Biodegradable Polymeric Membranes for Organic Solvent/Water Pervaporation Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Solvent Swelling Experiment
2.5. Pervaporation Experiment
3. Results and Discussion
3.1. Membrane Characterization
3.1.1. Membrane Morphology
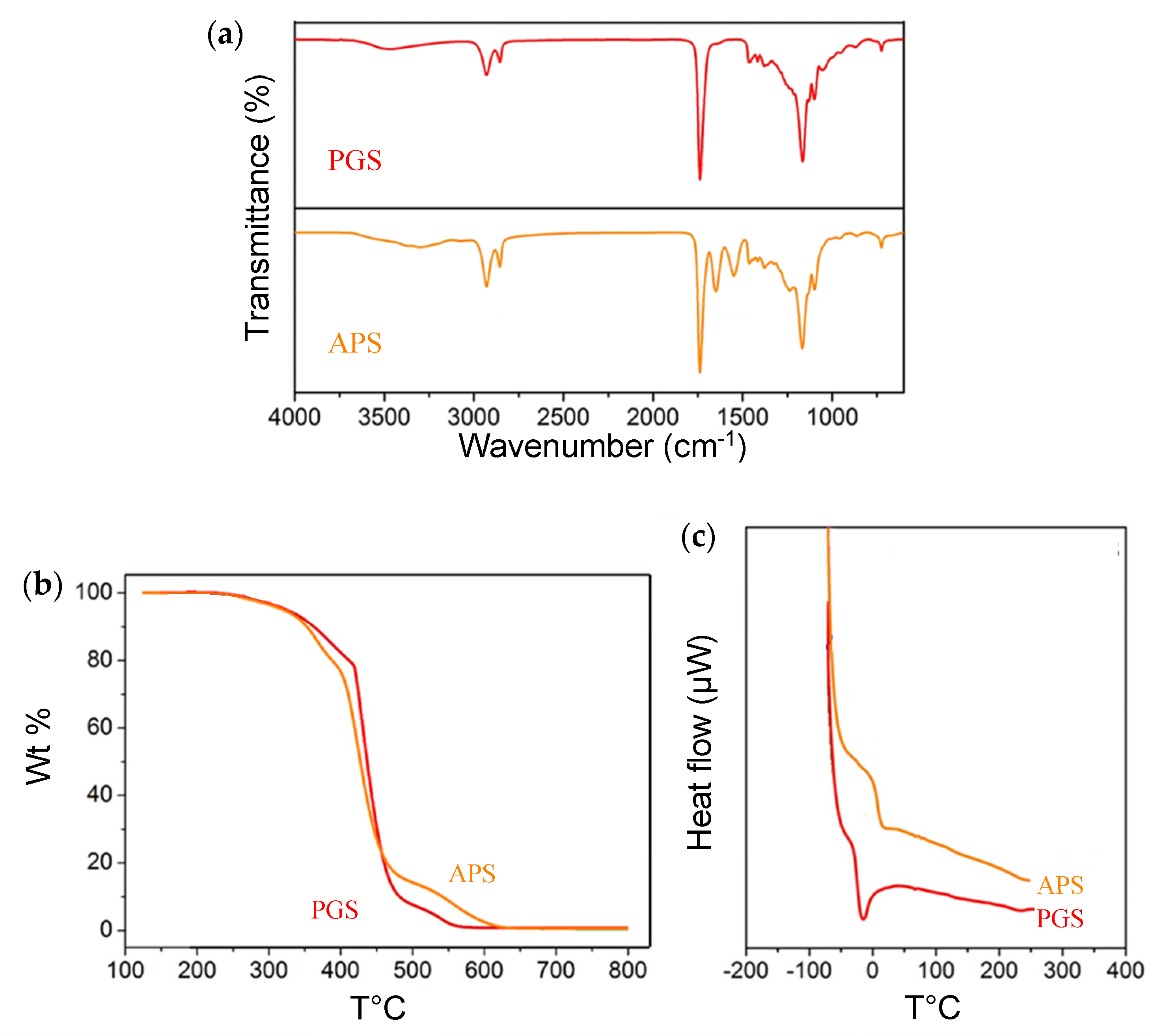
3.1.2. ATR-FTIR Results
3.1.3. Thermal Properties
3.1.4. Water Contact Angle Results
3.1.5. Swelling Behaviors
3.2. Pervaporation Performance
3.2.1. Organic Solvent/Water Systems
3.2.2. Acetone–Butanol–Ethanol (ABE) System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Tsuru, T. Progress in pervaporation membranes for dehydration of acetic acid. Sep. Purif. Technol. 2021, 262, 118338. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W. Pervaporation membrane materials: Recent trends and perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Knozowska, K.; Li, G.; Kujawski, W.; Kujawa, J. Novel heterogeneous membranes for enhanced separation in organic-organic pervaporation. J. Membr. Sci. 2020, 599, 117814. [Google Scholar] [CrossRef]
- Hassankhan, B.; Raisi, A. Separation of isobutanol/water mixtures by hybrid distillation-pervaporation process: Modeling, simulation and economic comparison. Chem. Eng. Process. 2020, 155, 108071. [Google Scholar] [CrossRef]
- Vatankhah, F.; Moheb, A.; Arjomand, M.Z. A study on the effects of feed temperature and concentration on design of a multi-stage pervaporation system for isopropanol-water separation using commercial available modules with inter-stage heating. J. Membr. Sci. 2021, 618, 118717. [Google Scholar] [CrossRef]
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for bioethanol production by pervaporation. Biotechnol. Biofuels 2021, 14, 10. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Highly hydrophobic ceramic membranes applied to the removal of volatile organic compounds in pervaporation. Chem. Eng. J. 2015, 260, 43–54. [Google Scholar] [CrossRef]
- Ramaiah, K.P.; Mishra, K.; Atkar, A.; Sridhar, S. Pervaporation separation of chlorinated environmental pollutants from aqueous solutions by castor oil based composite interpenetrating network membranes. Chem. Eng. J. 2020, 387, 124050. [Google Scholar] [CrossRef]
- Aouinti, L.; Roizard, D.; Belbachir, M. PVC–activated carbon based matrices: A promising combination for pervaporation membranes useful for aromatic-alkane separations. Sep. Purif. Technol. 2015, 147, 51–61. [Google Scholar] [CrossRef]
- Ribeiro, C.P.; Freeman, B.D.; Kalika, D.S.; Kalakkunnath, S. Aromatic polyimide and polybenzoxazole membranes for the fractionation of aromatic/aliphatic hydrocarbons by pervaporation. J. Membr. Sci. 2012, 390, 182–193. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, B.; Qu, L.; Ren, J.; Li, Y. A novel atmospheric dielectric barrier discharge (DBD) plasma graft-filling technique to fabricate the composite membranes for pervaporation of aromatic/aliphatic hydrocarbons. J. Membr. Sci. 2011, 371, 163–170. [Google Scholar] [CrossRef]
