Groundwater Remediation of Volatile Organic Compounds Using Nanofiltration and Reverse Osmosis Membranes—A Field Study
Abstract
1. Introduction
2. Experimental
2.1. Background Information and Preliminary Testing
2.1.1. Pilot-Scale Testing of Ceramic Microfiltration Membranes
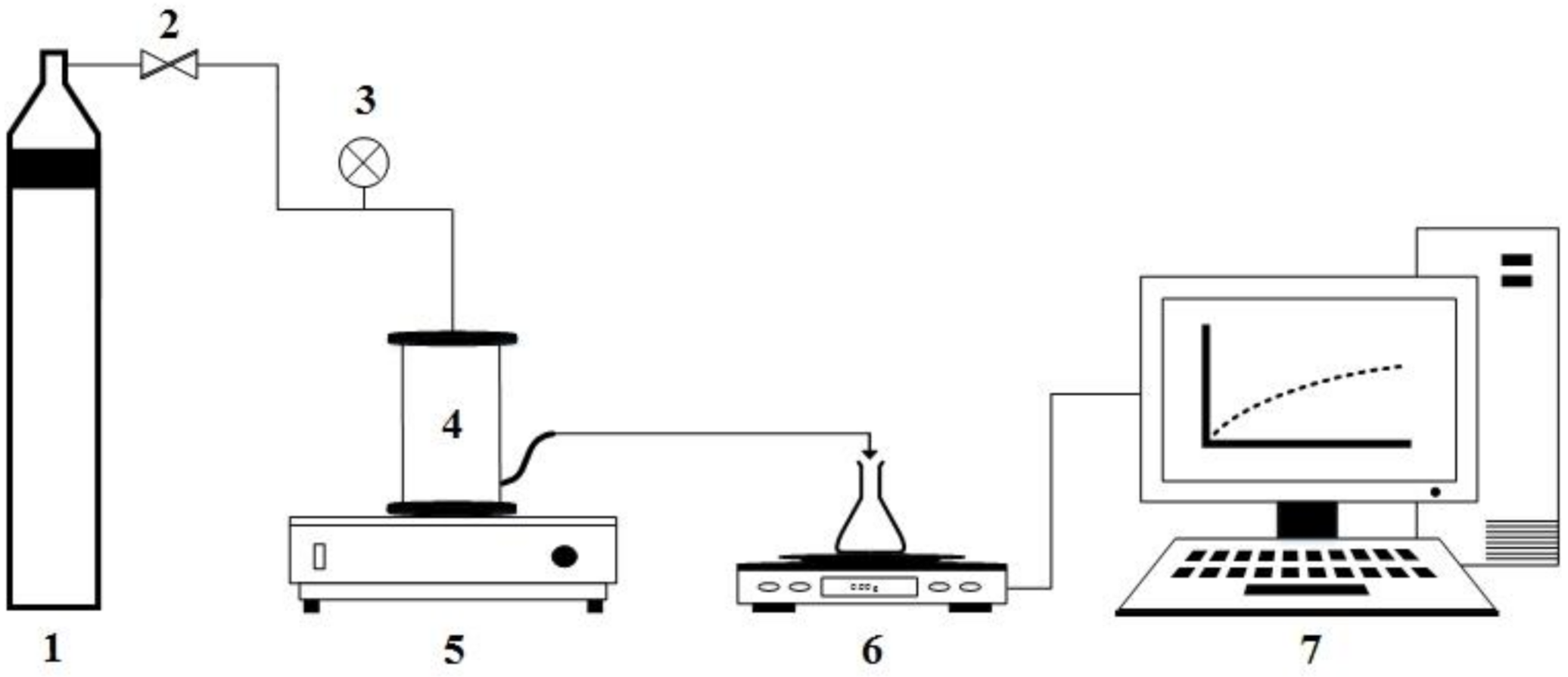
2.1.2. Small-Scale Laboratory Testing Equipment for Nanofiltration and Reverse Osmosis
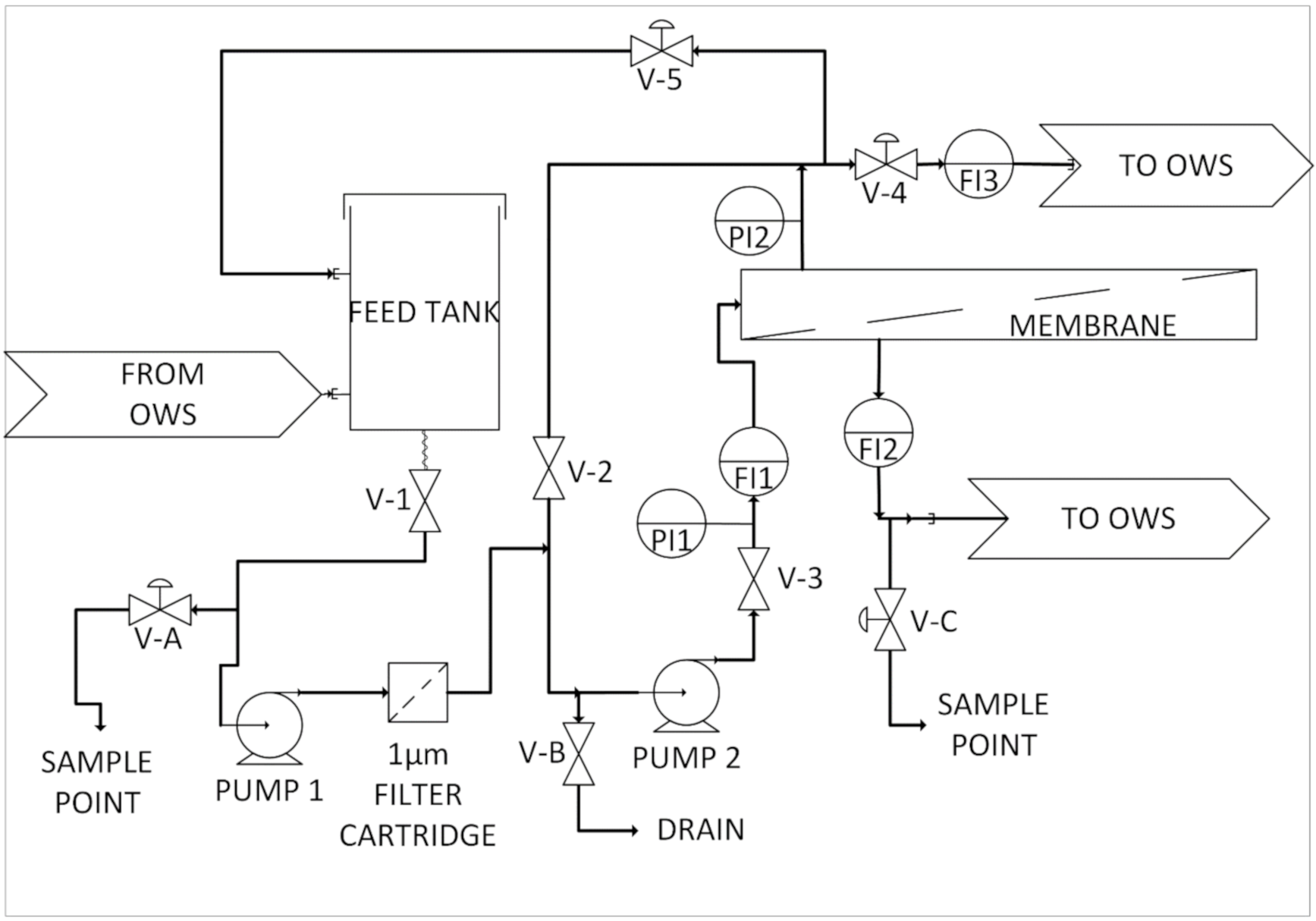
2.2. Nanofiltration/Reverse Osmosis Pilot for Deployment at the Bedfordshire Site
2.3. Laboratory Analysis
2.3.1. General Laboratory Filtration Trials
2.3.2. Contact Angle Measurements
2.3.3. Membranes Used
2.4. Rejection Theory
3. Results and Discussion
3.1. Preliminary Experiments
3.2. Pilot Scale
3.2.1. Nanofiltration
3.2.2. Reverse Osmosis
3.2.3. Contact Angle
3.3. Laboratory Scale
3.3.1. TOC Analysis
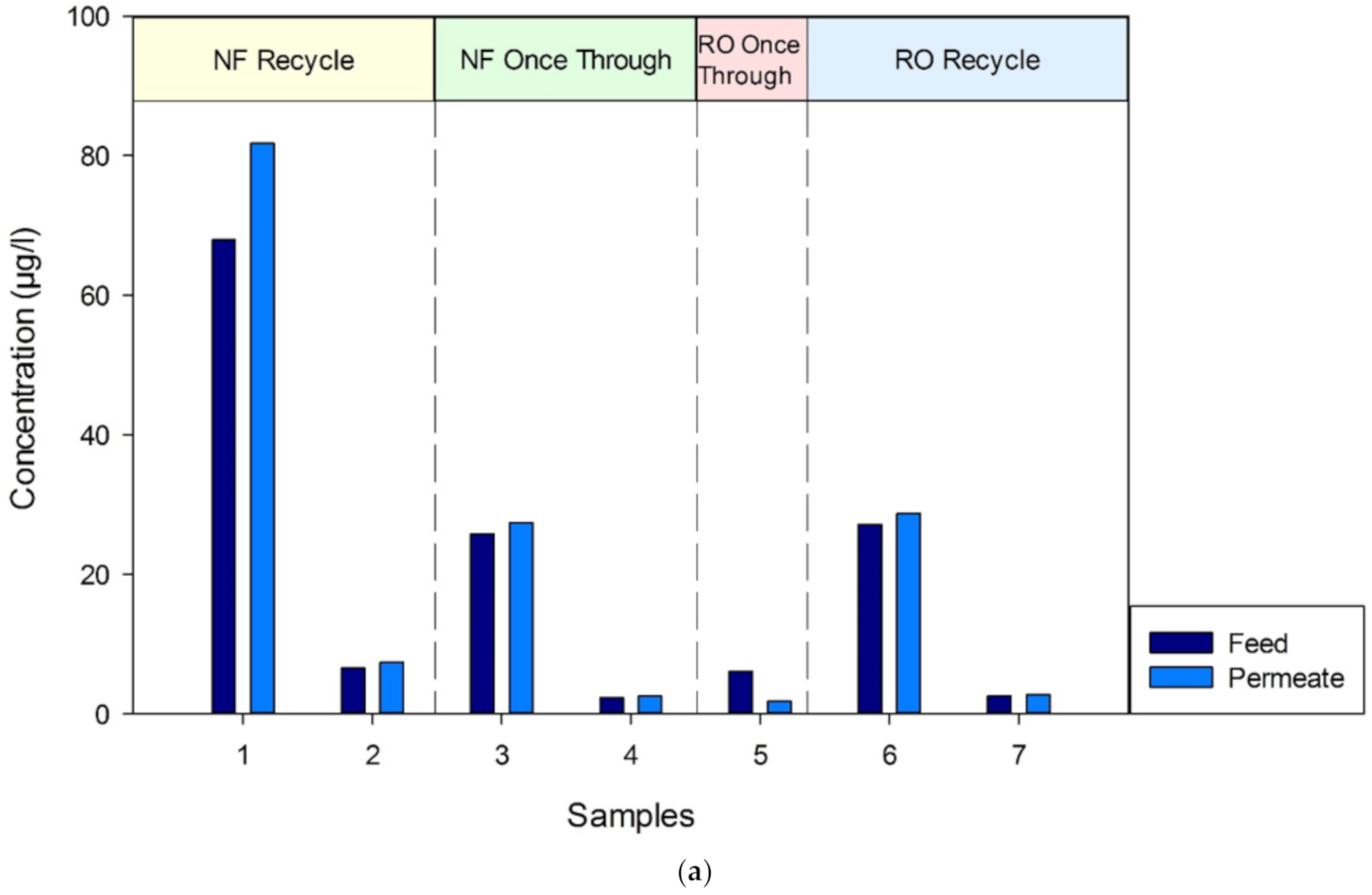
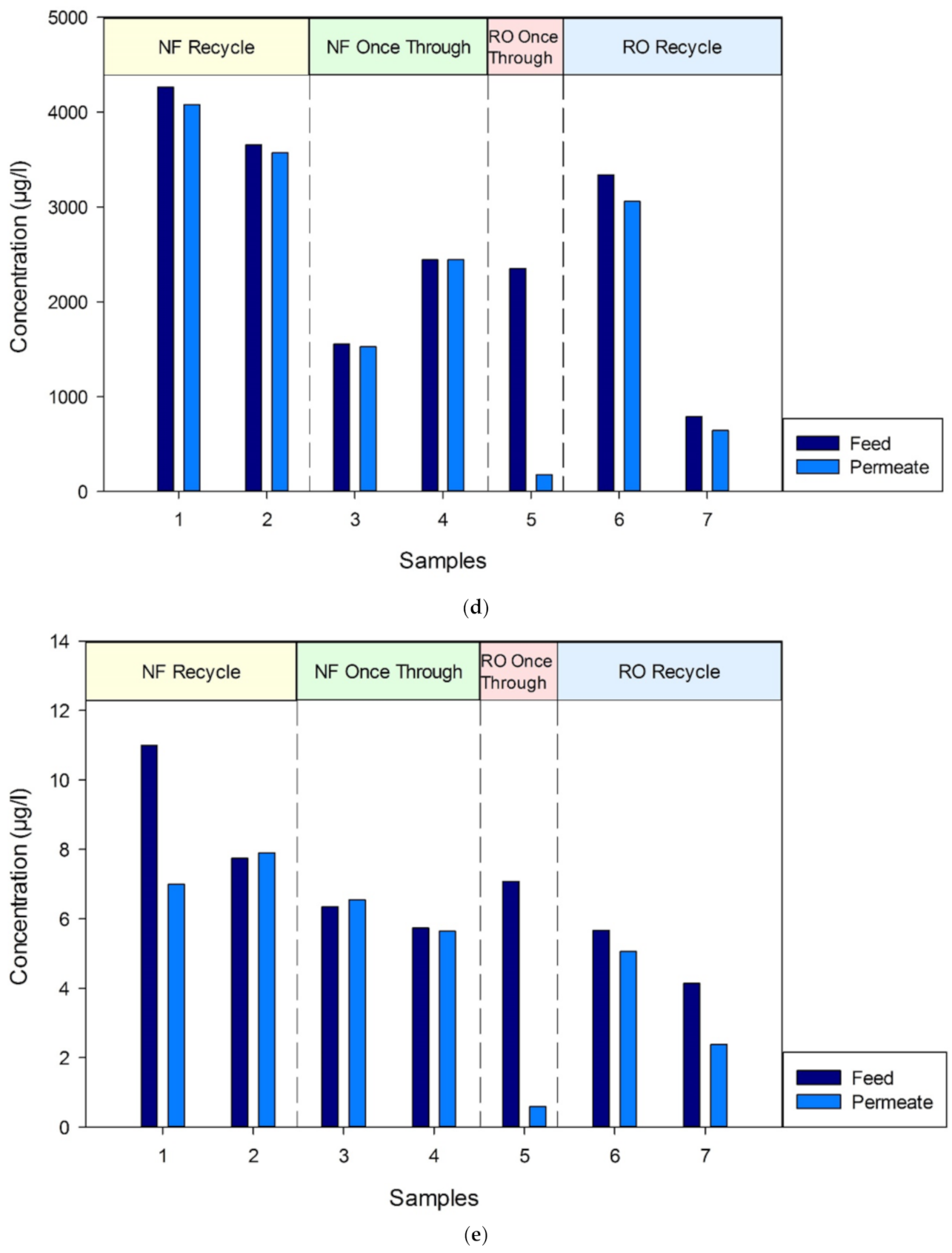
3.3.2. GC–MS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Houlihan, M.F.; Lucia, P.C. Remediation of Contaminated Groundwater. In The Handbook of Groundwater Engineering, 1st ed.; Delleur, J., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 29–39. [Google Scholar]
- Henze, M.; Harremoes, P.; la Cour Jansen, J.; Arvin, E. Wastewater Treatment: Biological and Chemical Processes, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Rahman, K.S.M.; Thahira-Rahman, J.; Lakshmanaperumalsamy, P.; Banat, I.M. Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresour. Technol. 2002, 85, 257–261. [Google Scholar] [CrossRef]
- Peña, A.A.; Hirasaki, A.G.J.; Miller, C.A. Chemically Induced Destabilization of Water-in-Crude Oil Emulsions. Ind. Eng. Chem. Res. 2005, 44, 1139–1149. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- KP Veerapandian, S.; De Geyter, N.; Giraudon, J.M.; Lamonier, J.F.; Morent, R. The use of zeolites for VOCs abatement by combining non-thermal plasma, adsorption, and/or catalysis: A review. Catalysts 2019, 9, 98. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatleyradcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Costa, A.R.; De Pinho, M.N. Performance and cost estimation of nanofiltration for surface water treatment in drinking water production. Desalination 2006, 196, 55–65. [Google Scholar] [CrossRef]
- Schaep, J.; Van Der Bruggen, B.; Uytterhoeven, S.; Croux, R.; Vandecasteele, C.; Wilms, D.; Van Houtte, E.; Vanlerberghe, F. Removal of hardness from groundwater by nanofiltration. Desalination 1998, 119, 295–301. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Vandecasteele, C. Removal of pollutants from surface water and groundwater by nanofiltration: Overview of possible applications in the drinking water industry. Environ. Pollut. 2003, 122, 435–445. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Everaert, K.; Wilms, D.; Vandecasteele, C. Application of nanofiltration for removal of pesticides, nitrate and hardness from ground water: Rejection properties and economic evaluation. J. Membr. Sci. 2001, 193, 239–248. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Darwish, N.A.; Mohamma, A.W.; Abu Arabi, M. A comprehensive review of nanofiltration membranes:Treatment, pretreatment, modelling, and atomic force microscopy. Desalination 2004, 170, 281–308. [Google Scholar] [CrossRef]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Mondal, S.; Wickramasinghe, S.R. