Layer-by-Layer Coating of MK-40 Heterogeneous Membrane with Polyelectrolytes Creates Samples with Low Electrical Resistance and Weak Generation of H+ and OH− Ions
Abstract
1. Introduction
2. Materials and Methods
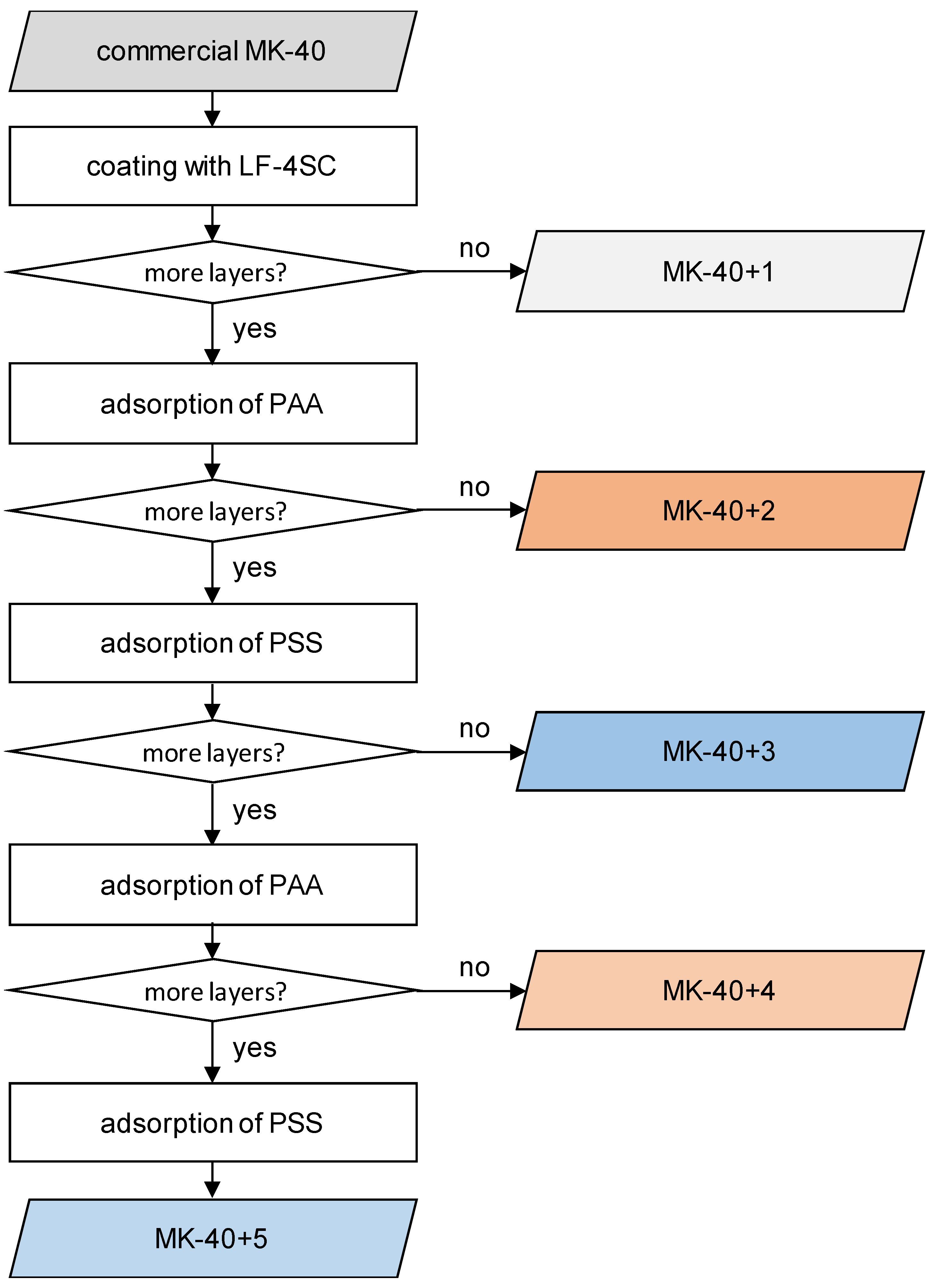
2.1. Sample Preparation
2.2. Thickness Measurement
2.3. Current–Voltage Curves
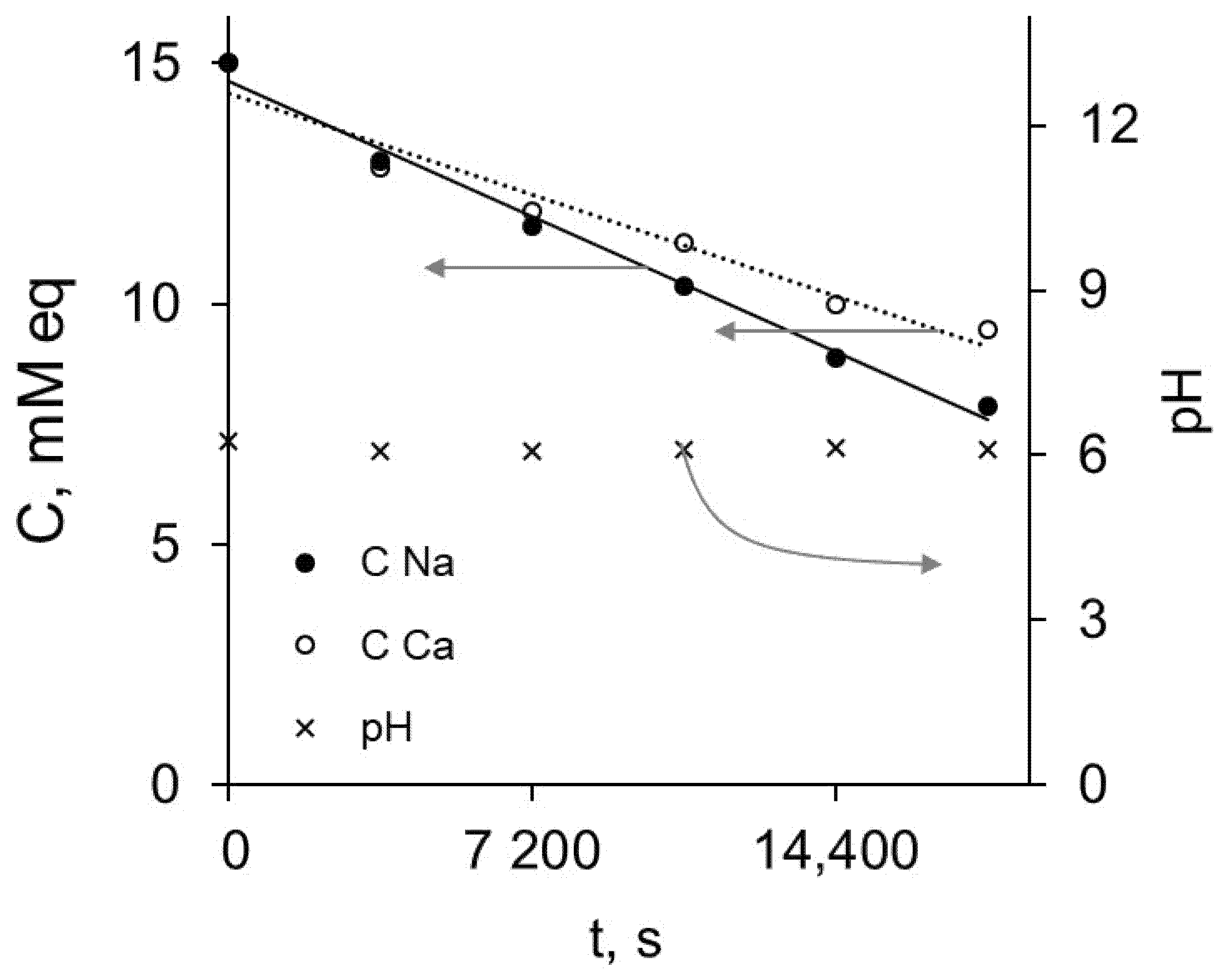
2.4. Electrodialysis of Mixed Solution
3. Results and Discussion
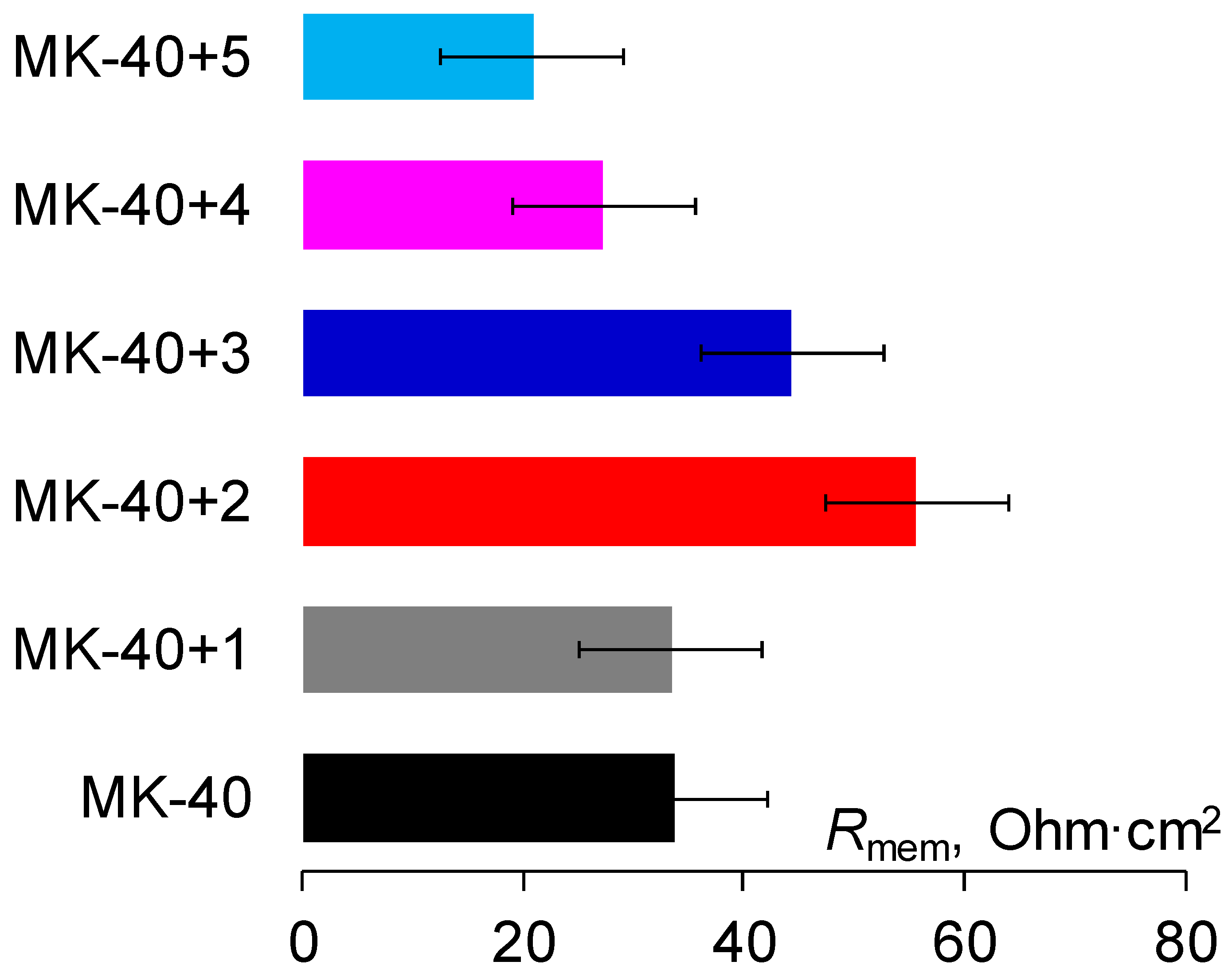
3.1. Thickness Measurement
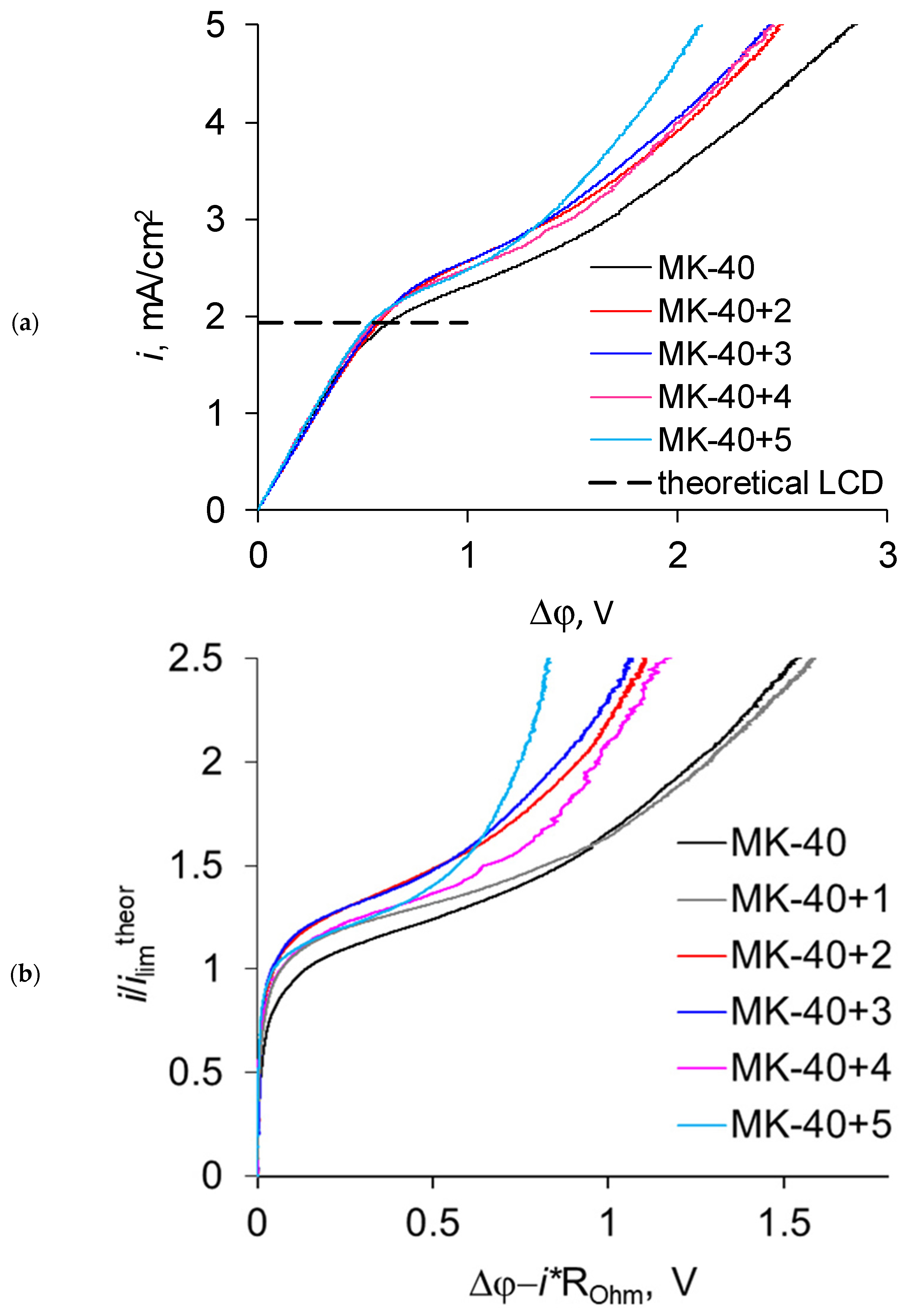
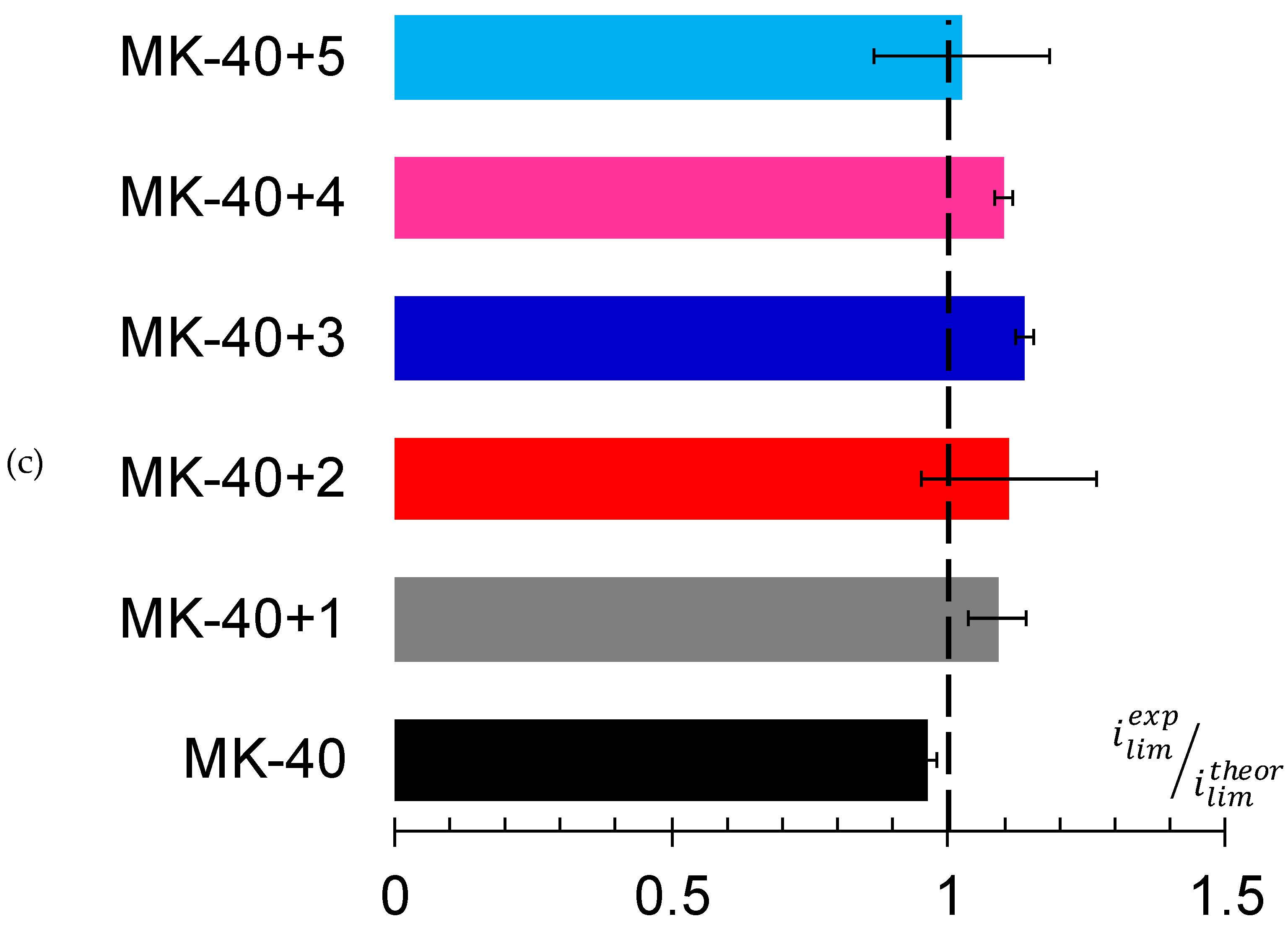
3.2. Current–Voltage Curves
3.3. Electrodialysis of Mixed Solution: Evaluation of Monovalent Selectivity
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Lopez, A.M.; Williams, M.; Paiva, M.; Demydov, D.; Do, T.D.; Fairey, J.L.; Lin, Y.J.; Hestekin, J.A. Potential of electrodialytic techniques in brackish desalination and recovery of industrial process water for reuse. Desalination 2017, 409, 108–114. [Google Scholar] [CrossRef]
- Goodman, N.B.; Taylor, R.J.; Xie, Z.; Gozukara, Y.; Clements, A. A feasibility study of municipal wastewater desalination using electrodialysis reversal to provide recycled water for horticultural irrigation. Desalination 2013, 317, 77–83. [Google Scholar] [CrossRef]
- Cohen, B.; Lazarovitch, N.; Gilron, J. Upgrading groundwater for irrigation using monovalent selective electrodialysis. Desalination 2018, 431, 126–139. [Google Scholar] [CrossRef]
