Electrodeposited Hybrid Biocathode-Based CO2 Reduction via Microbial Electro-Catalysis to Biofuels
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
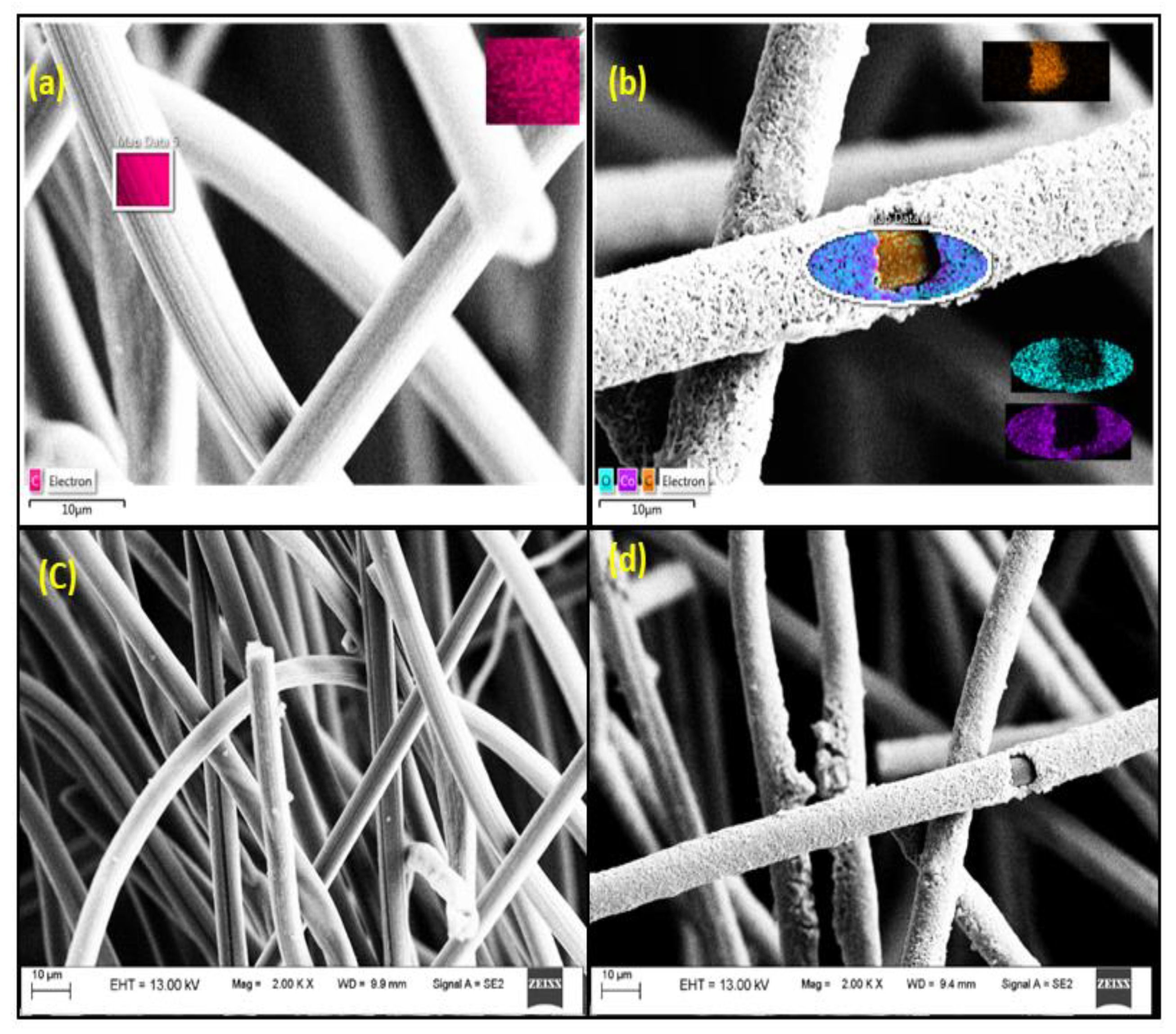
2.2. Electrode Preparations and Morphology, Elemental Characterization
2.3. Inoculation
2.4. MES Configuration and Design
2.5. Operation
2.6. Analysis
3. Results and Discussion
3.1. Characterization of Modified Carbon Felt
3.2. Responsive Reductive Current Generation
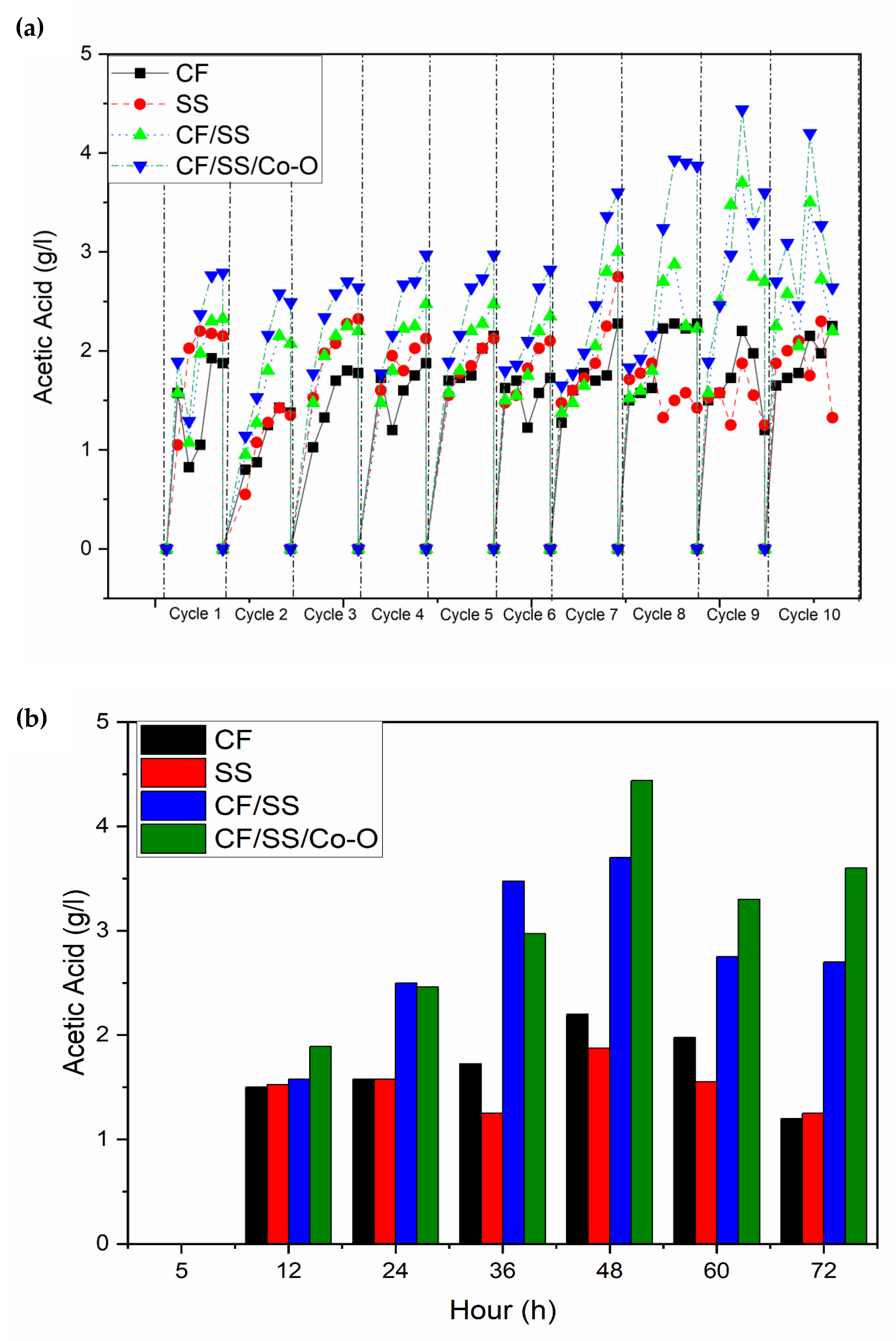
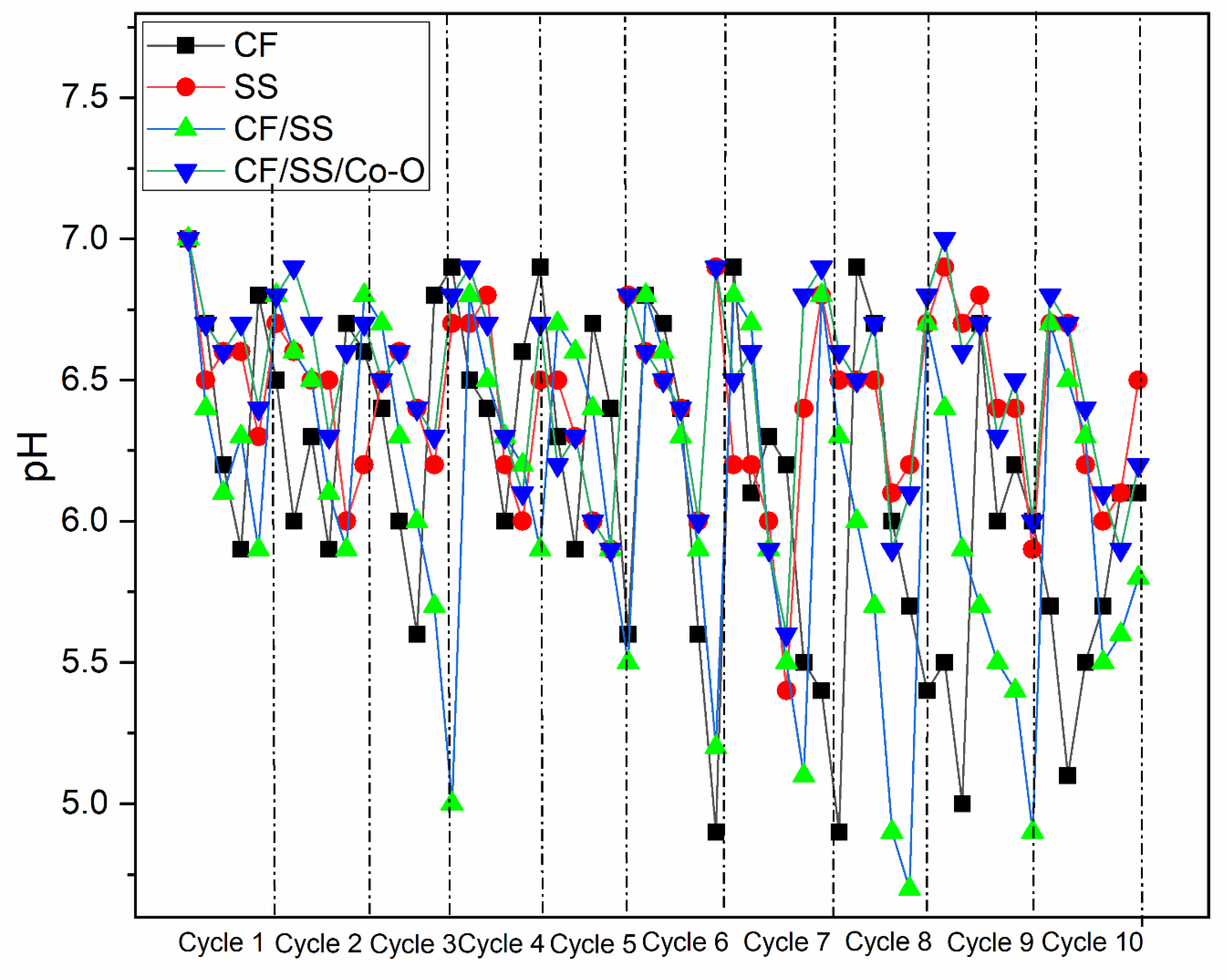
3.3. Acetic Acid
3.4. Ethanol
3.5. Redox Profile
3.6. Carbon Conversion Efficiency
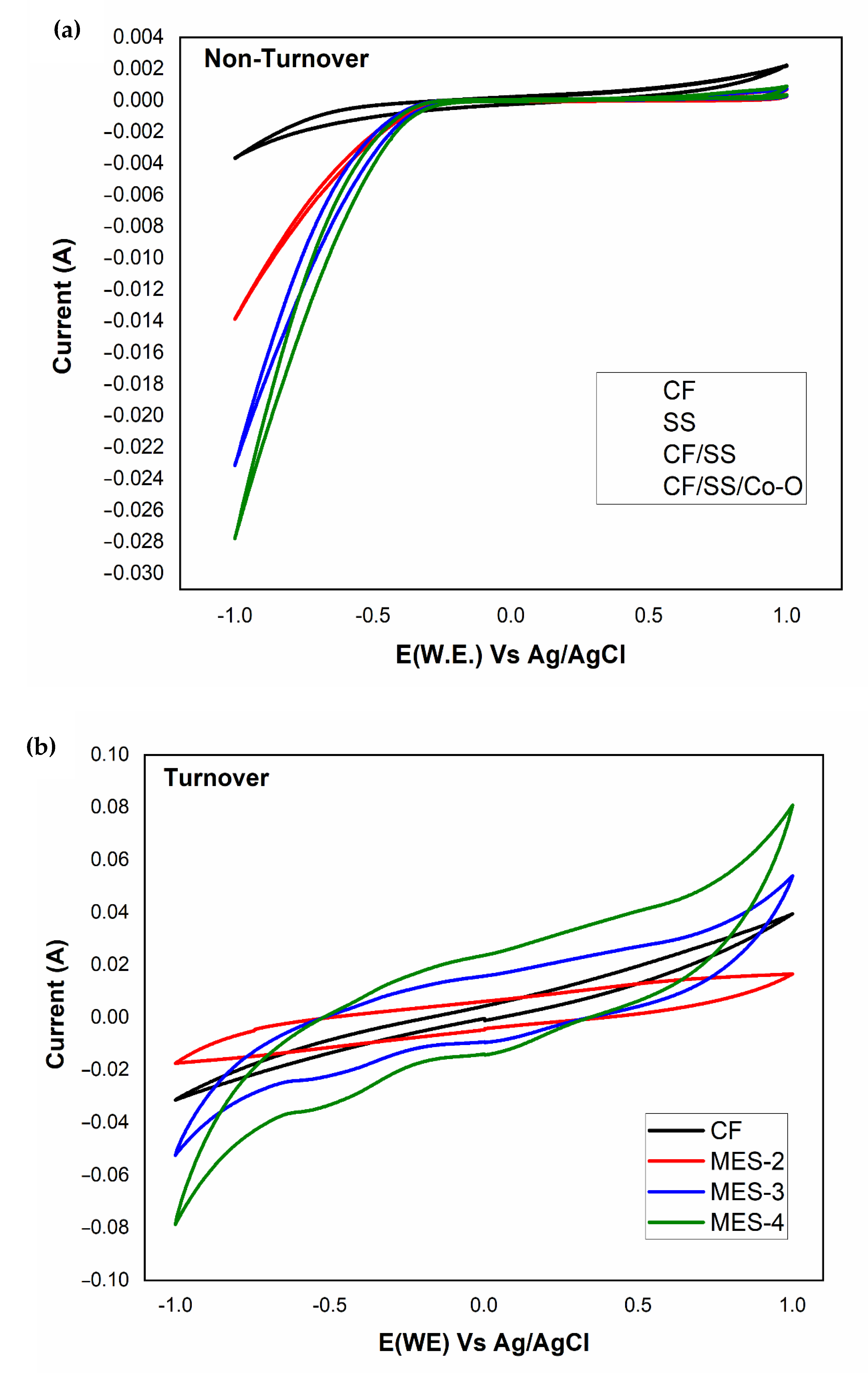
3.7. Bioelectrochemical (BEC) Behavior of MES
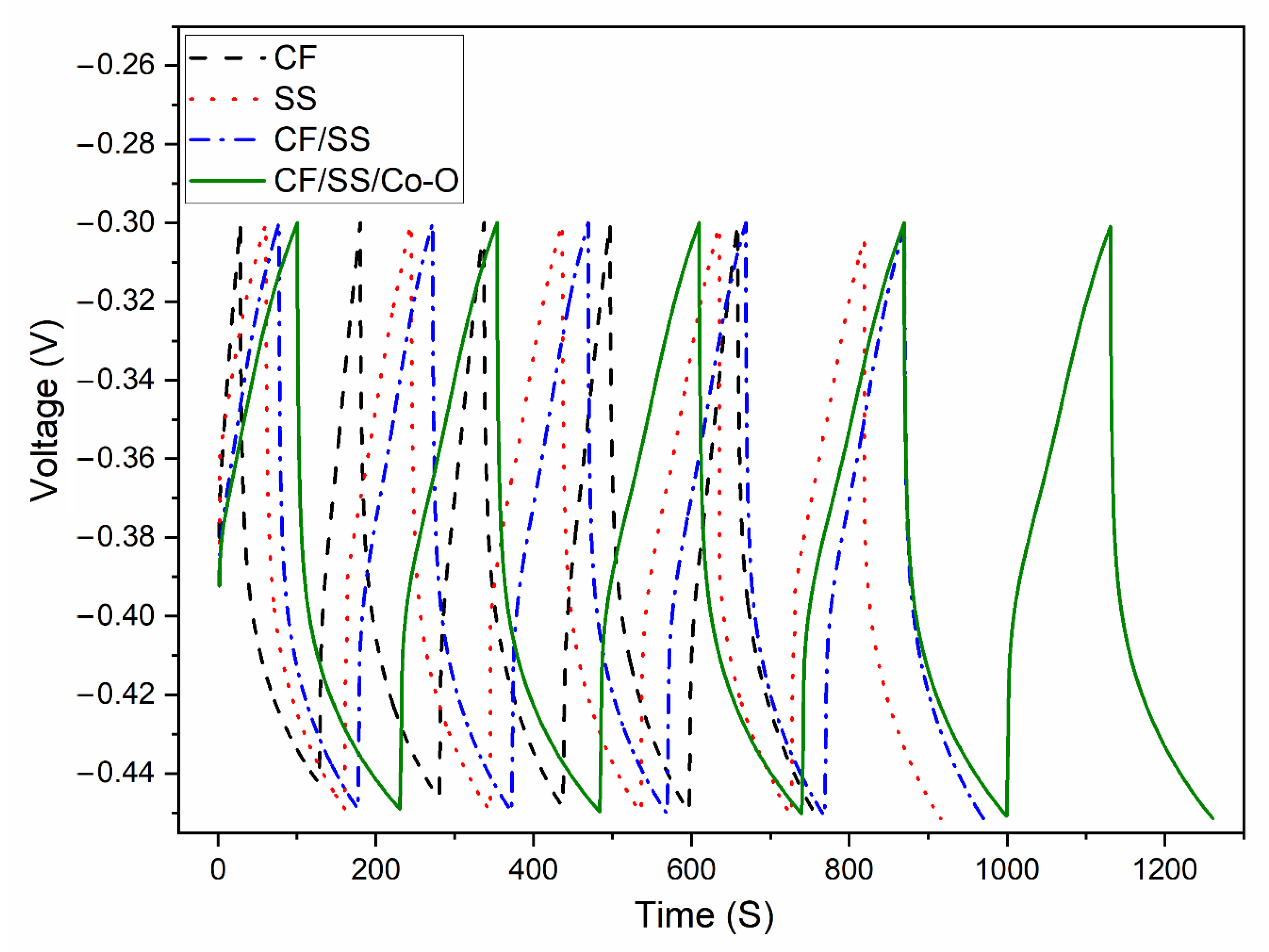
3.8. Energy Storage in MES
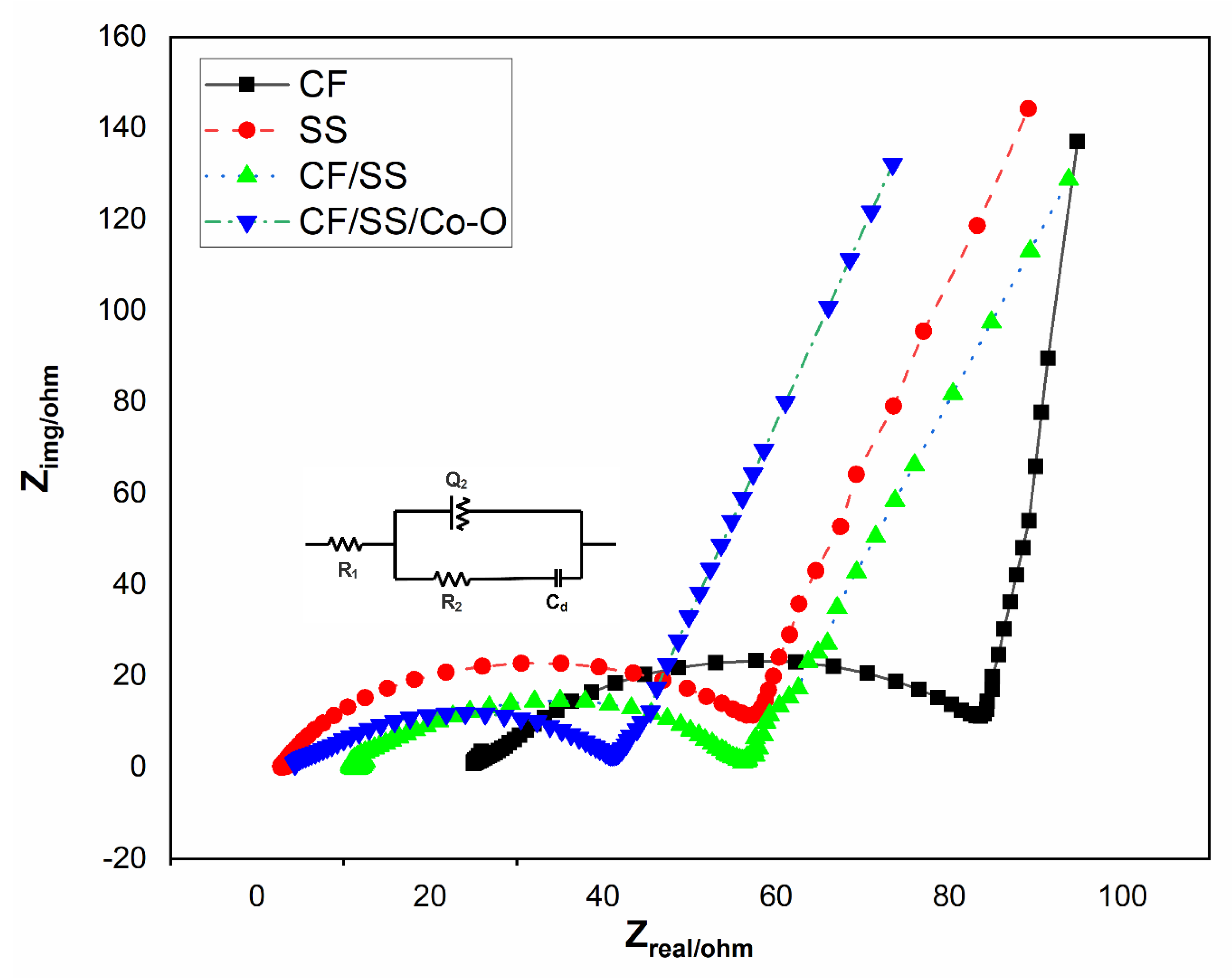
3.9. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Deng, J.; Han, J.; Imhanria, S.; Chen, K.; Wang, W. Effective tunable syngas generation via CO2 reduction reaction by non- precious Fe-N-C electrocatalyst. Chem. Eng. J. 2020, 389, 124323. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Andrew, R.M.; Rogelj, J.; Peters, G.P.; Canadell, J.G.; Knutti, R.; Luderer, G.; Raupach, M.R.; Schaeffer, M.; Van Vuuren, D.P.; et al. Persistent growth of CO2 emissions and implications for reaching climate targets. Nat. Geosci. 2014, 7, 709–715. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Mohanakrishna, G.; Elbaz, A.F.; Bulut, M.; Pant, D. Technological advances in CO2 conversion electro-biorefinery: A step toward commercialization. Bioresour. Technol. 2016, 215, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Tharak, A.; Venkata Mohan, S. Electrotrophy of biocathodes regulates microbial-electro-catalyzation of CO2 to fatty acids in single chambered system. Bioresour. Technol. 2021, 320, 124272. [Google Scholar] [CrossRef]
- Annie Modestra, J.; Katakojwala, R.; Venkata Mohan, S. CO2 fermentation to short chain fatty acids using selectively enriched chemolithoautotrophic acetogenic bacteria. Chem. Eng. J. 2020, 394, 124759. [Google Scholar] [CrossRef]
- Vamshi Krishna, K.; Venkata Mohan, S. Selective enrichment of electrogenic bacteria for fuel cell application: Enumerating microbial dynamics using MiSeq platform. Bioresour. Technol. 2016, 213, 146–154. [Google Scholar] [CrossRef]
- Annie Modestra, J.; Navaneeth, B.; Venkata Mohan, S. Bio-electrocatalytic reduction of CO2: Enrichment of homoacetogens and pH optimization towards enhancement of carboxylic acids biosynthesis. J. CO2 Util. 2015, 10, 78–87. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef]
