The Removal of Selected Inorganics from Municipal Membrane Bioreactor Wastewater Using UF/NF/RO Membranes for Water Reuse Application: A Pilot-Scale Study
Abstract
1. Introduction
2. Materials and Methods
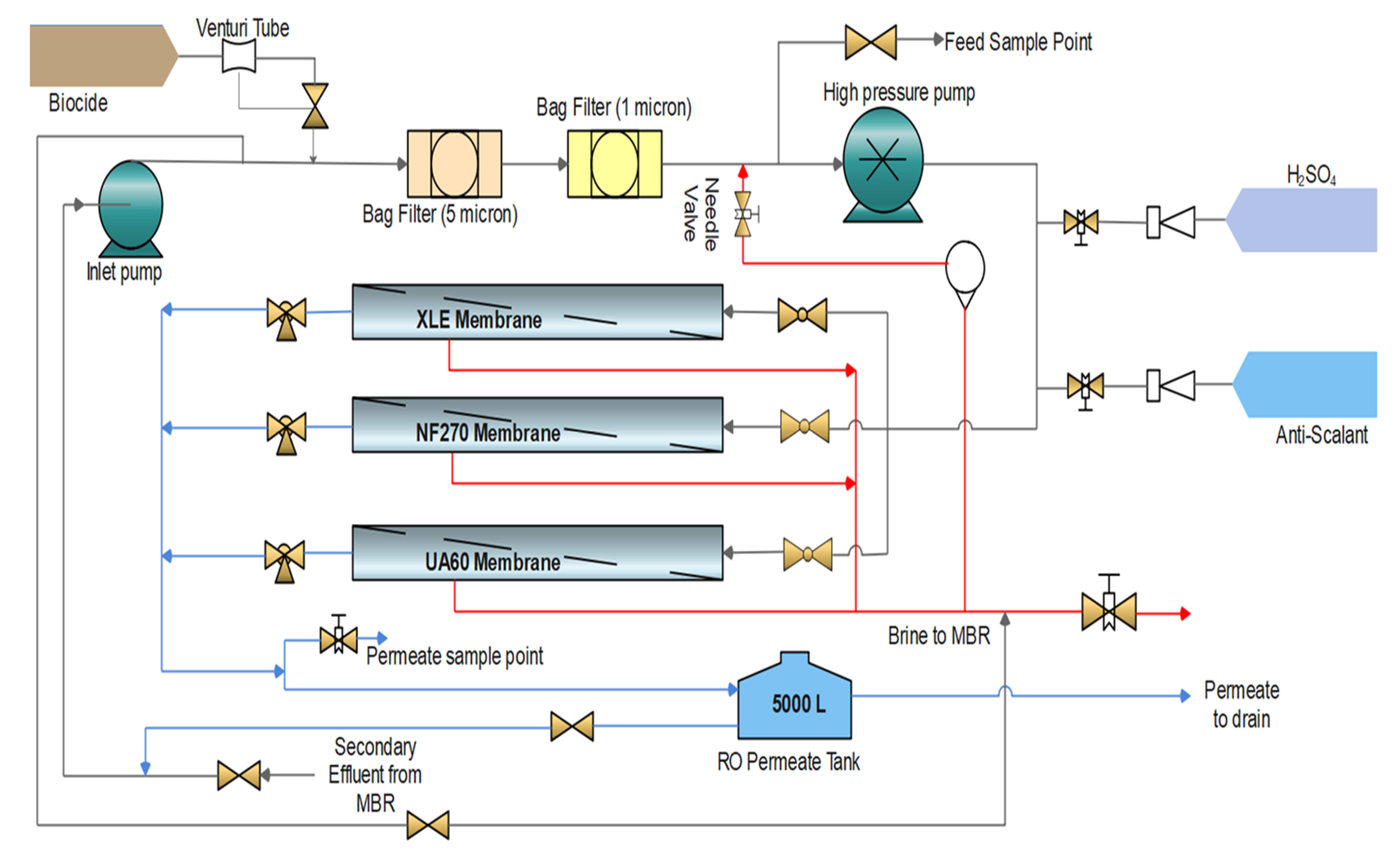
2.1. Full-Scale MBR and RO Pilot-Plant Hybrid System
2.2. UF/NF/RO Membranes
2.3. Membrane Energy Consumptions
2.4. Analytical Methods and Water Analysis
2.5. Statistical Analysis
3. Results and Discussion
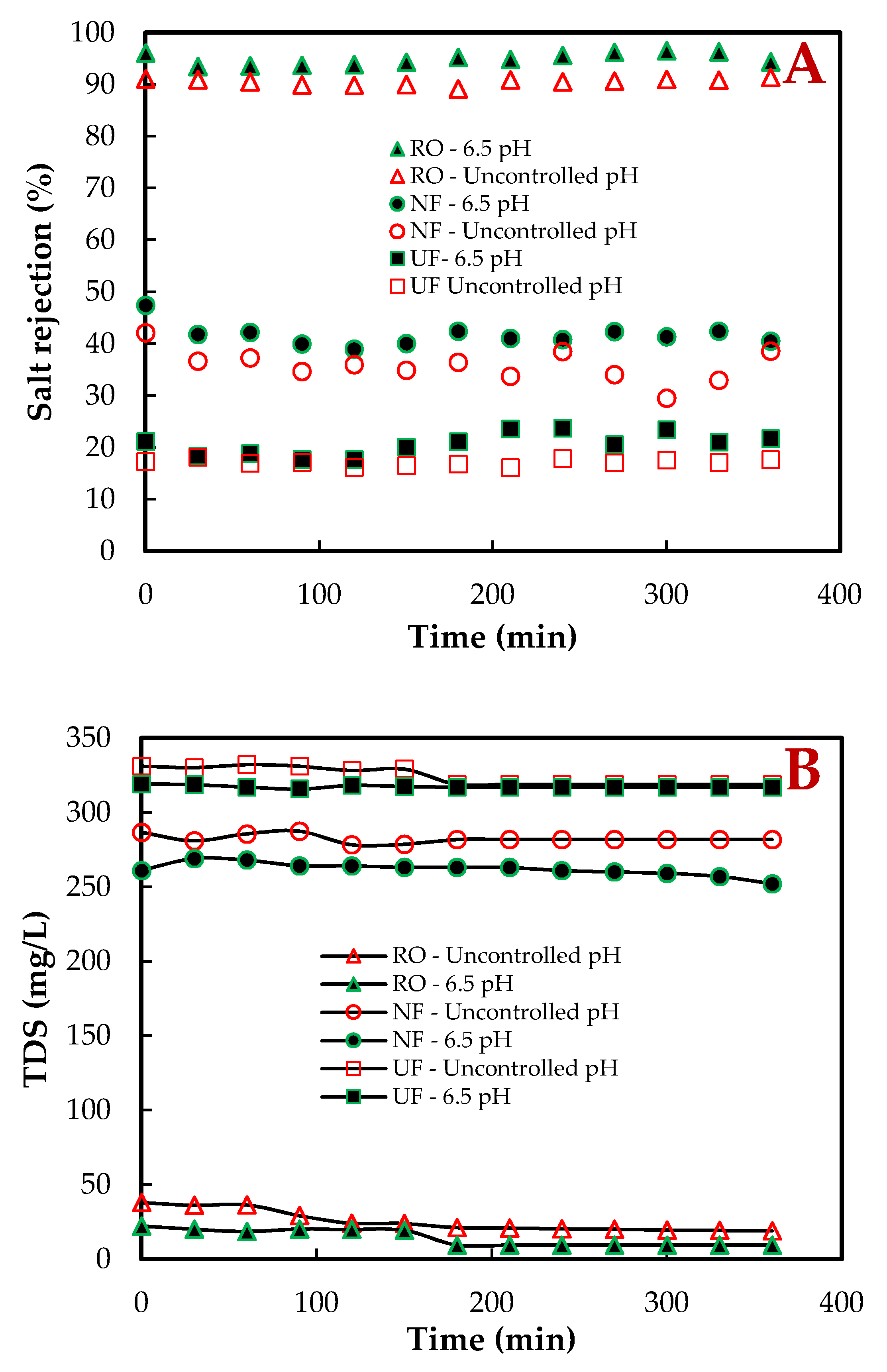
3.1. Salt Rejection and Total Dissolved Solids (TDS)
3.2. Chemical Oxygen Demand (COD)
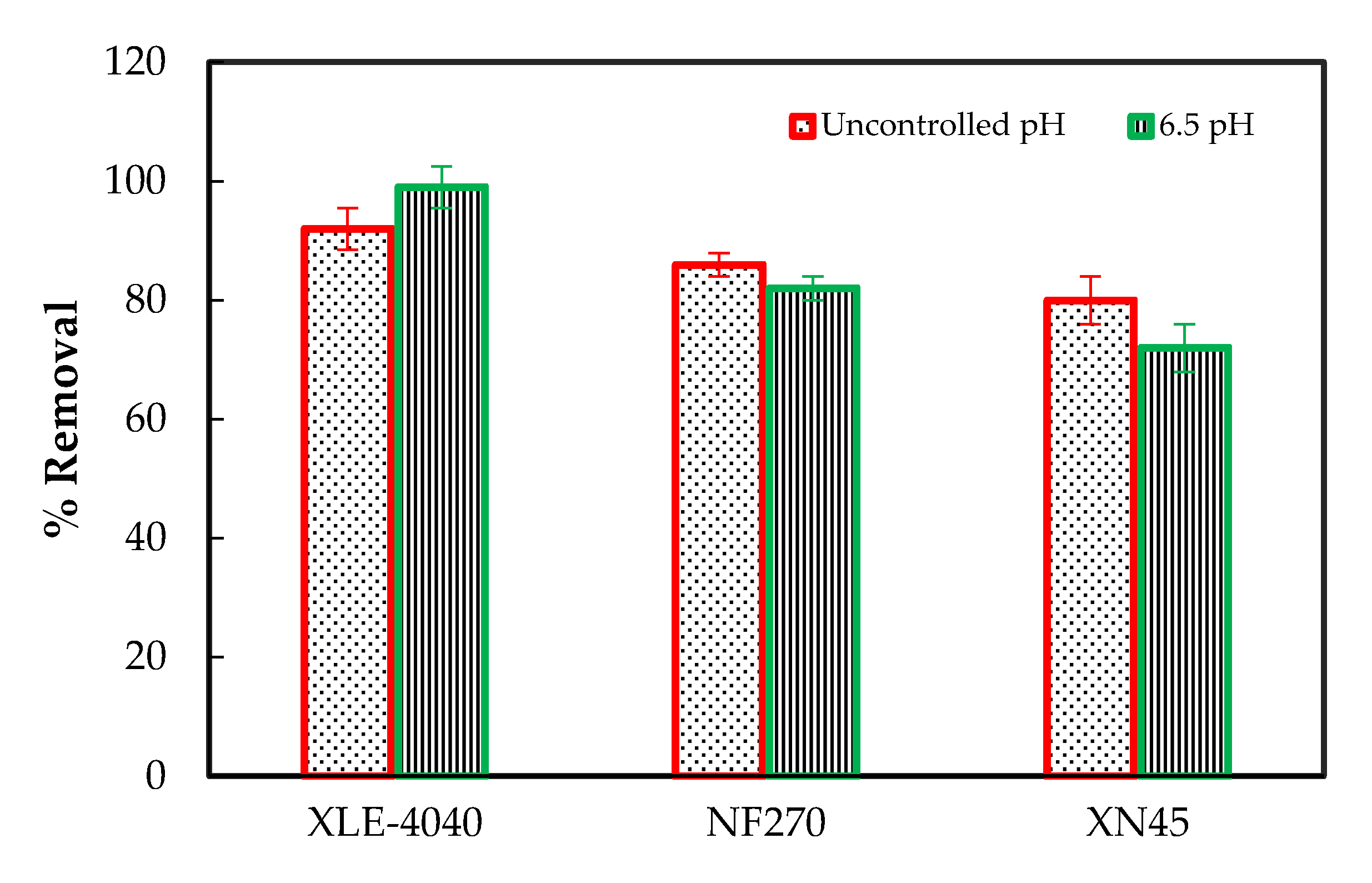
3.3. Inorganics Removal
3.4. Membranes Energy Consumption Comparison and Effect of RO Operating Conditions
3.5. Reuse Application for Wastewater Effluent
3.6. Effect of Operation Conditions on RO Membrane Rejection
3.6.1. Selected Inorganic Rejection
3.6.2. Chemical Oxygen Demand (COD) rejection
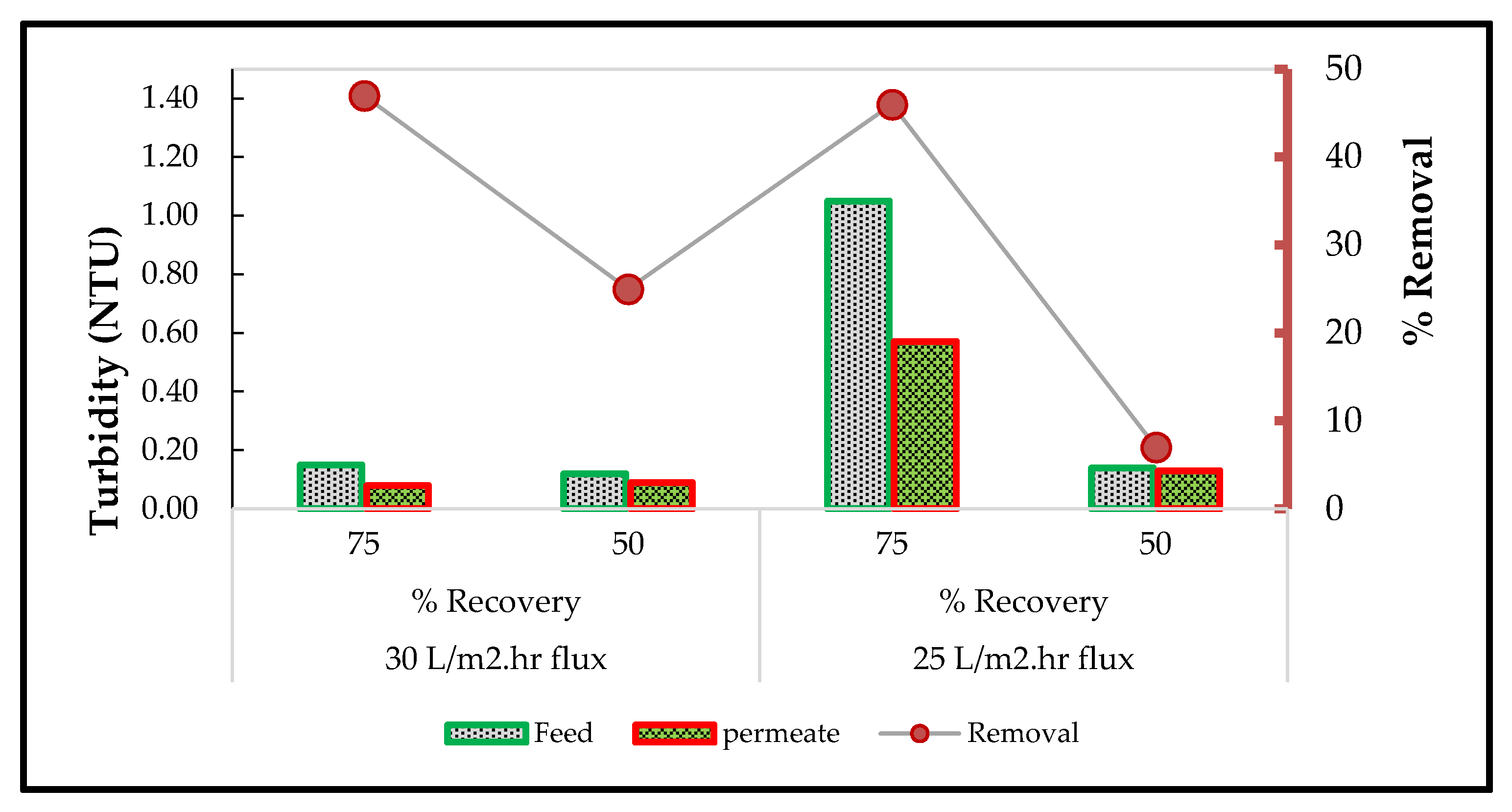
3.6.3. Water Turbidity Using the RO System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Teva, F.; Leal, A.I. Retention of emerging micropollutants from UP water and a municipal secondary effluent by ultrafiltration and nanofiltration. Chem. Eng. J. 2010, 163, 264–272. [Google Scholar] [CrossRef]
- Bunani, S.; Yörükoğlu, E.; Yüksel, Ü.; Kabay, N.; Yüksel, M.; Sert, G. Application of reverse osmosis for reuse of secondary treated urban wastewater in agricultural irrigation. Desalination 2015, 364, 68–74. [Google Scholar] [CrossRef]
- Dialynas, E.; Diamadopoulos, E. Integration of a membrane bioreactor coupled with reverse osmosis for advanced treatment of municipal wastewater. Desalination 2009, 238, 302–311. [Google Scholar] [CrossRef]