- Delgado, P.; Sanz, M.T.; Beltran, S. Pervaporation of the quaternary mixture present during the esterification of lactic acid with ethanol. J. Membr. Sci. 2009, 332, 113–120. [Google Scholar] [CrossRef]
- Zhu, M.H.; Kumakiri, I.; Tanaka, K.; Kita, H. Dehydration of acetic acid and esterification product by acid-stable ZSM-5 membrane. Micropor. Mesopor. Mat. 2013, 181, 47–53. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: Current state of the art. Rev. Chem. Eng. 2018, 57, 15998–16011. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on pervaporation: Theory, membrane performance, and application to intensification of esterification reaction. J. Eng. 2015, 2, 927068. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Martínez, J.; Mohsenpour, S.; Ameen, A.W.; Budd, P.M.; García-Payo, C.; Khayet, M.; Gorgojo, P. High-flux thin film composite PIM-1 membranes for butanol recovery: Experimental study and process simulations. ACS Appl. Mater. Interfaces 2021, 13, 42635–42649. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, Y.; Fan, S.; Liu, J.; Jian, S.; Qin, Y.; Xiao, Z.; Tang, X.; Wang, W. Ethanol mass transfer during pervaporation with PDMS membrane based on solution-diffusion model considering concentration polarization. Sep. Purif. Technol. 2019, 220, 276–282. [Google Scholar] [CrossRef]
- Guo, R.; Hu, C.; Li, B.; Jiang, Z. Pervaporation separation of ethylene glycol/water mixtures through surface crosslinked PVA membranes: Coupling effect and separation performance analysis. J. Membr. Sci. 2007, 289, 191–198. [Google Scholar] [CrossRef]
- Austria, H.F.M.; Leearos, R.L.G.; Hung, W.-S.; Tayo, L.L.; Hu, C.-C.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Investigation of salt penetration mechanism in hydrolyzed polyacrylonitrile asymmetric membranes for pervaporation desalination. Desalination 2019, 463, 32–39. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Zhang, P.; Sun, B.; Li, J. Pervaporation properties of polyimide membranes for separation of ethanol water mixtures. J. Chem. Eng. Data 2006, 51, 1841–1845. [Google Scholar] [CrossRef]
- Won, W.; Won, X.; Lawless, D. Pervaporation with chitosan membranes: Separation of dimethyl carbonate/methanol/water mixtures. J. Membr. Sci. 2002, 209, 493–508. [Google Scholar] [CrossRef]
- Bhat, S.D.; Aminabhavi, T.M. Pervaporation separation using sodium alginate and its modified membranes—A review. Sep. Purif. Rev. 2007, 36, 203–229. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Lahderanta, E.; Solovyev, N.; Penkova, A. Modification approaches to enhance dehydration properties of sodium alginate-based pervaporation membranes. Membranes 2021, 11, 255. [Google Scholar] [CrossRef]
