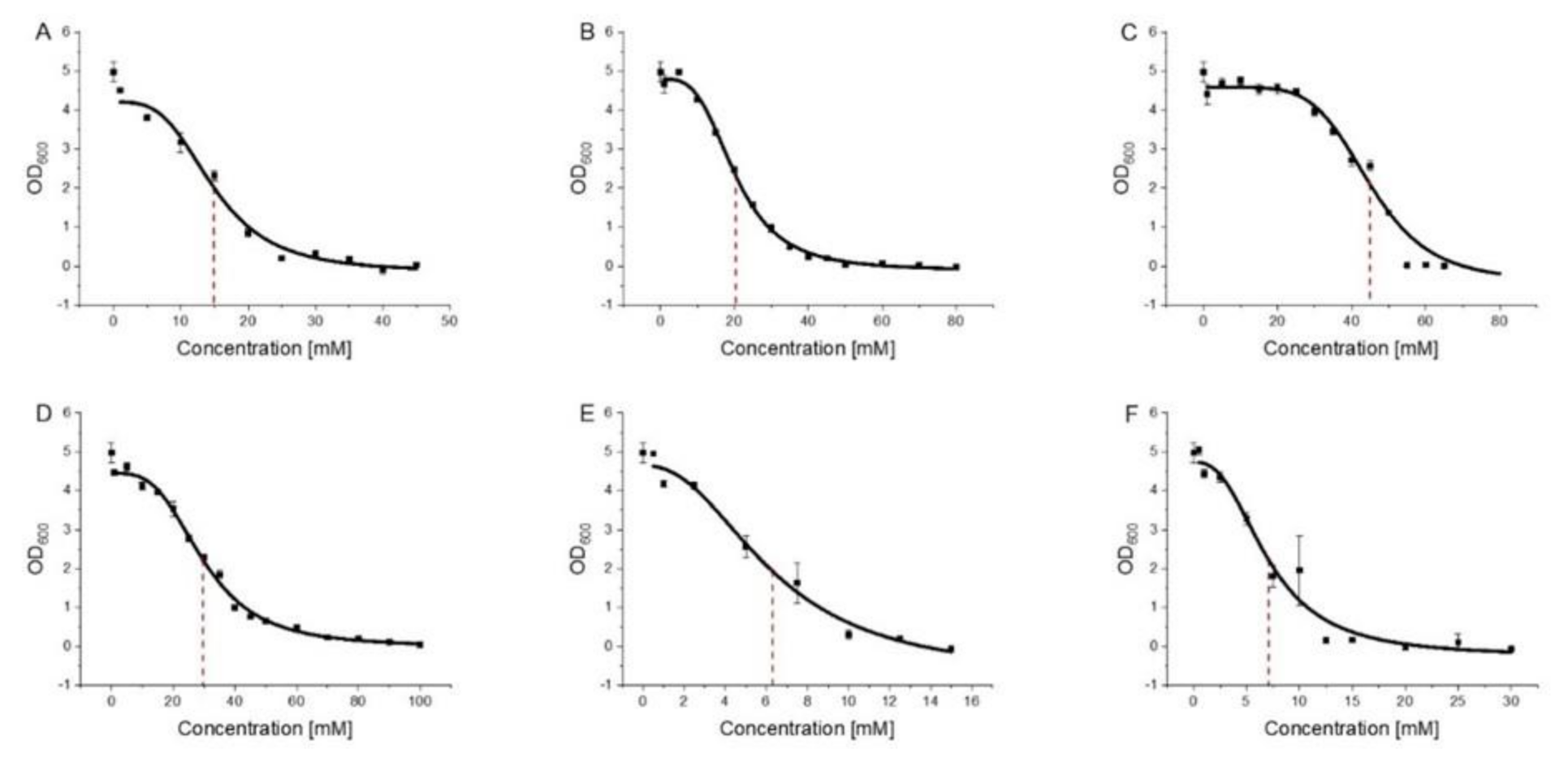
2.3. Growth Assay and Determination of Minimal Inhibitory Concentrations 50 (MIC50)
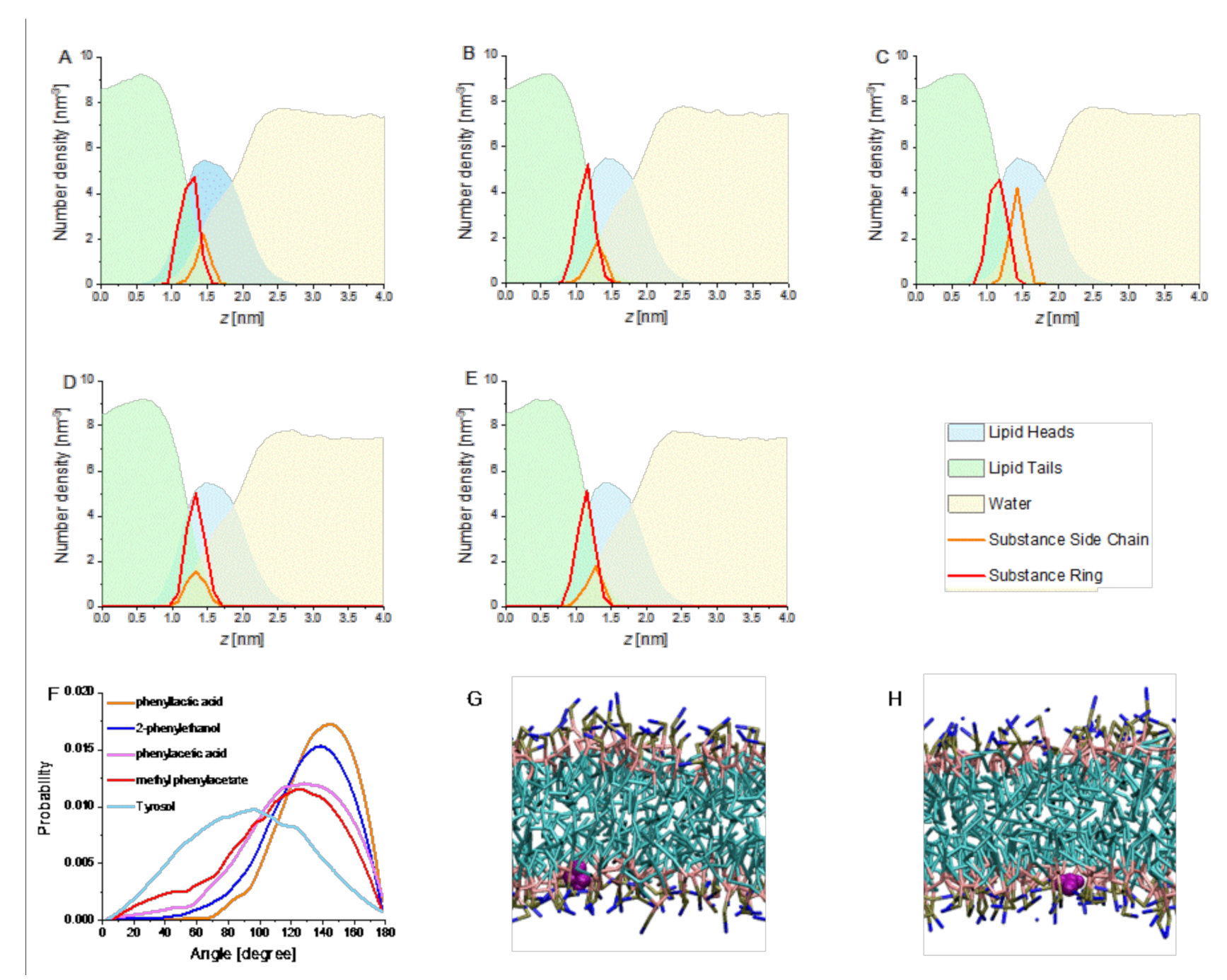
2.4. Computer Simulations
3. Results and Discussion
3.1. Membrane Partitioning and the Impact of 2-PEtOH Derivatives on the Membrane Structure
3.2. 2-PEtOH and Derivatives Are Bacteriostatic
3.3. Hydrophobicity, Membrane Fluidity, and Bacterial Growth Correlate
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingram, L.O. Adaptation of membrane lipids to alcohols. J. Bacteriol. 1976, 125, 670–678. [Google Scholar] [CrossRef]
- Ingram, L.O.; Vreeland, N.S. Differential effects of ethanol and hexanol on the Escherichia coli cell envelope. J. Bacteriol. 1980, 144, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, J.; Tosteson, D.C. Ionic permeability of thin lipid membranes: Effects of n-alkyl alcohols, polyvalent cations, and a secondary amine. J. Gen. Physiol. 1970, 55, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.; Wendt, L. Mechanism of action of phenethyl alcohol: Breakdown of the cellular permeability barrier. J. Bacteriol. 1967, 93, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Zhou, H.T.; Hu, Y.H.; Tang, J.Y.; Su, M.X.; Guo, Y.J.; Chen, Q.X.; Liu, B. Antityrosinase and antimicrobial activities of 2-phenylethanol, 2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem. 2011, 124, 298–302. [Google Scholar] [CrossRef]
- Lilley, B.D.; Brewer, J.H. The selective antibacterial action of phenylethyl alcohol. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1953, 42, 6–8. [Google Scholar] [CrossRef]
- Anbazhagan, V.; Munz, C.; Tome, L.; Schneider, D. Fluidizing the Membrane by a Local Anesthetic: Phenylethanol Affects Membrane Protein Oligomerization. J. Mol. Biol. 2010, 404, 773–777. [Google Scholar] [CrossRef]
- Stark, D.; Zala, D.; Münch, T.; Sonnleitner, B.; Marison, I.W.; Von Stockar, U. Inhibition aspects of the bioconversion of L-phenylalanine to 2-phenylethanol by Saccharomyces cerevisiae. Enzyme Microb. Technol. 2003, 32, 212–223. [Google Scholar] [CrossRef]