- Ahdab, Y.D.; Rehman, D.; Lienhard, J.H. Brackish water desalination for greenhouses: Improving groundwater quality for irrigation using monovalent selective electrodialysis reversal. J. Membr. Sci. 2020, 610, 118072. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Liu, L.; Du, J.; Fu, R.; Van Der Bruggen, B.; Zhang, Y. Fracsis: Ion fractionation and metathesis by a NF-ED integrated system to improve water recovery. J. Membr. Sci. 2017, 523, 385–393. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, C.; Wang, Y.; Fu, R.; Liu, Z.; Xu, T. Selectrodialysis with bipolar membrane for the reclamation of concentrated brine from RO plant. Desalination 2018, 442, 8–15. [Google Scholar] [CrossRef]
- Hell, F.; Lahnsteiner, J.; Frischherz, H.; Baumgartner, G. Experience with full-scale electrodialysis for nitrate and hardness removal. Desalination 1998, 117, 173–180. [Google Scholar] [CrossRef]
- Nie, X.-Y.; Sun, S.-Y.; Sun, Z.; Song, X.; Yu, J.-G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 2017, 403, 128–135. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Ji, Z.-Y.; Chen, Q.-B.; Liu, J.; Zhao, Y.-Y.; Li, F.; Liu, Z.-Y.; Yuan, J.-S. Prefractionation of LiCl from concentrated seawater/salt lake brines by electrodialysis with monovalent selective ion exchange membranes. J. Clean. Prod. 2018, 193, 338–350. [Google Scholar] [CrossRef]
- White, N.; Misovich, M.; Yaroshchuk, A.; Bruening, M.L. Coating of Nafion Membranes with Polyelectrolyte Multilayers to Achieve High Monovalent/Divalent Cation Electrodialysis Selectivities. ACS Appl. Mater. Interfaces 2015, 7, 6620–6628. [Google Scholar] [CrossRef]
