Poly(styrene-co-butadiene)/Maghnia-Organo-Montmorillonite Clay Nanocomposite. Preparation, Properties and Application as Membrane in the Separation of Methanol/Toluene Azeotropic Mixture by Pervaporation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Organophilic Clay
2.3. Preparation of CSBR/OMMT Nanocomposite
2.4. Membrane Characterisation
2.5. Swelling Experiments
2.6. Pervaporation Experiments
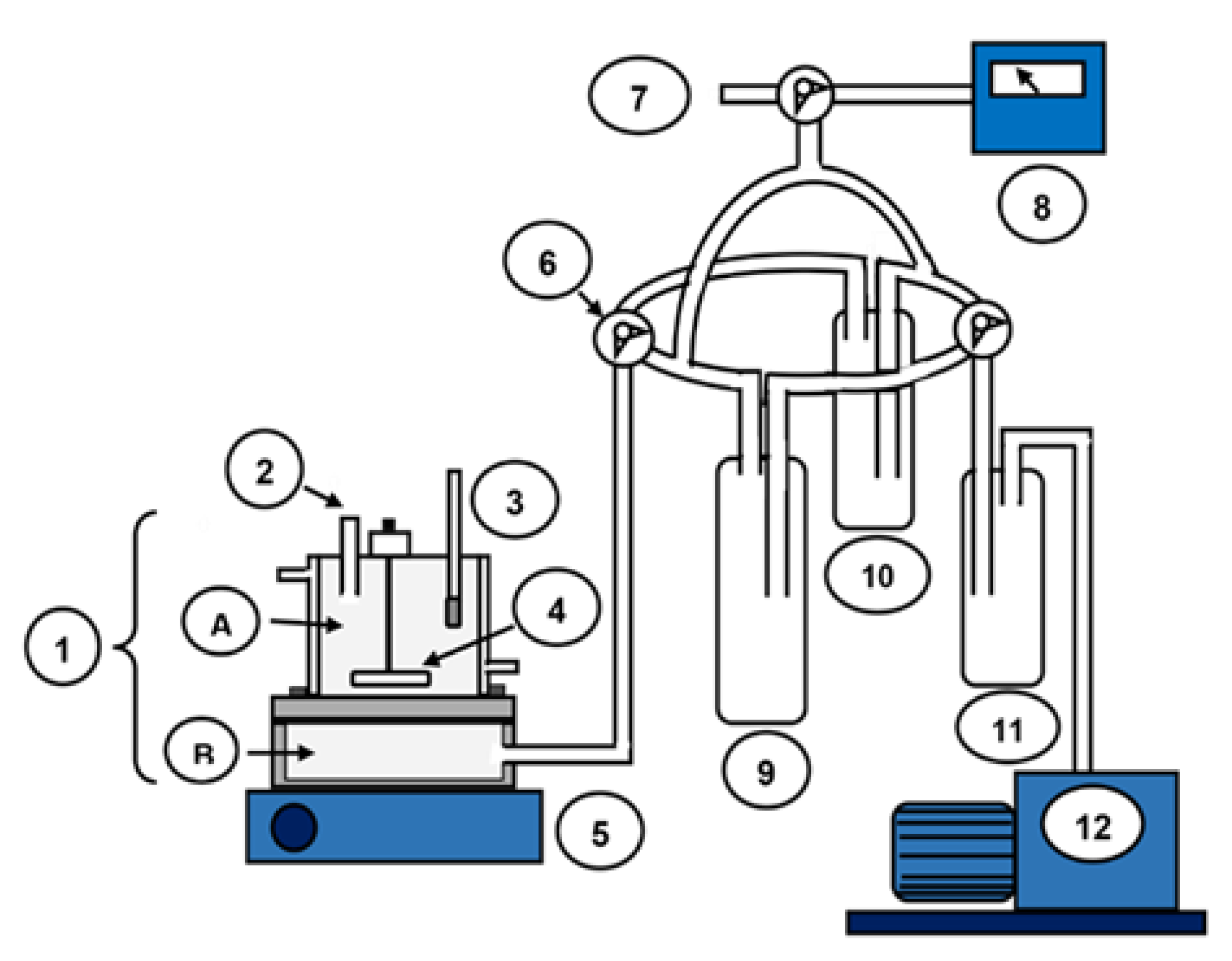
2.6.1. Pervaporation Setup
2.6.2. Pervaporative Parameters
3. Results and Discussion
3.1. Membrane Characterization
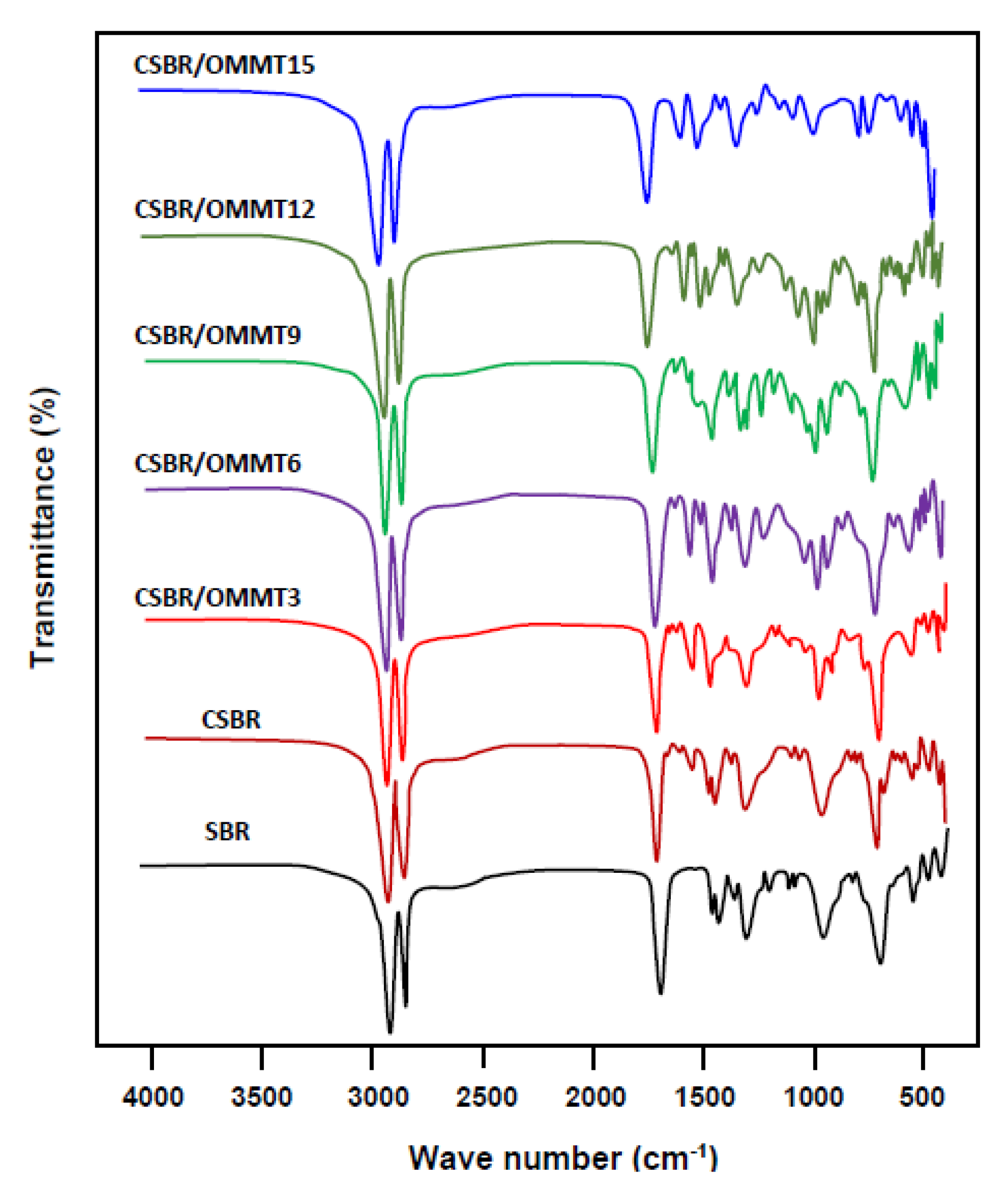
3.1.1. FTIR Analysis
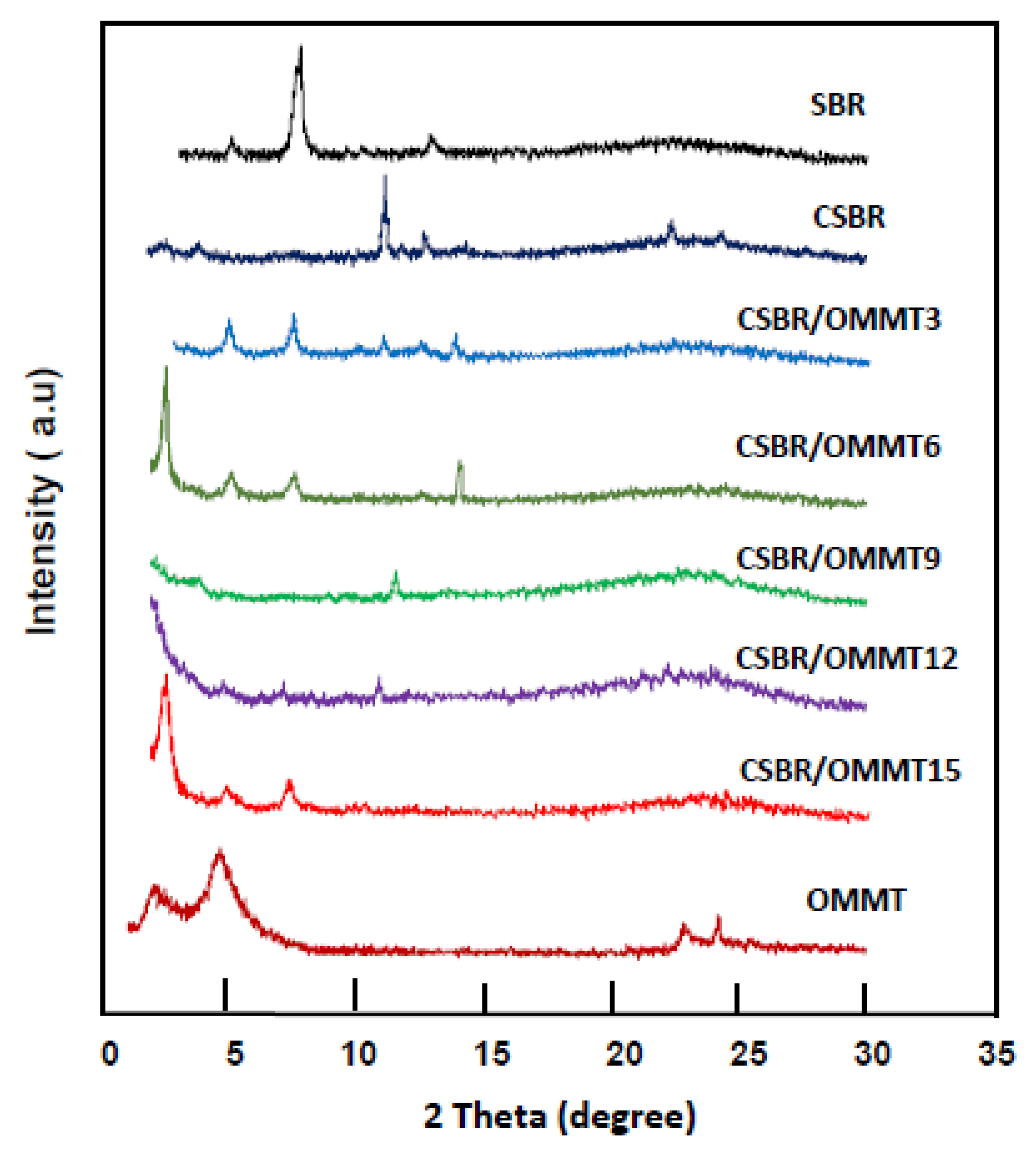
3.1.2. XRD Analysis
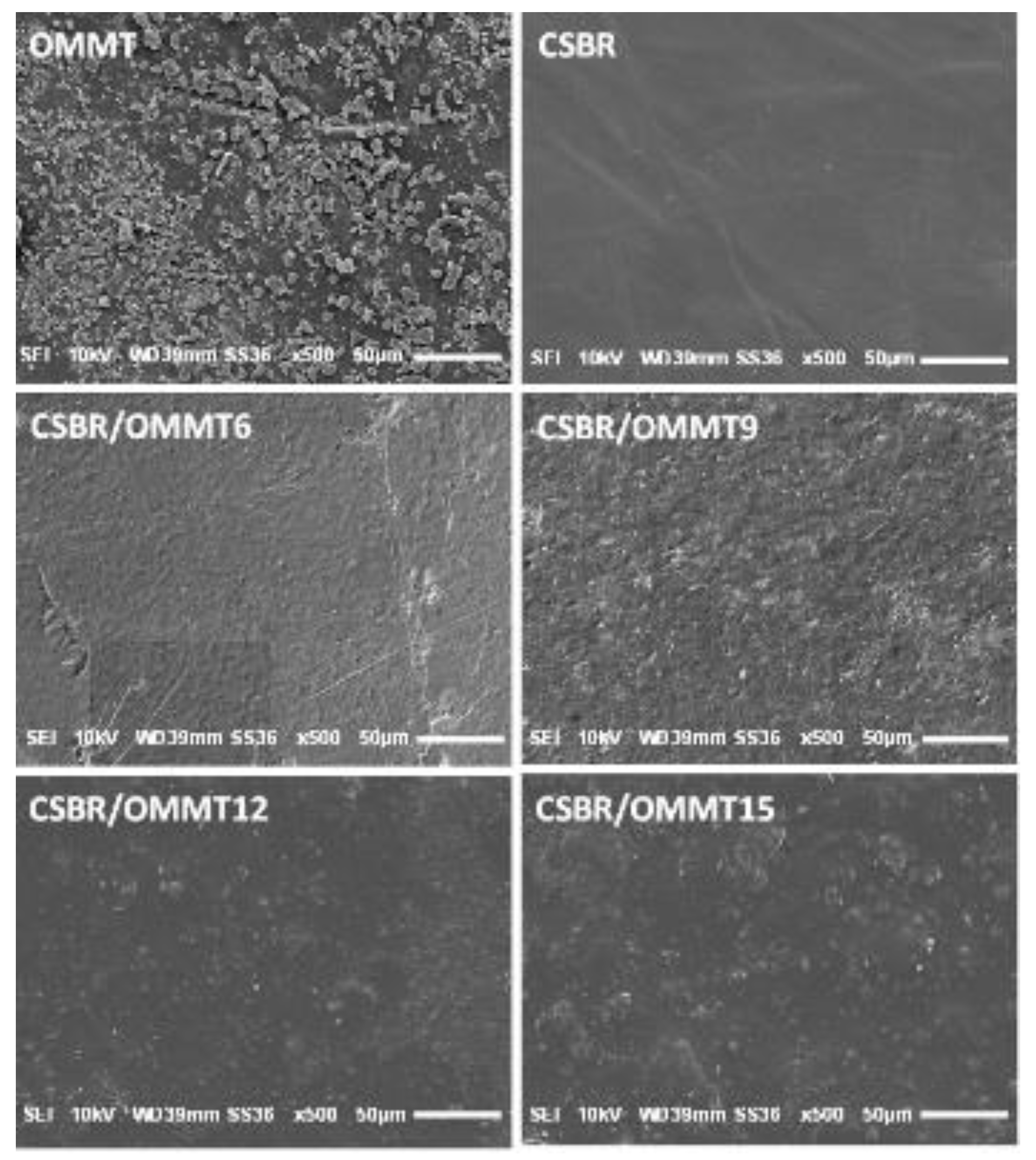
3.1.3. SEM Analysis
3.1.4. DLS Analysis
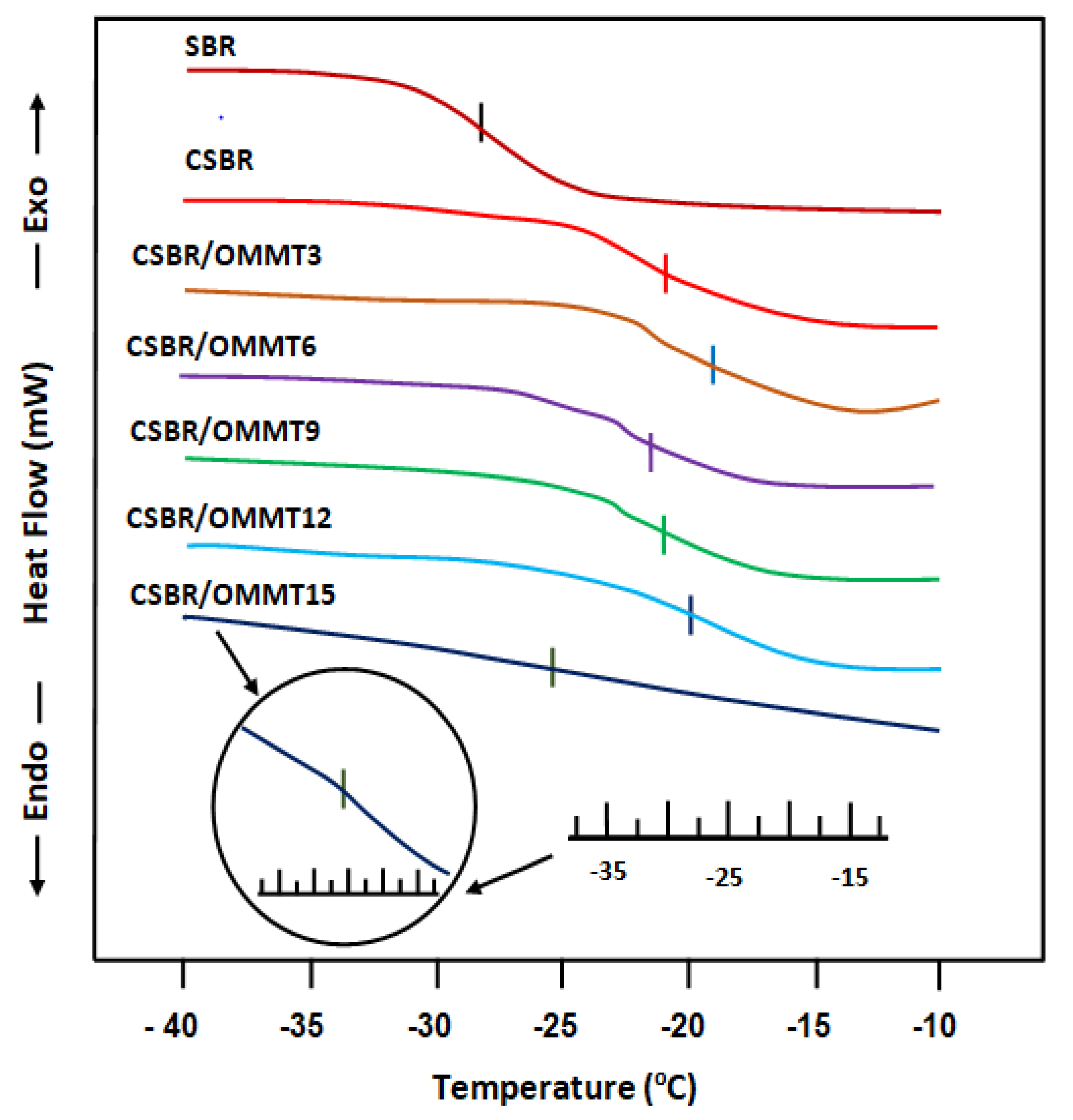
3.1.5. DSC Analysis
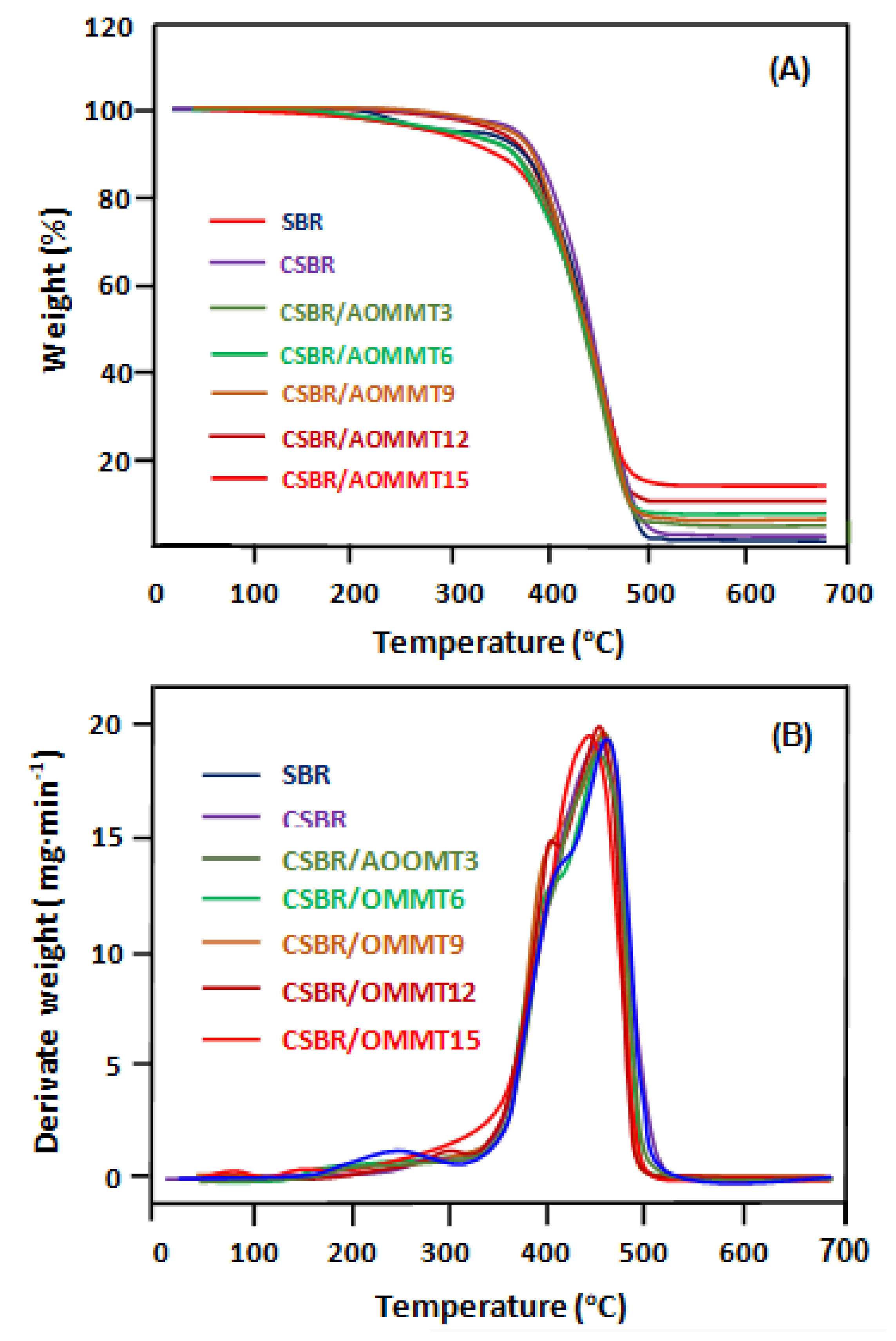
3.1.6. TGA/DTG Analysis
3.2. Mechanical Strength
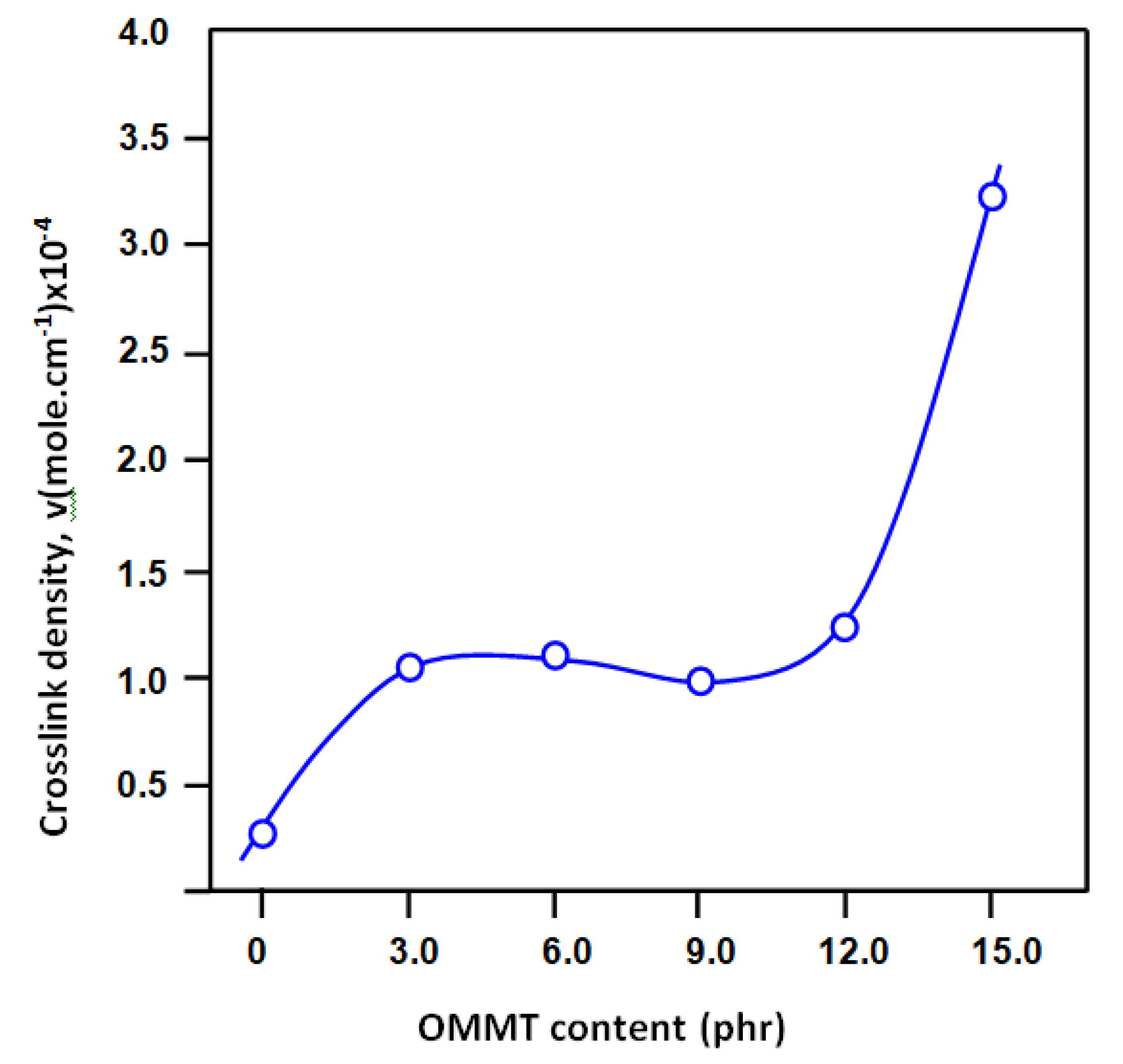
3.3. Crosslink Density
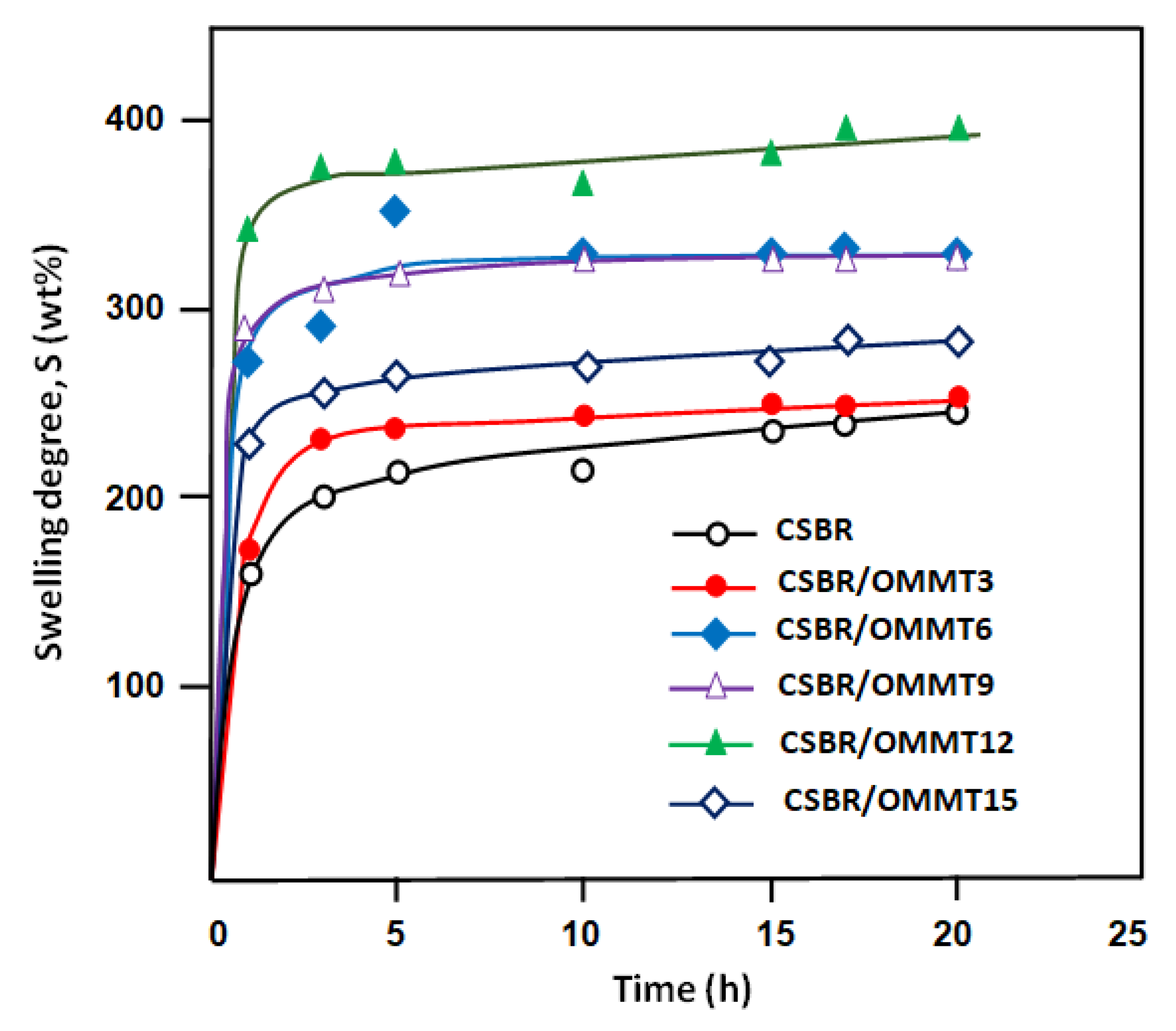
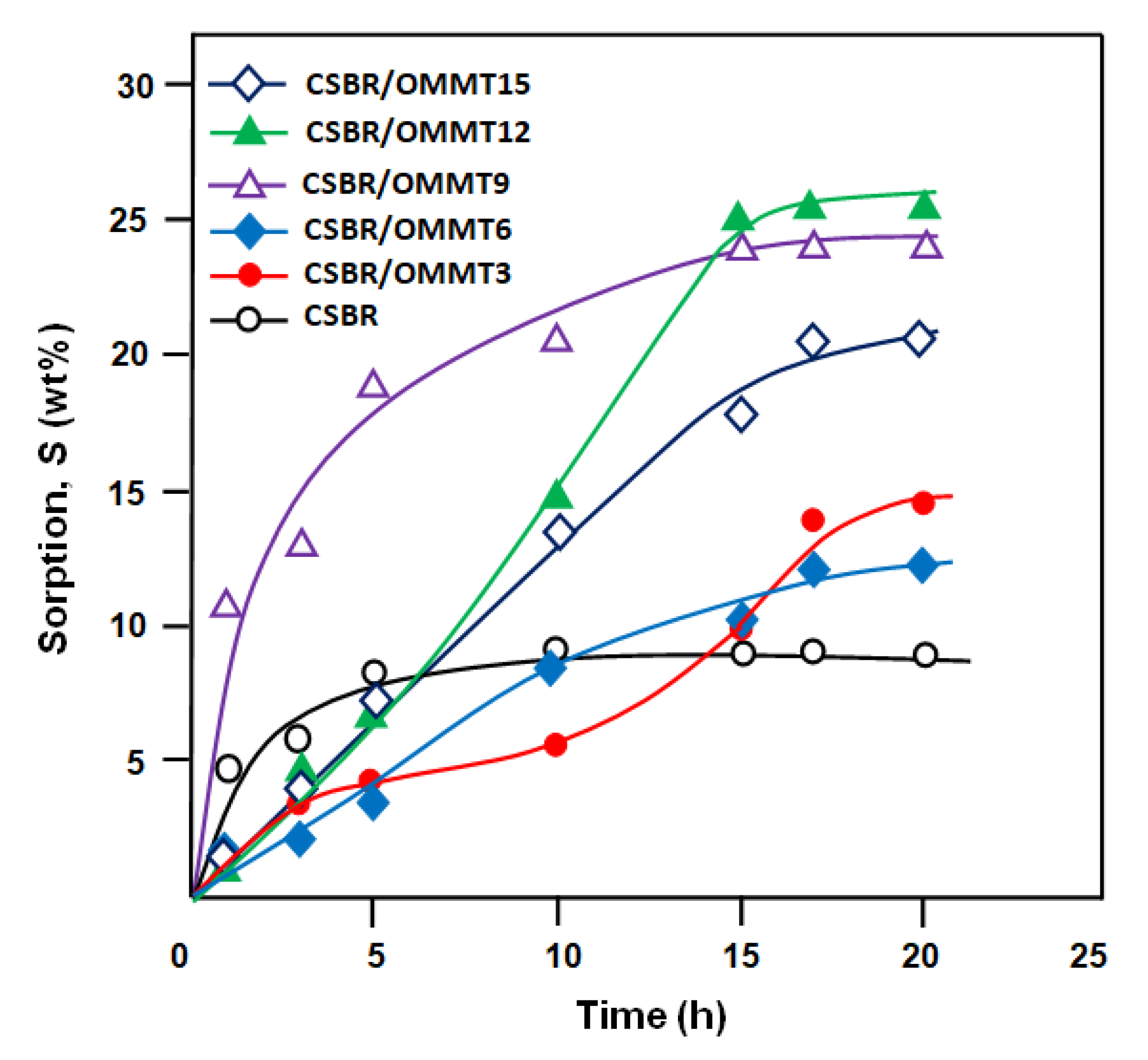
