Half-Cell State of Charge Monitoring for Determination of Crossover in VRFB—Considerations and Results Concerning Crossover Direction and Amount
Abstract
1. Introduction
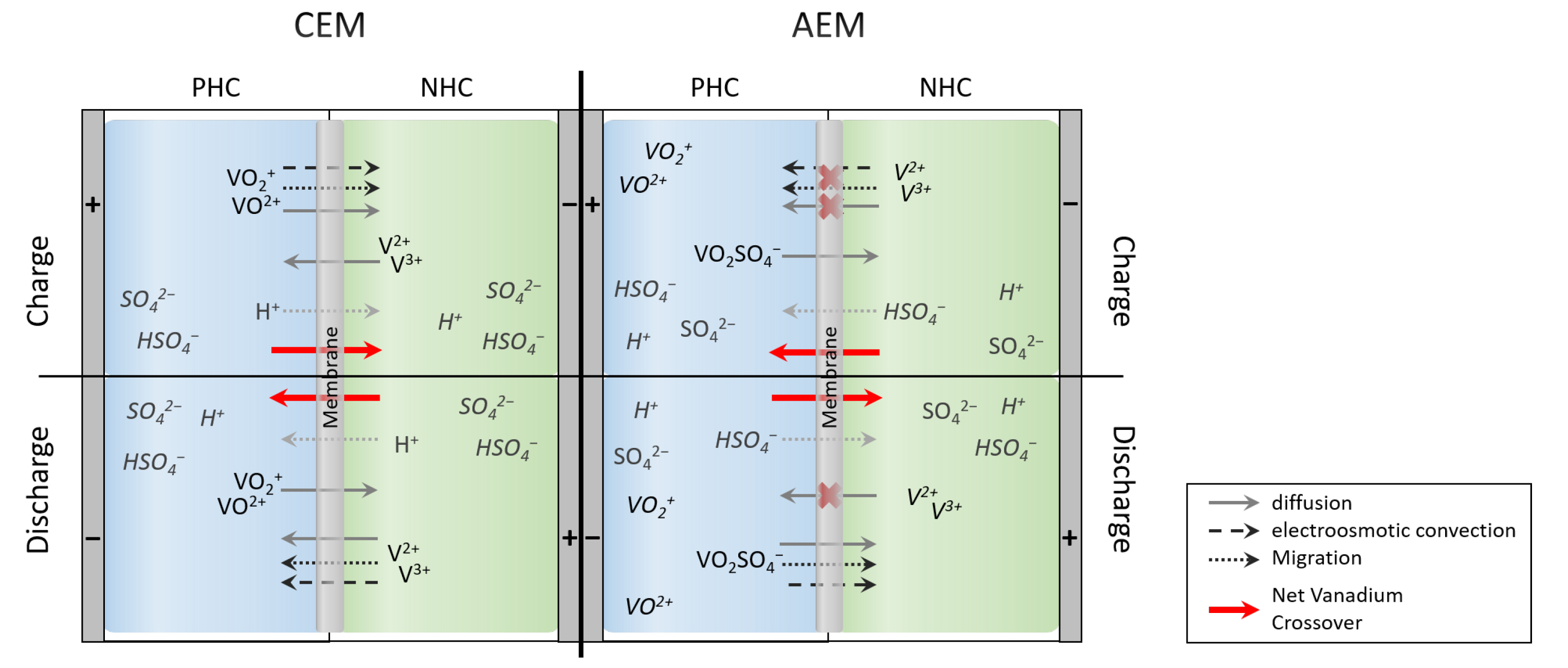
Crossover Processes through Ion Exchange Membranes
2. Materials and Methods
3. Impact of Vanadium Crossover on State of Charge: Case Studies
- Net crossover of vanadium ions results in self-discharge of one half-cell of the VFB predominantly; which half-cell is affected most depends on the type and material of the membrane and state of operation.
- Amount of vanadium ions crossing depends on current density and membrane thickness.
- Crossover of vanadium ions results from different processes (diffusion, migration and electroosmotic convection) which may coincide or counteract.
3.1. Vanadium Crossover: Assumptions
- Only V and VO ions are assumed to cross the membranes since the resulting self discharge is more explicit.
- Each vanadium ion crossing the membrane reacts stochiometrically in the other half-cell, other side reactions or crossing of other electrolyte components, e.g., H or HSO are neglected.
- The amount of vanadium crossover is defined and kept constant for each cycle within the calculated charge-discharge (cd) sequences if not otherwise claimed.
- For charging and discharging the VFB, it is assumed that a maximum of 100% and minimum of 0% is achievable.
- No distinction is made between current density or thickness dependent crossover respectively between migration, electroosmotic convection and diffusion driven crossover.
- Water transport is neglected and a constant volume in the half-cells is assumed.