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Membr. Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrilla, J.; Sánchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Lee, L.J.-H.; Chan, C.-C.; Chung, C.-W.; Ma, Y.-C.; Wang, G.-S.; Wang, J. Health risk assessment on residents exposed to chlorinated hydrocarbons contaminated in groundwater of a hazardous waste site. J. Toxicol. Environ. Health Part A 2002, 65, 219–235. [Google Scholar] [CrossRef]
- Fan, C.; Wang, G.-S.; Chen, Y.-C.; Ko, C.-H. Risk assessment of exposure to volatile organic compounds in groundwater in Taiwan. Sci. Total. Environ. 2009, 407, 2165–2174. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef]
- Cooney, D.O.; Nagerl, A.; Hines, A.L. Solvent regeneration of activated carbon. Water Res. 1983, 17, 403–410. [Google Scholar] [CrossRef]
- Tamon, H.; Saito, T.; Kishimura, M.; Okazaki, M.; Toei, R. Solvent regeneration of spent activated carbon in wastewater treatment. J. Chem. Eng. Jpn. 1990, 23, 426–432. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ryu, Y.-K.; Haam, S.; Lee, C.-H.; Kim, W.-S. Adsorption and steam regeneration of n-hexane, mek, and toluene on activated carbon fiber. Sep. Sci. Technol. 2001, 36, 263–281. [Google Scholar] [CrossRef]
- San Miguel, G.; Lambert, S.D.; Graham, N. The regeneration of field-spent granular-activated carbons. Water Res. 2001, 35, 2740–2748. [Google Scholar] [CrossRef]
- Suzuki, M.; Misic, D.M.; Koyama, O.; Kawazoe, K. Study of thermal regeneration of spent activated carbons: Thermogravimetric measurement of various single component organics loaded on activated carbons. Chem. Eng. Sci. 1978, 33, 271–279. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Rivera-Utrilla, J.; Joly, J.P.; López-Ramón, M.V.; Ferro-García, M.A.; Carrasco-Marín, F. Thermal regeneration of an activated carbon exhausted with different substituted phenols. Carbon 1995, 33, 1417–1423. [Google Scholar] [CrossRef]
- Sabio, E.; González, E.; González, J.F.; González-García, C.M.; Ramiro, A.; Ganan, J. Thermal regeneration of activated carbon saturated with p-nitrophenol. Carbon 2004, 42, 2285–2293. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Wastewater Technology Fact Sheet: Granular Activated Carbon Adsorption and Regeneration; EPA 832-F-00-017; UESPA: Washington, DC, USA, 2000. [Google Scholar]
- Álvarez, P.M.; Beltrán, F.J.; Gómez-Serrano, V.; Jaramillo, J.; Rodríguez, E.M. Comparison between thermal and ozone regenerations of spent activated carbon exhausted with phenol. Water Res. 2004, 38, 2155–2165. [Google Scholar] [CrossRef]
- Ledesma, B.; Román, S.; Álvarez-Murillo, A.; Sabio, E.; González, J. Cyclic adsorption/thermal regeneration of activated carbons. J. Anal. Appl. Pyrolysis 2014, 106, 112–117. [Google Scholar] [CrossRef]
- Maguire-Boyle, S.J.; Barron, A.R. A new functionalization strategy for oil/water separation membranes. J. Membr. Sci. 2011, 382, 107–115. [Google Scholar] [CrossRef]
- Maguire-Boyle, S.J.; Huszman, J.E.; Ainscough, T.J.; Oatley-Radcliffe, D.; Alabdulkarem, A.A.; Al-Mojil, S.F.; Barron, A.R. Superhydrophilic functionalization of micro-filtration ceramic membranes enables separation of hydrocarbons from frac and produced waters without fouling. Sci. Rep. 2017, 7, 12267. [Google Scholar] [CrossRef]
