Estradiol Removal by Adsorptive Coating of a Microfiltration Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
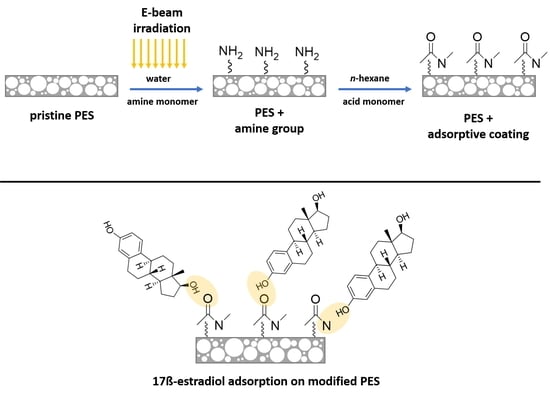
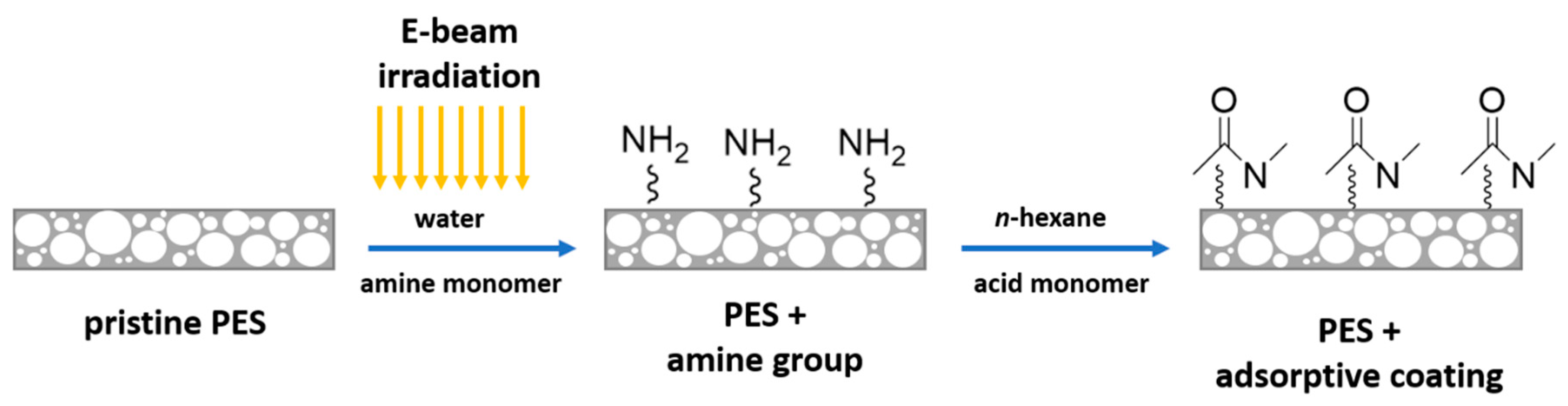
2.2. Membrane Modification
2.3. Membrane Characterization
2.3.1. Water Permeance
2.3.2. X-ray Photoelectron Spectroscopy
2.3.3. Scanning Electron Microscopy
2.3.4. Water Contact Angle
2.3.5. Adsorption of E2 on Modified Membranes
3. Results and Discussion
3.1. Membrane Characterization
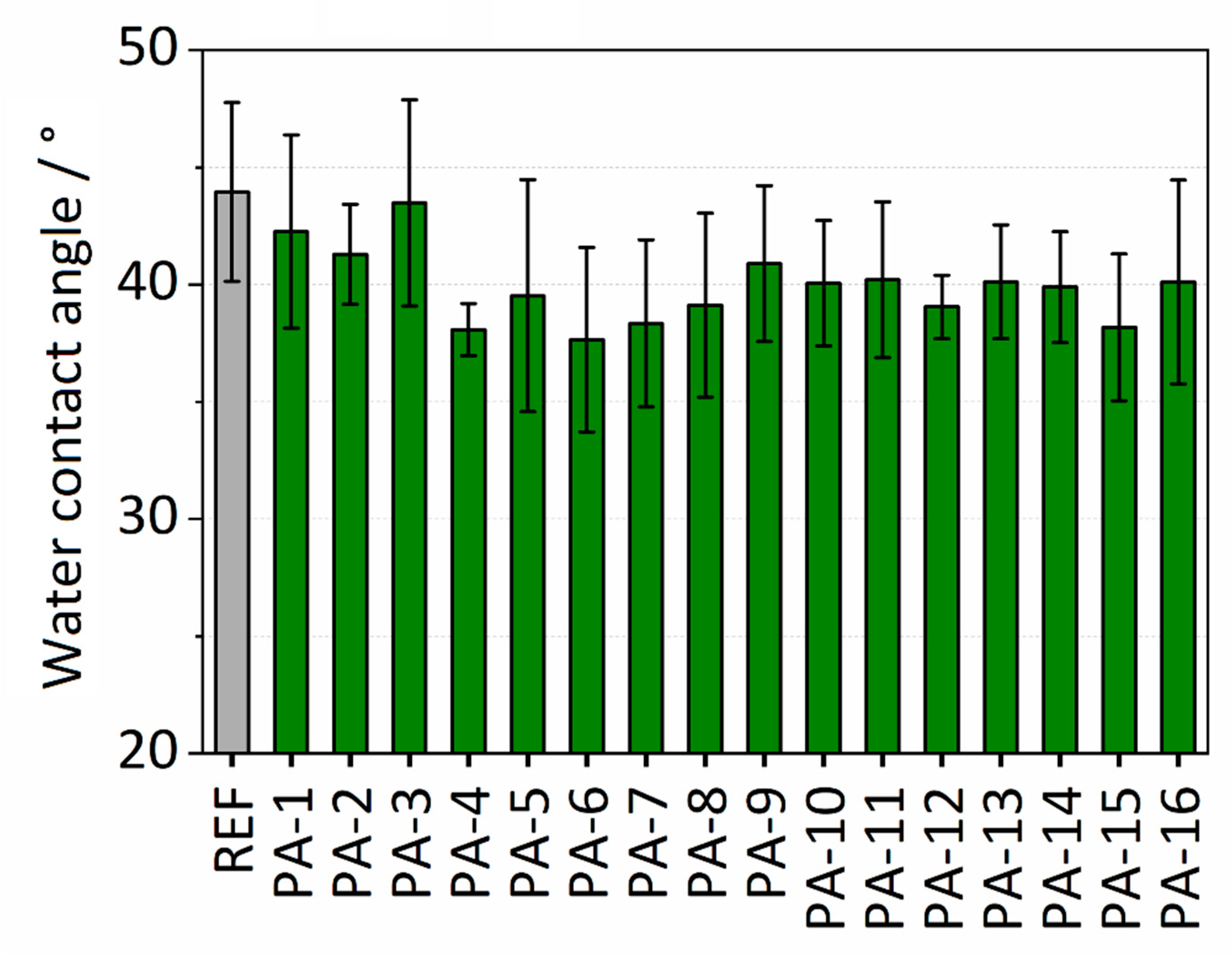
3.1.1. Water Contact Angle
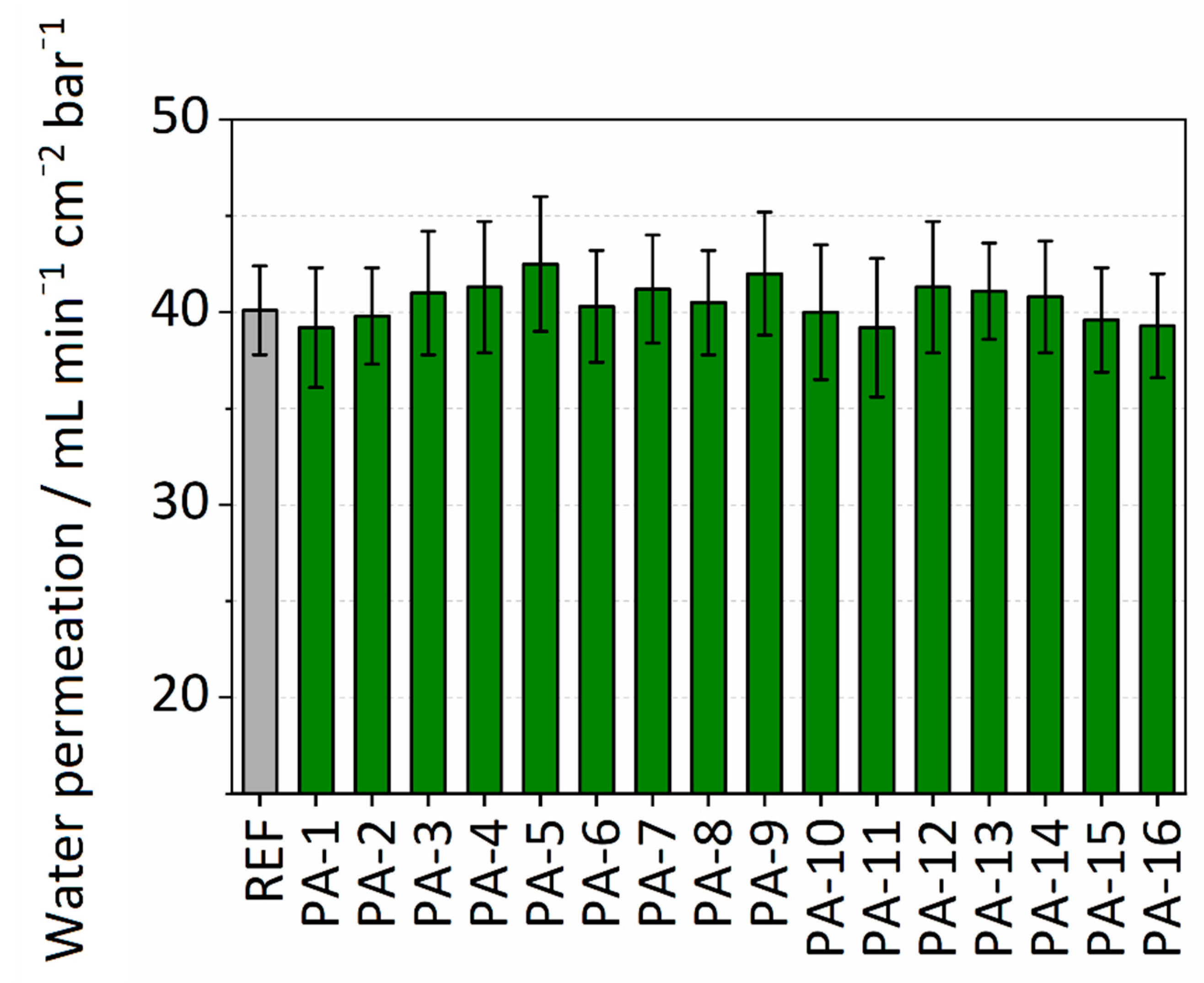
3.1.2. Water Permeance
3.1.3. Membrane Pore Structure
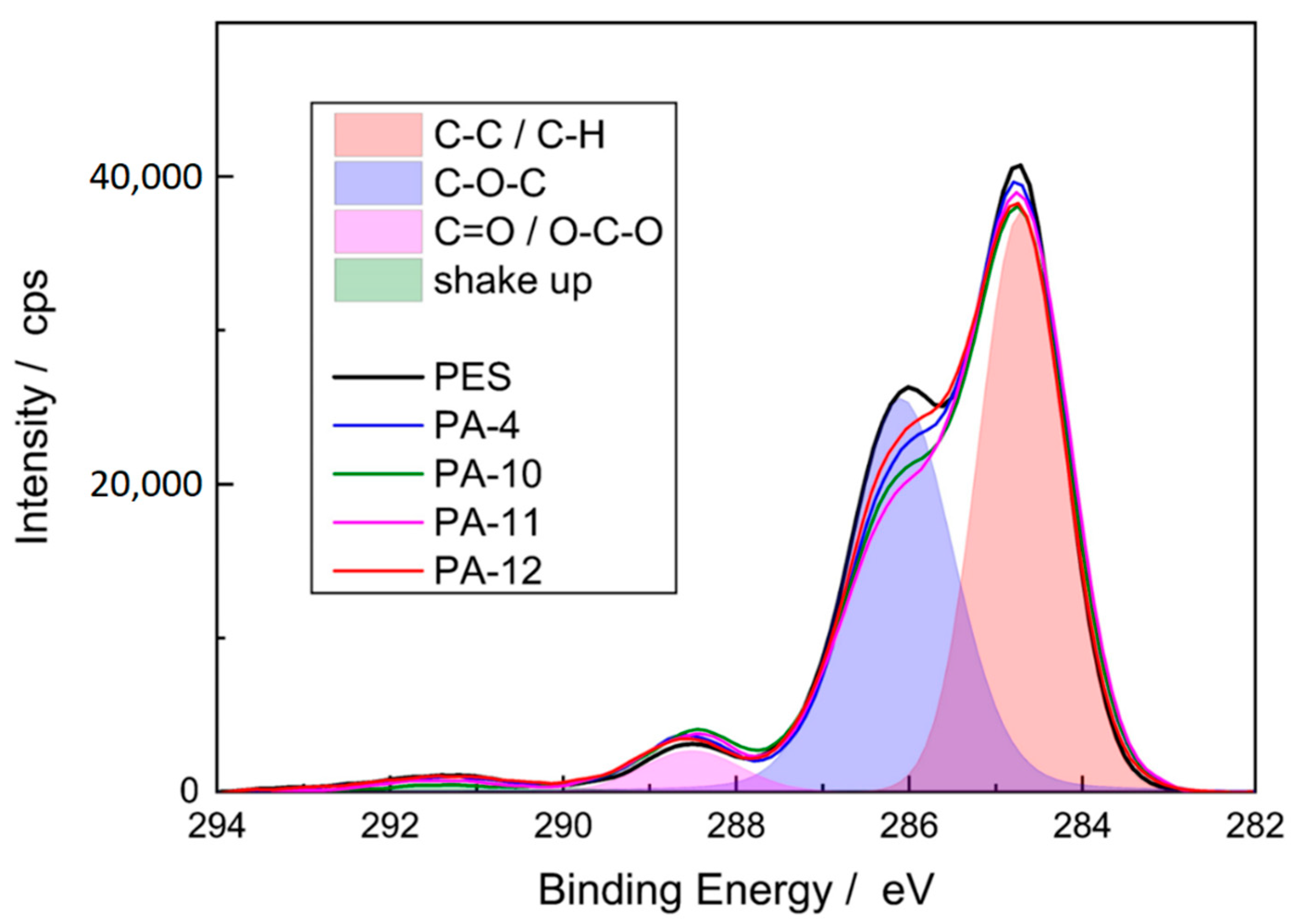
3.1.4. Membrane Chemical Composition
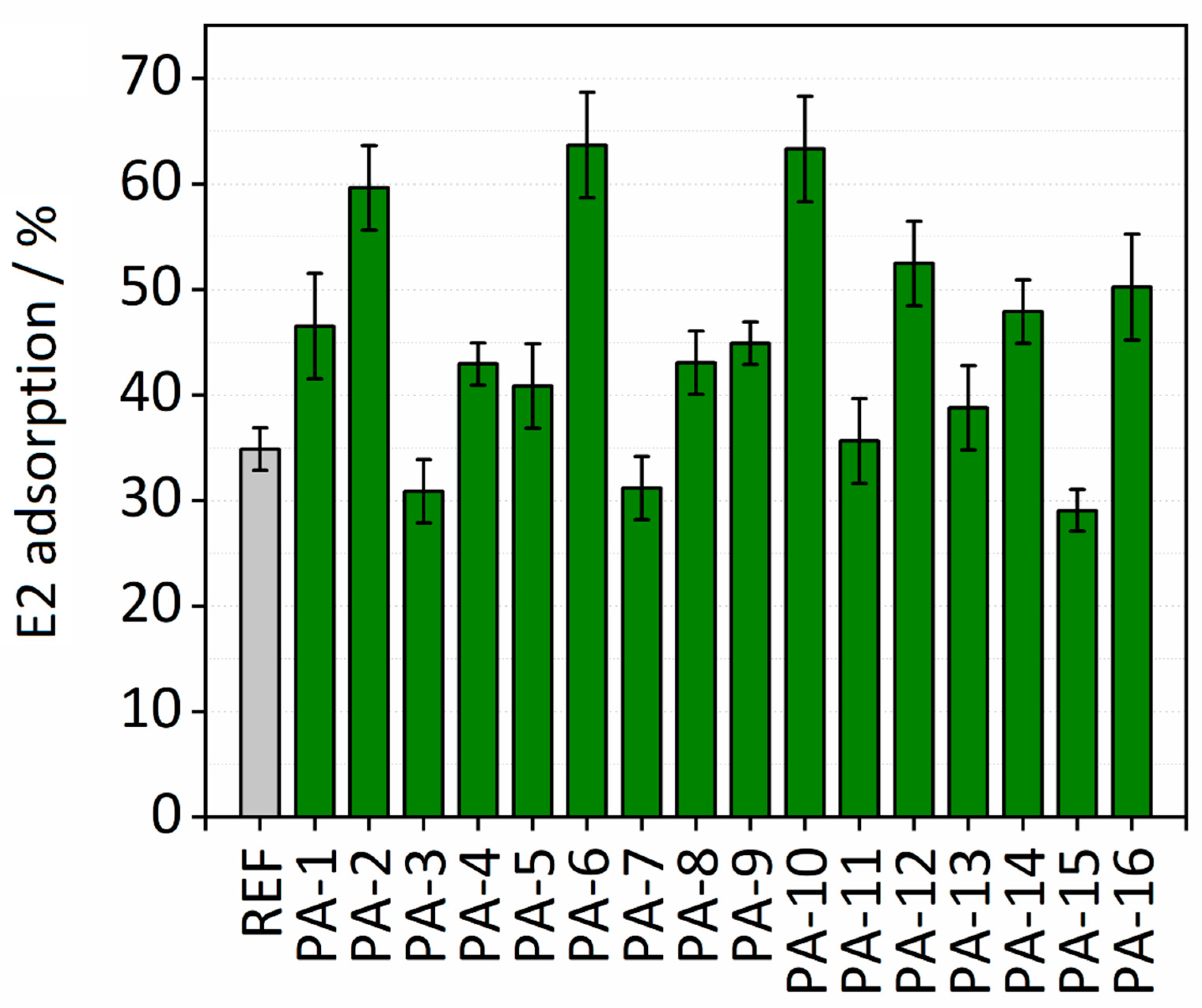
3.1.5. E2 Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Deich, C.; Kanwischer, M.; Jähne, M.; Waniek, J.J. Patterns of estrogenic activity in the Baltic Sea. Chemosphere 2020, 240, 124870. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Routledge, E.J.; Sheahan, D.; Desbrow, C.; Brighty, G.C.; Waldock, M.; Sumpter, J.P. Identification of Estrogenic Chemicals in STW Effluent. 2. In Vivo Responses in Trout and Roach. Environ. Sci. Technol. 1998, 32, 1559–1565. [Google Scholar] [CrossRef]
- Boulay, F.; Perdiz, D. 17β-Estradiol modulates UVB-induced cellular responses in estrogen receptors positive human breast cancer cells. J. Photochem. Photobiol. B, Biol. 2005, 81, 143–153. [Google Scholar] [CrossRef]