- Song, T.S.; Wang, G.; Wang, H.; Huang, Q.; Xie, J. Experimental evaluation of the influential factors of acetate production driven by a DC power system via CO2 reduction through microbial electrosynthesis. Bioresour. Bioprocess. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Batlle-Vilanova, P.; Ganigué, R.; Ramió-Pujol, S.; Bañeras, L.; Jiménez, G.; Hidalgo, M.; Balaguer, M.D.; Colprim, J.; Puig, S. Microbial electrosynthesis of butyrate from carbon dioxide: Production and extraction. Bioelectrochemistry 2017, 117, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Anwer, A.H.; Khan, M.D.; Khan, N.; Nizami, A.S.; Rehan, M.; Khan, M.Z. Development of novel MnO2 coated carbon felt cathode for microbial electroreduction of CO2 to biofuels. J. Environ. Manag. 2019, 249, 109376. [Google Scholar] [CrossRef]
- Alqahtani, M.F.; Katuri, K.P.; Bajracharya, S.; Yu, Y.; Lai, Z.; Saikaly, P.E. Porous Hollow Fiber Nickel Electrodes for Effective Supply and Reduction of Carbon Dioxide to Methane through Microbial Electrosynthesis. Adv. Funct. Mater. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Lovley, D.R.; Fraga, J.L.; Coates, J.D.; Blunt-Harris, E.L. Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1999, 1, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2011, 3, 27–35. [Google Scholar] [CrossRef]
- Summers, Z.M.; Gralnick, J.A.; Bond, D.R. Cultivation of an obligate Fe(II)-oxidizing lithoautotrophic bacterium using electrodes. MBio 2013, 4, e00420-12. [Google Scholar] [CrossRef]
- Patil, S.A.; Arends, J.B.A.; Vanwonterghem, I.; Van Meerbergen, J.; Guo, K.; Tyson, G.W.; Rabaey, K. Selective Enrichment Establishes a Stable Performing Community for Microbial Electrosynthesis of Acetate from CO2. Environ. Sci. Technol. 2015, 49, 8833–8843. [Google Scholar] [CrossRef]
- Modestra, J.A.; Chiranjeevi, P.; Mohan, S.V. Cathodic material effect on electron acceptance towards bioelectricity generation and wastewater treatment. Renew. Energy 2016, 98, 178–187. [Google Scholar] [CrossRef]
- Molenaar, S.D.; Saha, P.; Mol, A.R.; Sleutels, T.H.J.A.; ter Heijne, A.; Buisman, C.J.N. Competition between methanogens and acetogens in biocathodes: A comparison between potentiostatic and galvanostatic control. Int. J. Mol. Sci. 2017, 18, 204. [Google Scholar] [CrossRef]