- Sahar, E.; David, I.; Gelman, Y.; Chikurel, H.; Aharoni, A.; Messalem, R.; Brenner, A. The use of RO to remove emerging micropollutants following CAS/UF or MBR treatment of municipal wastewater. DES 2011, 273, 142–147. [Google Scholar] [CrossRef]
- Aziz, M.; Kasongo, G. Scaling prevention of thin film composite polyamide Reverse Osmosis membranes by Zn ions. Desalination 2019, 464, 76–83. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Rana, H.H.; Saha, N.K.; Jewrajka, S.K.; Reddy, A.V.R. Low fouling and improved chlorine resistant thin film composite reverse osmosis membranes by cerium(IV)/polyvinyl alcohol mediated surface modification. Desalination 2015, 357, 93–103. [Google Scholar] [CrossRef]
- Kasongo, G.; Steenberg, C.; Morris, B.; Kapenda, G.; Jacobs, N.; Aziz, M. Surface grafting of polyvinyl alcohol (Pva) cross-linked with glutaraldehyde (ga) to improve resistance to fouling of aromatic polyamide thin film composite reverse osmosis membranes using municipal membrane bioreactor effluent. Water Pract. Technol. 2019, 14, 614–624. [Google Scholar] [CrossRef]
- Sert, G.; Bunani, S.; Yörüko, E.; Kabay, N.; Egemen, Ö.; Yüksel, M.; Falizi, N.J.; Hacıfazlıoğlu, M.C.; Parlar, İ.; Kabay, N.; et al. Challenges of municipal wastewater reclamation for irrigation by MBR and NF/RO: Physico-chemical and microbiological parameters, and emerging contaminants. Chem. Eng. J. 2018, 22, 137959. [Google Scholar]