- Kujawska, A.; Knozowska, K.; Kujawa, J.; Li, G.; Kujawski, W. Fabrication of PDMS based membranes with improved separation efficiency in hydrophobic pervaporation. Sep. Purif. Technol. 2020, 234, 116092. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.S.; Lee, J.H. High performance and thermally stable PDMS pervaporation membranes prepared using a phenyl-containing tri-functional crosslinker for n-butanol recovery. Sep. Purif. Technol. 2020, 235, 116142. [Google Scholar] [CrossRef]
- Yang, Y.; Si, Z.; Cai, D.; Teng, X.; Li, G.; Wang, Z.; Li, S.; Qin, P. High-hydrophobic-CF3 groups within PTFPMS membrane for enhancing the furfural pervaporation performance. Sep. Purif. Technol. 2020, 235, 116144. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Volkov, A.V. High-selectivity polysiloxane membranes for gases and liquids separation (a review). Pet. Chem. 2021, 61, 959–976. [Google Scholar] [CrossRef]
- Guan, K.; Liu, G.; Matsuyama, H.; Jin, W. Graphene-based membranes for pervaporation processes. Chin. J. Chem. Eng. 2020, 28, 1755–1766. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Ikeda, A. Pervaporative dehydration of organic solvents using high-silica CHA-type zeolite membrane. Membranes 2021, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.L.; Chang, C.K.; Kang, Y.H.; Chen, J.J.; Kang, D.Y. Enhanced pervaporation performance of zeolite membranes treated by atmospheric-pressure plasma. J. Taiwan Inst. Chem. Eng. 2020, 116, 112–120. [Google Scholar] [CrossRef]
- Xu, X.; Nikolaeva, D.; Hartanto, Y.; Luis, P. MOF-based membranes for pervaporation. Sep. Purif. Technol. 2021, 278, 119233. [Google Scholar] [CrossRef]
- Saw, E.T.; Ang, K.L.; He, W.; Dong, X.; Ramakrishna, S. Molecular sieve ceramic pervaporation membranes in solvent recovery: A comprehensive review. J. Environ. Chem. Eng. 2019, 7, 103367. [Google Scholar] [CrossRef]
- Okumuş, E.; Gürkan, T.; Yilmaz, L. Effect of fabrication and process parameters on the morphology and performance of a PAN-based zeolite-filled pervaporation membran. J. Membr. Sci. 2003, 223, 23–38. [Google Scholar] [CrossRef]
- Adoor, S.G.; Manjeshwar, L.S.; Bhat, S.D. Aluminum-rich zeolite beta incorporated sodium alginate mixed matrix membranes for pervaporation dehydration and esterification of ethanol and acetic acid. J. Membr. Sci. 2008, 318, 233–246. [Google Scholar] [CrossRef]
