Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Membrane Fabrication
2.3. Membranes Characterization
2.4. Gas Permeation Study
3. Results and Discussions
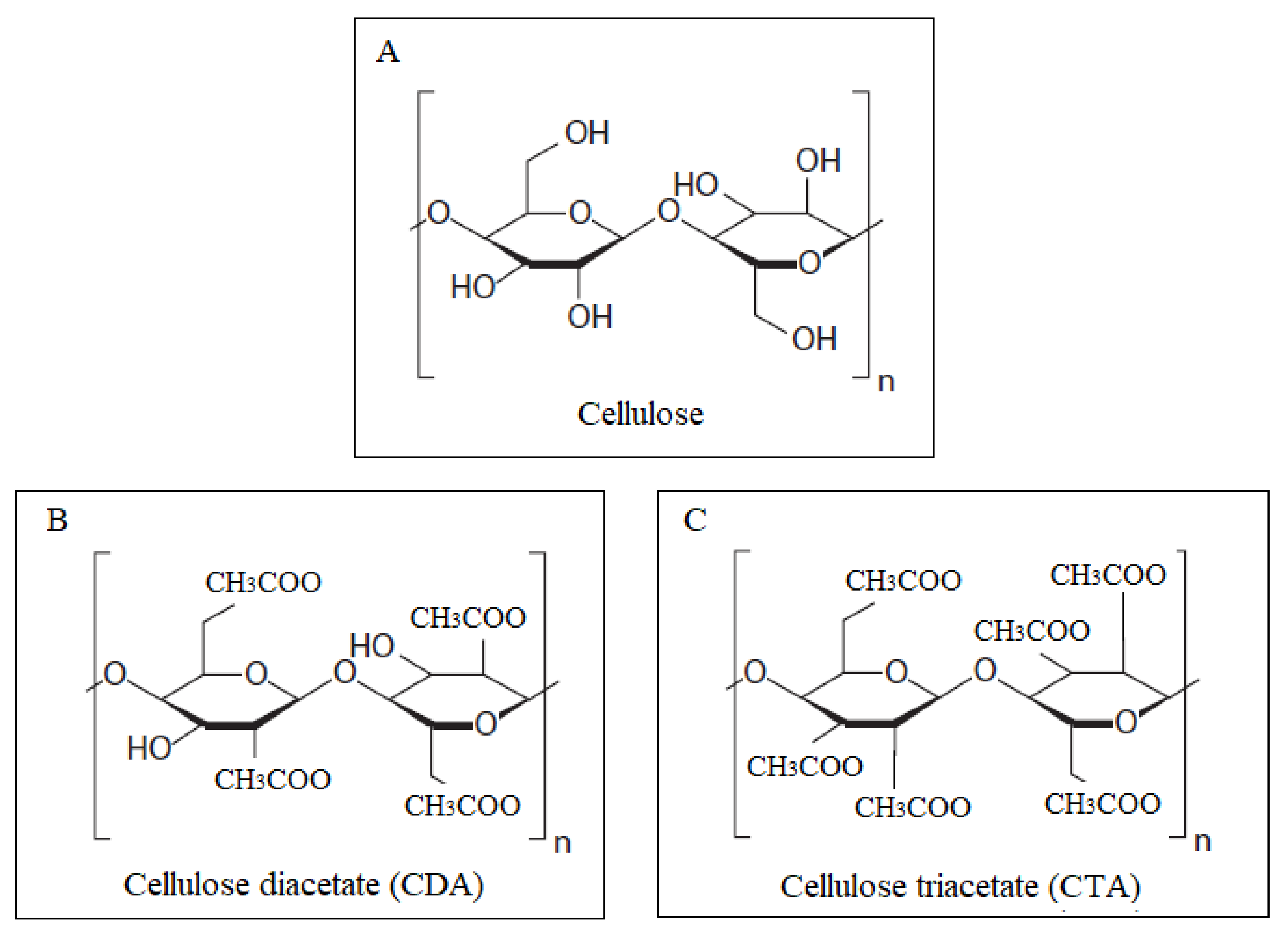
3.1. Membrane Characterization
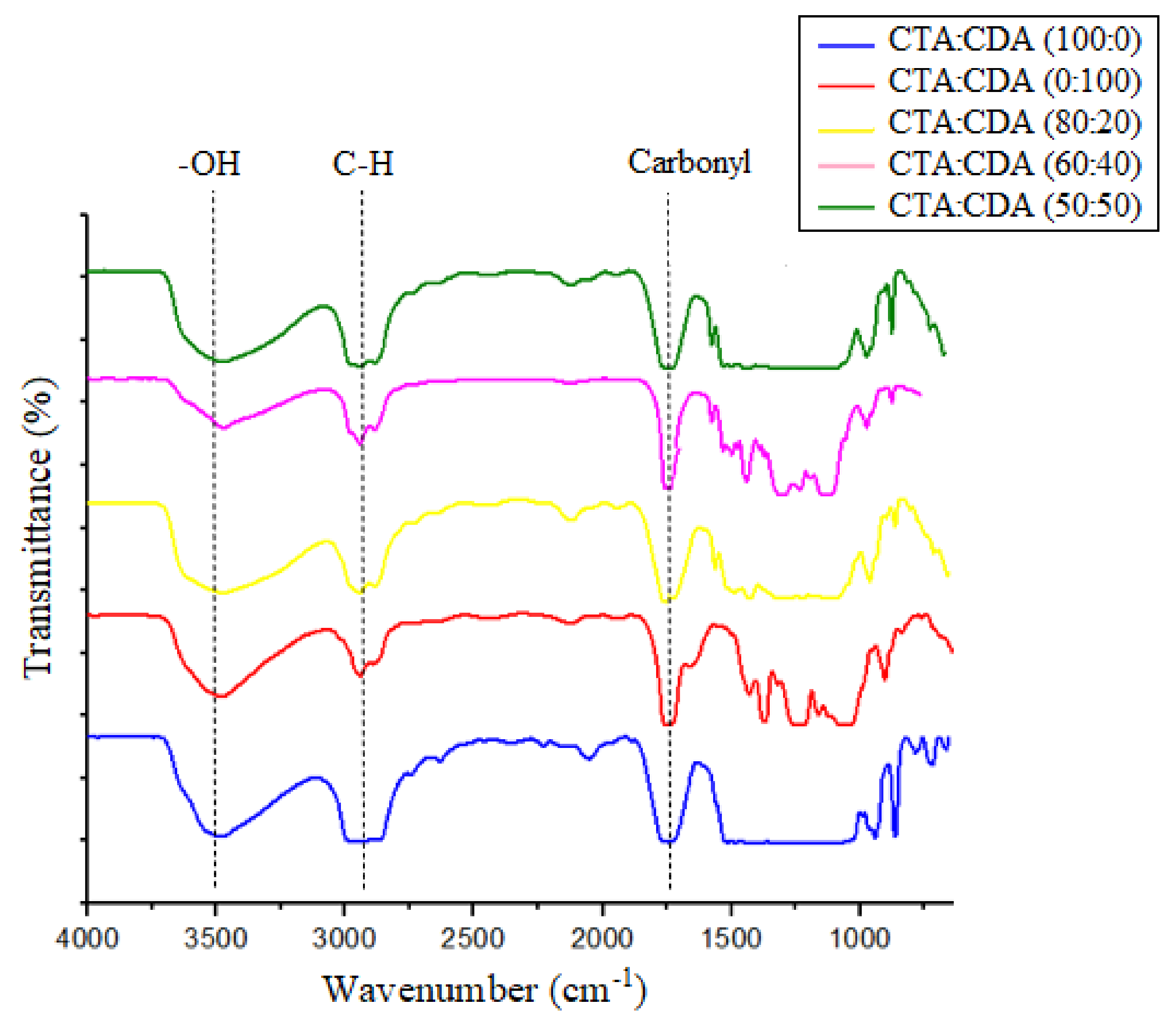
3.1.1. FTIR Analysis
3.1.2. XRD Analysis
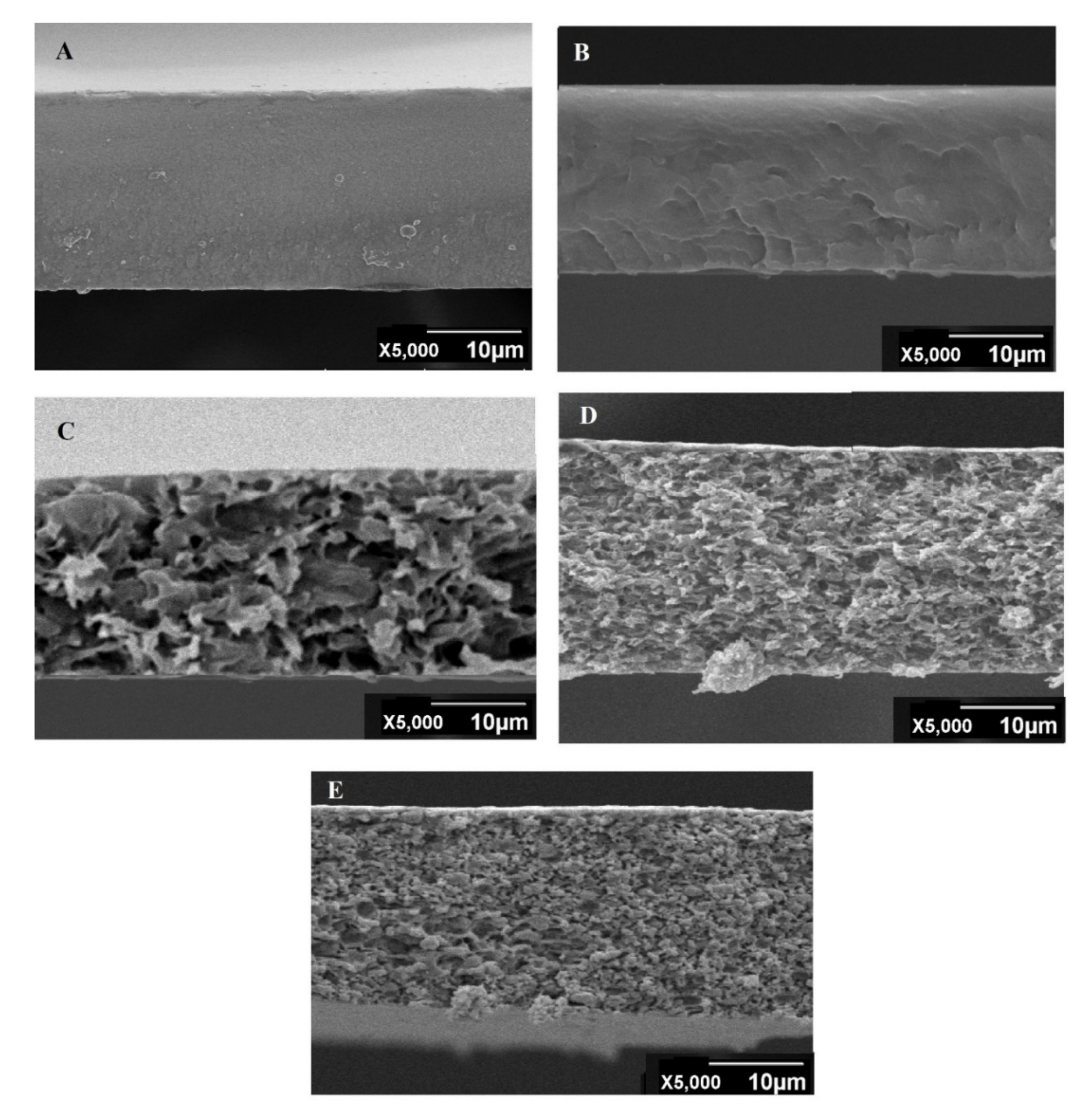
3.1.3. SEM Analysis
3.1.4. Mechanical Properties
3.2. Gas Permeation Study
3.3. Performance Comparison of Fabricated Membranes
3.4. Plasticization Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Baker, R.W.; Freeman, B.; Kniep, J.; Wei, X.; Merkel, T. CO2 capture from natural gas power plants using selective exhaust gas recycle membrane designs. Int. J. Greenh. Gas Control. 2017, 66, 35–47. [Google Scholar] [CrossRef]
- Lin, H.; Yavari, M. Upper bound of polymeric membranes for mixed-gas CO2/CH4 separations. J. Membr. Sci. 2015, 475, 101–109. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Bhide, B.; Stern, S. Membrane processes for the removal of acid gases from natural gas. II. Effects of operating conditions, economic parameters, and membrane properties. J. Membr. Sci. 1993, 81, 239–252. [Google Scholar] [CrossRef]
- Othman, M.; Tan, S.; Bhatia, S. Separability of carbon dioxide from methane using MFI zeolite–silica film deposited on gamma-alumina support. Microporous Mesoporous Mater. 2009, 121, 138–144. [Google Scholar] [CrossRef]