- Stair, J.L.; Harris, J.J.; Bruening, M.L. Enhancement of the Ion-Transport Selectivity of Layered Polyelectrolyte Membranes through Cross-Linking and Hybridization. Chem. Mater. 2001, 13, 2641–2648. [Google Scholar] [CrossRef]
- García-Morales, V.; Silva, T.; Moura, C.; Manzanares, J.A.; Silva, F. Ion transport through polyelectrolyte multilayers under steady-state conditions. J. Electroanal. Chem. 2004, 569, 111–119. [Google Scholar] [CrossRef]
- Abdu, S.; Martí-Calatayud, M.-C.; Wong, J.E.; García-Gabaldón, M.; Wessling, M. Layer-by-Layer Modification of Cation Exchange Membranes Controls Ion Selectivity and Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, B.; Tong, X.; Chen, Y. Monovalent-anion selective and antifouling polyelectrolytes multilayer anion exchange membrane for reverse electrodialysis. J. Membr. Sci. 2018, 567, 68–75. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, H.-J.; Choi, S.-J.; Geckeler, K.E.; Cho, J.; Moon, S.-H. Fouling mitigation of anion exchange membrane by zeta potential control. J. Colloid Interface Sci. 2003, 259, 293–300. [Google Scholar] [CrossRef]
- Xiangguo, T.; Wu, Z.; Teng, X.; Zhao, Y.; Chen, L.; Qiu, X. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries. J. Mater. Chem. 2008, 18, 1232–1238. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, H.; Shi, S.; Li, Y.; Yao, L. Characterization of anion exchange membrane modified by electrodeposition of polyelectrolyte containing different functional groups. Desalination 2016, 386, 58–66. [Google Scholar] [CrossRef]