- Chen, W.; Wu, D.; Wan, H.; Tang, R.; Li, C.; Wang, G.; Feng, C. Carbon-based cathode as an electron donor driving direct bioelectrochemical denitrification in biofilm-electrode reactors: Role of oxygen functional groups. Carbon N. Y. 2017, 118, 310–318. [Google Scholar] [CrossRef]
- Bajracharya, S.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.T.B.; Pant, D. Bioelectrochemical conversion of CO2 to chemicals: CO2 as a next generation feedstock for electricity-driven bioproduction in batch and continuous modes. Faraday Discuss. 2017, 202, 433–449. [Google Scholar] [CrossRef]
- Jourdin, L.; Grieger, T.; Monetti, J.; Flexer, V.; Freguia, S.; Lu, Y.; Chen, J.; Romano, M.; Wallace, G.G.; Keller, J. High Acetic Acid Production Rate Obtained by Microbial Electrosynthesis from Carbon Dioxide. Environ. Sci. Technol. 2015, 49, 13566–13574. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Anwer, A.H.; Khan, M.D.; Azam, A.; Ibhadon, A.; Khan, M.Z. Magnesium ferrite spinels as anode modifier for the treatment of Congo red and energy recovery in a single chambered microbial fuel cell. J. Hazard. Mater. 2020, 124561. [Google Scholar] [CrossRef]
- Lai, B.; Tang, X.; Li, H.; Du, Z.; Liu, X.; Zhang, Q. Power production enhancement with a polyaniline modified anode in microbial fuel cells. Biosens. Bioelectron. 2011, 28, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Bao, S.; Li, C.M.; Cui, X.; Lu, Z.; Guo, J. Nanostructured Polyaniline/Titanium Fuel Cells. ACS Nano 2008, 2, 113–119. [Google Scholar] [CrossRef]
- Khan, N.; Anwer, A.H.; Ahmad, A.; Sabir, S.; Sevda, S.; Khan, M.Z. Investigation of CNT/PPy-Modified Carbon Paper Electrodes under Anaerobic and Aerobic Conditions for Phenol Bioremediation in Microbial Fuel Cells. ACS Omega 2020, 5, 471–480. [Google Scholar] [CrossRef]
- Ganesana, M.; Istarnboulie, G.; Marty, J.L.; Noguer, T.; Andreescu, S. Site-specific immobilization of a (His)6-tagged acetylcholinesterase on nickel nanoparticles for highly sensitive toxicity biosensors. Biosens. Bioelectron. 2011, 30, 43–48. [Google Scholar] [CrossRef]
- Cui, M.; Nie, H.; Zhang, T.; Lovley, D.; Russell, T.P. Three-dimensional hierarchical metal oxide-carbon electrode materials for highly efficient microbial electrosynthesis. Sustain. Energy Fuels 2017, 1, 1171–1176. [Google Scholar] [CrossRef]
- Li, M.; Garg, S.; Chang, X.; Ge, L.; Li, L.; Konarova, M.; Rufford, T.E.; Rudolph, V.; Wang, G. Toward Excellence of Transition Metal-Based Catalysts for CO2 Electrochemical Reduction: An Overview of Strategies and Rationales. Small Methods 2020, 4, 2000033. [Google Scholar] [CrossRef]