- Solomon, G.M.; Schettler, T. Environment and health: 6. Endocrine disruption and potential human health implications. CMAJ 2000, 163, 1471–1476. [Google Scholar]
- Hess, R.A.; Bunick, D.; Lee, K.-H.; Bahr, J.; Taylor, J.A.; Korach, K.S.; Lubahn, D.B. A role for oestrogens in the male reproductive system. Nature 1997, 390, 509–512. [Google Scholar] [CrossRef]
- Canesi, L.; Fabbri, E. Environmental Effects of BPA: Focus on Aquatic Species. Dose-Response 2015, 13, 1559325815598304. [Google Scholar] [CrossRef]
- Watabe, Y.; Kubo, T.; Nishikawa, T.; Fujita, T.; Kaya, K.; Hosoya, K. Fully automated liquid chromatography–mass spectrometry determination of 17β-estradiol in river water. J. Chromatogr. A 2006, 1120, 252–259. [Google Scholar] [CrossRef]
- Desbrow, C.; Routledge, E.J.; Brighty, G.C.; Sumpter, J.P.; Waldock, M. Identification of Estrogenic Chemicals in STW Effluent. 1. Chemical Fractionation and in Vitro Biological Screening. Environ. Sci. Technol. 1998, 32, 1549–1558. [Google Scholar] [CrossRef]
- Huang, C.-H.; Sedlak, D.L. Analysis of estrogenic hormones in municipal wastewater effluent and surface water using enzyme-linked immunosorbent assay and gas chromatography/tandem mass spectrometry. Environ. Toxicol. Chem. 2001, 20, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environmen. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Hohenblum, P.; Gans, O.; Moche, W.; Scharf, S.; Lorbeer, G. Monitoring of selected estrogenic hormones and industrial chemicals in groundwaters and surface waters in Austria. Sci. Total Environ. 