- Sun, H.; Lu, L.; Chen, X.; Jiang, Z. Pervaporation dehydration of aqueous ethanol solution using H-ZSM-5 filled chitosan membranes. Sep. Purif. Technol. 2008, 58, 429–436. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Gebreslase, G.A.; Bruggen, B.V. Incorporation of Al2O3 into cellulose triacetate membranes to enhance the performance of pervaporation for desalination of hypersaline solutions. Desalination 2020, 474, 114198. [Google Scholar] [CrossRef]
- Hsieh, C.-W.; Li, B.-X.; Suen, S.-Y. Alicyclic polyimide/SiO2 mixed matrix membranes for water/ n-butanol pervaporation. Membranes 2021, 11, 564. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-González, J.; de laIglesia, Ó.; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly (vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415–416, 577–586. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadatnia, B. Poly (vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 469, 927068. [Google Scholar] [CrossRef]
- Casado-Coterillo, C. Mixed matrix membranes. Membranes 2019, 9, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.-K.; Chen, S.-C.; Huang, W.-L.; Hsu, K.-P.; Gorday, K.A.V.; Wang, T.; Wang, J. Direct micromachining of microfluidic channels on biodegradable materials using laser ablation. Polymers 2017, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.-K.; Hsu, K.-P.; Hsiao, S.-K.; Gorday, K.A.V.; Wang, T.; Wang, J. Laser-pattern induced contact guidance in biodegradable microfluidic channels for vasculature regeneration. J. Mater. Chem. 2018, 6, 3684–3691. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Feng, X.S. Dehydration of isopropanol by pervaporation using aromatic polyetherimide membranes. Sep. Sci. Technol. 1993, 28, 2035–2048. [Google Scholar] [CrossRef]
- He, X.; Wang, T.; Huang, J.; Chen, J.; Li, J. Fabrication and characterization of superhydrophobic PDMS composite membranes for efficient ethanol recovery via pervaporation. Sep. Sci. Technol. 2020, 241, 116675. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, R.; Jin, W. Novel organic-inorganic pervaporation membrane with a superhydrophobic surface for the separation of ethanol from an aqueous solution. Sep. Purif. Technol. 2014, 127, 61–69. [Google Scholar] [CrossRef]
- Magalad, V.T.; Gokavi, G.S.; Ranganathaiah, C.; Burshe, M.H.; Han, C.; Dionysiou, D.D.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric blend nanocomposite membranes for ethanol dehydration—Effect of morphology and membrane-solvent interactions. J. Membr. Sci. 2013, 430, 321–329. [Google Scholar] [CrossRef]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Bruggeman, J.P.; Borenstein, J.T.; Langer, R.S. Amino alcohol-based biodegradable poly(ester amide) elastomers. Biomaterials 2008, 29, 2315–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çalhan, A.; Deniz, S.; Kujawski, W.; Kujawa, J.; Knozowska, K.; Hasanoglu, A. Silica filled polyphenylsulfone/polydimethylsiloxane composite membranes for pervaporation separation of biobutanol from ABE mixtures. Chem. Eng. Process. Process Intensif. 