- Liu, C.; Colón, B.C.; Ziesack, M.; Silver, P.A.; Nocera, D.G. Water splitting–biosynthetic system with CO 2 reduction efficiencies exceeding photosynthesis. Science 2016, 352, 1210–1213. [Google Scholar] [CrossRef]
- Rodrigues, R.M.; Guan, X.; Iñiguez, J.A.; Estabrook, D.A.; Chapman, J.O.; Huang, S.; Sletten, E.M.; Liu, C. Perfluorocarbon nanoemulsion promotes the delivery of reducing equivalents for electricity-driven microbial CO2 reduction. Nat. Catal. 2019, 2, 407–414. [Google Scholar] [CrossRef]
- Kabeer, H.; Hanif, S.; Arsalan, A.; Asmat, S.; Younus, H.; Shakir, M. Structural-Dependent N,O-Donor Imine-Appended Cu(II)/Zn(II) Complexes: Synthesis, Spectral, and in Vitro Pharmacological Assessment. ACS Omega 2020, 5, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Asmat, S.; Anwer, A.H.; Husain, Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int. J. Biol. Macromol. 2019, 140, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.F.; Abbas, S.Z.; Mohamad Ibrahim, M.N.; Ismail, N.; Rafatullah, M. Insights into advancements and electrons transfer mechanisms of electrogens in benthic microbial fuel cells. Membranes 2020, 10, 205. [Google Scholar] [CrossRef]
- Sarkar, O.; Venkata Mohan, S. Synergy of anoxic microenvironment and facultative anaerobes on acidogenic metabolism in a self-induced electrofermentation system. Bioresour. Technol. 2020, 313, 123604. [Google Scholar] [CrossRef]
- Zaybak, Z.; Pisciotta, J.M.; Tokash, J.C.; Logan, B.E. Enhanced start-up of anaerobic facultatively autotrophic biocathodes in bioelectrochemical systems. J. Biotechnol. 2013, 168, 478–485. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Lalit Babu, V.; Sarma, P.N. Effect of various pretreatment methods on anaerobic mixed microflora to enhance biohydrogen production utilizing dairy wastewater as substrate. Bioresour. Technol. 2008, 99, 59–67. [Google Scholar] [CrossRef]
- Das, S.; Das, S.; Ghangrekar, M.M. Application of TiO2 and Rh as cathode catalyst to boost the microbial electrosynthesis of organic compounds through CO2 sequestration. Process. Biochem. 2021, 101, 237–246. [Google Scholar] [CrossRef]
- Bajracharya, S.; Ter Heijne, A.; Dominguez Benetton, X.; Vanbroekhoven, K.; Buisman, C.J.N.; Strik, D.P.B.T.B.; Pant, D. Carbon dioxide reduction by mixed and pure cultures in microbial electrosynthesis using an assembly of graphite felt and stainless steel as a cathode. Bioresour. Technol. 2015, 195, 14–24. [Google Scholar] [CrossRef]