2004, 333, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Troya, D.; Shang, C.; Hildreth, S.; Helm, R.; Xia, K. Surface Catalyzed Oxidative Oligomerization of 17β-Estradiol by Fe3+-Saturated Montmorillonite. Environ. Sci. Technol. 2015, 49, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.; Wang, Q.; Wang, C.; Wang, P.; Mao, K. Performance evaluation and application of surface-molecular-imprinted polymer-modified TiO2 nanotubes for the removal of estrogenic chemicals from secondary effluents. Environ. Sci. Pollut. Res. Int. 2012, 20. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.M.; Barber, L.B.; Chapelle, F.H.; Gray, J.L.; Kolpin, D.W.; McMahon, P.B. Biodegradation of 17β-Estradiol, Estrone and Testosterone in Stream Sediments. Environ. Sci. Technol. 2009, 43, 1902–1910. [Google Scholar] [CrossRef]
- Hu, C.-L.; Wang, W.-F.; Hsieh, Y.-H. Study on 17β-Estradiol (E2) Removal in Wastewater by Continuous-Flow Advanced Treatment and Economic Benefit Evaluation. J. Chem. Eng. Jpn. 2015, 48, 458–462. [Google Scholar] [CrossRef]
- Ben Fredj, S.; Nobbs, J.; Tizaoui, C.; Monser, L. Removal of estrone (E1), 17β-estradiol (E2), and 17α-ethinylestradiol (EE2) from wastewater by liquid–liquid extraction. Chem. Eng. J. 2015, 262, 417–426. [Google Scholar] [CrossRef]
- Ben Fredj, S.; Novakoski, R.T.; Tizaoui, C.; Monser, L. Two-Phase Ozonation for the Removal of Estrone, 17β-Estradiol and 17α-Ethinylestradiol in Water Using Ozone-Loaded Decamethylcyclopentasiloxane. Ozone: Sci. Eng. 2017, 39, 343–356. [Google Scholar] [CrossRef]