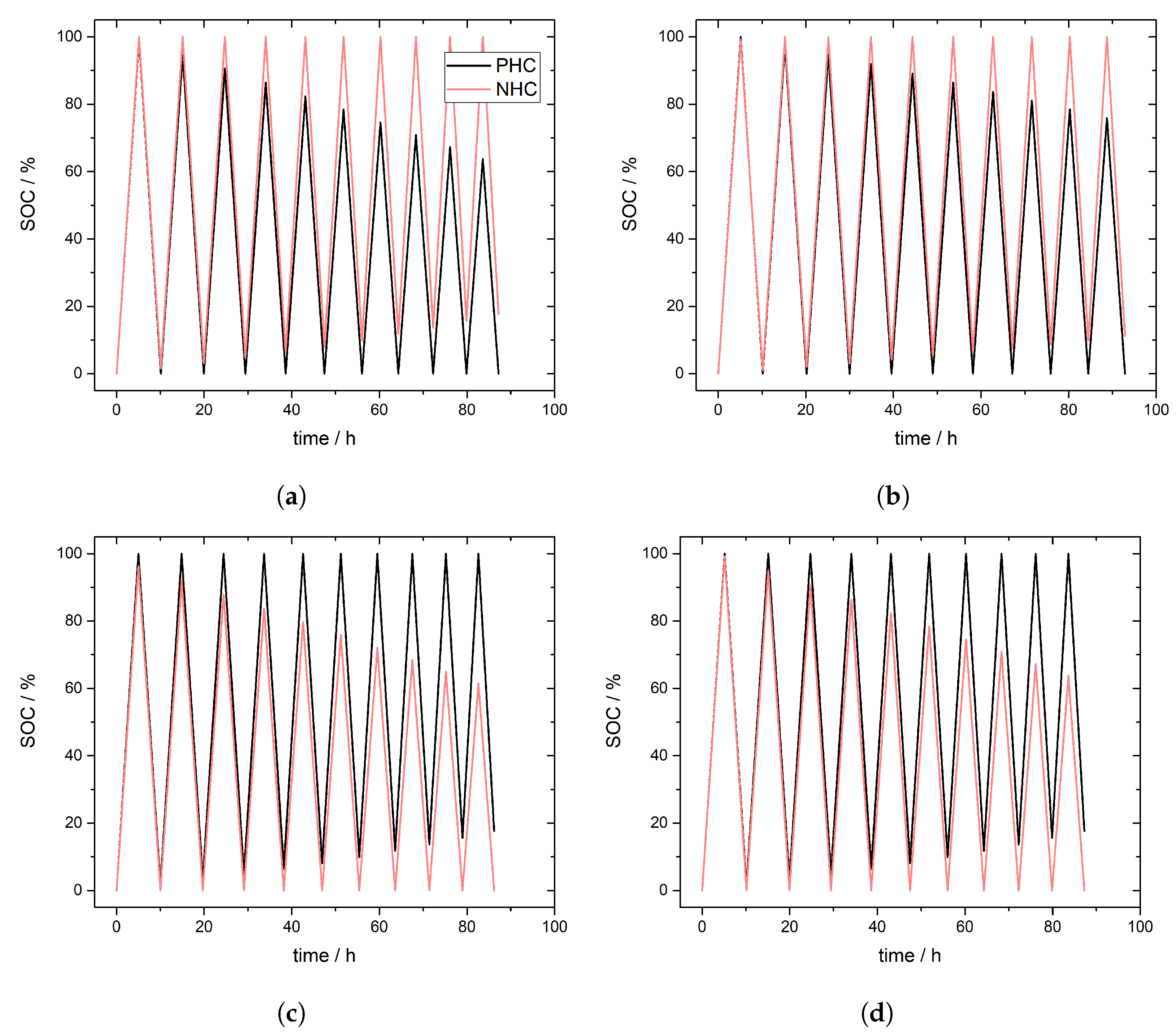
3.2. Simulation of Crossover Direction
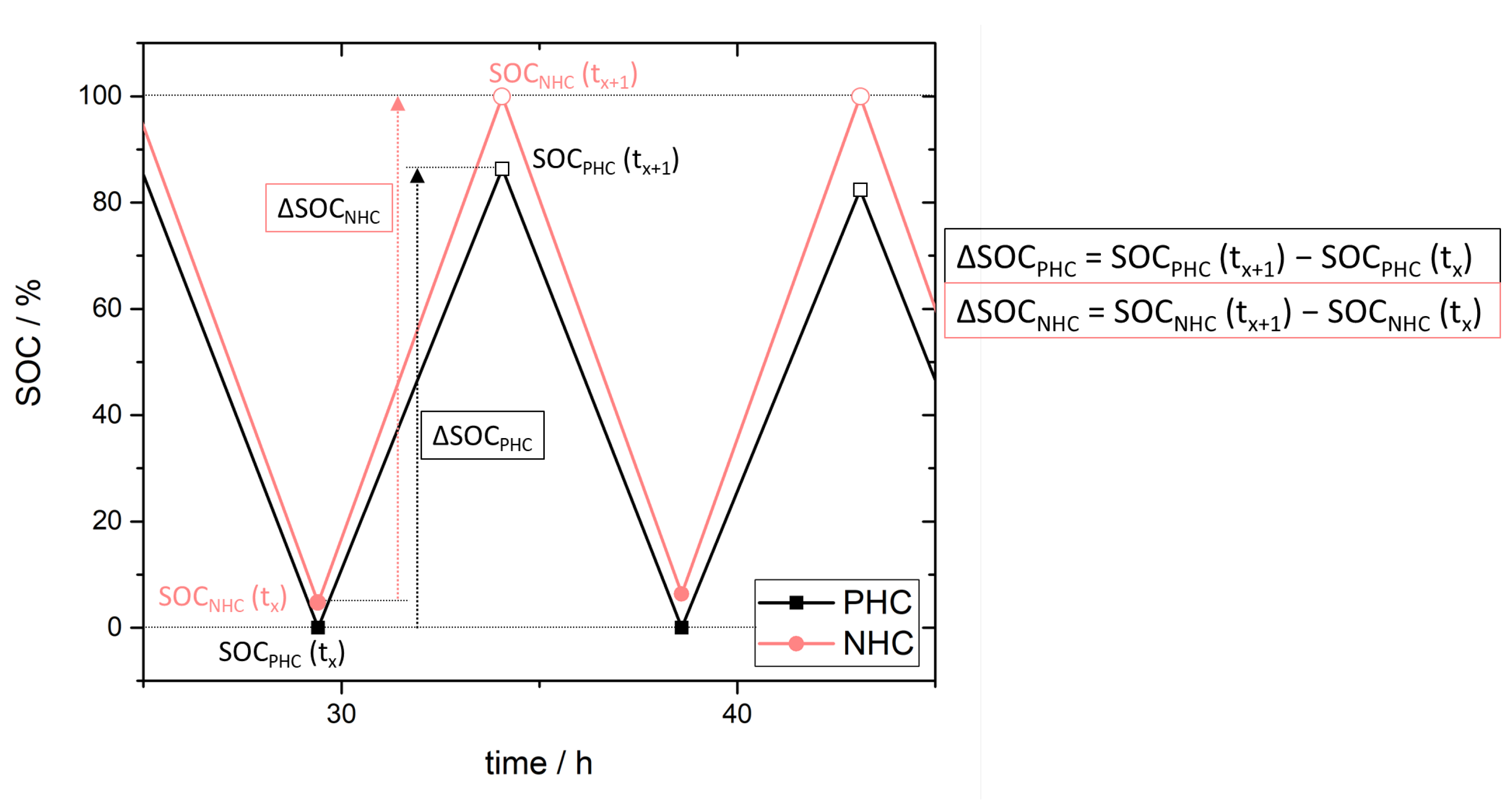
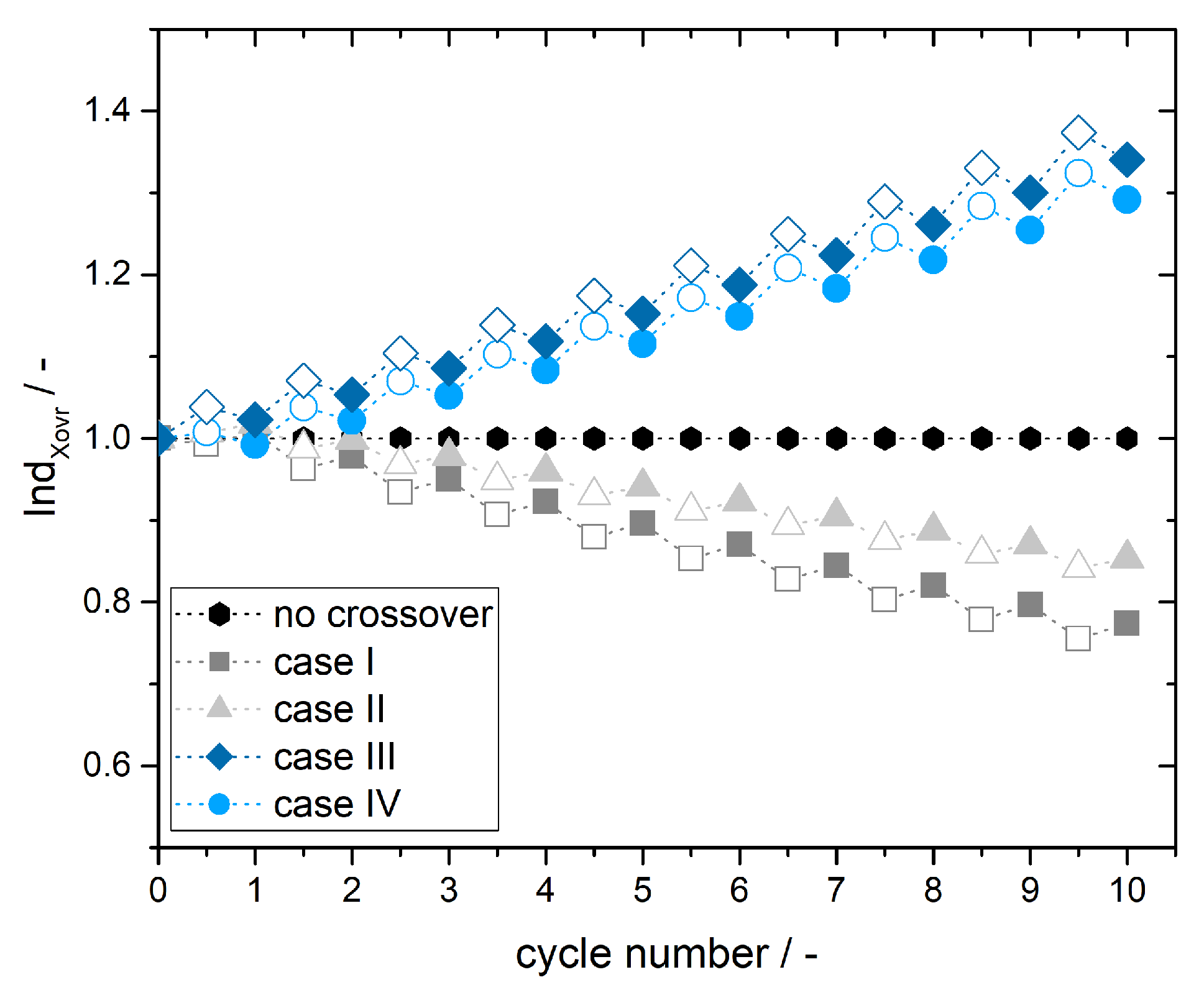
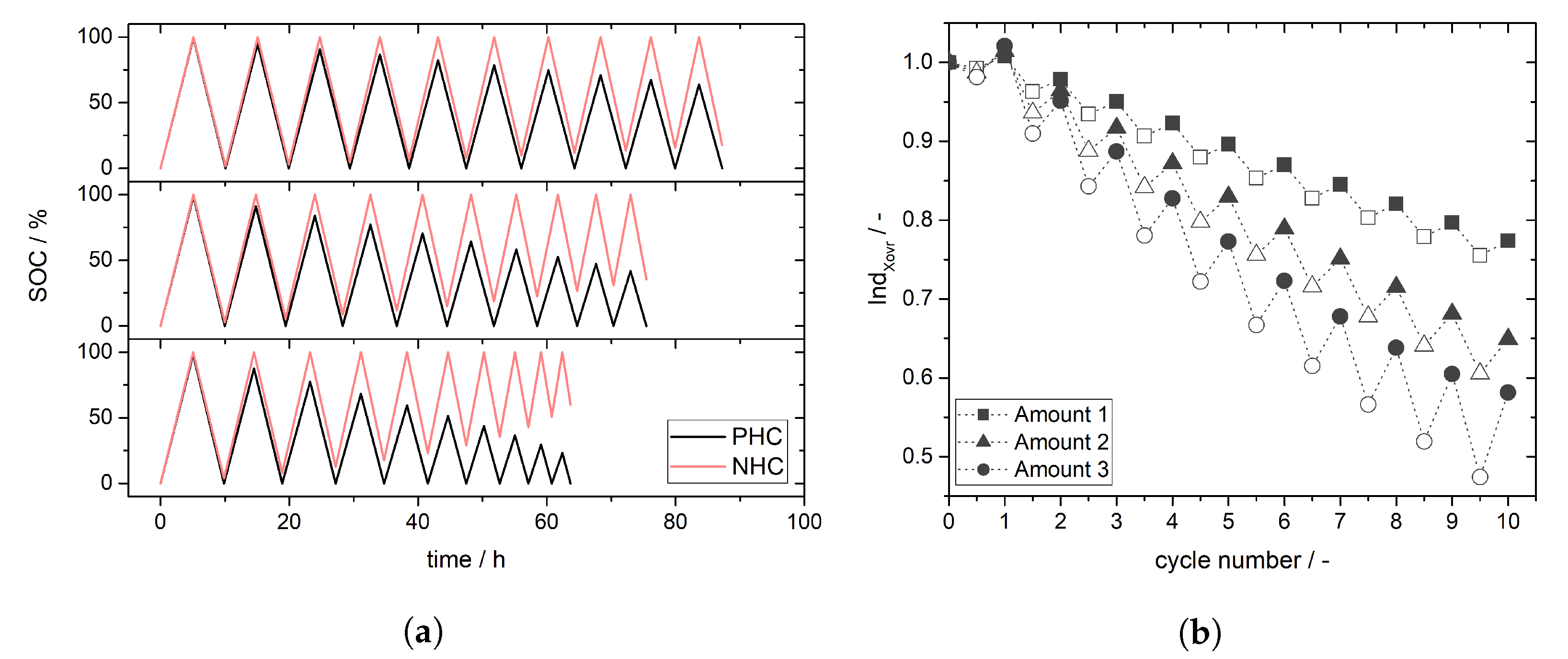
3.3. Simulation of Crossover Amount
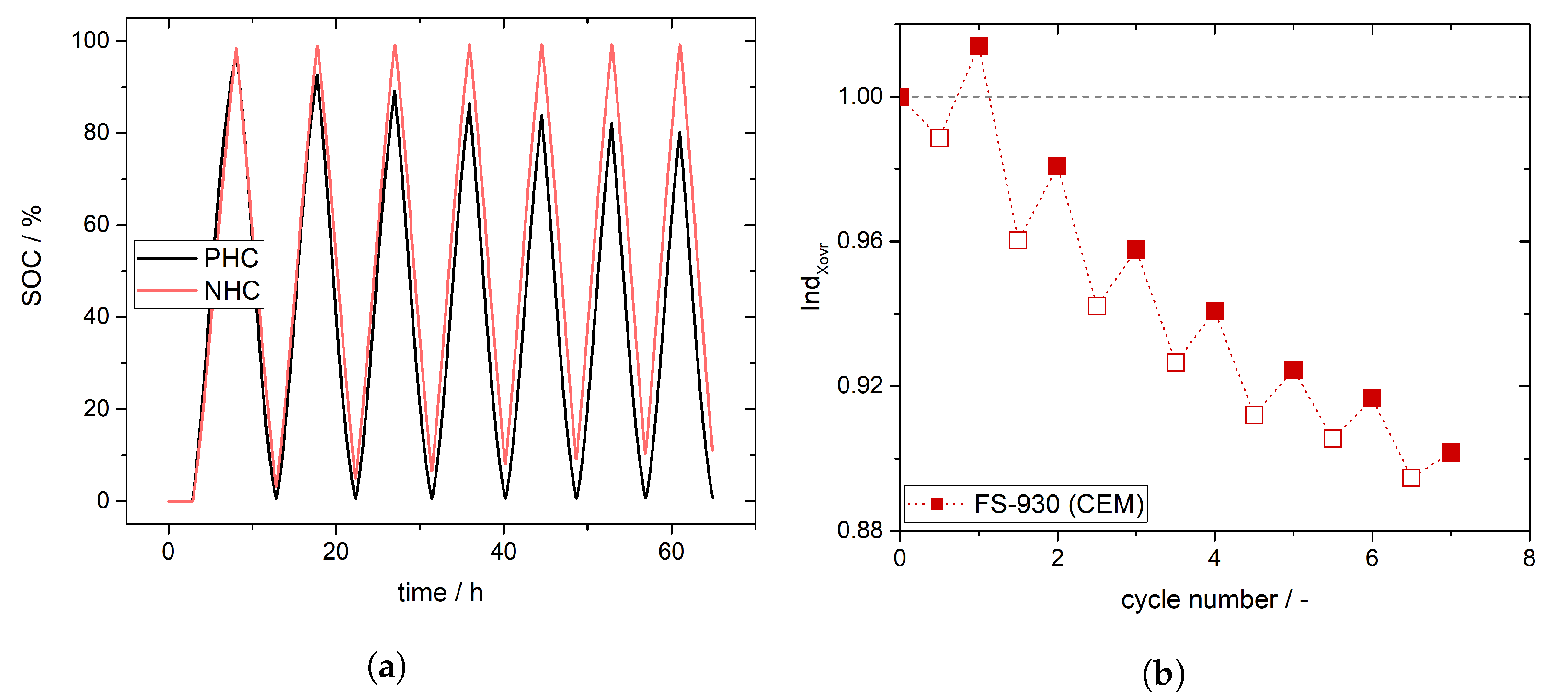
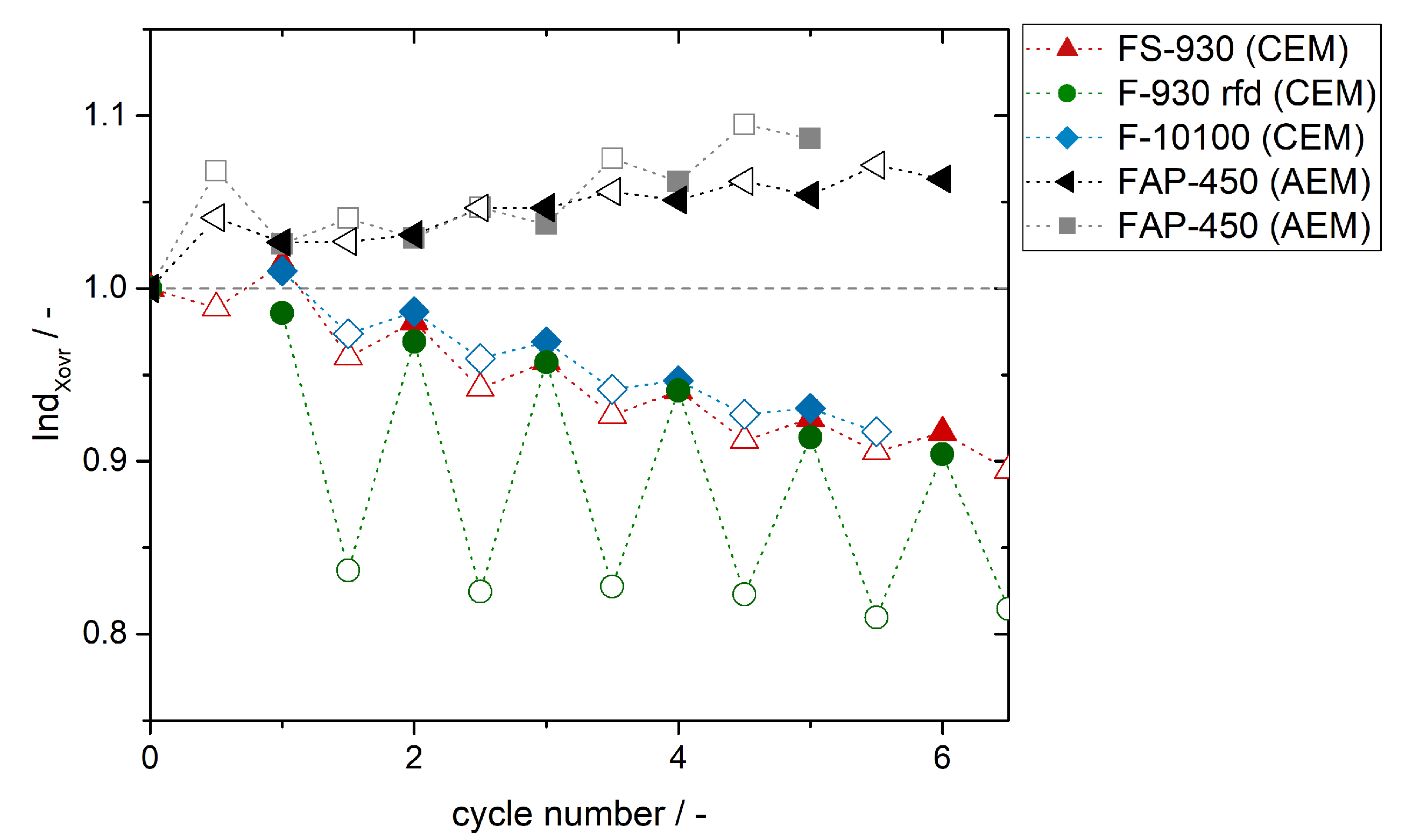
4. Experimental Results and Discussion: Determination of Crossover Direction and Amount
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, A.Z.; Mench, M.M.; Meyers, J.P.; Ross, P.N.; Gostick, J.T.; Liu, Q. Redox flow batteries: A review. J. Appl. Electrochem. 2011, 41, 1137. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. ChemSusChem 2016, 9, 1521–1543. [Google Scholar] [CrossRef]
- Arenas, L.; De León, C.P.; Walsh, F. Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef]
- Agar, E.; Benjamin, A.; Dennison, C.; Chen, D.; Hickner, M.; Kumbur, E. Reducing capacity fade in vanadium redox flow batteries by altering charging and discharging currents. J. Power Sources 2014, 246, 767–774. [Google Scholar] [CrossRef]
- Nourani, M.; Dennison, C.R.; Jin, X.; Liu, F.; Agar, E. Elucidating Effects of Faradaic Imbalance on Vanadium Redox Flow Battery Performance: Experimental Characterization. J. Electrochem. Soc. 2019, 166, A3844. [Google Scholar] [CrossRef]
- Pugach, M.; Kondratenko, M.; Briola, S.; Bischi, A. Zero dimensional dynamic model of vanadium redox flow battery cell incorporating all modes of vanadium ions crossover. Appl. Energy 2018, 226, 560–569. [Google Scholar] [CrossRef]
- Piller, S.; Perrin, M.; Jossen, A. Methods for state-of-charge determination and their applications. J. Power Sources 2001, 96, 113–120. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Kazacos, M. State of charge monitoring methods for vanadium redox flow battery control. J. Power Sources 2011, 196, 8822–8827. [Google Scholar] [CrossRef]