- Malamis, S.; Katsou, E.; Takopoulos, K.; Demetriou, P.; Loizidou, M. Assessment of metal removal, biomass activity and RO concentrate treatment in an MBR-RO system. J. Hazard. Mater. 2012, 209–210, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dolar, D.; Gros, M.; Rodriguez-Mozaz, S.; Moreno, J.; Comas, J.; Rodriguez-Roda, I.; Barceló, D. Removal of emerging contaminants from municipal wastewater with an integrated membrane system, MBR-RO. J. Hazard. Mater. 2012, 239–240, 64–69. [Google Scholar] [CrossRef] [PubMed]
- de Souza, D.I.; Giacobbo, A.; Fernandes, E.d.S.; Rodrigues, M.A.S.; de Pinho, M.N.; Bernardes, A.M. Experimental design as a tool for optimizing and predicting the nanofiltration performance by treating antibiotic-containing wastewater. Membranes 2020, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Bunani, S.; Yörükoğlu, E.; Sert, G.; Kabay, N.; Yüksel, Ü.; Yüksel, M.; Egemen, Ö.; Pek, T.Ö. Utilization of reverse osmosis (RO) for reuse of MBR-treated wastewater in irrigation-preliminary tests and quality analysis of product water. Environ. Sci. Pollut. Res. 2018, 25, 3030–3037. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Drewes, J.E.; Kim, T.U.; Bellona, C.; Amy, G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J. Membr. Sci. 2006, 279, 165–175. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Agoro, M.A.; Okoh, O.O.; Adefisoye, M.A.; Okoh, A.I. Physicochemical Properties of Wastewater in Three Typical South African Sewage Works. Pol. J. Environ. Stud. 2018, 27, 491–499. [Google Scholar] [CrossRef]
- Aziz, M.; Ojumu, T. Exclusion of estrogenic and androgenic steroid hormones from municipal membrane bioreactor wastewater using UF/NF/RO membranes for water reuse application. Membranes 2020, 10, 37. [Google Scholar] [CrossRef]