- Bila, D.; Montalvão, A.F.; Azevedo, D.d.A.; Dezotti, M. Estrogenic activity removal of 17β-estradiol by ozonation and identification of by-products. Chemosphere 2007, 69, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.L. Removal of estrone and 17β-estradiol from water by adsorption. Water Res. 2005, 39, 3991–4003. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Esparza, M. HPLC-fluorescence detection and adsorption of bisphenol A, 17β-estradiol, and 17α-ethynyl estradiol on powdered activated carbon. Water Res. 2003, 37, 3530–3537. [Google Scholar] [CrossRef]
- Tagliavini, M.; Engel, F.; Weidler, P.G.; Scherer, T.; Schäfer, A.I. Adsorption of steroid micropollutants on polymer-based spherical activated carbon (PBSAC). J. Hazard. Mater. 2017, 337, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Zaib, Q.; Khan, I.A.; Saleh, N.B.; Flora, J.R.V.; Park, Y.-G.; Yoon, Y. Removal of Bisphenol A and 17β-Estradiol by Single-Walled Carbon Nanotubes in Aqueous Solution: Adsorption and Molecular Modeling. Water Air Soil Pollut. 2012, 223, 3281–3293. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, K. Adsorption of 17β-estradiol by multi-walled carbon nanotubes in natural waters with or without aquatic colloids. Chem. Eng. J. 2014, 258, 185–193. [Google Scholar] [CrossRef]
- McCallum, E.A.; Hyung, H.; Do, T.A.; Huang, C.-H.; Kim, J.-H. Adsorption, desorption, and steady-state removal of 17β-estradiol by nanofiltration membranes. J. Membr. Sci. 2008, 319, 38–43. [Google Scholar] [CrossRef]