- Oatley, D.L.; Williams, S.R.; Barrow, M.S.; Williams, P.M. Critical appraisal of current nanofiltration modelling strategies for seawater desalination and further insights on dielectric exclusion. Desalination 2014, 343, 154–161. [Google Scholar] [CrossRef]
- Oatley-Radcliffe, D.L.; Williams, S.R.; Ainscough, T.J.; Lee, C.; Johnson, D.J.; Williams, P.M. Experimental determination of the hydrodynamic forces within nanofiltration membranes and evaluation of the current theoretical descriptions. Sep. Purif. Technol. 2015, 149, 339–348. [Google Scholar] [CrossRef][Green Version]
- Nicolas, S.; Balannec, B.; Béline, F.; Bariou, B. Ultrafiltration and reverse osmosis of small non-charged molecules: A comparison study of rejection in a stirred and an unstirred batch cell. J. Membr. Sci. 2000, 164, 141–155. [Google Scholar] [CrossRef]
- Nakao, S.-I.; Kimura, S. Analysis of solutes rejection in ultrafiltration. J. Chem. Eng. Jpn. 1981, 14, 32–37. [Google Scholar] [CrossRef]
- Spiegler, K.S.; Kedem, O. Thermodynamics of hyperfiltration (reverse osmosis): Criteria for efficient membranes. Desalination 1966, 1, 311–326. [Google Scholar] [CrossRef]
- Verliefde, A.R.; Cornelissen, E.R.; Heijman, S.G.J.; Petrinić, I.; Luxbacher, T.; Amy, G.L.; Van Der Bruggen, B.; Van Dijk, J.C. Influence of membrane fouling by (pretreated) surface water on rejection of pharmaceutically active compounds (PhACs) by nanofiltration membranes. J. Membr. Sci. 2009, 330, 90–103. [Google Scholar] [CrossRef]
- Bellona, C.; Marts, M.; Drewes, J.E. The effect of organic membrane fouling on the properties and rejection characteristics of nanofiltration membranes. Sep. Purif. Technol. 2010, 74, 44–54. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Kim, T.-U.; Bellona, C.; Amy, G. Effect of membrane fouling on transport of organic contaminants in NF/RO membrane applications. J. Membr. Sci. 2006, 279, 165–175. [Google Scholar] [CrossRef]
- Agenson, K.O.; Urase, T. Change in membrane performance due to organic fouling in nanofiltration (NF)/reverse osmosis (RO) applications. Sep. Purif. Technol. 2007, 55, 147–156. [Google Scholar] [CrossRef]
- Chang, E.E.; Chang, Y.C.; Liang, C.H.; Huang, C.P.; Chiang, P.C. Identifying the rejection mechanism for nanofiltration membranes fouled by humic acid and calcium ions exemplified by acetaminophen, sulfamethoxazole, and triclosan. J. Hazard. Mater. 2012, 221, 19–27. [Google Scholar] [CrossRef]
- Hilal, N.; Al-Zoubi, H.; Mohammad, A.W.; Darwish, N.A. Nanofiltration of highly concentrated salt solutions up to seawater salinity. Desalination 2005, 184, 315–326. [Google Scholar] [CrossRef]
- Bowen, W.R.; Mohammad, A.W.; Hilal, N. Characterisation of nanofiltration membranes for predictive purposes—Use of salts, uncharged solutes and atomic force microscopy. J. Membr. Sci. 1997, 126, 91–105. [Google Scholar] [CrossRef]
- Oatley, D.L.; Llenas, L.; Pérez, R.; Williams, P.M.; Martínez-Lladó, X.; Rovira, M. Review of the dielectric properties of nanofiltration membranes and verification of the single oriented layer approximation. Adv. Colloid Interface Sci. 2012, 173, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Oatley, D.L.; Llenas, L.; Aljohani, N.H.; Williams, P.M.; Martínez-Lladó, X.; Rovira, M.; De Pablo, J. Investigation of the dielectric properties of nanofiltration membranes. Desalination 2013, 315, 100–106. [Google Scholar] [CrossRef]