- Brossmer, R.; Bohn, B.; Schlicker, H. Influence of 2-Phenylethanol and 1,1’Dimethylphenylethanol on metabolic activity and cell membrane function in ehrlich ascites tumor cells. FEBS Lett. 1973, 35, 191–194. [Google Scholar] [CrossRef]
- Prevost, C.; Moses, V. Action of Phenethyl Alcohol on the Sythesis of Macromolecules in E. coli. J. Bacteriol. 1966, 91, 1446–1452. [Google Scholar] [CrossRef]
- Masker, W.E.; Eberle, H. Effect of phenethyl alcohol on deoxyribonucleic acid-membrane association in Escherichia coli. J. Bacteriol. 1972, 109, 1170–1174. [Google Scholar] [CrossRef]
- Lucchini, J.J.; Bonnaveiro, N.; Cremieux, A.; Le Goffic, F. Mechanism of bactericidal action of phenethyl alcohol in Escherichia coli. Curr. Microbiol. 1993, 27, 295–300. [Google Scholar] [CrossRef]
- Lovrien, R.; Hart, G.; Anderson, K.J. Quantitative aspects of phenyl substituted alcohol and ether bacteriostatic interaction with Escherichia coli B/5. Microbios 1977, 20, 153–172. [Google Scholar] [PubMed]
- Dieuleveux, V.; Lemarinier, S.; Guéguen, M. Antimicrobial spectrum and target site of D-3-phenyllactic acid. Int. J. Food Microbiol. 1998, 40, 177–183. [Google Scholar] [CrossRef]
- Prema, P.; Smila, D.; Palavesam, A.; Immanuel, G. Production and characterization of an antifungal compound (3-phenyllactic acid) produced by Lactobacillus plantarum strain. Food Bioprocess. Technol. 2010, 3, 379–386. [Google Scholar] [CrossRef]
- Lopez, S.; Bermudez, B.; Montserrat-De La Paz, S.; Jaramillo, S.; Varela, L.M.; Ortega-Gomez, A.; Abia, R.; Muriana, F.J.G. Membrane composition and dynamics: A target of bioactive virgin olive oil constituents. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1638–1656. [Google Scholar] [CrossRef] [PubMed]
- Sandler, M.; Ruthven, C.R.J.; Goodwin, B.L.; Lees, A.; Stern, G.M. Phenylacetic acid in human body fluids: High correlation between plasma and cerebrospinal fluid concentration values. J. Neurol. Neurosurg. Psychiatry 1982, 45, 366–368. [Google Scholar] [CrossRef]
- Sherwin, C.; Kennard, K. Toxicity of phenylacetic acid. J. Inherit. Metab. Dis. 1919, 40, 259. [Google Scholar]