- Zhongbo, Z.; Hu, J. Selective removal of estrogenic compounds by molecular imprinted polymer (MIP). Water Res. 2008, 42, 4101–4108. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I. Adsorption and Transport of Trace Contaminant Estrone in NF/RO Membranes. Environ. Eng. Sci. 2002, 19, 441–451. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Manis, A.; Soldenhoff, K.; Schäfer, A.I. Estrogenic hormone removal from wastewater using NF/RO membranes. J. Membr. Sci. 2004, 242, 37–45. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Removal of Natural Hormones by Nanofiltration Membranes: Measurement, Modeling, and Mechanisms. Environ. Sci. Technol. 2004, 38, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Westerhoff, P.; Yoon, J.; Snyder, S.A. Removal of 17ß estradiol and fluoranthene by nanofiltration and ultrafiltration. J. Environ. Eng., ASCE 2004, 130. [Google Scholar] [CrossRef]
- Ng, H.; Elimelech, M. Influence of Colloidal Fouling on Rejection of Trace Organic Contaminants by Reverse Osmosis. J. Membr. Sci. 2004, 244, 215–226. [Google Scholar] [CrossRef]
- Han, J.; Meng, S.; Dong, Y.; Hu, J.; Gao, W. Capturing hormones and bisphenol A from water via sustained hydrogen bond driven sorption in polyamide microfiltration membranes. Water Res. 2013, 47, 197–208. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Waite, T.D. Adsorptive interactions between membranes and trace contaminants. Desalination 2002, 147, 269–274. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Nghiem, L.D.; Waite, T.D. Removal of the Natural Hormone Estrone from Aqueous Solutions Using Nanofiltration and Reverse Osmosis. Environ. Sci. Technol. 2003, 37, 182–188. [Google Scholar] [CrossRef]