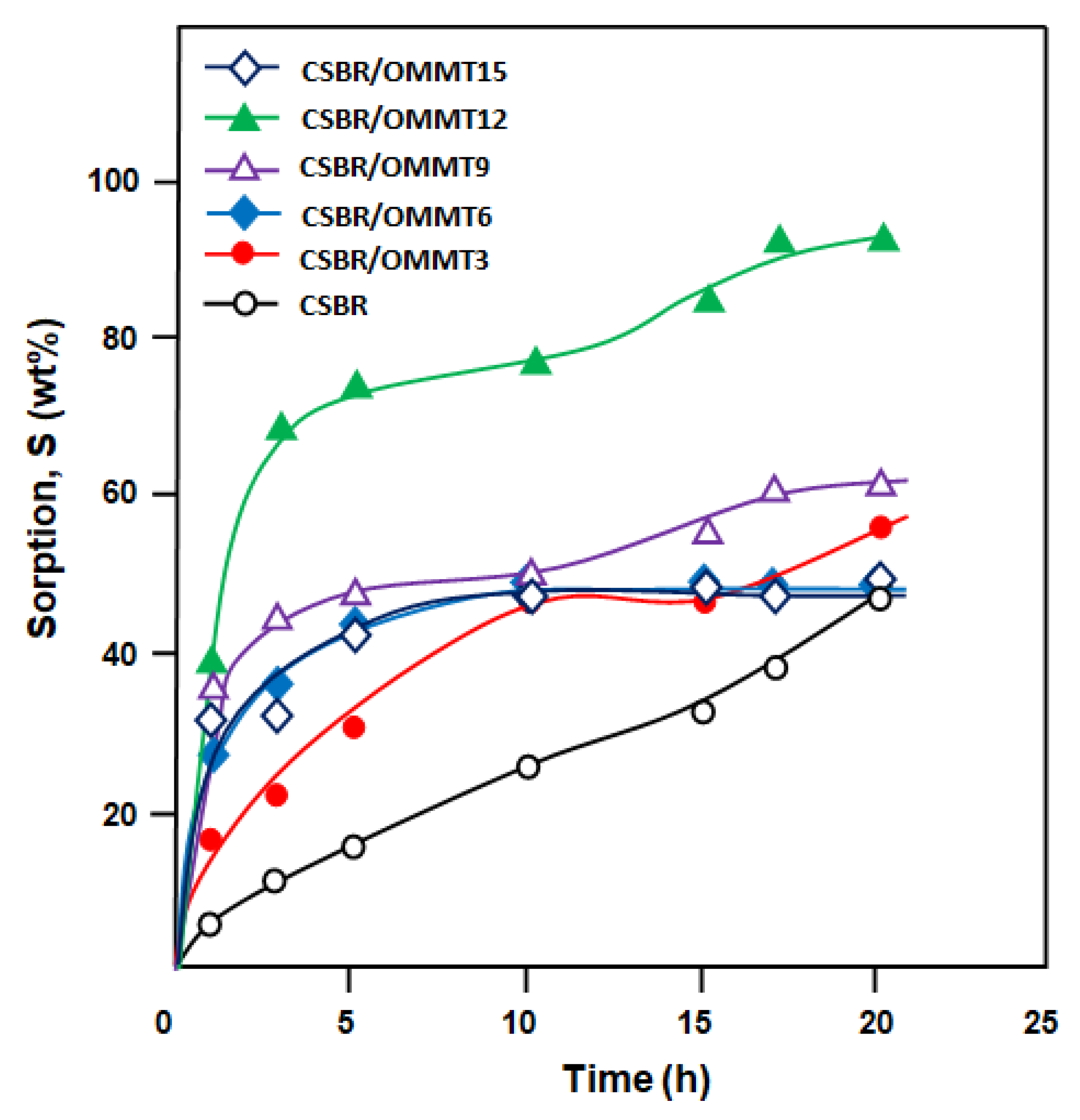
3.4. Swelling Performance
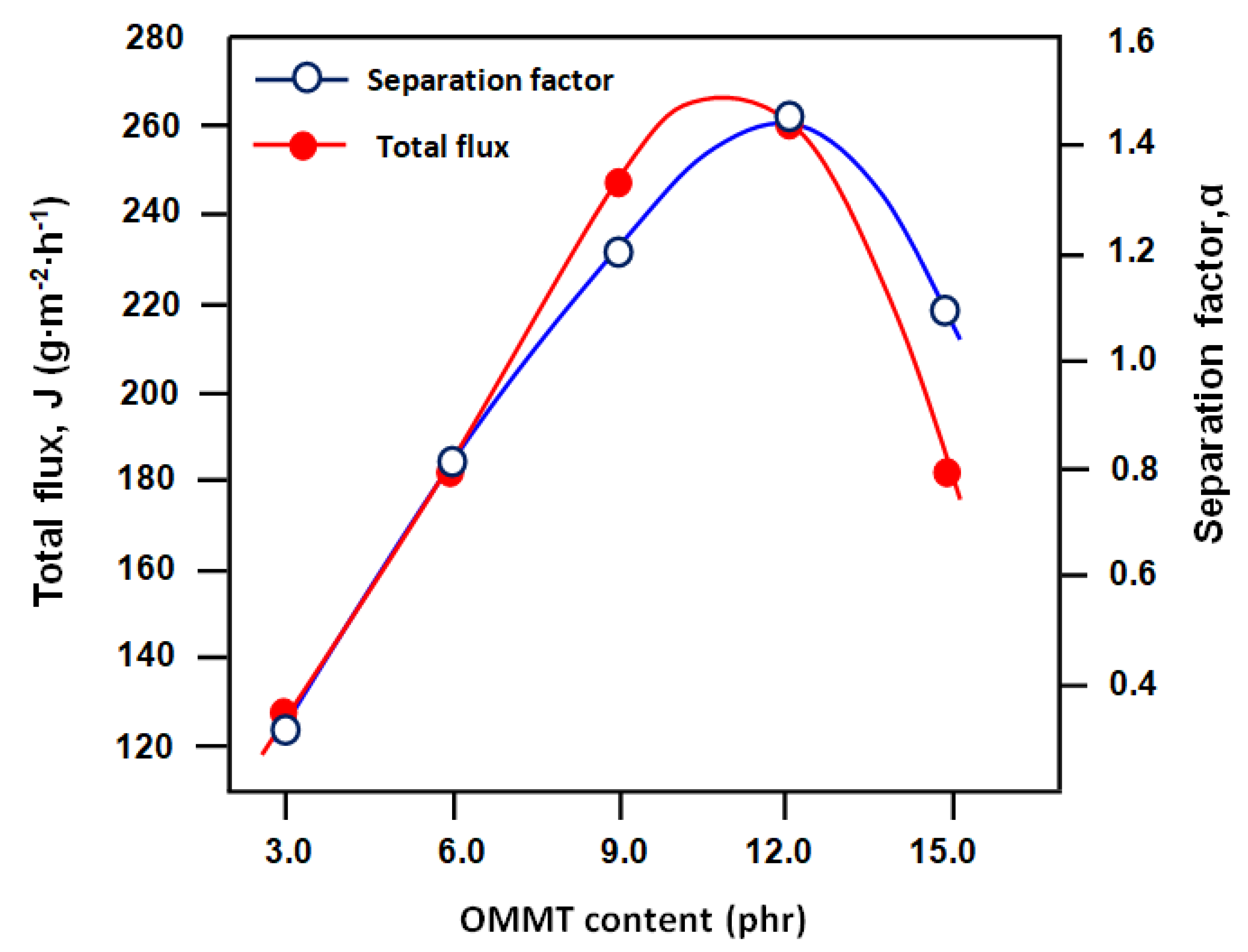
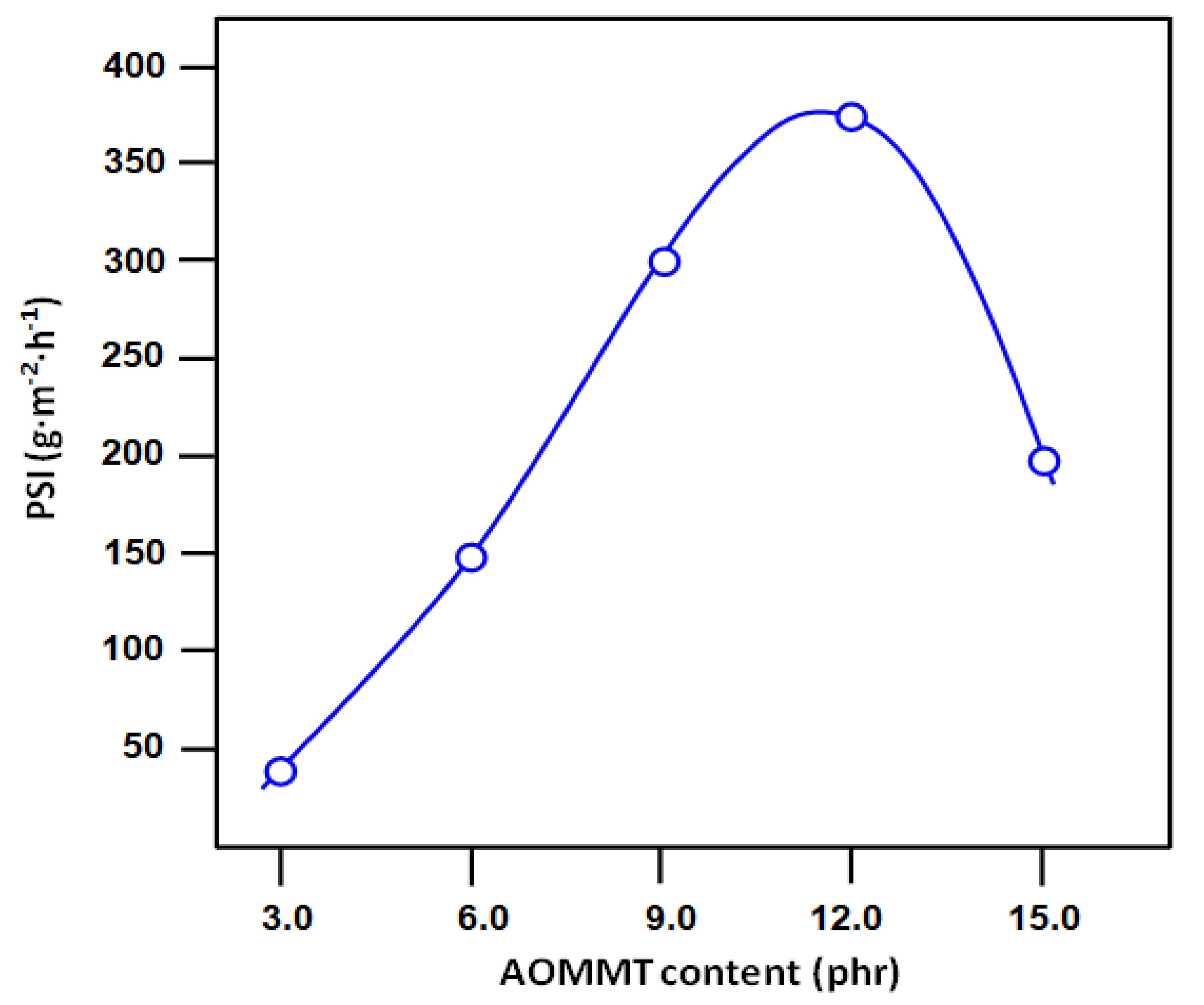
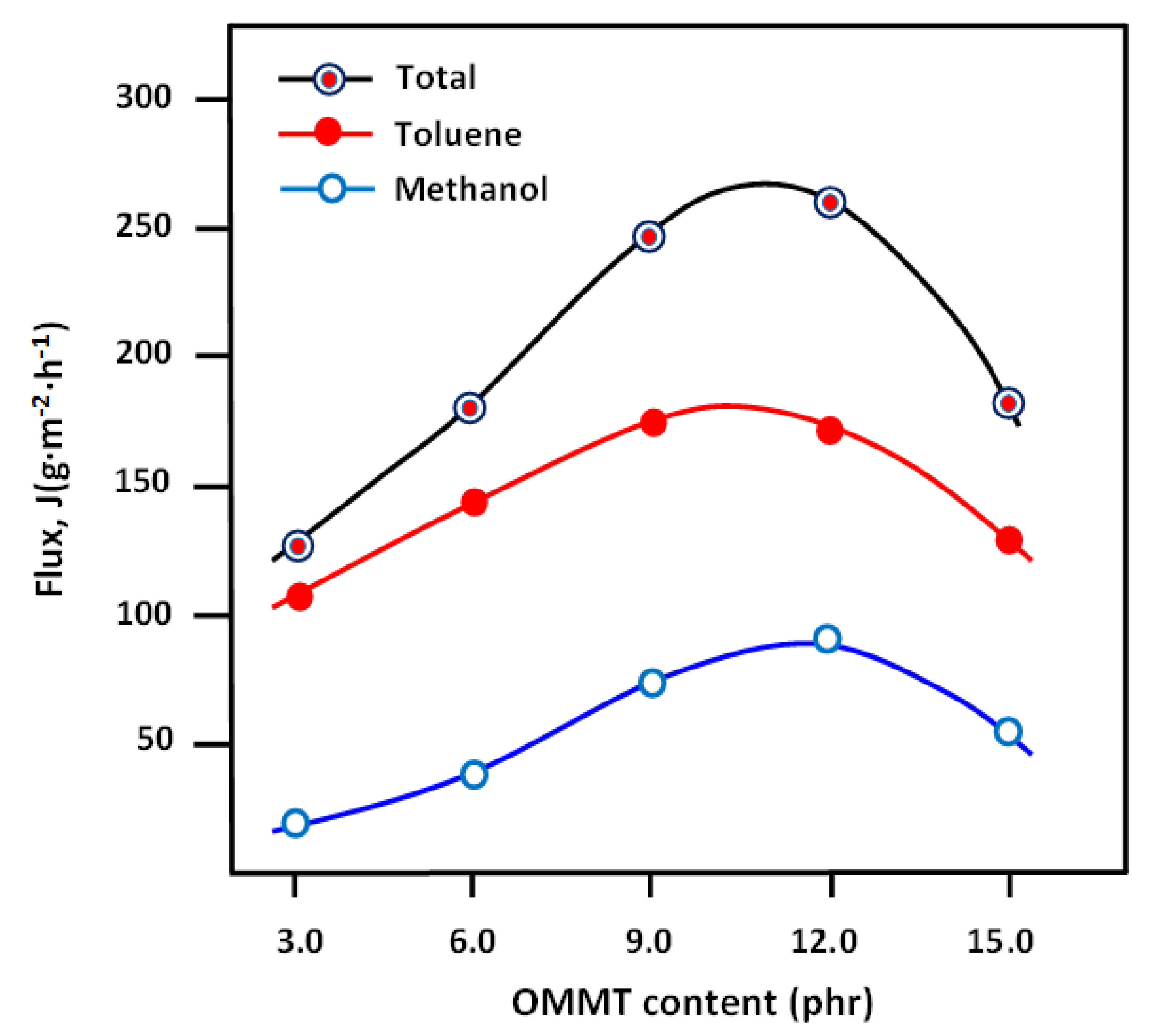
3.5. Pervaporation Performance
3.6. Diffusion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabeharitsara, A.T.; Hanitriniaina, R.M.; Fabrice, R.A.; Rijalalaina, R.; Randriana, N.R. Synthesis of Glues with Citric Acid and Sulfuric Acid Protonic Acid-H+ as Catalysts Using Banana Peel and Kaki as Valorised Raw Materials. Am. J. Appl. Chem. 2021, 9, 21–35. [Google Scholar] [CrossRef]
- Brandt, H.D.; Nentwig, W.; Rooney, N.; LaFlair, R.T.; Wolf, U.U.; Duffy, J.; Puskas, J.E.; Kaszas, G.; Drewitt, M.; Glander, S. Rubber, 5. Solution Rubbers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Mansilla, M.A.; Valentín, J.; López-Manchado, M.; González-Jiménez, A.; Marzocca, A.J. Effect of entanglements in the microstructure of cured NR/SBR blends prepared by solution and mixing in a two-roll mill. Eur. Polym. J. 2016, 81, 365–375. [Google Scholar] [CrossRef]