- Ducom, G.; Cabassud, C. Interests and limitations of nanofiltration for the removal of volatile organic compounds in drinking water production. Desalination 1999, 124, 115–123. [Google Scholar] [CrossRef]
- Altalyan, H.N.; Jones, B.; Bradd, J.; Nghiem, L.D.; Alyazichi, Y.M. Removal of volatile organic compounds (VOCs) from groundwater by reverse osmosis and nanofiltration. J. Water Process. Eng. 2016, 9, 9–21. [Google Scholar] [CrossRef]
- Kiso, Y.; Nishimura, Y.; Kitao, T.; Nishimura, K. Rejection properties of non-phenylic pesticides with nanofiltration membranes. J. Membr. Sci. 2000, 171, 229–237. [Google Scholar] [CrossRef]





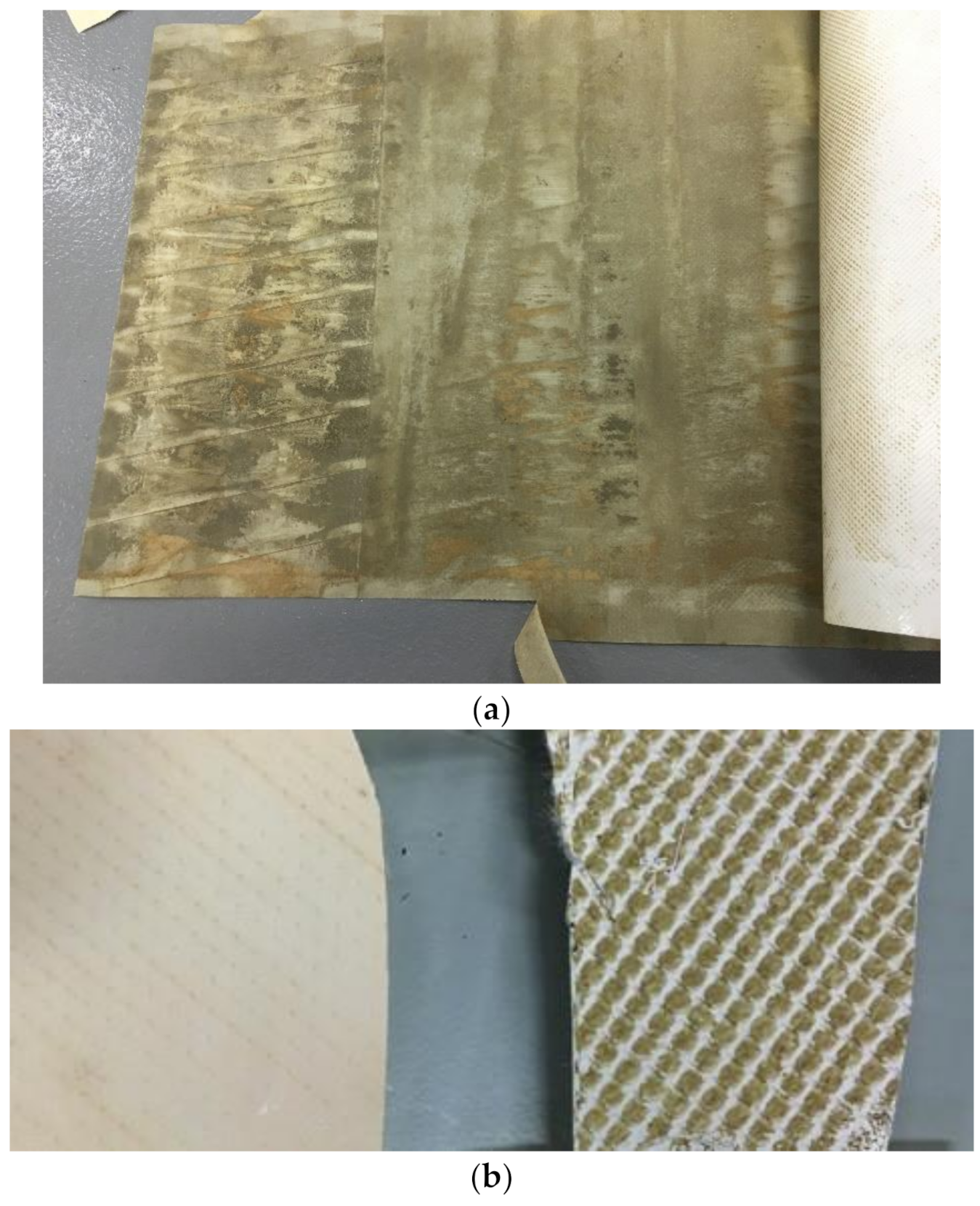

| Compound | CAS # | Formula | MW (g/mol) | XLogP3 |
|---|---|---|---|---|
| Vinyl Chloride | 75-01-4 | C2H3Cl | 62.50 | 1.5 |
| Cis-1,2-Dichloroethylene | 156-59-2 | C2H2CL2 | 96.94 | 1.9 |
| 2,2-Dichloropropane | 594-20-7 | C3H6Cl2 | 112.981 | 2.1 |
| Trichloroethylene | 79-01-6 | C2HCl3 | 131.39 | 2.6 |
| Toluene | 108-88-3 | C7H8 | 92.141 | 2.7 |
| Tetrachloroethylene | 127-18-4 | C2Cl4 | 165.83 | 3.4 |
| Headspace Parameters | Agilent 7697A HS—12 vial | ||
|---|---|---|---|
| Temperature settings | |||
| Oven temperature | 70 °C | ||
| Loop temperature | 85 °C | ||
| Transfer line temperature | 120 °C | ||
| Timing settings | |||
| Vial equilibration | 10 min | ||
| Injection duration | 0.3 min | ||
| GC cycle time | 22 min | ||
| Vial settings | |||
| Vial size | 20 mL | ||
| Vial pressurization | 15 psi | ||
| Loop size | 1.0 mL | ||
| Extraction time | 0.3 min | ||
| Mode | Single extraction | ||
| Transfer line flow | 20 mL/min | ||
| Transfer line | Agilent p/n 160-2535-5 | ||
| Line type | Fused silica, deactivated | ||
| Line diameter | 0.53 mm | ||
| Vial and cap | 20 mL, PTFE/silicone septa | ||
| GC Parameters | Agilent 6850 series II GC | ||
| Inlet settings | |||
| Heater | On—150 °C | ||
| Pressure | On—6.4 psi | ||
| Total flow | On—42.6 mL/min | ||
| Run time | 6.8 min | ||
| Gas saver | Off | ||
| Split ratio | 40:1 | ||
| Split flow | 40 mL/min | ||
| Oven settings | |||
| Oven ramp | °C/min | Next °C | Hold |
| Initial | 30 | 0.3 min | |
| Ramp 1 | 5 | 55 | 0 min |
| Ramp 2 | 10 | 70 | 0 min |
| Total run time | 15.8 min | ||
| Equilibration time | 0.5 min | ||
| Oven Max temperature | 260 °C | ||
| Column | Agilent J & W HP-5 | ||
| Length | 30 m | ||
| Diameter | 0.25 mm | ||
| Film thickness | 0.25 μm | ||
| Mode | Constant flow | ||
| Pressure | 6.4 psi | ||
| Nominal initial flow | 1 mL/min | ||
| Inlet | Front | ||
| Outlet | MSD | ||
| Outlet pressure | Vacuum | ||
| MSD Parameters | Agilent 5977A MSD | ||
| MSD settings | |||
| Solvent delay | 0.0 min | ||
| Sim ions | 4 | ||
| Quantitation ions | 61, 77, 130, 166 M/Z | ||
| Sim dwell | 50 msec/ion | ||
| Quad temperature | 150 | ||
| Source temperature | 230 | ||
| Transfer line temperature | 250 | ||
| Gain factor | 5 | ||
| a. Properties of NF membranes used in this study. | |||||||||
| Membrane | DK | DL | NF90 | NF270 | |||||
| Manufacturer | GE Osmonics | GE Osmonics | Dow Filmtec | Dow Filmtec | |||||
| Support material | Polysulfone | Polysulfone | Polysulfone | Polysulfone | |||||