- Nasir, R.; Mukhtar, H.; Man, Z.; Mohshim, D.F. Material Advancements in Fabrication of Mixed-Matrix Membranes. Chem. Eng. Technol. 2013, 36, 717–727. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea water demineralization by means of an osmotic membrane. Adv. Chem. Ser. ACS 1963, 38, 117–132. [Google Scholar]
- Schell, W.; Wensley, C.; Chen, M.; Venugopal, K.; Miller, B.; Stuart, J. Recent advances in cellulosic membranes for gas separation and pervaporation. Gas Sep. Purif. 1989, 3, 162–169. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gasseparation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Buonomenna, M.; Yave, W.; Golemme, G. Some approaches for high performance polymer based membranes for gas separation: Block copolymers, carbon molecular sieves and mixed matrix membranes. RSC Adv. 2012, 2, 10745–10773. [Google Scholar] [CrossRef]
- Kamide, K.; Saito, M. Cellulose and cellulose derivatives: Recent advances in physical chemistry. In Biopolymers; Springer: Berlin/Heidelberg, Germany, 1987; pp. 1–56. [Google Scholar]
- Puleo, A.; Paul, D.; Kelley, S. The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J. Membr. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]
- Li, G.S. Cellulosic Semipermeable Membranes Containing Silicon Compounds. U.S. Patent 4,428,776, 31 January 1984. [Google Scholar]
- Sada, E.; Kumazawa, H.; Xu, P.; Wang, S.-T. Permeation of pure carbon dioxide and methane and binary mixtures through cellulose acetate membranes. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 113–125. [Google Scholar] [CrossRef]