- Gude, V.G. Energy consumption and recovery in reverse osmosis. Desalin. Water Treat. 2011, 36, 239–260. [Google Scholar] [CrossRef]
- Mazlan, N.M.; Peshev, D.; Livingston, A.G. Energy consumption for desalination—A comparison of forward osmosis with reverse osmosis, and the potential for perfect membranes. Desalination 2016, 377, 138–151. [Google Scholar] [CrossRef]
- Qin, M.; Deshmukh, A.; Epsztein, R.; Patel, S.K.; Owoseni, O.M.; Walker, W.S.; Elimelech, M. Comparison of energy consumption in desalination by capacitive deionization and reverse osmosis. Desalination 2019, 455, 100–114. [Google Scholar] [CrossRef]
- Garcia-Aleman, J.; Dickson, J.M. Mathematical modeling of nanofiltration membranes with mixed electrolyte solutions. J. Membr. Sci. 2004, 235, 1–13. [Google Scholar] [CrossRef]
- Üstün, G.E.; Solmaz, S.K.A.; Çiner, F.; Bažkaya, H.S. Tertiary treatment of a secondary effluent by the coupling of coagulation-flocculation-disinfection for irrigation reuse. Desalination 2011, 277, 207–212. [Google Scholar] [CrossRef]
- Emongor, V.E.; Khonga, E.B.; Ramolemana, G.M.; Marumo, K.; Machacha, S.; Motsamai, T. Suitability of treated secondary sewage effluent for irrigation of horticultural crops in Botswana. J. Appl. Sci. 2005, 5, 451–454. [Google Scholar] [CrossRef][Green Version]
- Van Voorthuizen, E.M.; Zwijnenburg, A.; Wessling, M. Nutrient removal by NF and RO membranes in a decentralized sanitation system. Water Res. 2005, 39, 3657–3667. [Google Scholar] [CrossRef]