- Rijnaarts, T.; Reurink, D.M.; Radmanesh, F.; De Vos, W.M.; Nijmeijer, K. Layer-by-layer coatings on ion exchange membranes: Effect of multilayer charge and hydration on monovalent ion selectivities. J. Membr. Sci. 2019, 570, 513–521. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Matsuyama, H. Simultaneous improvement of the monovalent anion selectivity and antifouling properties of an anion exchange membrane in an electrodialysis process, using polyelectrolyte multilayer deposition. J. Membr. Sci. 2013, 431, 113–120. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Improvement of the antifouling potential of an anion exchange membrane by surface modification with a polyelectrolyte for an electrodialysis process. J. Membr. Sci. 2012, 137–143. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis—Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Membr. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Zabolotskii, V.; Sheldeshov, N.; Melnikov, S. Effect of cation-exchange layer thickness on electrochemical and transport characteristics of bipolar membranes. J. Appl. Electrochem. 2013, 43, 1117–1129. [Google Scholar] [CrossRef]
- Zabolotskii, V.; Sheldeshov, N.; Melnikov, S. Heterogeneous bipolar membranes and their application in electrodialysis. Desalination 2014, 342, 183–203. [Google Scholar] [CrossRef]
- Tanaka, Y. Bipolar Membrane Electrodialysis. In Ion Exchange Membranes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 369–392. [Google Scholar]
- Mani, K. Electrodialysis water splitting technology. J. Membr. Sci. 1991, 58, 117–138. [Google Scholar] [CrossRef]
- Zabolotsky, V.; Achoh, A.; Lebedev, K.; Melnikov, S. Permselectivity of bilayered ion-exchange membranes in ternary electrolyte. J. Membr. Sci. 2020, 608, 118152. [Google Scholar] [CrossRef]
- Titorova, V.; Sabbatovskiy, K.; Sarapulova, V.; Kirichenko, E.; Sobolev, V.; Kirichenko, K. Characterization of MK-40 Membrane Modified by Layers of Cation Exchange and Anion Exchange Polyelectrolytes. Membranes 2020, 10, 20. [Google Scholar] [CrossRef]
- Monopolar Membranes—Innovative Company Shchekinoazot Ltd. Available online: https://www.azotom.ru/monopolyarnye-membrany/ (accessed on 17 December 2020).
- Volodina, E.; Pismenskaya, N.; Nikonenko, V.; Larchet, C.; Pourcelly, G. Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J. Colloid Interface Sci. 2005, 285, 247–258. [Google Scholar] [CrossRef]
- Vasil’Eva, V.I.; Akberova, E.M.; Zhiltsova, A.V.; Chernykh, E.I.; Sirota, E.A.; Agapov, B.L. SEM diagnostics of the surface of MK-40 and MA-40 heterogeneous ion-exchange membranes in the swollen state after thermal treatment. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2013, 7, 833–840. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Melnik, N.; Nevakshenova, E.; Nebavskaya, K.; Nikonenko, V.V. Enhancing Ion Transfer in Overlimiting Electrodialysis of Dilute Solutions by Modifying the Surface of Heterogeneous Ion-Exchange Membranes. Int. J. Chem. Eng. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Berezina, N.; Timofeev, S.; Kononenko, N. Effect of conditioning techniques of perfluorinated sulphocationic membranes on their hydrophylic and electrotransport properties. J. Membr. Sci. 2002, 209, 509–518. [Google Scholar] [CrossRef]
- Newman, J.; Thomas-Alyea, K.E. Electrochemical Systems, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0471477567. [Google Scholar]
- Kharkats, Y.; Sokirko, A. Theory of the effect of migration current exaltation taking into account dissociation-recombination reactions. J. Electroanal. Chem. Interfacial Electrochem. 1991, 303, 27–44. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Electro-osmotically induced convection at a permselective membrane. Phys. Rev. E 2000, 62, 2238–2251. [Google Scholar] [CrossRef]
- Filippov, A. Asymmetry of current–voltage characteristics: A bilayer model of a modified ion-exchange membrane. Colloid J. 2016, 78, 397–406. [Google Scholar] [CrossRef]
- Guzmán, E.; Rubio, R.G.; Ortega, F. A closer physico-chemical look to the Layer-by-Layer electrostatic self-assembly of polyelectrolyte multilayers. Adv. Colloid Interface Sci. 2020, 282, 102197. [Google Scholar] [CrossRef]