- Xue, X.; Yin, Q.; Jia, H.; Zhang, X.; Wen, Y.; Ji, Q.; Xu, Z. Enhancing mechanical and thermal properties of styrene-butadiene rubber/carboxylated acrylonitrile butadiene rubber blend by the usage of graphene oxide with diverse oxidation degrees. Appl. Surf. Sci. 2017, 423, 584–591. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Luo, Y.; Jia, Z.; Chen, Y.; Jia, D. Inorganic and organic hybrid nanoparticles as multifunctional crosslinkers for rubber vulcanization with high-filler rubber interaction. Polymers 2018, 10, 1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, L.; Wang, Q. Reinforcement of natural rubber with carbon black/nanoclay hybrid filler. Plast. Rubber Compos. 2010, 39, 370–376. [Google Scholar] [CrossRef]
- Ravikumar, K.; Joseph, R.; Ravichandran, K. Effect of organo clay on curing, mechanical and dielectric properties of NR/SBR blends. J. Phys. Conf. Ser. 2018, 1000, 012116. [Google Scholar] [CrossRef]
- El-Sabbagh, S.H.; Mahmoud, D.S.; Ahmed, N.M.; Ward, A.; Sabaa, M.W. Composites of styrene butadiene rubber/modified clay: Mechanical, dielectric and morphological properties. Pigment. Resin Technol. 2017, 46, 161–171. [Google Scholar] [CrossRef]
- Singha, N.; Ray, S. Removal of pyridine from water by pervaporation using crosslinked and filled natural rubber membranes. J. Appl. Polym. Sci. 2012, 124, E99–E107. [Google Scholar] [CrossRef]
- Gu, Z.; Song, G.; Liu, W.; Li, P.; Gao, L.; Li, H.; Hu, X. Preparation and properties of styrene butadiene rubber/natural rubber/organo-bentonite nanocomposites prepared from latex dispersions. Appl. Clay Sci. 2009, 46, 241–244. [Google Scholar] [CrossRef]
- Kuila, S.; Ray, S. Sorption and permeation studies of tetrahydrofuran–water mixtures using full interpenetrating network membranes. Sep. Purif. Technol. 2012, 89, 39–50. [Google Scholar] [CrossRef]
- Bergaya, F.; Detellier, C.; Lambert, J.-F.; Lagaly, G. Introduction to clay–polymer nanocomposites (CPN). In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 655–677. [Google Scholar]