- Falizi, N.J.; Hacıfazlıoğlu, M.C.; Parlar, İ.; Kabay, N.; Pek, T.; Yüksel, M. Evaluation of MBR treated industrial wastewater quality before and after desalination by NF and RO processes for agricultural reuse. J. Water Process Eng. 2018, 22, 103–108. [Google Scholar] [CrossRef]
- Xu, R.; Qin, W.; Zhang, B.; Wang, X.; Li, T.; Zhang, Y.; Wen, X. Nanofiltration in pilot scale for wastewater reclamation: Long-term performance and membrane biofouling characteristics. Chem. Eng. J. 2020, 395, 125087. [Google Scholar] [CrossRef]
- Saichek, R.E.; Reddy, K.R. Effect of pH control at the anode for the electrokinetic removal of phenanthrene from kaolin soil. Chemosphere 2003, 51, 273–287. [Google Scholar] [CrossRef]
- Chan, G.Y.S.; Chang, J.; Kurniawan, T.A.; Fu, C.X.; Jiang, H.; Je, Y. Removal of non-biodegradable compounds from stabilized leachate using VSEPRO membrane filtration. Desalination 2007, 202, 310–317. [Google Scholar] [CrossRef]
- Farrokh Shad, M.; Juby, G.J.G.; Delagah, S.; Sharbatmaleki, M. Evaluating occurrence of contaminants of emerging concerns in MF/RO treatment of primary effluent for water reuse—Pilot study. J. Water Reuse Desalin. 2019, 9, 350–371. [Google Scholar] [CrossRef]
- Chu, K.H.; Fathizadeh, M.; Yu, M.; Flora, J.R.V.; Jang, A.; Jang, M.; Park, C.M.; Yoo, S.S.; Her, N.; Yoon, Y. Evaluation of Removal Mechanisms in a Graphene Oxide-Coated Ceramic Ultrafiltration Membrane for Retention of Natural Organic Matter, Pharmaceuticals, and Inorganic Salts. ACS Appl. Mater. Interfaces 2017, 9, 40369–40377. [Google Scholar] [CrossRef] [PubMed]