- Steinle-Darling, E.; Litwiller, E.; Reinhard, M. Effects of Sorption on the Rejection of Trace Organic Contaminants During Nanofiltration. Environ. Sci. Technol. 2010, 44, 2592–2598. [Google Scholar] [CrossRef]
- Semião, A.J.C.; Schäfer, A.I. Removal of adsorbing estrogenic micropollutants by nanofiltration membranes. Part A—Experimental evidence. J. Membr. Sci. 2013, 431, 244–256. [Google Scholar] [CrossRef]
- Liu, Y.-l.; Wang, X.-m.; Yang, H.-w.; Xie, Y.F. Adsorption of pharmaceuticals onto isolated polyamide active layer of NF/RO membranes. Chemosphere 2018, 200, 36–47. [Google Scholar] [CrossRef]
- Hu, J.Y.; Jin, X.; Ong, S.L. Rejection of estrone by nanofiltration: Influence of solution chemistry. J. Membr. Sci. 2007, 302, 188–196. [Google Scholar] [CrossRef]
- Kimura, K.; Amy, G.; Drewes, J.; Watanabe, Y. Adsorption of hydrophobic compounds onto NF/RO membranes: An artifact leading to overestimation of rejection. J. Membr. Sci. 2003, 221, 89–101. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Yang, P. Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J. Membr. Sci. 2007, 303, 267–277. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Kühnert, M.; Schulze, A. Reduction of Biofouling of a Microfiltration Membrane Using Amide Functionalities—Hydrophilization without Changes in Morphology. Polymers 2020, 12, 1379. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Sumpter, J.P. Removal of Endocrine-Disrupting Chemicals in Activated Sludge Treatment Works. Environ. Sci. Technol. 2001, 35, 4697–4703. [Google Scholar] [CrossRef]