- Overman, D.C., III; Kau, J.I.; Mahoney, R.D. Preparing Cellulose Ester Membranes for Gas Separation. U.S. Patent 5,011,637, 30 April 1991. [Google Scholar]
- Houde, A.Y.; Krishnakumar, B.; Charati, S.G.; Stern, S.A. Permeability of dense (homogeneous) cellulose acetate membranes to methane, carbon dioxide, and their mixtures at elevated pressures. J. Appl. Polym. Sci. 1996, 62, 2181–2192. [Google Scholar] [CrossRef]
- Bos, A.; Punt, I.G.; Wessling, M.; Strathmann, H. CO2-induced plasticization phenomena in glassy polymers. J. Membr. Sci. 1999, 155, 67–78. [Google Scholar] [CrossRef]
- Liu, C.; Wilson, S.T.; Kulprathipanja, S. Crosslinked Organic-Inorganic Hybrid Membranes and Their Use in Gas Separation. U.S. Patent 7,790,803, 7 September 2010. [Google Scholar]
- Kebiche-Senhadji, O.; Bey, S.; Clarizia, G.; Mansouri, L.; Benamor, M. Gas permeation behavior of CTA polymer inclusion membrane (PIM) containing an acidic carrier for metal recovery (DEHPA). Sep. Purif. Technol. 2011, 80, 38–44. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L.; Norwahyu, J. Efficient CO2/N2 and CO2/CH4 separation using NH2-MIL-53(Al)/cellulose acetate (CA) mixed matrix membranes. Sep. Purif. Technol. 2018, 199, 140–151. [Google Scholar] [CrossRef]