- Pagès, N.; Reig, M.; Gibert, O.; Cortina, J.L. Trace ions rejection tunning in NF by selecting solution composition: Ion permeances estimation. Chem. Eng. J. 2017, 308, 126–134. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.; Rodrigues, M.A.S.; Aquim, P.M. de Wastewater reuse in a cascade based system of a petrochemical industry for the replacement of losses in cooling towers. J. Environ. Manag. 2016, 181, 157–162. [Google Scholar] [CrossRef]
- Asano, T.; Mujeriego, R.; Parker, J.D. Evaluation of industrial cooling systems using reclaimed municipal wastewater. Water Sci. Technol. 1988, 20, 163–174. [Google Scholar] [CrossRef]
- Paugam, L.; Taha, S.; Dorange, G.; Jaouen, P.; Quéméneur, F. Mechanism of nitrate ions transfer in nanofiltration depending on pressure, pH, concentration and medium composition. J. Membr. Sci. 2004, 231, 37–46. [Google Scholar] [CrossRef]
- Egea-Corbacho Lopera, A.; Gutiérrez Ruiz, S.; Quiroga Alonso, J.M. Removal of emerging contaminants from wastewater using reverse osmosis for its subsequent reuse: Pilot plant. J. Water Process Eng. 2019, 29, 100800. [Google Scholar] [CrossRef]
- Osode, A.N.; Okoh, A.I. Impact of discharged wastewater final effluent on the physicochemical qualities of a receiving watershed in a suburban community of the eastern Cape Province. Clean Soil Air Water 2009, 37, 938–944. [Google Scholar] [CrossRef]



| Parameters | Operating Conditions | ||
|---|---|---|---|
| Membrane module | XLE | NF270 | UA60 |
| Recovery (%) | 50; 75 | 75 | 75 |
| Flux (L·m−2h−1) | 25; 30 | 30 | 30 |
| pH | uncontrolled; 6.5 | uncontrolled | uncontrolled |
| Parameter | Units | Average MBR Effluent | Limit 1 |
|---|---|---|---|
| Electron conductivity (EC) | mS/m | 56 | 75 1 |
| pH | 6.5 | 5.5–9.5 1 | |
| Total Dissolved Solids (TDS) | mg/L | 360 | 450 1 |