- Corcuera, S.; Skyllas-Kazacos, M. State-of-charge monitoring and electrolyte rebalancing methods for the vanadium redox flow battery. Eur. Chem. Bull. 2012, 1, 511–519. [Google Scholar]
- Ressel, S.; Bill, F.; Holtz, L.; Janshen, N.; Chica, A.; Flower, T.; Weidlich, C.; Struckmann, T. State of charge monitoring of vanadium redox flow batteries using half cell potentials and electrolyte density. J. Power Sources 2018, 378, 776–783. [Google Scholar] [CrossRef]
- Haisch, T.; Ji, H.; Weidlich, C. Monitoring the state of charge of all-vanadium redox flow batteries to identify crossover of electrolyte. Electrochim. Acta 2020, 336, 135573. [Google Scholar] [CrossRef]
- Knehr, K.; Kumbur, E. Open circuit voltage of vanadium redox flow batteries: Discrepancy between models and experiments. Electrochem. Commun. 2011, 13, 342–345. [Google Scholar] [CrossRef]
- Schubert, U.S.; Nolte, O.; Volodin, I.; Stolze, C.; Hager, M.D. Trust is Good, Control is Better: A Review on Monitoring and Characterization Techniques for Flow Battery Electrolytes. Mater. Horizons 2021. [Google Scholar] [CrossRef]
- Struckmann, T.; Kuhn, P.; Ressel, S. A combined in situ monitoring approach for half cell state of charge and state of health of vanadium redox flow batteries. Electrochim. Acta 2020, 362, 137174. [Google Scholar] [CrossRef]
- Parasuraman, A.; Lim, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, H.; Li, X.; Mai, Z.; Zhang, H. Poly (tetrafluoroethylene) reinforced sulfonated poly (ether ether ketone) membranes for vanadium redox flow battery application. J. Power Sources 2012, 208, 421–425. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Selective anion exchange membranes for high coulombic efficiency vanadium redox flow batteries. Electrochem. Commun. 2013, 26, 37–40. [Google Scholar] [CrossRef]