- Annie Modestra, J.; Venkata Mohan, S. Capacitive biocathodes driving electrotrophy towards enhanced CO2 reduction for microbial electrosynthesis of fatty acids. Bioresour. Technol. 2019, 294, 122181. [Google Scholar] [CrossRef]
- Pandit, S.; Khilari, S.; Roy, S.; Pradhan, D.; Das, D. Improvement of power generation using Shewanella putrefaciens mediated bioanode in a single chambered microbial fuel cell: Effect of different anodic operating conditions. Bioresour. Technol. 2014, 166, 451–457. [Google Scholar] [CrossRef]
- Saheb-Alam, S.; Singh, A.; Hermansson, M.; Persson, F.; Schnürer, A.; Wilén, B.M.; Modin, O. Effect of start-up strategies and electrode materials on carbon dioxide reduction on biocathodes. Appl. Environ. Microbiol. 2018, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Roca-Puigros, M.; Geppert, F.; Caizán-Juanarena, L.; Na Ayudthaya, S.P.; Buisman, C.; Heijne, A. Granular carbon-based electrodes as cathodes in methane-producing bioelectrochemical systems. Front. Bioeng. Biotechnol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Prévoteau, A.; Patil, S.A.; Rabaey, K. Engineering electrodes for microbial electrocatalysis. Curr. Opin. Biotechnol. 2015, 33, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Donose, B.C.; Soeriyadi, A.H.; Prévoteau, A.; Patil, S.A.; Freguia, S.; Gooding, J.J.; Rabaey, K. Flame oxidation of stainless steel felt enhances anodic biofilm formation and current output in bioelectrochemical systems. Environ. Sci. Technol. 2014, 48, 7151–7156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Nie, H.; Bain, T.S.; Lu, H.; Cui, M.; Snoeyenbos-West, O.L.; Franks, A.E.; Nevin, K.P.; Russell, T.P.; Lovley, D.R. Improved cathode materials for microbial electrosynthesis. Energy Environ. Sci. 2013, 6, 217–224. [Google Scholar] [CrossRef]
- Jourdin, L.; Raes, S.M.T.; Buisman, C.J.N.; Strik, D.P.B.T.B. Critical biofilm growth throughout unmodified carbon felts allows continuous bioelectrochemical chain elongation from CO2 up to caproate at high current density. Front. Energy Res. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Marshall, C.W.; Ross, D.E.; Fichot, E.B.; Norman, R.S.; May, H.D. Long-term operation of microbial electrosynthesis systems improves acetate production by autotrophic microbiomes. Environ. Sci. Technol. 2013, 47, 6023–6029. [Google Scholar] [CrossRef]
- Zeppilli, M.; Mattia, A.; Villano, M.; Majone, M. Three-chamber Bioelectrochemical System for Biogas Upgrading and Nutrient Recovery. Fuel Cells 2017, 17, 593–600. [Google Scholar] [CrossRef]