| Surface material | TFC PA | TFC PA | PA | PA | |||||
| Maximum operating temperature | 50 °C | 50 °C | 45 °C | 45 °C | |||||
| Maximum operating pressure | 41 bar | 41 bar | 41 bar | 41 bar | |||||
| pH range | 3–9 | 3–9 | 2–11 | 2–11 | |||||
| Flux (GFD)/psi | 22/100 | 28/220 | 46–60/130 | 72–98/130 | |||||
| MWCO | ~150–300 | ~150–300 | ~200–400 | ~200–400 | |||||
| Contact angle | 26.36 ± 0.48° | 27.60 ± 0.38° | 27.40 ± 0.89° | 21.22 ± 0.88° | |||||
| b. Properties of RO membranes used in this study. | |||||||||
| Membrane | AK | AG | BW30 | BW30LE | BW30XFR | ||||
| Manufacturer | GE Osmonics | GE Osmonics | Dow Filmtec | Dow Filmtec | Dow Filmtec | ||||
| Support material | Polysulfone | Polysulfone | Polysulfone | Polysulfone | Polysulfone | ||||
| Surface material | TFC PA | TFC PA | PA | PA | PA | ||||
| Maximum operatingtemperature | 50 °C | 50 °C | 45 °C | 45 °C | 45 °C | ||||
| Maximum operatingpressure | 27 bar | 41 bar | 41 bar | 41 bar | 41 bar | ||||
| pH range | 4–11 | 4–11 | 2–11 | 2–11 | 2–11 | ||||
| Flux (GFD)/psi | 26/115 | 26/225 | 26/225 | 37–46/225 | 28–33/225 | ||||
| MWCO | ~0 | ~0 | ~100 | ~100 | ~100 | ||||
| Contact angle | 70.21 ± 1.09° | 72.21 ± 2.52° | 55.86 ± 0.67° | 67.58 ± 0.30° | 56.61 ± 0.66° | ||||
| 1 g/L Toluene–Water Dead-End Filtration | ||
|---|---|---|
| Sample Name | TOC (mg/L) | Rejection |
| Desal DK feed | 508.6 | |
| Desal DK 2.5 bar permeate | 34.15 | 93.29% |
| Desal DK 5 bar permeate | 108.5 | 78.67% |
| Desal DK 10 bar permeate | 127.1 | 75.01% |
| Desal DK 20 bar permeate | 154.2 | 69.68% |
| Company a Water Dead-End Filtration | ||
|---|---|---|
| Sample Name | TOC (µg/L) | Rejection |
| Desal DK feed | 3913 | |
| Desal DK 2.5 bar permeate | 316.4 | 91.91% |
| Desal DK 5 bar permeate | 37.5 | 99.04% |
| Desal DK 10 bar permeate | 0 | 100.00% |
| Desal DK 20 bar permeate | 0.11 | ~100.00% |
| Compound | Feed 1 (µg/L) | Feed 2 (µg/L) | Permeate 1 (µg/L) | Permeate 2 (µg/L) |
|---|---|---|---|---|
| Total | 5476 | 5684 | 5662 | 5639 |
| VC | 6.6 | 6.5 | 7.5 | 7.3 |
| DCE | 1687 | 1758 | 1974 | 1749 |
| DCP | 177.4 | 188.6 | 212 | 180.1 |
| TCE | 3594 | 3720 | 3457 | 3691 |
| PCE | 7.9 | 7.6 | 8.1 | 7.7 |
| Date | Sample | Total Carbon (µg/L) | Inorganic Carbon (µg/L) | Total Organic Carbon (µg/L) | Average (µg/L) | Rejection |
|---|---|---|---|---|---|---|
| 13/05/2015 | Feed | 89,796 | 82,635 | 7161 | 7598 | 57.19% |
| Feed | 90,388 | 82,353 | 8035 | |||
| Permeate | 64,208 | 60,934 | 3274 | 3253 | ||
| Permeate | 63,759 | 60,527 | 3232 | |||
| 27/05/2015 | Feed | 84,059 | 76,947 | 7112 | 7314 | 70.30% |
| Feed | 83,069 | 75,553 | 7516 | |||
| Permeate | 65,731 | 63,566 | 2165 | 2172 | ||
| Permeate | 65,714 | 63,535 | 2179 | |||
| 09/06/2015 | Feed | 80,894 | 74,534 | 6360 | 6360 | 78.30% |
| Permeate | 62,659 | 61,279 | 1380 | 1380 | ||
| Feed | 79,405 | 73,265 | 6140 | 6140 | 79.10% | |
| Permeate | 59,334 | 58,051 | 1283 | 1283 | ||
| 23/06/2015 | Feed | 75,762 | 70,445 | 5317 | 5317 | 71.96% |
| Permeate | 60,341 | 58,850 | 1491 | 1491 |
| Date | Inlet (bar) | Outlet (bar) | TMP (bar) | Recycle (l/s) | Permeate Flow (usgph) | Permeate Flow (l/h) | Flux (LMH) | Specific Flux (LMH/bar) |
|---|---|---|---|---|---|---|---|---|
| 13th May | 2.85 | 1.65 | 2.25 | 1.04 | 4.50 | 17.03 | 10.65 | 4.73 |