- Hudgins, W.R.; Shack, S.; Myers, C.E.; Samid, D. Cytostatic activity of phenylacetate and derivatives against tumor cells. Biochem. Pharmacol. 1995, 50, 1273–1279. [Google Scholar] [CrossRef]
- Mu, W.; Yu, S.; Zhu, L.; Zhang, T.; Jiang, B. Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl. Microbiol. Biotechnol. 2012, 95, 1155–1163. [Google Scholar] [CrossRef]
- Dieuleveux, V.; Guéguen, M. Antimicrobial effects of D-3-phenyllactic acid on Listeria monocytogenes in TSB-YE medium, milk, and cheese. J. Food Prot. 1998, 61, 1281–1285. [Google Scholar] [CrossRef]
- Mu, W.; Chen, C.; Li, X.; Zhang, T.; Jiang, B. Optimization of culture medium for the production of phenyllactic acid by Lactobacillus sp. SK007. Bioresour. Technol. 2009, 100, 1366–1370. [Google Scholar] [CrossRef]
- Ning, Y.; Yan, A.; Yang, K.; Wang, Z.; Li, X.; Jia, Y. Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem. 2017, 228, 533–540. [Google Scholar] [CrossRef]
- Hillenga, D.J.; Versantvoort, H.J.M.; Van der Molen, S.; Driessen, A.J.M.; Konings, W.N. Penicillium chrysogenum takes up the penicillin G precursor phenylacetic acid by passive diffusion. Appl. Environ. Microbiol. 1995, 61, 2589–2595. [Google Scholar] [CrossRef]
- Bittenbender, W.A.; Bittenbender, E.F.D. The effect of para-substituents on the bacteriostatic properties of phenylacetic acid. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1972, 28, 514–519. [Google Scholar]
- Giovannini, C.; Straface, E.; Modesti, D.; Coni, E.; Cantafora, A.; De Vincenzi, M.; Malorni, W.; Masella, R. Tyrosol, the Major Olive Oil Biophenol, Protects Against Oxidized-LDL-Induced Injury in Caco-2 Cells. J. Nutr. 1999, 129, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Covas, M.; Miró-Casas, E.; Fitó, M.; Farré-Albadalejo, M.; Gimeno, E.; Marrugat, J.; De LA Torre, R. Bioavalability of tyrosol, an antioxidant phenolic compound present in wine and olive oil, in humans. Drugs Exp. Clin. Res. 2003, 29, 203–206. [Google Scholar] [PubMed]
- Fujita, T.; Winkler, D.A. Understanding the Roles of the “two QSARs”. J. Chem. Inf. Model. 2016, 56, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; De Stasio, G.; d’Ubaldo, A.; Gratton, E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 1990, 57, 1179–1186. [Google Scholar] [CrossRef]