- Jourdin, L.; Freguia, S.; Donose, B.C.; Chen, J.; Wallace, G.G.; Keller, J.; Flexer, V. A novel carbon nanotube modified scaffold as an efficient biocathode material for improved microbial electrosynthesis. J. Mater. Chem. A 2014, 2, 13093–13102. [Google Scholar] [CrossRef]
- Song, T.S.; Fei, K.; Zhang, H.; Yuan, H.; Yang, Y.; Ouyang, P.; Xie, J. High efficiency microbial electrosynthesis of acetate from carbon dioxide using a novel graphene–nickel foam as cathode. J. Chem. Technol. Biotechnol. 2018, 93, 457–466. [Google Scholar] [CrossRef]
- Min, S.; Jiang, Y.; Li, D. Production of acetate from carbon dioxide in bioelectrochemical systems based on autotrophic mixed culture. J. Microbiol. Biotechnol. 2013, 23, 1140–1146. [Google Scholar]
- Mohanakrishna, G.; Seelam, J.S.; Vanbroekhoven, K.; Pant, D. An enriched electroactive homoacetogenic biocathode for the microbial electrosynthesis of acetate through carbon dioxide reduction. Faraday Discuss. 2015, 183, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Singh, D.; Vanbroekhoven, K.; Pant, D.; Kumar, M.; Puri, S.K.; Ramakumar, S.S.V. Electro-biocatalytic conversion of carbon dioxide to alcohols using gas diffusion electrode. Bioresour. Technol. 2018, 265, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, O.; Butti, S.K.; Venkata Mohan, S. Acidogenesis driven by hydrogen partial pressure towards bioethanol production through fatty acids reduction. Energy 2017, 118, 425–434. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Butti, S.K.; Kannaiah Goud, R.; Venkata Mohan, S. Spatiometabolic stratification of anoxic biofilm in prototype bioelectrogenic system. Bioelectrochemistry 2017, 115, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Raghavulu, S.V.; Goud, R.K.; Sarma, P.N.; Mohan, S.V. Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: Influence of redox condition and substrate load. Bioresour. Technol. 2011, 102, 2751–2757. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Modestra, J.A.; Amulya, K.; Butti, S.K.; Velvizhi, G. A Circular Bioeconomy with Biobased Products from CO2 Sequestration. Trends Biotechnol. 2016, 34, 506–519. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Abu Reesh, I.M.; Vanbroekhoven, K.; Pant, D. Microbial electrosynthesis feasibility evaluation at high bicarbonate concentrations with enriched homoacetogenic biocathode. Sci. Total Environ. 2020, 715, 137003. [Google Scholar] [CrossRef]
- Jin, Q.; Kirk, M.F. Thermodynamic and kinetic response of microbial reactions to high CO2. Front. Microbiol. 2016, 7, 1696. [Google Scholar] [CrossRef]