- Xing, D.; Zhang, S.; Yin, C.; Zhang, B.; Jian, X. Effect of amination agent on the properties of quaternized poly (phthalazinone ether sulfone) anion exchange membrane for vanadium redox flow battery application. J. Membr. Sci. 2010, 354, 68–73. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for redox flow battery applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef]
- Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T.; Ukil, A.; Zhao, J. Recent development of membrane for vanadium redox flow battery applications: A review. Appl. Energy 2019, 238, 202–224. [Google Scholar] [CrossRef]
- Nibel, O.; Rojek, T.; Schmidt, T.J.; Gubler, L. Amphoteric Ion-Exchange Membranes with Significantly Improved Vanadium Barrier Properties for All-Vanadium Redox Flow Batteries. ChemSusChem 2017, 10, 2767–2777. [Google Scholar] [CrossRef] [PubMed]
- Chae, I.S.; Luo, T.; Moon, G.H.; Ogieglo, W.; Kang, Y.S.; Wessling, M. Ultra-high proton/vanadium selectivity for hydrophobic polymer membranes with intrinsic nanopores for redox flow battery. Adv. Energy Mater. 2016, 6, 1600517. [Google Scholar] [CrossRef]
- Wei, X.; Nie, Z.; Luo, Q.; Li, B.; Chen, B.; Simmons, K.; Sprenkle, V.; Wang, W. Nanoporous polytetrafluoroethylene/silica composite separator as a high-performance all-vanadium redox flow battery membrane. Adv. Energy Mater. 2013, 3, 1215–1220. [Google Scholar] [CrossRef]