- Sapalidis, A.A.; Katsaros, F.K.; Kanellopoulos, N.K. PVA/montmorillonite nanocomposites: Development and properties. In Nanocomposites and Polymers with Analytical Methods; IntechOpen: London, UK, 2011; pp. 29–50. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Ullah, H.; Ali, Z.; Rahim, F.; Khan, F.; Rehman, Z.U. Polymer-clay nanocomposites, preparations and current applications: A review. Curr. Nanomater. 2016, 1, 83–95. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wang, Y.; Sui, Y.; Yu, D. Morphology and mechanical properties of clay/styrene-butadiene rubber nanocomposites. J. Appl. Polym. Sci. 2000, 78, 1873–1878. [Google Scholar] [CrossRef]
- Sampranpiboon, P.; Jiraratananon, R.; Uttapap, D.; Feng, X.; Huang, R. Pervaporation separation of ethyl butyrate and isopropanol with polyether block amide (PEBA) membranes. J. Membr. Sci. 2000, 173, 53–59. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sengupta, R.; Dasgupta, S.; Mukhopadhyay, R.; Bandyopadhyay, S.; Joshi, M.; Ameta, S.C. Synthesis and characterization of styrene butadiene rubber—bentonite clay nanocomposites. Polym. Eng. Sci. 2009, 49, 1279–1290. [Google Scholar] [CrossRef]
- Zha, C.; Wang, W.; Lu, Y.; Zhang, L. Constructing covalent interface in rubber/clay nanocomposite by combining structural modification and interlamellar silylation of montmorillonite. ACS Appl. Mater. Interfaces 2014, 6, 18769–18779. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Meertens, R.; Mulder, M.; Smolders, C. Pervaporation of alcohol-toluene mixtures through polymer blend membranes of poly (acrylic acid) and poly (vinyl alcohol). J. Membr. Sci. 1994, 90, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Chovau, S.; Dobrak, A.; Figoli, A.; Galiano, F.; Simone, S.; Drioli, E.; Sikdar, S.; Van der Bruggen, B. Pervaporation performance of unfilled and filled PDMS membranes and novel SBS membranes for the removal of toluene from diluted aqueous solutions. Chem. Eng. J. 2010, 159, 37–46. [Google Scholar] [CrossRef]
- Porter, M.C. Handbook of Industrial Membrane Technology. 1989. Available online: https://www.osti.gov/biblio/6379997 (accessed on 10 October 2021).