| Chemical oxygen demand (COD) | mg/L | <20 | 75 1 |
| Ammonium (NH42−) | mg/L | <0.4 | 3.0 1 |
| Phosphate (PO4) | mg/L | 2.6 | 10 1 |
| Nitrate (NO3) | mg/L | 13 | 15 1 |
| Turbidity | NTU | 0.25 | - |
| Temperature | °C | 25 | 35 1 |
| Membrane Component 1 | Texture 1 | Type 1 | Rejection 1 % | Effective Area 1 (m2) | MWCO 1 (Da) | Maximum Pressure 1 (bar) | Maximum 1 Temperature (°C) | Maximum Permeate Flowrate 1 (m3/h) |
|---|---|---|---|---|---|---|---|---|
| RO | TFC Polyamide | Filmtec XLE−4040 | 99% NaCl | 8.1 | <200 | 6.9 | 45 | 9.8 |
| NF | TFC Polyamide | Filmtec NF270-4040 | >97% MgSO4 | 7.6 | 400 | 4.8 | 45 | 9.5 |
| UF | TFC Piperazine | TriSep 4040-UA60-TSA | 80% MgSO4 | 8.2 | 1000 | 7.6 | 45 | 11.4 |
| RO | NF | UF | ||||
|---|---|---|---|---|---|---|
| 30 L·m−2 h−1 Flux | 25 L·m−2 h−1 Flux | 30 L·m−2 h−1 Flux | ||||
| % Recovery (ϒ) | 75% Recovery (ϒ) | |||||
| Parameter | 75 | 50 | 75 | 50 | ||
| ∆P (kPa) | 384.2 | 423.7 | 385.7 | 389.2 | 298.7 | 269.7 |
| QB (m3·h−1) | 0.081 | 0.243 | 0.068 | 0.204 | 0.063 | 0.205 |
| QP (m3·h−1) | 0.243 | 0.243 | 0.2024 | 0.204 | 0.19 | 0.205 |
| QF (m3·h−1) | 0.324 | 0.486 | 0.270 | 0.405 | 0.253 | 0.41 |
| SEC (kWh·m−3) | 0.142 | 0.235 | 0.143 | 0.216 | 0.111 | 0.100 |
| Parameter | Irrigation [23,24] | Cooling System [34,35] | UF | NF | RO |
|---|---|---|---|---|---|
| COD (mg·L−1) | <50 | <30 | 16 | 10 | 2 |
| NH3 (mg·L−1) | <6.08 | <1 | 0.62 | 0.28 | 0.17 |
| P (mg·L−1) | <1.5 | - | 1.8 | 0.79 | 0.21 |
| PO4 (mg·L−1) | <2 | <7 | 2.07 | 0.91 | 0.45 |
| TDS (mg·L−1) | <200 | - | 300 | 255 | 19 |
| pH | 6.5–8.4 | 6.8–7.2 | 6.5–7.05 | ||