2020, 156, 108099. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Ting, Y.-S.; Chen, B.-Y.; Cheng, Y.-W.; Liu, T.-Y. Bionic shark skin replica and zwitterionic polymer brushes functionalized PDMS membrane for anti-fouling and wound dressing applications. Surf. Coat. Technol. 2020, 391, 125663. [Google Scholar] [CrossRef]
- Lin, Y.F.; Wu, C.Y.; Liu, T.Y.; Lin, K.Y.A.; Tung, K.L.; Chung, T.W. Synthesis of mesoporous SiO2 xerogel/chitosan mixed-matrix membranes for butanol dehydration. J. Ind. Eng. Chem. 2018, 57, 297–303. [Google Scholar] [CrossRef]
- Ham, H.T.; Choi, Y.S.; Chung, I.J. An explanation of dispersion states of single-walled carbon nanotubes in solvents and aqueous surfactant solutions using solubility parameters. J. Colloid Interface Sci. 2005, 286, 216–223. [Google Scholar] [CrossRef]
- Vebber, G.C.; Pranke, P.; Pereira, C.N. Calculating Hansen solubility parameters of polymers with genetic algorithms. J. Appl. Polym. Sci. 2014, 131, 39696. [Google Scholar] [CrossRef]
- Ishihara, K.; Matsui, K. Pervaporation of ethanol-water mixture through composite membranes composed of styrene-fluoroalkyl acrylate graft copolymers and cross-linked polydimethylsiloxane membrane. J. Appl. Polym. Sci. 1987, 34, 437–440. [Google Scholar] [CrossRef]
- Ahmed, I.; Pa, N.F.C.; Nawawi, M.G.M.; Rahman, W.A.W.A. Modified polydimethylsiloxane/polystyrene blended IPN pervaporation membrane for ethanol/water separation. J. Appl. Polym. Sci. 2011, 122, 2666–2679. [Google Scholar] [CrossRef]
- Krea, M.; Roizard, D.; Moulai-Mostefa, N.; Sacco, D. New copolyimide membranes with high siloxane content designed to remove polar organics from water by pervaporation. J. Membr. Sci. 2004, 241, 55–64. [Google Scholar] [CrossRef]
- Liu, W.; Guo, H.X.; Ji, S.L.; Niu, H.J.; Li, J.R. A new PDMS-b-PPO block copolymer membrane with novel non-perforated structure towards high flux for alcohol permselective pervaporation. Express Polym. Lett. 2015, 9, 372–383. [Google Scholar] [CrossRef]
- Volkov, V.V.; Fadeev, A.G.; Khotimsky, V.S.; Litvinova, E.G.; Selinskaya, Y.A.; McMillan, J.D.; Kelley, S.S. Effects of synthesis conditions on the pervaporation properties of poly[1-(trimethylsilyl)-1-propyne] useful for membrane bioreactors. J. Appl. Polym. Sci. 2004, 91, 2271–2277. [Google Scholar] [CrossRef]
- Liu, F.F.; Liu, L.; Feng, X.S. Separation of acetone–butanol–ethanol (ABE) from dilute aqueous solutions by pervaporation. Sep. Purif. Technol. 2005, 42, 273–282. [Google Scholar] [CrossRef]
- Wang, X.P.; Shen, Z.Q.; Zhang, F.Y.; Zhang, Y.F. Preferential separation of ethanol from aqueous solution through hydrophilic polymer membranes. J. Appl. Polym. Sci. 1999, 73, 1145–1151. [Google Scholar] [CrossRef]