- Qiao, X.; Chung, T.-S.; Guo, W.F.; Matsuura, T.; Teoh, M.M. Dehydration of isopropanol and its comparison with dehydration of butanol isomers from thermodynamic and molecular aspects. J. Membr. Sci. 2005, 252, 37–49. [Google Scholar] [CrossRef]
- Ibrahim, A.; Lin, Y. Pervaporation separation of organic mixtures by MOF-5 membranes. Ind. Eng. Chem. Res. 2016, 55, 8652–8658. [Google Scholar] [CrossRef]
- Polotskaya, G.; Pulyalina, A.; Goikhman, M.; Podeshvo, I.; Rostovtseva, V.; Shugurov, S.; Gofman, I.; Saprykina, N.; Gulii, N.; Loretsyan, N. Novel Polyheteroarylene Membranes for Separation of Methanol-Hexane Mixture by Pervaporation. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Singha, N.; Ray, S.; Ray, S.; Konar, B. Removal of pyridine from water by pervaporation using filled SBR Membranes. J. Appl. Polym. Sci. 2011, 121, 1330–1334. [Google Scholar] [CrossRef]
- Mahapatra, M.; Karmakar, M.; Dutta, A.; Singha, N.R. Fabrication of composite membranes for pervaporation of tetrahydrofuran-water: Optimization of intrinsic property by response surface methodology and studies on vulcanization mechanism by density functional theory. Korean J. Chem. Eng. 2018, 35, 1889–1910. [Google Scholar] [CrossRef]
- Burke, D.; Williams, G.; Plank, C. Vapor-Liquid Equilibria for the Methanol-Toluene System. J. Chem. Eng. Data 1964, 9, 212–214. [Google Scholar] [CrossRef]
- Singha, N.; Kar, S.; Ray, S.; Ray, S. Separation of isopropyl alcohol–water mixtures by pervaporation using crosslink IPN membranes. Chem. Eng. Process. Process. Intensif. 2009, 48, 1020–1029. [Google Scholar] [CrossRef]
- Mandal, S.; Pangarkar, V.G. Separation of methanol–benzene and methanol–toluene mixtures by pervaporation: Effects of thermodynamics and structural phenomenon. J. Membr. Sci. 2002, 201, 175–190. [Google Scholar] [CrossRef]
- Bhat, A.A.; Pangarkar, V.G. Methanol-selective membranes for the pervaporative separation of methanol–toluene mixtures. J. Membr. Sci. 2000, 167, 187–201. [Google Scholar] [CrossRef]
- Sommer, S.; Melin, T. Influence of operation parameters on the separation of mixtures by pervaporation and vapor permeation with inorganic membranes. Part 1: Dehydration of solvents. Chem. Eng. Sci. 2005, 60, 4509–4523. [Google Scholar] [CrossRef]
- Park, H.-c.; Meertens, R.M.; Mulder, M.H. Sorption of alcohol−toluene mixtures in poly (acrylic acid)-poly (vinyl alcohol) blend membranes and its role on pervaporation. Ind. Eng. Chem. Res. 1998, 37, 4408–4417. [Google Scholar] [CrossRef]
- Das, P.; Ray, S.K. Separation of toluene–methanol mixtures by pervaporation using filled elastomeric membranes. J. Taiwan Inst. Chem. Eng. 2016, 64, 89–105. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S. Separation of organic mixtures by pervaporation using crosslinked rubber membranes. J. Membr. Sci. 2006, 270, 132–145. [Google Scholar] [CrossRef]
- Moulik, S.; Bukke, V.; Sajja, S.C.; Sridhar, S. Chitosan-polytetrafluoroethylene composite membranes for separation of methanol and toluene by pervaporation. Carbohydr. Polym. 2018, 193, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.; Karmakar, M.; Mondal, B.; Singha, N.R. Role of ZDC/S ratio for pervaporative separation of organic liquids through modified EPDM membranes: Rational mechanistic study of vulcanization. RSC Adv. 2016, 6, 69387–69403. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Kuzminova, A.; Atta, R.; Zolotarev, A.; Mazur, A.; Vezo, O.; Lahderanta, E.; Markelov, D.; Ermakov, S. Development and investigation of novel polyphenylene isophthalamide pervaporation membranes modified with various fullerene derivatives. Sep. Purif. Technol. 2019, 226, 241–251. [Google Scholar] [CrossRef]
- Lue, S.J.; Ou, J.S.; Kuo, C.H.; Chen, H.Y.; Yang, T.-H. Pervaporative separation of azeotropic methanol/toluene mixtures in polyurethane–poly (dimethylsiloxane)(PU–PDMS) blend membranes: Correlation with sorption and diffusion behaviors in a binary solution system. J. Membr. Sci. 2010, 347, 108–115. [Google Scholar] [CrossRef]
- Abraham, J.; Jose, T.; Moni, G.; George, S.C.; Kalarikkal, N.; Thomas, S. Ionic liquid modified multiwalled carbon nanotube embedded styrene butadiene rubber membranes for the selective removal of toluene from toluene/methanol mixture via pervaporation. J. Taiwan Inst. Chem. Eng. 2019, 95, 594–601. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Aadhar, M. Studies on preparation and analysis of organoclay nano particles. Res. J. Eng. Sci. 2014, 2278, 9472. [Google Scholar]
- Khalaf, H.; Bouras, O.; Perrichon, V. Synthesis and characterization of Al-pillared and cationic surfactant modified Al-pillared Algerian bentonite. Microporous Mater. 1997, 8, 141–150. [Google Scholar] [CrossRef]
- Allan, F.B.; Barton, M. Handbook of Solubility Parameters and Other Cohesion Parameters; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Kulkarni, A.; Pugh, C.; Jana, S.C.; Wims, D.T.; Gawad, A.A. Crosslinking of SBR compounds for tire tread using benzocyclobutene chemistry. Rubber Chem. Technol. 2019, 92, 25–42. [Google Scholar] [CrossRef]
- Allel, A.; Naceur, M.W.; Benguergoura, H.; Ledoux, A.; Saeed, W.S.; Al-Odayni, A.-B.; Aouak, T. Pervaporative separation of water–ethanol mixtures using an Algerian Na+ montmorillonite nanoclay-incorporated poly (vinyl alcohol) nanocomposite membrane. RSC Adv. 2020, 10, 39531–39541. [Google Scholar] [CrossRef]
- Benguergoura, H.; Moulay, S. Styrene–butadiene rubber membranes for the pervaporative separation of benzene/cyclohexane mixtures. J. Appl. Polym. Sci. 2012, 123, 1455–1467. [Google Scholar] [CrossRef]
- Toth, A.J.; Andre, A.; Haaz, E.; Mizsey, P. New horizon for the membrane separation: Combination of organophilic and hydrophilic pervaporations. Sep. Purif. Technol. 2015, 156, 432–443. [Google Scholar] [CrossRef]
- Choudhari, S.K.; Kariduraganavar, M.Y. Development of novel composite membranes using quaternized chitosan and Na+-MMT clay for the pervaporation dehydration of isopropanol. J. Colloid Interface Sci. 2009, 338, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, S.; Samaržija-Jovanović, S.; Marković, G.; Jovanović, V.; Adamović, T.; Marinović-Cincović, M. Mechanical properties and thermal aging behaviour of polyisoprene/polybutadiene/styrene-butadiene rubber ternary blend reinforced with carbon black. Compos. Part B Eng. 2016, 98, 126–133. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Separation of ethanol from water by pervaporation using mixed matrix copolymer membranes. Sep. Purif. Technol. 2015, 146, 176–186. [Google Scholar] [CrossRef]
- Sadhu, S.; Bhowmick, A.K. Preparation and properties of nanocomposites based on acrylonitrile–butadiene rubber, styrene–butadiene rubber, and polybutadiene rubber. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1573–1585. [Google Scholar] [CrossRef]