| 19th May | 2.60 | 1.40 | 2.00 | 1.05 | 3.40 | 12.87 | 8.04 | 5.75 |
| 19th May | 3.00 | 1.80 | 2.40 | 1.05 | 4.20 | 15.90 | 9.94 | 5.52 |
| 27th May | 2.60 | 1.40 | 2.00 | 1.04 | 3.20 | 12.11 | 7.57 | 5.41 |
| 27th May | 3.00 | 1.80 | 2.40 | 1.05 | 4.60 | 17.41 | 10.88 | 6.05 |
| 1st June | 2.95 | 2.40 | 2.68 | 1.04 | 4.00 | 15.14 | 9.46 | 3.94 |
| 9th June | 2.80 | 1.60 | 2.20 | 1.02 | 4.00 | 15.14 | 9.46 | 5.91 |
| 9th June | 3.00 | 1.80 | 2.40 | 1.03 | 4.20 | 15.90 | 9.94 | 5.52 |
| 23rd June | 2.50 | 1.30 | 1.90 | 1.02 | 3.00 | 11.36 | 7.10 | 5.46 |
| 23rd June | 3.00 | 1.80 | 2.40 | 1.03 | 4.40 | 16.66 | 10.41 | 5.78 |
| 8th July | 2.00 | 0.80 | 1.40 | 1.03 | 2.00 | 7.57 | 4.73 | 5.91 |
| Compound | Feed (µg/L) | Permeate (µg/L) | Rejection (%) |
|---|---|---|---|
| Total | 3670.0 | 264.0 | 92.8 |
| VC | 6.1 | 1.8 | 70.3 |
| DCE | 1165.0 | 43.5 | 96.3 |
| DCP | 137.2 | 5.1 | 96.3 |
| TCE | 2351.7 | 175.8 | 92.5 |
| PCE | 7.1 | 0.6 | 91.7 |
| Compound | Feed (µg/L) | Permeate (µg/L) |
|---|---|---|
| Total | 6552 | 6613 |
| VC | 27.1 | 28.7 |
| DCE | 2636.9 | 2881.8 |
| DCP | 539.8 | 635.2 |
| TCE | 3340.8 | 3058.7 |
| PCE | 5.7 | 5.1 |
| Time | Inlet (bar) | Outlet (bar) | TMP (bar) | Recycle Flow (L/s) | Permeate Flow (L/h) | Flux (LMH) | Specific Flux (LMH/bar) |
|---|---|---|---|---|---|---|---|
| 8th July | 2.00 | 1.40 | 1.70 | 0.67 | 2398 | 2.12 | 1.51 |
| 21st July | 3.00 | 1.60 | 2.30 | 1.03 | 3708 | 2.42 | 1.51 |
| 04th Aug | 3.00 | 1.40 | 2.20 | 0.68 | 2441 | 1.97 | 1.41 |
| 18th Aug | 3.00 | 1.40 | 2.20 | * | * | 1.51 | 1.08 |
| 1st Sept | 2.60 | 0.70 | 1.65 | * | * | 0.30 | 0.43 |
| 1st Sept | 3.00 | 1.50 | 2.25 | * | * | 1.67 | 1.11 |
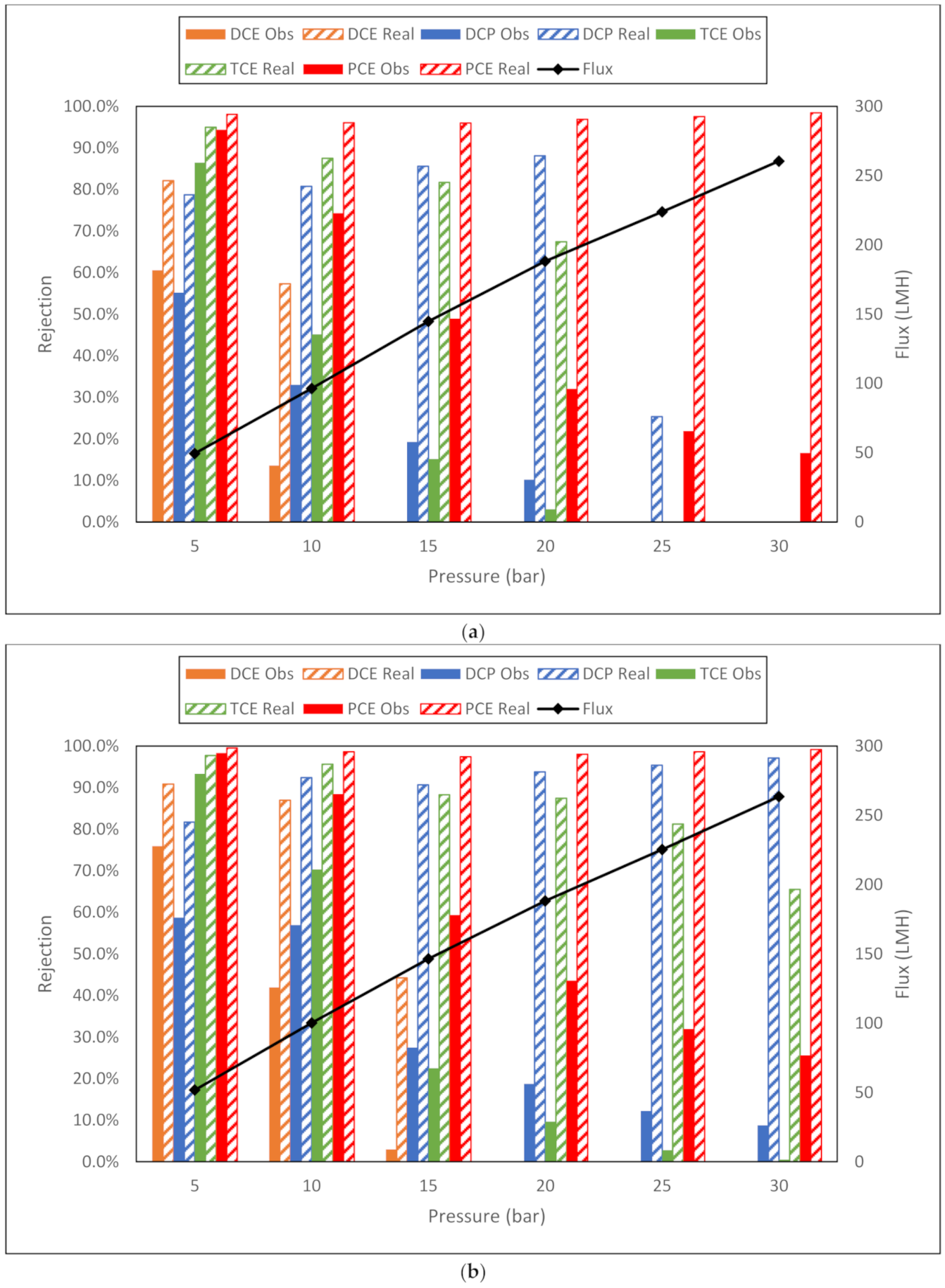
| a. Dow NF90 membrane flux (LMH) and observed and real rejection data for NaCl, toluene and trichloroethylene from conductivity and TOC measurements. | |||||||||
| NaCl | Toluene | TCE | |||||||
| Membrane | Flux | Obsd | Real | Flux | Obsd | Real | Flux | Obsd | Real |
| NF90 5 bar | 35.5 | 50.5% | 69.2% | 40.2 | 90.4% | 95.8% | 45.9 | 72.7% | 88.1% |
| NF90 10 bar | 80.4 | 58.6% | 89.4% | 85.8 | 70.5% | 94.1% | 86.1 | 42.6% | 83.4% |
| NF90 15 bar | 123 | 60.6% | 95.9% | 131.1 | 58.6% | 96.3% | 126.3 | 32.3% | 88.7% |
| NF90 20 bar | 172.2 | 52.4% | 98.1% | 179.5 | 49.7% | 98.1% | 163.9 | 32.6% | 94.8% |
| NF90 25 bar | 213.1 | 54.8% | 99.3% | 225.6 | 49.5% | 99.3% | 203.8 | 39.3% | 98.4% |
| NF90 30 bar | 236.5 | 32.1% | 98.9% | 275.6 | 51.7% | 99.8% | 241.1 | 32.7% | 99.0% |
| b. Dow NF270 membrane flux (LMH) and observed and real rejection data for NaCl, toluene and trichloroethylene from conductivity and TOC measurements. | |||||||||
| NaCl | Toluene | TCE | |||||||
| Membrane | Flux | Obsd | Real | Flux | Obsd | Real | Flux | Obsd | Real |
| NF270 5 bar | 45.9 | 48.6% | 72.3% | 54.5 | 83.5% | 94.4% | 47.8 | 53.9% | 77.2% |
| NF270 10 bar | 94.7 | 49.2% | 88.8% | 108.4 | 57.1% | 93.6% | 98.8 | 36.4% | 83.7% |
| NF270 15 bar | 137.8 | 50.4% | 95.6% | 157.7 | 45.6% | 96.5% | 155 | 29.2% | 92.8% |
| NF270 20 bar | 186 | 48.4% | 98.3% | 215.3 | 36.4% | 98.6% | 199.3 | 23.2% | 96.2% |
| NF270 25 bar | 230.4 | 38.1% | 99.0% | 259.4 | 36.0% | 99.4% | 258.4 | 21.5% | 98.8% |
| NF270 30 bar | 282.9 | 41.8% | 99.7% | 303.2 | 39.9% | 99.8% | 296.3 | 28.5% | 99.7% |
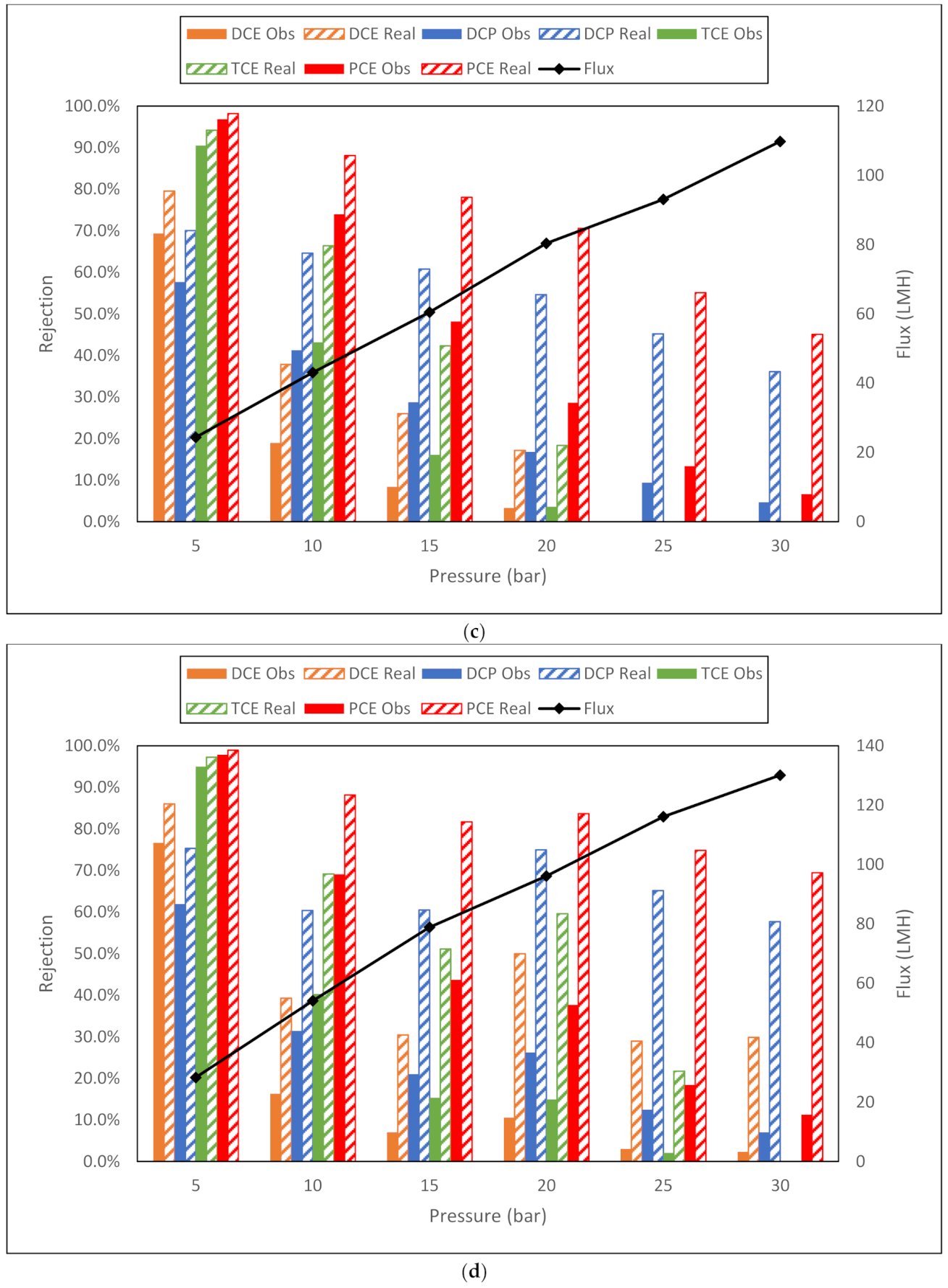