- Guzmán, E.; Ritacco, H.A.; Ortega, F.; Rubio, R.G. Growth of Polyelectrolyte Layers Formed by Poly(4-styrenesulfonate sodium salt) and Two Different Polycations: New Insights from Study of Adsorption Kinetics. J. Phys. Chem. C 2012, 116, 15474–15483. [Google Scholar] [CrossRef]
- Cheng, C.; Yaroshchuk, A.; Bruening, M.L. Fundamentals of Selective Ion Transport through Multilayer Polyelectrolyte Membranes. Langmuir 2013, 29, 1885–1892. [Google Scholar] [CrossRef]
- Lösche, M.; Schmitt, J.; Decher, G.; Bouwman, W.G.; Kjaer, K. Detailed Structure of Molecularly Thin Polyelectrolyte Multilayer Films on Solid Substrates as Revealed by Neutron Reflectometry. Macromolecules 1998, 31, 8893–8906. [Google Scholar] [CrossRef]
- Schmitt, J.; Gruenewald, T.; Decher, G.; Pershan, P.S.; Kjaer, K.; Loesche, M. Internal structure of layer-by-layer adsorbed polyelectrolyte films: A neutron and x-ray reflectivity study. Macromolecules 1993, 26, 7058–7063. [Google Scholar] [CrossRef]
- Guzmán, E.; Fernández-Peña, L.; Ortega, F.; Rubio, R.G. Equilibrium and kinetically trapped aggregates in polyelectrolyte–oppositely charged surfactant mixtures. Curr. Opin. Colloid Interface Sci. 2020, 48, 91–108. [Google Scholar] [CrossRef]
- Belashova, E.; Melnik, N.; Pismenskaya, N.; Shevtsova, K.; Nebavsky, A.; Lebedev, K.; Nikonenko, V. Overlimiting mass transfer through cation-exchange membranes modified by Nafion film and carbon nanotubes. Electrochim. Acta 2012, 59, 412–423. [Google Scholar] [CrossRef]