- Kim, W.-G.; Lee, J.S.; Bucknall, D.G.; Koros, W.J.; Nair, S. Nanoporous layered silicate AMH-3/cellulose acetate nanocomposite membranes for gas separations. J. Membr. Sci. 2013, 441, 129–136. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Morisato, A.; Bhuwania, N.; Chinn, D.; Koros, W.J. Natural gas sweetening using a cellulose triacetate hollow fiber membrane illustrating controlled plasticization benefits. J. Membr. Sci. 2020, 601, 117910. [Google Scholar] [CrossRef]
- Nguyen, H.; Wang, M.; Hsiao, M.-Y.; Nagai, K.; Ding, Y.; Lin, H. Suppression of crystallization in thin films of cellulose diacetate and its effect on CO2/CH4 separation properties. J. Membr. Sci. 2019, 586, 7–14. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.J. Cellulose Esters, Organic. Encycl. Polym. Sci. Technol. 2004. [Google Scholar] [CrossRef]
- Li, X.-G.; Kresse, I.; Springer, J.; Nissen, J.; Yang, Y.-L. Morphology and gas permselectivity of blend membranes of polyvinylpyridine with ethylcellulose. Polymer 2001, 42, 6859–6869. [Google Scholar] [CrossRef]
- Lam, B.; Wei, M.; Zhu, L.; Luo, S.; Guo, R.; Morisato, A.; Alexandridis, P.; Lin, H. Cellulose triacetate doped with ionic liquids for membrane gas separation. Polymer 2016, 89, 1–11. [Google Scholar] [CrossRef]
- Dyer, C.; Bozell, J.; Rials, T.; Jiang, Z.; Heller, W.T.; Dadmun, M. Effect of chain structure on the miscibility of cellulose acetate blends: A small-angle neutron scattering study. Soft Matter 2013, 9, 3402–3411. [Google Scholar] [CrossRef]