| c. GE DK membrane flux (LMH) and observed and real rejection data for NaCl, toluene and trichloroethylene from conductivity and TOC measurements. | |||||||||
| NaCl | Toluene | TCE | |||||||
| Membrane | Flux | Obsd | Real | Flux | Obsd | Real | Flux | Obsd | Real |
| DK 5 bar | 27.8 | 38.3% | 53.5% | 28.5 | 87.8% | 93.1% | 26.6 | 72.8% | 82.9% |
| DK 10 bar | 49.8 | 42.0% | 68.6% | 55.2 | 66.5% | 87.1% | 49.7 | 58.8% | 81.2% |
| DK 15 bar | 72.6 | 48.2% | 82.4% | 80.4 | 59.0% | 89.6% | 75.7 | 47.7% | 83.1% |
| DK 20 bar | 103.3 | 50.4% | 91.0% | 109.6 | 53.3% | 92.8% | 96.2 | 47.4% | 88.4% |
| DK 25 bar | 120.6 | 50.8% | 93.8% | 129.2 | 53.0% | 95.2% | 115.6 | 52.2% | 93.4% |
| DK 30 bar | 146.4 | 51.4% | 96.5% | 155 | 51.8% | 97.1% | 137.8 | 48.2% | 95.2% |
| d. GE DL membrane flux (LMH) and observed and real rejection data for NaCl, toluene and trichloroethylene from conductivity and TOC measurements. | |||||||||
| NaCl | Toluene | TCE | |||||||
| Membrane | Flux | Obsd | Real | Flux | Obsd | Real | Flux | Obsd | Real |
| DL 5 bar | 31.6 | 33.7% | 50.6% | 32.7 | 77.5% | 87.7% | 29.4 | 77.8% | 87.0% |
| DL 10 bar | 64.6 | 34.7% | 69.0% | 60.8 | 58.4% | 84.4% | 59.2 | 50.9% | 79.4% |
| DL 15 bar | 91.7 | 39.1% | 83.1% | 90 | 47.7% | 87.0% | 84.9 | 38.9% | 80.7% |
| DL 20 bar | 117.6 | 40.7% | 90.3% | 114.4 | 50.2% | 92.7% | 111.6 | 35.5% | 86.7% |
| DL 25 bar | 140.3 | 41.2% | 94.0% | 136.4 | 50.5% | 95.5% | 137.8 | 35.6% | 92.2% |
| DL 30 bar | 174.5 | 40.9% | 97.1% | 160.7 | 53.1% | 97.6% | 155 | 34.5% | 94.3% |
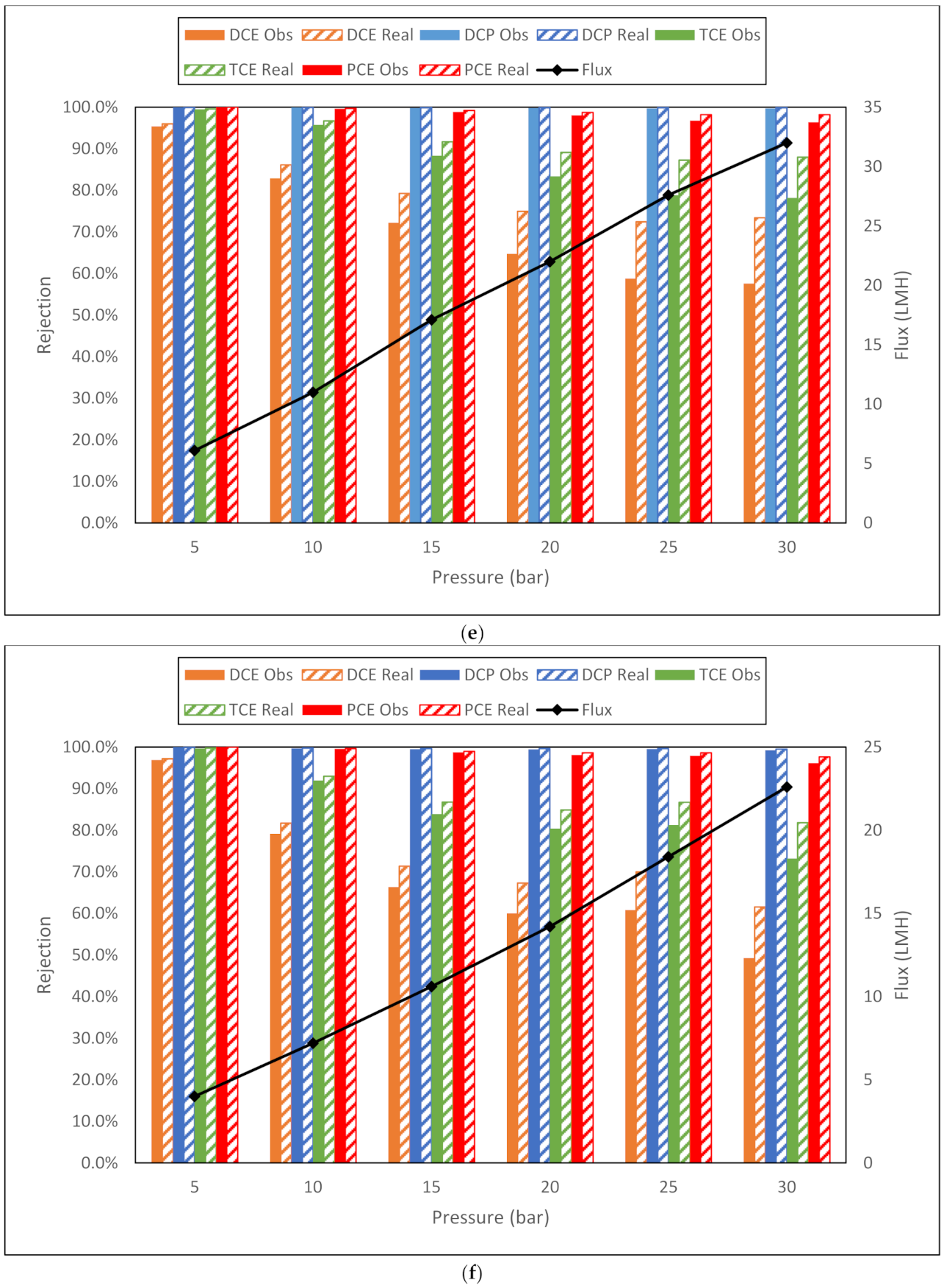
| e. GE AK membrane flux (LMH) and observed and real rejection data for NaCl, toluene and trichloroethylene from conductivity and TOC measurements. | |||||||||
| NaCl | Toluene | TCE | |||||||
| Membrane | Flux | Obsd | Real | Flux | Obsd | Real | Flux | Obsd | Real |
| AK 5 bar | 6.9 | 93.2% | 94.1% | 9.2 | 98.3% | 98.6% | 8.5 | 79.1% | 82.1% |
| AK 10 bar | 15.3 | 95.3% | 96.6% | 16.7 | 94.9% | 96.4% | 17.2 | 73.4% | 80.2% |
| AK 15 bar | 21.8 | 95.8% | 97.3% | 24.1 | 96.5% | 97.9% | 26.3 | 70.4% | 81.0% |
| AK 20 bar | 30.7 | 96.6% | 98.3% | 32.1 | 95.4% | 97.7% | 34.4 | 73.1% | 85.4% |
| AK 25 bar | 38.2 | 97.0% | 98.7% | 40.6 | 94.0% | 97.5% | 41.2 | 72.2% | 86.6% |
| AK 30 bar | 47.1 | 97.0% | 98.9% | 48.1 | 93.8% | 97.8% | 51.7 | 69.7% | 87.9% |
| f. GE AG membrane flux (LMH) and observed and real rejection data for NaCl, toluene and trichloroethylene from conductivity and TOC measurements. | |||||||||
| NaCl | Toluene | TCE | |||||||
| Membrane | Flux | Obsd | Real | Flux | Obsd | Real | Flux | Obsd | Real |
| AG 5 bar | 10.9 | 96.8% | 97.5% | 15.4 | 98.9% | 99.3% | 14.4 | 78.2% | 83.2% |
| AG 10 bar | 27.8 | 98.9% | 99.4% | 28.8 | 95.0% | 97.3% | 28.5 | 63.1% | 76.3% |
| AG 15 bar | 46.5 | 99.2% | 99.7% | 41.7 | 95.9% | 98.3% | 42.3 | 68.9% | 85.0% |
| AG 20 bar | 60.3 | 99.3% | 99.8% | 56.5 | 94.1% | 98.2% | 57.4 | 69.9% | 89.3% |
| AG 25 bar | 76.8 | 99.4% | 99.9% | 70.2 | 92.9% | 98.4% | 70.1 | 69.8% | 91.6% |
| AG 30 bar | 95.5 | 99.3% | 99.9% | 85.1 | 92.3% | 98.8% | 84 | 68.0% | 93.2% |
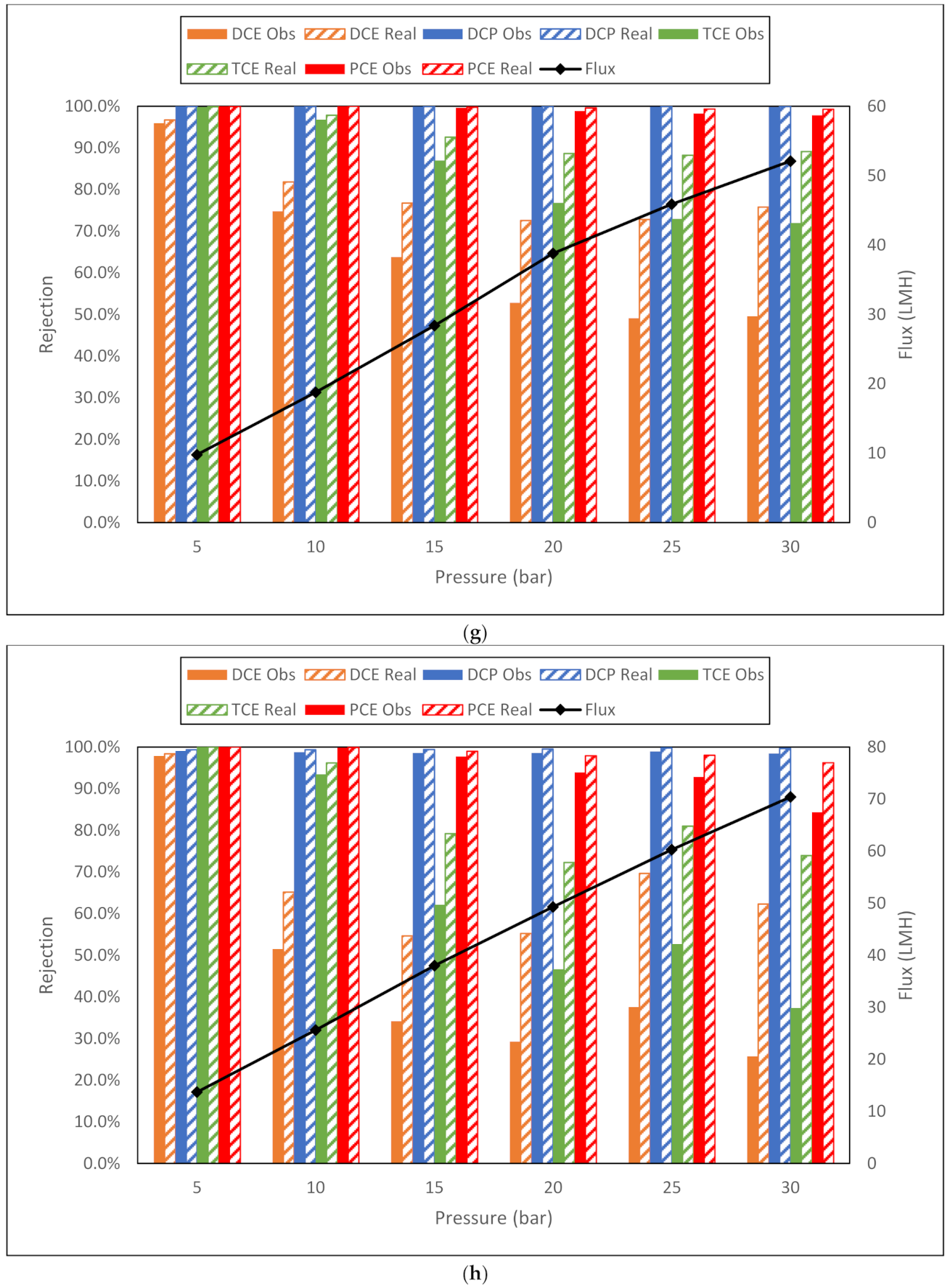
| g. Dow BW30 membrane flux (LMH) and observed and real rejection data for NaCl, toluene and trichloroethylene from conductivity and TOC measurements. | |||||||||