- Shirazi, Y.; Ghadimi, A.; Mohammadi, T. Recovery of alcohols from water using polydimethylsiloxane-silica nanocomposite membranes: Characterization and pervaporation performance. J. Appl. Polym. Sci. 2012, 124, 2871–2882. [Google Scholar] [CrossRef]
- Jee, K.Y.; Lee, Y.T. Preparation and characterization of siloxane composite membranes for n-butanol concentration from ABE solution by pervaporation. J. Membr. Sci. 2014, 456, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Qi, B.; Luo, J.; Zhuang, X.; Su, Y.; Wan, Y. Continuous acetone-butanol-ethanol (ABE) fermentation with in situ solvent recovery by silicalite-1 filled PDMS/PAN composite membrane. Energy Fuels 2014, 28, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Wu, Y.; Zhou, Y.; Liu, Z.; Li, T. Pervaporative recovery of n-butanol from aqueous solutions with MCM-41 filled PEBA mixed matrix membrane. J. Membr. Sci. 2014, 453, 302–311. [Google Scholar] [CrossRef]
- Huang, J.; Meagher, M.M. Pervaporative recovery of n-butanol from aqueous solutions and ABE fermentation broth using thin-film silicalite-filled silicone composite membranes. J. Membr. Sci. 2001, 192, 231–242. [Google Scholar] [CrossRef]
- Kim, D.-G.; Takigawa, T.; Kashino, T.; Burtovyy, O.; Bell, A.; Register, R.A. Hydroxyhexafluoroisopropylnorbornene block and random copolymers via vinyl addition polymerization and their application as biobutanol pervaporation membranes. Chem. Mater. 2015, 27, 6791–6801. [Google Scholar] [CrossRef]
- Tong, C.; Bai, Y.; Wu, J.; Zhang, L.; Yang, L.; Qian, J. Pervaporation recovery of acetone-butanol from aqueous solution and fermentation broth using HTPB-based polyurethaneurea membranes. Sep. Sci. Technol. 2010, 45, 751–761. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Pervaporative recovery of acetone from water using mixed matrix blend membranes. Sep. Purif. Technol. 2015, 143, 52–63. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Chiu, C.-P.; Huang, H.-Y. Pervaporation of acetic acid/water mixtures through silicalite filled polydimethylsiloxane membranes. J. Membr. Sci. 2000, 176, 159–167. [Google Scholar] [CrossRef]
- Hong, H.; Chen, L.; Zhang, Q.; He, F. The structure and pervaporation properties for acetic acid/water of polydimethylsiloxane composite membranes. Mater. Des. 2012, 34, 732–738. [Google Scholar] [CrossRef]
- Wang, J.; Bettinger, C.J.; Langer, R.S.; Borenstein, J.T. Biodegradable microfluidic scaffolds for tissue engineering from amino alcohol-based poly(ester amide) elastomers. Organogenesis 2010, 6, 212–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Boutin, K.G.; Abdulhadi, O.; Personnat, L.D.; Shazly, T.; Langer, R.; Channick, C.L.; Borenstein, J.T. Fully biodegradable airway stents using amino alcohol-based poly(ester amide) elastomers. Adv. Healthc. Mater. 2013, 2, 1329–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Membrane | DS (%) | |||||