- Oldenburg, F.J.; Schmidt, T.J.; Gubler, L. Tackling capacity fading in vanadium flow batteries with amphoteric membranes. J. Power Sources 2017, 368, 68–72. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Zhang, H.; Han, X.; Luo, Q. Investigations on transfer of water and vanadium ions across Nafion membrane in an operating vanadium redox flow battery. J. Power Sources 2010, 195, 890–897. [Google Scholar] [CrossRef]
- Knehr, K.; Agar, E.; Dennison, C.; Kalidindi, A.; Kumbur, E. A transient vanadium flow battery model incorporating vanadium crossover and water transport through the membrane. J. Electrochem. Soc. 2012, 159, A1446. [Google Scholar] [CrossRef]
- Knehr, K.; Kumbur, E. Role of convection and related effects on species crossover and capacity loss in vanadium redox flow batteries. Electrochem. Commun. 2012, 23, 76–79. [Google Scholar] [CrossRef]
- Agar, E.; Knehr, K.; Chen, D.; Hickner, M.; Kumbur, E. Species transport mechanisms governing capacity loss in vanadium flow batteries: Comparing Nafion® and sulfonated Radel membranes. Electrochim. Acta 2013, 98, 66–74. [Google Scholar] [CrossRef]
- Darling, R.M.; Weber, A.Z.; Tucker, M.C.; Perry, M.L. The influence of electric field on crossover in redox-flow batteries. J. Electrochem. Soc. 2015, 163, A5014. [Google Scholar] [CrossRef]
- Vrána, J.; Charvát, J.; Mazúr, P.; Bělskỳ, P.; Dundálek, J.; Pocedič, J.; Kosek, J. Commercial perfluorosulfonic acid membranes for vanadium redox flow battery: Effect of ion-exchange capacity and membrane internal structure. J. Membr. Sci. 2018, 552, 202–212. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Xi, X.; Lai, Q.; Liu, T.; Zhang, H. The transfer behavior of different ions across anion and cation exchange membranes under vanadium flow battery medium. J. Power Sources 2014, 271, 1–7. [Google Scholar] [CrossRef]