- Nakai, Y.; Yoshimizu, H.; Tsujita, Y. Enhancement of Gas Permeability in HPC, CTA and PMMA under Microwave Irradiation. Polym. J. 2006, 38, 376–380. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Kamide, K. Cellulose and Cellulose Derivatives; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Liu, J.; Zhang, G.; Clark, K.; Lin, H. Maximizing Ether Oxygen Content in Polymers for Membrane CO2 Removal from Natural Gas. ACS Appl. Mater. Interfaces 2019, 11, 10933–10940. [Google Scholar] [CrossRef]
- Koros, W.J.; Fleming, G.K. Membrane-based gas separation. J. Membr. Sci. 1993, 83, 1–80. [Google Scholar] [CrossRef]
- Krol, J.; Boerrigter, M.; Koops, G. Polyimide hollow fiber gas separation membranes: Preparation and the suppression of plasticization in propane/propylene environments. J. Membr. Sci. 2001, 184, 275–286. [Google Scholar] [CrossRef]
- Chen, C.-C.; Miller, S.J.; Koros, W.J. Characterization of Thermally Cross-Linkable Hollow Fiber Membranes for Natural Gas Separation. Ind. Eng. Chem. Res. 2012, 52, 1015–1022. [Google Scholar] [CrossRef]
- Wessling, M.; Schoeman, S.; Boomgaard, A.V.D.; Smolders, C. Plasticization of gas separation membranes. Gas Sep. Purif. 1991, 5, 222–228. [Google Scholar] [CrossRef]



| Properties | CDA | CTA |
|---|---|---|
| Glass transition temp. (Tg °C) | 187 | 185 |
| Melting temp. (Tm °C) | 233 | 293 |
| Crystallinity (%) | 37 | 52 |
| Tensile strength (103 MPa) | 12.7 | 14 |
| Elongation at break (%) | 14 | 17 |
| Name of Sample | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|
| CTA:CDA (100:0) | 38.55 ± 0.22 | 5.56 ± 0.32 |
| CTA:CDA (0:100) | 32.89 ± 0.41 | 6.47 ± 0.56 |