|---|---|---|---|---|---|---|
| Ethanol | Isopropanol | N-Butanol | Acetone | Acetic Acid | Water | |
| PGS | 85.2 | 103.6 | 91.8 | 98.0 | 199.8 | 4.3 |
| APS | 89.6 | 107.5 | 98.4 | 31.6 | 109.2 | 5.6 |
| PDMS | 4.2 | 22.9 | 20.6 | 20.6 | 4.8 | 0.6 |
| Membrane | DSo/DSw | |||||
| Ethanol/Water | Isopropanol/Water | N-Butanol/Water | Acetone/Water | Acetic Acid/Water | ||
| PGS | 19.8 | 24.1 | 21.3 | 22.8 | 46.5 | |
| APS | 16.0 | 19.2 | 17.6 | 5.6 | 19.5 | |
| PDMS | 7.0 | 38.2 | 34.3 | 34.3 | 8.0 | |
| Feed Mixture | Organic Solvent wt% in Feed | Membrane | J (g/m2h) | JN (g/m2h) | α | PSI (g/m2h) |
|---|---|---|---|---|---|---|
| Ethanol/water | 5 | PGS | 65 ± 3 | 130 | 11.6 ± 1.1 | 1378 |
| APS | 50 ± 3 | 100 | 8.2 ± 0.4 | 720 | ||
| PDMS | 89 ± 2 | 178 | 6.5 ± 0.7 | 979 | ||
| 10 | PGS | 77 ± 1 | 154 | 8.7 ± 0.8 | 1186 | |
| APS | 69 ± 3 | 138 | 6.4 ± 0.2 | 745 | ||
| PDMS | 102 ± 1 | 204 | 5.2 ± 0.2 | 857 | ||
| Isopropanol/water | 5 | PGS | 75 ± 1 | 150 | 6.8 ± 0.1 | 870 |
| APS | 22 ± 2 | 44 | 3.1 ± 0.2 | 92 | ||
| PDMS | 64 ± 1 | 128 | 10.2 ± 0.7 | 1178 | ||
| 10 | PGS | 82 ± 3 | 164 | 5.9 ± 0.2 | 804 | |
| APS | 35 ± 3 | 70 | 2.7 ± 0.2 | 119 | ||
| PDMS | 69 ± 1 | 138 | 9.4 ± 0.6 | 1159 | ||
| n-Butanol/water | 1 | PGS | 53 ± 2 | 106 | 18.4 ± 1.0 | 1844 |
| APS | 49 ± 1 | 98 | 15.4 ± 0.5 | 1411 | ||
| PDMS | 66 ± 3 | 132 | 31.2 ± 0.8 | 3986 | ||
| 2 | PGS | 66 ± 3 | 132 | 16.7 ± 0.2 | 2072 | |
| APS | 52 ± 2 | 104 | 14.5 ± 0.4 | 1404 | ||
| PDMS | 72 ± 4 | 144 | 30.1 ± 0.2 | 4190 | ||
| Acetone/water | 0.5 | PGS | 51 ± 2 | 102 | 39.0 ± 0.7 | 3876 |
| APS | 48 ± 2 | 96 | 27.0 ± 0.6 | 2496 | ||
| PDMS | 63 ± 4 | 126 | 48.2 ± 0.5 | 5947 | ||
| Acetic acid/water | 10 | PGS | 118 ± 4 | 236 | 3.6 ± 0.5 | 614 |
| APS | 96 ± 4 | 192 | 2.9 ± 0.3 | 365 | ||
| PDMS | 82 ± 3 | 164 | 1.5 ± 0.2 | 82 |
| Mixture | Membrane | Thickness (µm) | T (°C) | Organic Solvent wt% in Feed | J (g/m2h) | JN (g/m2h) | α | PSI (g/m2h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ethanol/ water | PDMS | 100 | 30 | 8 | 25 | 25 | 10.8 | 245 | [57] |
| PDMS | 34 | 30 | 10 | 179 | 61 | 1.8 | 49 | [58] | |
| PDMS | 9 | 37 | 6 | ~700 | 63 | ~8.3 | 460 | [47] | |
| PSI (PD 5000, 94% PDMS) | 10 | 60 | 10 | 560 | 56 | 10.6 | 538 | [59] | |
| PDMS-b-PPO | - | 60 | 5 | 3816 | - | 8.5 | - | [60] | |
| PTMSP-2 | 14 | 30 | 6 | 500 | 70 | 16.5 | 1085 | [61] | |
| PTMSP-4 | 25 | 30 | 6 | 340 | 85 | 19.9 | 1607 | [61] | |
| Pebax 2533 | 30 | 23 | 5 | 117.5 | 35 | 2.5 | 53 | [62] | |
| PEO/CS (8 wt%) | 20 | 20 | 8 | 900 | 180 | 4.4 | 612 | [63] | |