- Sarkar, S.; SenGupta, A.K.; Prakash, P. The Donnan membrane principle: Opportunities for sustainable engineered processes and materials. Environ. Sci. Technol. 2010, 44, 1161–1166. [Google Scholar] [CrossRef]
- Kausar, N.; Howe, R.; Skyllas-Kazacos, M. Raman spectroscopy studies of concentrated vanadium redox battery positive electrolytes. J. Appl. Electrochem. 2001, 31, 1327–1332. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.; Kim, S.; Li, L.; Liu, J.; Yang, Z. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef]
- Kim, D.K.; Yoon, S.J.; Kim, S. Transport phenomena associated with capacity loss of all-vanadium redox flow battery. Int. J. Heat Mass Transf. 2020, 148, 119040. [Google Scholar] [CrossRef]
- Song, Y.; Li, X.; Xiong, J.; Yang, L.; Pan, G.; Yan, C.; Tang, A. Electrolyte transfer mechanism and optimization strategy for vanadium flow batteries adopting a Nafion membrane. J. Power Sources 2020, 449, 227503. [Google Scholar] [CrossRef]
- Sukkar, T.; Skyllas-Kazacos, M. Water transfer behaviour across cation exchange membranes in the vanadium redox battery. J. Membr. Sci. 2003, 222, 235–247. [Google Scholar] [CrossRef]
- Sukkar, T.; Skyllas-Kazacos, M. Modification of membranes using polyelectrolytes to improve water transfer properties in the vanadium redox battery. J. Membr. Sci. 2003, 222, 249–264. [Google Scholar] [CrossRef]
- Poli, N.; Schäffer, M.; Trovò, A.; Noack, J.; Guarnieri, M.; Fischer, P. Novel electrolyte rebalancing method for vanadium redox flow batteries. Chem. Eng. J. 2021, 405, 126583. [Google Scholar] [CrossRef]
- Rudolph, S.; Schröder, U.; Bayanov, I. On-line controlled state of charge rebalancing in vanadium redox flow battery. J. Electroanal. Chem. 2013, 703, 29–37. [Google Scholar] [CrossRef]