- Nweke, C.O.; Ogbonna, C.J. Statistical models for biphasic dose-response relationships (hormesis) in toxicological studies. Ecotoxicol. Environ. Contam. 2017, 12, 39–55. [Google Scholar] [CrossRef]
- Sezonov, G.; Joseleau-Petit, D.; D’Ari, R. Escherichia coli physiology in Luria-Bertani broth. J. Bacteriol. 2007, 189, 8746–8749. [Google Scholar] [CrossRef]
- Gray, W.T.; Govers, S.K.; Xiang, Y.; Parry, B.R.; Campos, M.; Kim, S.; Jacobs-wagner, C. Nucleoid size scaling and intracellular organization of translation across bacteria. Cell 2019, 177, 1632–1648. [Google Scholar] [CrossRef]
- Petrache, H.I.; Dodd, S.W.; Brown, M.F. Area per lipid and acyl length distributions in fluid phosphatidylcholines determined by 2H NMR spectroscopy. Biophys. J. 2000, 79, 3172–3192. [Google Scholar] [CrossRef]
- Centi, A.; Dutta, A.; Parekh, S.H.; Bereau, T. Inserting Small Molecules across Membrane Mixtures: Insight from the Potential of Mean Force. Biophys. J. 2020, 118, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Centi, A.; Menichetti, R.; Bereau, T. Molecular dynamics trajectories for 630 coarse-grained drug-membrane permeations. Sci. Data 2020, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Kutzner, C.; Van Der Spoel, D.; Lindahl, E. GRGMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- De Jong, D.H.; Singh, G.; Bennett, W.F.D.; Arnarez, C.; Wassenaar, T.A.; Schäfer, L.V.; Periole, X.; Tieleman, D.P.; Marrink, S.J. Improved parameters for the martini coarse-grained protein force field. J. Chem. Theory Comput. 2013, 9, 687–697. [Google Scholar] [CrossRef]
- Bereau, T.; Kremer, K. Automated Parametrization of the Coarse-Grained Martini Force Field for Small Organic Molecules. J. Chem. Theory Comput. 2015, 11, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Sardessai, Y.; Bhosle, S. Tolerance of bacteria to organic solvents. Res. Microbiol. 2002, 153, 263–268. [Google Scholar] [CrossRef]