| Isopropanol/ water | PDMS | - | 30 | 4 | 306 | - | 13 | - | [64] |
| PDMS-b-PPO | - | 60 | 5 | 3650 | - | 13.5 | - | [60] | |
| n-Butanol/ water | PDMS/PVDF | 10 | 30 | 1 | 160 | 16 | 43.1 | 674 | [65] |
| PPhS/PDMS/PVDF | 10 | 30 | 1 | 261 | 26 | 46.8 | 1191 | [65] | |
| PDMS-PhTMS/PVDF | 12 | 40 | 1 | 704 | 84 | 41.5 | 3402 | [27] | |
| PDMS/PAN/silicatite-1 | 7 | 37 | 1 | 708 | 50 | 30 | 1450 | [66] | |
| Pebax 2533 | 100 | 23 | 5 | 65 | 65 | 8.2 | 468 | [62] | |
| Pebax 2533 | - | 35 | 2.5 | ~300 | - | ~25 | - | [67] | |
| Thin-film silicone | 50 | 30 | 1 | 52.8 | 26.4 | 42 | 1082 | [68] | |
| PolyHFANB-base a-BCP81 | 1.7 | 60 | 1 | ~3500 | 60 | ~22 | 1260 | [69] | |
| HTPB-based PUU | 140 | 35 | 1 | ~10.5 | 14.7 | ~9 | 118 | [70] | |
| Acetone/ water | Pebax 2533 | 30 | 23 | 5 | 140 | 42 | 3.3 | 97 | [62] |
| HTPB-based PUU | 140 | 35 | 0.5 | ~6 | 8.4 | ~12.5 | 97 | [70] | |
| PVC/PS-F2.0 | 40 | 30 | 5 | 42 | 17 | 11 | 170 | [71] | |
| Acetic acid/ water | PDMS | 95 | 35 | 10 | ~57 | 54 | ~1.35 | 19 | [72] |
| PDMS-AMEO/PES | - | 40 | 10 | 90 | - | 2.1 | - | [73] |
| Membrane | Thickness (µm) | T (°C) | Organic Solvent wt% in Feed | J (g/m2h) | JN (g/m2h) | α | PSI (g/m2h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PGS | 200 | 37 | Acetone 0.07 Ethanol 0.04 n-Butanol 0.25 Acetic acid 0.05 | 29 ± 2 | 58 | 37.1 ± 0.5 13.7 ± 0.5 16.6 ± 0.6 2.5 ± 0.2 | 2094 737 905 87 | This study |
| APS | 200 | 37 | Acetone 0.07 Ethanol 0.04 n-Butanol 0.25 Acetic acid 0.05 | 22 ± 3 | 44 | 33.5 ± 0.3 9.9 ± 1.0 14.5 ± 0.7 1.2 ± 0.6 | 1430 392 594 8.8 | This study |
| PDMS/PAN/ silicatite-1 | 7 | 37 | Acetone 0.067 Ethanol 0.043 n-Butanol 0.196 Acetic acid 0.026 | 491 | 34 | 41.4 9.8 31.6 - | 1374 299 1040 - | [66] |
| HTPB-based PUU | 140 | 40 | Acetone 0.5 Ethanol 0.1 n-Butanol 1.1 | 9.7 | 13.6 | 15.3 - 13.7 | 194 - 173 | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, P.-Y.; Wang, J.; Li, S.-Y.; Suen, S.-Y. Biodegradable Polymeric Membranes for Organic Solvent/Water Pervaporation Applications. Membranes 2021, 11, 970. https://doi.org/10.3390/membranes11120970
Chang P-Y, Wang J, Li S-Y, Suen S-Y. Biodegradable Polymeric Membranes for Organic Solvent/Water Pervaporation Applications. Membranes. 2021; 11(12):970. https://doi.org/10.3390/membranes11120970
Chicago/Turabian StyleChang, Pao-Yueh, Jane Wang, Si-Yu Li, and Shing-Yi Suen. 2021. "Biodegradable Polymeric Membranes for Organic Solvent/Water Pervaporation Applications" Membranes 11, no. 12: 970. https://doi.org/10.3390/membranes11120970
APA StyleChang, P.-Y., Wang, J., Li, S.-Y., & Suen, S.-Y. (2021). Biodegradable Polymeric Membranes for Organic Solvent/Water Pervaporation Applications. Membranes, 11(12), 970. https://doi.org/10.3390/membranes11120970