- Ullah, N.; McArthur, M.A.; Omanovic, S. Iridium-ruthenium-oxide coatings for supercapacitors. Can. J. Chem. Eng. 2015, 93, 1941–1948. [Google Scholar] [CrossRef]
- Jiang, F.; Li, W.; Zou, R.; Liu, Q.; Xu, K.; An, L.; Hu, J. MoO3/PANI coaxial heterostructure nanobelts by in situ polymerization for high performance supercapacitors. Nano Energy 2014, 7, 72–79. [Google Scholar] [CrossRef]
- Khilari, S.; Pandit, S.; Varanasi, J.L.; Das, D.; Pradhan, D. Bifunctional Manganese Ferrite/Polyaniline Hybrid as Electrode Material for Enhanced Energy Recovery in Microbial Fuel Cell. ACS Appl. Mater. Interfaces 2015, 7, 20657–20666. [Google Scholar] [CrossRef] [PubMed]
- Sekar, N.; Ramasamy, R.P. Electrochemical impedance spectroscopy for microbial fuel cell characterization. J. Microb. Biochem. Technol. 2013, 6, 139–146. [Google Scholar]
- Lv, Z.; Chen, Y.; Wei, H.; Li, F.; Hu, Y.; Wei, C.; Feng, C. One-step electrosynthesis of polypyrrole/graphene oxide composites for microbial fuel cell application. Electrochim. Acta 2013, 111, 366–373. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Luhana, C.; Wang, H.; Li, M.; Guo, L. A partially reduced C60-grafted macroporous carbon composite for the enhanced electrocatalysis of nitroaromatic compounds. RSC Adv. 2013, 3, 17300–17306. [Google Scholar] [CrossRef]
- Sun, J.J.; Zhao, H.Z.; Yang, Q.Z.; Song, J.; Xue, A. A novel layer-by-layer self-assembled carbon nanotube-based anode: Preparation, characterization, and application in microbial fuel cell. Electrochim. Acta 2010, 55, 3041–3047. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhu, J.; Ren, P. Electrochemical in situ polymerization of reduced graphene oxide/polypyrrole composite with high power density. J. Power Sources 2012, 208, 138–143. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, L.; Li, J.; Zeng, J.; Zeng, B. Electrocatalytic oxidation and voltammetric determination of nitrite on hydrophobic ionic liquid-carbon nanotube gel-chitosan composite modified electrodes. Electroanalysis 2008, 20, 2047–2054. [Google Scholar] [CrossRef]


| Cathode Material | Applied Potential (V vs. SHE) | Biocatalyst | Acetate Production Rate (g m−2 day−1) | Coulombic Efficiency (%) | Reference |
|---|---|---|---|---|---|
| Gas diffusion activated carbon | −1.00 | Enriched anaerobic sludge | 36.6 | 35.46 | [49] |
| MWCNT-RVC | −1.10 | WWTP sludge | 1330 | 84 ± 2 | [50] |
| Nanoweb 3D RVC | −0.85 | WWTP sludge | 195 ± 30 | 70 ± 11 | [51] |
| Carbon felt | −0.90 | WWTP sludge | 9.75 | 89.5 | [52] |