| NaCl | Toluene | TCE | |||||||
| Membrane | Flux | Obsd | Real | Flux | Obsd | Real | Flux | Obsd | Real |
| BW30 5 bar | 11.1 | 93.4% | 94.7% | 14 | 95.4% | 96.6% | 14.6 | 84.2% | 88.1% |
| BW30 10 bar | 24.4 | 94.9% | 96.9% | 27.7 | 96.7% | 98.2% | 27.3 | 79.0% | 87.3% |
| BW30 15 bar | 37.3 | 96.1% | 98.2% | 40.2 | 93.0% | 97.0% | 40.3 | 63.6% | 81.1% |
| BW30 20 bar | 52.6 | 96.6% | 98.9% | 53.5 | 92.0% | 97.4% | 54.5 | 52.2% | 78.6% |
| BW30 25 bar | 64.9 | 96.7% | 99.2% | 66 | 91.3% | 97.9% | 66 | 66.7% | 89.7% |
| BW30 30 bar | 77.5 | 96.9% | 99.4% | 78.9 | 90.7% | 98.2% | 79.6 | 65.1% | 91.6% |
| h. Dow BW30LE membrane flux (LMH) and observed and real rejection data for NaCl, toluene and trichloroethylene from conductivity and TOC measurements. | |||||||||
| NaCl | Toluene | TCE | |||||||
| Membrane | Flux | Obsd | Real | Flux | Obsd | Real | Flux | Obsd | Real |
| BW30LE 5 bar | 15.2 | 91.5% | 93.8% | 20.7 | 96.0% | 97.5% | 22.6 | 64.9% | 75.3% |
| BW30LE 10 bar | 35.9 | 94.3% | 97.4% | 41.1 | 91.2% | 96.3% | 43.2 | 47.6% | 70.3% |
| BW30LE 15 bar | 53.9 | 96.1% | 98.8% | 60.5 | 84.3% | 95.4% | 63.2 | 48.8% | 79.5% |
| BW30LE 20 bar | 77.5 | 96.1% | 99.3% | 79.8 | 83.1% | 96.7% | 87.2 | 48.5% | 86.7% |
| BW30LE 25 bar | 94.7 | 96.4% | 99.5% | 103.3 | 82.0% | 97.8% | 105.3 | 49.3% | 91.0% |
| BW30LE 30 bar | 112 | 96.6% | 99.7% | 112 | 77.0% | 97.6% | 127.4 | 65.2% | 96.9% |
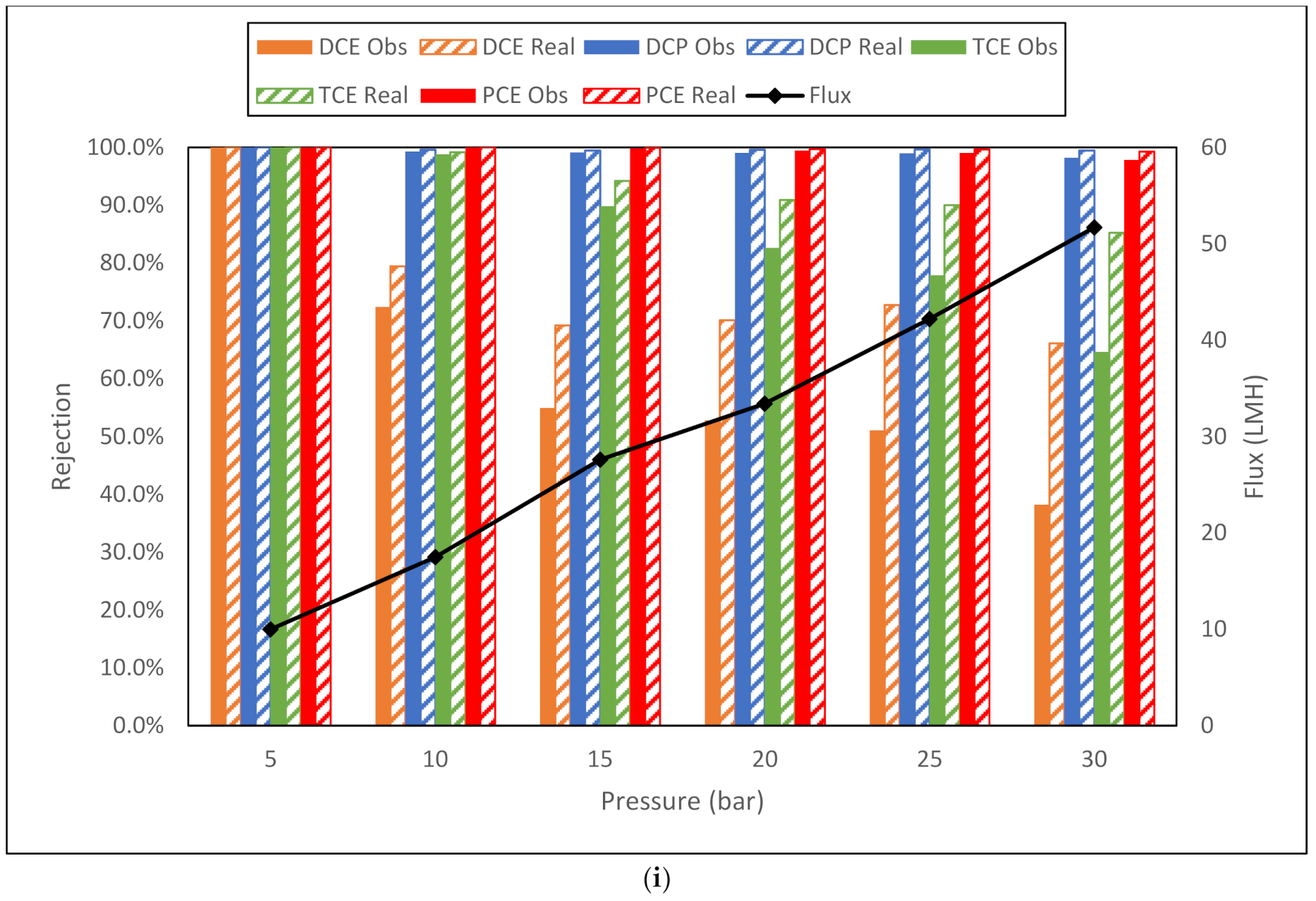
| i. Dow BW30XFR membrane flux (LMH) and observed and real rejection data for NaCl, toluene and trichloroethylene from conductivity and TOC measurements. | |||||||||
| NaCl | Toluene | TCE | |||||||
| Membrane | Flux | Obsd | Real | Flux | Obsd | Real | Flux | Obsd | Real |
| BW30XFR 5 bar | 10 | 94.8% | 95.8% | 13.6 | 95.4% | 96.5% | 13 | 68.9% | 74.7% |
| BW30XFR 10 bar | 23 | 96.0% | 97.6% | 26.9 | 98.1% | 98.9% | 26.6 | 83.6% | 90.2% |
| BW30XFR 15 bar | 35.7 | 96.8% | 98.5% | 40.5 | 97.0% | 98.8% | 40.2 | 76.0% | 88.5% |
| BW30XFR 20 bar | 48.4 | 96.9% | 98.9% | 53.9 | 95.7% | 98.7% | 51.7 | 74.3% | 90.1% |
| BW30XFR 25 bar | 60.3 | 97.0% | 99.2% | 66 | 95.0% | 98.8% | 63.2 | 76.5% | 93.0% |
| BW30XFR 30 bar | 68.9 | 97.1% | 99.4% | 80.4 | 94.7% | 99.1% | 75.2 | 76.8% | 94.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ainscough, T.J.; Oatley-Radcliffe, D.L.; Barron, A.R. Groundwater Remediation of Volatile Organic Compounds Using Nanofiltration and Reverse Osmosis Membranes—A Field Study. Membranes 2021, 11, 61. https://doi.org/10.3390/membranes11010061
Ainscough TJ, Oatley-Radcliffe DL, Barron AR. Groundwater Remediation of Volatile Organic Compounds Using Nanofiltration and Reverse Osmosis Membranes—A Field Study. Membranes. 2021; 11(1):61. https://doi.org/10.3390/membranes11010061
Chicago/Turabian StyleAinscough, Thomas J., Darren L. Oatley-Radcliffe, and Andrew R. Barron. 2021. "Groundwater Remediation of Volatile Organic Compounds Using Nanofiltration and Reverse Osmosis Membranes—A Field Study" Membranes 11, no. 1: 61. https://doi.org/10.3390/membranes11010061
APA StyleAinscough, T. J., Oatley-Radcliffe, D. L., & Barron, A. R. (2021). Groundwater Remediation of Volatile Organic Compounds Using Nanofiltration and Reverse Osmosis Membranes—A Field Study. Membranes, 11(1), 61. https://doi.org/10.3390/membranes11010061