- Rodby, K.E.; Carney, T.J.; Gandomi, Y.A.; Barton, J.L.; Darling, R.M.; Brushett, F.R. Assessing the levelized cost of vanadium redox flow batteries with capacity fade and rebalancing. J. Power Sources 2020, 460, 227958. [Google Scholar] [CrossRef]
- Cao, L.; Skyllas-Kazacos, M.; Menictas, C.; Noack, J. A review of electrolyte additives and impurities in vanadium redox flow batteries. J. Energy Chem. 2018, 27, 1269–1291. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, T.; An, L.; Zeng, Y.; Wei, L. Modeling of ion transport through a porous separator in vanadium redox flow batteries. J. Power Sources 2016, 327, 67–76. [Google Scholar] [CrossRef]
- Won, S.; Oh, K.; Ju, H. Numerical analysis of vanadium crossover effects in all-vanadium redox flow batteries. Electrochim. Acta 2015, 177, 310–320. [Google Scholar] [CrossRef]
- Gandomi, Y.A.; Aaron, D.; Mench, M. Coupled membrane transport parameters for ionic species in all-vanadium redox flow batteries. Electrochim. Acta 2016, 218, 174–190. [Google Scholar] [CrossRef]
- Oh, K.; Won, S.; Ju, H. A comparative study of species migration and diffusion mechanisms in all-vanadium redox flow batteries. Electrochim. Acta 2015, 181, 238–247. [Google Scholar] [CrossRef]
| Membrane | Thickness | Electrolyte | Electrode | Potential | Current | Electrolyte |
|---|---|---|---|---|---|---|
| Volume | Area | Limits | Density | Flow | ||
| (m) | (mL) | (cm) | (V) | (mA cm) | (L h) | |
| CEM | ||||||
| FS-930 | 30 | 60 | 10 | 0.8–1.65 | 50 | 1.5 |
| F-930 rfd | 30 | 100 | 10 | 0.8–1.70 | 60 | 3 |
| F-10100 | 100 | 100 | 10 | 0.8–1.70 | 35 | 3 |
| AEM | ||||||
| FAP-450 1 | 50 | 100 | 10 | 0.8–1.70 | 60 | 3 |