- Bhal, S.; Log, P. Making Sense of the Value. Adv. Chem. Dev Inc. (ACD/Labs). Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjwpJ2c0tfvAhXzh_0HHahMA6AQFjABegQIBhAD&url=https%3A%2F%2Fwww.acdlabs.com%2Fdownload%2Fapp%2Fphyschem%2Fmaking_sense.pdf&usg=AOvVaw3rpY_xb_4mMyKpgWCWb3bm (accessed on 21 June 2018).
- Paiva-Martins, F.; Gordon, M.H.; Gameiro, P. Activity and location of olive oil phenolic antioxidants in liposomes. Chem. Phys. Lipids 2003, 124, 23–36. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef]
- Parasassi, T.; Di Stefano, M.; Loiero, M.; Ravagnan, G.; Gratton, E. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys. J. 1994, 66, 120–132. [Google Scholar] [CrossRef]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and Pharmacodymanics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23. [Google Scholar] [CrossRef] [PubMed]


| Substance | logP | MIC50 |
|---|---|---|
| phenyllactic acid | 0.72 | 44.97 |
| Tyrosol | 1.00 | 29.74 |
| phenylacetic acid | 1.36 | 20.28 |
| 2-phenylethanol | 1.49 | 14.89 |
| methyl phenylacetate | 1.98 | 6.30 |
| 1-hexanol | 2.13 | 7.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kleinwächter, I.S.; Pannwitt, S.; Centi, A.; Hellmann, N.; Thines, E.; Bereau, T.; Schneider, D. The Bacteriostatic Activity of 2-Phenylethanol Derivatives Correlates with Membrane Binding Affinity. Membranes 2021, 11, 254. https://doi.org/10.3390/membranes11040254
Kleinwächter IS, Pannwitt S, Centi A, Hellmann N, Thines E, Bereau T, Schneider D. The Bacteriostatic Activity of 2-Phenylethanol Derivatives Correlates with Membrane Binding Affinity. Membranes. 2021; 11(4):254. https://doi.org/10.3390/membranes11040254
Chicago/Turabian StyleKleinwächter, Isabel S., Stefanie Pannwitt, Alessia Centi, Nadja Hellmann, Eckhard Thines, Tristan Bereau, and Dirk Schneider. 2021. "The Bacteriostatic Activity of 2-Phenylethanol Derivatives Correlates with Membrane Binding Affinity" Membranes 11, no. 4: 254. https://doi.org/10.3390/membranes11040254
APA StyleKleinwächter, I. S., Pannwitt, S., Centi, A., Hellmann, N., Thines, E., Bereau, T., & Schneider, D. (2021). The Bacteriostatic Activity of 2-Phenylethanol Derivatives Correlates with Membrane Binding Affinity. Membranes, 11(4), 254. https://doi.org/10.3390/membranes11040254