| CTA:CDA (80:20) | 10.04 ± 0.03 | 10.06 ± 0.32 |
| CTA:CDA (60:40) | 11.98 ± 1.2 | 8.24 ± 0.99 |
| CTA:CDA (50:50) | 12.68 ± 0.82 | 7.50 ± 0.20 |
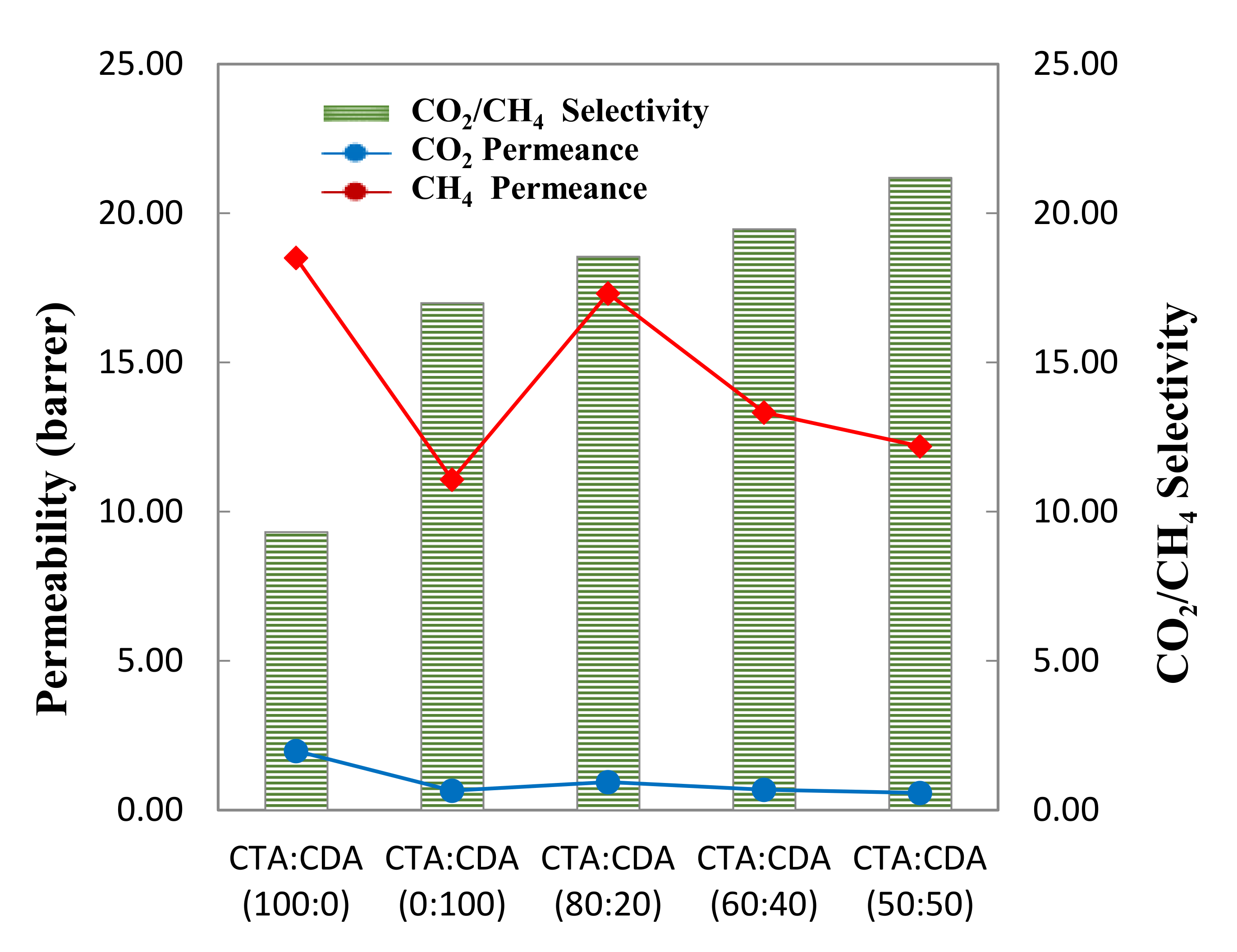
| Sample Name | CO2 Permeability (Barrer) | CH4 Permeability (Barrer) | CO2/CH4 Selectivity |
|---|---|---|---|
| CTA/CA (100:0) | 18.50 ± 0.12 | 1.98 ± 0.14 | 9.33 |
| CTA/CA (0:100) | 11.08 ± 0.42 | 0.65 ± 0.22 | 17.00 |
| CTA/CA (80:20) | 17.32 ± 0.09 | 0.93 ± 0.18 | 18.55 |
| CTA/CA (60:40) | 13.33 ± 0.52 | 0.68 ± 0.62 | 19.48 |
| CTA/CA (50:50) | 12.20 ± 0.25 | 0.58 ± 0.17 | 21.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Farrukh, S.; Hussain, A.; Khan, I.; Othman, M.H.D.; Ahsan, M. Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membranes 2021, 11, 245. https://doi.org/10.3390/membranes11040245
Raza A, Farrukh S, Hussain A, Khan I, Othman MHD, Ahsan M. Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membranes. 2021; 11(4):245. https://doi.org/10.3390/membranes11040245
Chicago/Turabian StyleRaza, Ayesha, Sarah Farrukh, Arshad Hussain, Imranullah Khan, Mohd Hafiz Dzarfan Othman, and Muhammad Ahsan. 2021. "Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation" Membranes 11, no. 4: 245. https://doi.org/10.3390/membranes11040245
APA StyleRaza, A., Farrukh, S., Hussain, A., Khan, I., Othman, M. H. D., & Ahsan, M. (2021). Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membranes, 11(4), 245. https://doi.org/10.3390/membranes11040245