| FAP-450 2 | 50 | 500 | 40 | 0.8–1.65 | 50 | 3 |
| Case I | Case II | Case III | Case IV | |
|---|---|---|---|---|
| Membrane Type | CEM | CEM | AEM | AEM |
| Crossover charge during | ||||
| NHCPHC | PHCNHC | PHCNHC | PHCNHC |
| V | VO | VOSO | VOSO |
| 0.004 M | 0.004 M | 0.02 M | 0.004 M |
| Crossover discharge during | ||||
| NHCPHC | NHCPHC | PHCNHC | PHCNHC |
| V | V | VOSO | VOSO |
| 0.02 M [45] | 0.02 M | 0.004 M | 0.02 M |
| Predominant during | discharge [28] | discharge [28] | charge | discharge |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haisch, T.; Ji, H.; Holtz, L.; Struckmann, T.; Weidlich, C. Half-Cell State of Charge Monitoring for Determination of Crossover in VRFB—Considerations and Results Concerning Crossover Direction and Amount. Membranes 2021, 11, 232. https://doi.org/10.3390/membranes11040232
Haisch T, Ji H, Holtz L, Struckmann T, Weidlich C. Half-Cell State of Charge Monitoring for Determination of Crossover in VRFB—Considerations and Results Concerning Crossover Direction and Amount. Membranes. 2021; 11(4):232. https://doi.org/10.3390/membranes11040232
Chicago/Turabian StyleHaisch, Theresa, Hyunjoon Ji, Lucas Holtz, Thorsten Struckmann, and Claudia Weidlich. 2021. "Half-Cell State of Charge Monitoring for Determination of Crossover in VRFB—Considerations and Results Concerning Crossover Direction and Amount" Membranes 11, no. 4: 232. https://doi.org/10.3390/membranes11040232
APA StyleHaisch, T., Ji, H., Holtz, L., Struckmann, T., & Weidlich, C. (2021). Half-Cell State of Charge Monitoring for Determination of Crossover in VRFB—Considerations and Results Concerning Crossover Direction and Amount. Membranes, 11(4), 232. https://doi.org/10.3390/membranes11040232