- Mishchuk, N.A.; Verbich, S.V.; Gonzales-Caballero, F. Concentration Polarization and Specific Selectivity of Membranes in Pulse Mode. Colloid J. 2001, 63, 586–595. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsygurina, K.; Rybalkina, O.; Sabbatovskiy, K.; Kirichenko, E.; Sobolev, V.; Kirichenko, K. Layer-by-Layer Coating of MK-40 Heterogeneous Membrane with Polyelectrolytes Creates Samples with Low Electrical Resistance and Weak Generation of H+ and OH− Ions. Membranes 2021, 11, 145. https://doi.org/10.3390/membranes11020145
Tsygurina K, Rybalkina O, Sabbatovskiy K, Kirichenko E, Sobolev V, Kirichenko K. Layer-by-Layer Coating of MK-40 Heterogeneous Membrane with Polyelectrolytes Creates Samples with Low Electrical Resistance and Weak Generation of H+ and OH− Ions. Membranes. 2021; 11(2):145. https://doi.org/10.3390/membranes11020145
Chicago/Turabian StyleTsygurina, Kseniia, Olesya Rybalkina, Konstantin Sabbatovskiy, Evgeniy Kirichenko, Vladimir Sobolev, and Ksenia Kirichenko. 2021. "Layer-by-Layer Coating of MK-40 Heterogeneous Membrane with Polyelectrolytes Creates Samples with Low Electrical Resistance and Weak Generation of H+ and OH− Ions" Membranes 11, no. 2: 145. https://doi.org/10.3390/membranes11020145
APA StyleTsygurina, K., Rybalkina, O., Sabbatovskiy, K., Kirichenko, E., Sobolev, V., & Kirichenko, K. (2021). Layer-by-Layer Coating of MK-40 Heterogeneous Membrane with Polyelectrolytes Creates Samples with Low Electrical Resistance and Weak Generation of H+ and OH− Ions. Membranes, 11(2), 145. https://doi.org/10.3390/membranes11020145