- Khorshidi, B.; Thundat, T.; Fleck, B.A.; Sadrzadeh, M. Thin film composite polyamide membranes: Parametric study on the influence of synthesis conditions. RSC Adv. 2015, 5, 54985–54997. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes: I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Koyuncu, I.; Arikan, O.A.; Wiesner, M.R.; Rice, C. Removal of hormones and antibiotics by nanofiltration membranes. J. Membr. Sci. 2008, 309, 94–101. [Google Scholar] [CrossRef]
- Jermann, D.; Pronk, W.; Boller, M.; Schäfer, A.I. The role of NOM fouling for the retention of estradiol and ibuprofen during ultrafiltration. J. Membr. Sci. 2009, 329, 75–84. [Google Scholar] [CrossRef]

| Amine Monomer 2 wt.%, 30 min | Acid Monomer 0.2 wt.%, 2 min | Radiation Dose (kGy) | Toluene | |
|---|---|---|---|---|
| PA 1-1 | PIP 2 | TMC 3 | 150 | - |
| PA-2 | PIP | TMC | 150 | 10 mL |
| PA-3 | PIP | TMC | 200 | - |
| PA-4 | PIP | TMC | 200 | 10 mL |
| PA-5 | PIP | ADC | 150 | - |
| PA-6 | PIP | ADC | 150 | 10 mL |
| PA-7 | PIP | ADC | 200 | - |
| PA-8 | PIP | ADC | 200 | 10 mL |
| PA-9 | HMD | TMC | 150 | - |
| PA-10 | HMD | TMC | 150 | 10 mL |
| PA-11 | HMD | TMC | 200 | - |
| PA-12 | HMD | TMC | 200 | 10 mL |
| PA-13 | HMD | ADC | 150 | - |
| PA-14 | HMD | ADC | 150 | 10 mL |
| PA-15 | HMD | ADC | 200 | - |
| PA-16 | HMD | ADC | 200 | 10 mL |
| Chemical Composition (Relative Atom %) | ||||