| Activated carbon VITO-CoRE™d | −0.40 | Mix culture | 9.49 | 29.9 | [53] |
| Carbon felt (CF) | −0.8 V | Enriched anaerobic sludge | 339.16 | 40 ± 0.6 | Present study |
| Stainless steel (SS) | −0.8 V | Enriched anaerobic sludge | 300.8 | 36 ± 0.9 | Present study |
| Carbon felt/stainless steel (CF/SS) | −0.8 V | Enriched anaerobic sludge | 556.6 | 52 ± 0.2 | Present study |
| Carbon felt/stainless steel/cobalt oxide (CF/SS/Co-O) | −0.8 V | Enriched anaerobic sludge | 622.5 | 60 ± 0.2 | Present study |
| Parameters | MES-1 | MES-2 | MES-3 | MES-3 |
|---|---|---|---|---|
| Working electrode (cathode) | Carbon felt (CC) | Stainless steel mesh (SS) | Hybrid (CF/SS) | Electrodeposited hybrid (CF/SS/Co-O) |
| Counter electrode (anode) | Carbon felt | Carbon felt | Carbon felt | Carbon felt |
| Reductive catalytic current (mA) | −5.9 | −3.2 | −7.8 | −9.2 |
| Acetate (g/L) | 2.2 ± 0.2 | 1.8 ± 0.4 | 3.7 ± 0.4 | 4.4 ± 0.4 |
| Ethanol (g/L) | 0.2 ± 0.02 | 0.14 ± 0.02 | 0.32 ± 0.02 | 0.35 ± 0.02 |
| CO2 to acetate conversion (%) | 40 ± 0.6 | 36 ± 0.9 | 52 ± 0.2 | 60 ± 0.2 |
| Cumulative carbon conversion (%) | 56 ± 0.5 | 39 ± 0.5 | 69 ± 0.5 | 75.9 ± 0.5 |
| Specific capacitance (F/cm2) | 0.147 | 0.131 | 0.157 | 0.215 |
| Modified Cathode MES | Solution Resistance (Rs Ω) | Charge Transfer Resistance (Rct, Ω) |
|---|---|---|
| CF (MES-1) | 25.4 | 65.7 |
| SS (MES-2) | 2.78 | 63.2 |
| CF/SS (MES-3) | 11.1 | 45.9 |
| CF/SS/Co-O (MES-4) | 3.92 | 40.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwer, A.H.; Khan, N.; Umar, M.F.; Rafatullah, M.; Khan, M.Z. Electrodeposited Hybrid Biocathode-Based CO2 Reduction via Microbial Electro-Catalysis to Biofuels. Membranes 2021, 11, 223. https://doi.org/10.3390/membranes11030223
Anwer AH, Khan N, Umar MF, Rafatullah M, Khan MZ. Electrodeposited Hybrid Biocathode-Based CO2 Reduction via Microbial Electro-Catalysis to Biofuels. Membranes. 2021; 11(3):223. https://doi.org/10.3390/membranes11030223
Chicago/Turabian StyleAnwer, Abdul Hakeem, Nishat Khan, Mohammad Faisal Umar, Mohd Rafatullah, and Mohammad Zain Khan. 2021. "Electrodeposited Hybrid Biocathode-Based CO2 Reduction via Microbial Electro-Catalysis to Biofuels" Membranes 11, no. 3: 223. https://doi.org/10.3390/membranes11030223
APA StyleAnwer, A. H., Khan, N., Umar, M. F., Rafatullah, M., & Khan, M. Z. (2021). Electrodeposited Hybrid Biocathode-Based CO2 Reduction via Microbial Electro-Catalysis to Biofuels. Membranes, 11(3), 223. https://doi.org/10.3390/membranes11030223