| EC (μS·cm−1) | <250 | <1445 | 471 | 355 | 37 |
| Turbidity (NTU) | <2 | <36 | - | - | 0.08 |
| Operating Conditions | 30 L/m2·h Flux | 25 L/m2·h Flux | |||
|---|---|---|---|---|---|
| % Recovery | |||||
| Water pH | Inorganic | 75 | 50 | 75 | 50 |
| 6.5 | NH3 | 92 | 97 | 98 | 97 |
| NO3 | 100 | 87 | 80 | 100 | |
| NO2 | 63 | 60 | 83 | 82 | |
| PO4 | 90 | 97 | 90 | 97 | |
| P | 92 | 97 | 98 | 97 | |
| Uncontrolled | NH3 | 80 | 87 | 94 | 92 |
| NO3 | 68 | 76 | 63 | 63 | |
| NO2 | 61 | 55 | 86 | 71 | |
| PO4 | 98 | 86 | 98 | 88 | |
| P | 80 | 87 | 94 | 92 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, M.; Kasongo, G. The Removal of Selected Inorganics from Municipal Membrane Bioreactor Wastewater Using UF/NF/RO Membranes for Water Reuse Application: A Pilot-Scale Study. Membranes 2021, 11, 117. https://doi.org/10.3390/membranes11020117
Aziz M, Kasongo G. The Removal of Selected Inorganics from Municipal Membrane Bioreactor Wastewater Using UF/NF/RO Membranes for Water Reuse Application: A Pilot-Scale Study. Membranes. 2021; 11(2):117. https://doi.org/10.3390/membranes11020117
Chicago/Turabian StyleAziz, Mujahid, and Godwill Kasongo. 2021. "The Removal of Selected Inorganics from Municipal Membrane Bioreactor Wastewater Using UF/NF/RO Membranes for Water Reuse Application: A Pilot-Scale Study" Membranes 11, no. 2: 117. https://doi.org/10.3390/membranes11020117
APA StyleAziz, M., & Kasongo, G. (2021). The Removal of Selected Inorganics from Municipal Membrane Bioreactor Wastewater Using UF/NF/RO Membranes for Water Reuse Application: A Pilot-Scale Study. Membranes, 11(2), 117. https://doi.org/10.3390/membranes11020117