|---|---|---|---|---|
| Label | C | N | O | S |
| REF 1 | 71.6 | - | 24.4 | 3.9 |
| PA-1 | 71.8 | 0.3 | 23.9 | 3.8 |
| PA-2 | 71.5 | 0.3 | 24.2 | 3.9 |
| PA-3 | 70.5 | 0.2 | 25.4 | 3.8 |
| PA-4 | 71.3 | 0.1 | 24.6 | 3.9 |
| PA-5 | 71.7 | 0.2 | 24.3 | 3.7 |
| PA-6 | 69.9 | 0.3 | 25.9 | 3.8 |
| PA-7 | 70.6 | 0.3 | 25.5 | 3.6 |
| PA-8 | 71.0 | 0.3 | 24.9 | 3.7 |
| PA-9 | 71.4 | 0.2 | 24.5 | 3.8 |
| PA-10 | 69.9 | 0.2 | 26,0 | 3.9 |
| PA-11 | 70.9 | 0.2 | 24.8 | 3.9 |
| PA-12 | 70.1 | 0.2 | 25.7 | 3.9 |
| PA-13 | 69.9 | 0.2 | 26.0 | 3.8 |
| PA-14 | 70.6 | 0.2 | 25.3 | 3.8 |
| PA-15 | 70.9 | 0.1 | 25.1 | 3.8 |
| PA-16 | 71.23 | 0.1 | 24.7 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niavarani, Z.; Breite, D.; Prager, A.; Abel, B.; Schulze, A. Estradiol Removal by Adsorptive Coating of a Microfiltration Membrane. Membranes 2021, 11, 99. https://doi.org/10.3390/membranes11020099
Niavarani Z, Breite D, Prager A, Abel B, Schulze A. Estradiol Removal by Adsorptive Coating of a Microfiltration Membrane. Membranes. 2021; 11(2):99. https://doi.org/10.3390/membranes11020099
Chicago/Turabian StyleNiavarani, Zahra, Daniel Breite, Andrea Prager, Bernd Abel, and Agnes Schulze. 2021. "Estradiol Removal by Adsorptive Coating of a Microfiltration Membrane" Membranes 11, no. 2: 99. https://doi.org/10.3390/membranes11020099
APA StyleNiavarani, Z., Breite, D., Prager, A., Abel, B., & Schulze, A. (2021). Estradiol Removal by Adsorptive Coating of a Microfiltration Membrane. Membranes, 11(2), 99. https://doi.org/10.3390/membranes11020099