- Santiago, F.; Mucientes, A.E.; Osorio, M.; Rivera, C. Preparation of composites and nanocomposites based on bentonite and poly (sodium acrylate). Effect of amount of bentonite on the swelling behaviour. Eur. Polym. J. 2007, 43, 1–9. [Google Scholar] [CrossRef]
- Sadek, E.; El-Nashar, D.; Ahmed, S. Effect of organoclay reinforcement on the curing characteristics and technological properties of styrene–butadiene rubber. Polym. Compos. 2015, 36, 1293–1302. [Google Scholar] [CrossRef]
- Long, L.; Yu, X.; Wu, L.; Li, J.; Li, X. Nano-CdS confined within titanate nanotubes for efficient photocatalytic hydrogen production under visible light illumination. Nanotechnology 2013, 25, 035603. [Google Scholar] [CrossRef]
- Radhakrishnan, C.; Sujith, A.; Unnikrishnan, G. Thermal behaviour of styrene butadiene rubber/poly (ethylene-co-vinyl acetate) blends. J. Therm. Anal. Calorim. 2007, 90, 191–199. [Google Scholar] [CrossRef]
- Torres, J.; Nealey, P.; De Pablo, J. Molecular simulation of ultrathin polymeric films near the glass transition. Phys. Rev. Lett. 2000, 85, 3221. [Google Scholar] [CrossRef] [PubMed]
- Rittigstein, P.; Torkelson, J.M. Polymer–nanoparticle interfacial interactions in polymer nanocomposites: Confinement effects on glass transition temperature and suppression of physical aging. J. Polym. Sci. Part. B Polym. Phys. 2006, 44, 2935–2943. [Google Scholar] [CrossRef]
- Moll, J.; Kumar, S.K. Glass transitions in highly attractive highly filled polymer nanocomposites. Macromolecules 2012, 45, 1131–1135. [Google Scholar] [CrossRef]
- Starr, F.W.; Douglas, J.F.; Meng, D.; Kumar, S.K. Bound layers “cloak” nanoparticles in strongly interacting polymer nanocomposites. ACS Nano 2016, 10, 10960–10965. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Tan, Y.Q.; Zhang, L. Effect of Filler on Glass Transition of Asphalt Mastics. Adv. Eng. Forum 2012, 5, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Xie, S.-J.; Carrillo, J.-M.Y.; Carroll, B.; Martin, H.; Cao, P.-F.; Dadmun, M.D.; Sumpter, B.G.; Novikov, V.N.; Schweizer, K.S. Big effect of small nanoparticles: A shift in paradigm for polymer nanocomposites. ACS Nano 2017, 11, 752–759. [Google Scholar] [CrossRef]
- Iqbal, S.; Iqbal, N.; Jamil, T.; Bashir, A.; Shahid, M. Experimental thermal transport evolution of silane activated nano-clay reinforced styrene butadiene elastomeric nanocomposites. IOP Conf. Ser. Mater. Sci. Eng. 2016, 146, 012039. [Google Scholar] [CrossRef] [Green Version]
- Noriman, N.; Ismail, H.; Rashid, A. Characterization of styrene butadiene rubber/recycled acrylonitrile-butadiene rubber (SBR/NBRr) blends: The effects of epoxidized natural rubber (ENR-50) as a compatibilizer. Polym. Test. 2010, 29, 200–208. [Google Scholar] [CrossRef]
- Diez, J.; Bellas, R.; Ramirez, C.; Rodriguez, A. Effect of organoclay reinforcement on the curing characteristics and technological properties of SBR sulphur vulcanizates. J. Appl. Polym. Sci. 2010, 118, 566–573. [Google Scholar] [CrossRef]
- Choi, S.S.; Park, B.H.; Song, H. Influence of filler type and content on properties of styrene-butadiene rubber (SBR) compound reinforced with carbon black or silica. Polym. Adv. Technol. 2004, 15, 122–127. [Google Scholar] [CrossRef]
- Helaly, F.; El Sabbagh, S.; El Kinawy, O.; El Sawy, S. Effect of synthesized zinc stearate on the properties of natural rubber vulcanizates in the absence and presence of some fillers. Mater. Des. 2011, 32, 2835–2843. [Google Scholar] [CrossRef]
- Ray, S.; Ray, S. Separation of organic mixtures by pervaporation using crosslinked and filled rubber membranes. J. Membr. Sci. 2006, 285, 108–119. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the crosslink characteristics and mechanical properties of natural rubber compound via accelerators and reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef] [PubMed]
- Marzocca, A. Evaluation of the polymer–solvent interaction parameter χ for the system cured styrene butadiene rubber and toluene. Eur. Polym. J. 2007, 43, 2682–2689. [Google Scholar] [CrossRef]
- Patil, M.B.; Aminabhavi, T.M. Pervaporation separation of toluene/alcohol mixtures using silicalite zeolite embedded chitosan mixed matrix membranes. Sep. Purif. Technol. 2008, 62, 128–136. [Google Scholar] [CrossRef]
- Garg, P.; Singh, R.; Choudhary, V. Pervaporation separation of organic azeotrope using poly(dimethyl siloxane)/clay nanocomposite membranes. Sep. Purif. Technol. 2011, 80, 435–444. [Google Scholar] [CrossRef]
- Polotskaya, G.; Meleshko, T.; Sushchenko, I.; Yakimansky, A.; Pulyalina, A.Y.; Toikka, A.; Pientka, Z. Membranes based on polyimide–polyaniline nanocomposites for pervaporation of organic mixtures. J. Appl. Polym. Sci. 2010, 117, 2175–2182. [Google Scholar] [CrossRef]
- Khayet, M.; Nasef, M.; Mengual, J. Radiation grafted poly (ethylene terephthalate)-graft-polystyrene pervaporation membranes for organic/organic separation. J. Membr. Sci. 2005, 263, 77–95. [Google Scholar] [CrossRef]


| Species | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | As | L.O.I |
|---|---|---|---|---|---|---|---|---|---|---|
| Percentage (wt%) | 69.4 | 14.7 | 1.2 | 1.1 | 0.3 | 0.5 | 0.8 | 0.2 | 0.05 | 11 |
| Compound | Molar Volume (cm3 mol−1) | Molar Mass (g·mol−1) | Density (g·cm−3) | Vapor Pressure at 25 °C | δ * (J·cm−3)0.5 |
|---|---|---|---|---|---|
| Methanol | 40.4 | 32.04 | 0.792 | 94.0 | 29.7 |
| Toluene | 107.1 | 92.14 | 0.867 | 28.4 | 18.2 |
| SBR | - | 140,000 | 0.980 | - | 16.5 |
| Membrane | SBR (phr) | Sulfur (phr) | ZDC (phr) | OMMT (phr) |
|---|---|---|---|---|
| pure SBR | 100 | 0 | 0 | 0 |
| CSBR | 100 | 1.5 | 6.0 | 0 |
| CSBR/OMMT-3 | 100 | 1.5 | 6.0 | 3 |
| CSBR/OMMT-6 | 100 | 1.5 | 6.0 | 6 |
| CSBR/OMMT-9 | 100 | 1.5 | 6.0 | 9 |
| CSBR/OMMT-12 | 100 | 1.5 | 6.0 | 12 |
| CSBR/OMMT-15 | 100 | 1.5 | 6.0 | 15 |
| Membrane | |
|---|---|
| SBR pure | −27.8 |
| CSBR | −21.5 |
| CSBR/OMMT3 | −20.0 |
| CSBR/OMMT6 | −22.0 |
| CSBR/OMMT9 | −22.5 |
| CSBR/OMMT12 | −21.5 |
| CSBR/OMMT15 | −25.5 |
| Membrane | Tensile Strength (MPa) | Elongation at Break (%) | Modulus at 100% Elongation (MPa) |
|---|---|---|---|
| CSBR | 3.45 ± 0.14 | 162.17 ± 13 | 1.75 ± 0.02 |
| CSBR/OMMT3 | 3.54 ± 0.27 | 176.48 ± 03 | 2.08 ± 0.38 |
| CSBR/OMMT6 | 3.63 ± 1.17 | 188.21 ± 27 | 2.37 ± 0.09 |
| CSBR/OMMT9 | 4.02 ± 0.32 | 223.30 ± 17 | 2.47 ± 0.11 |
| CSBR/OMMT12 | 4.89 ± 0.37 | 257.59 ± 09 | 2.71 ± 0.14 |
| CSBR/OMMT15 | 6.25 ± 0.29 | 347.20 ± 26 | 3.00 ± 0.15 |
| Film Sample | Crosslink Density (mol·cm−3) × 10−4 |
|---|---|
| pure SBR | 0 |
| CSBR | 0.29 |
| CSBR/OMMT3 | 1.04 |
| CSBR/OMMT6 | 1.09 |
| CSBR/OMMT9 | 0.99 |
| CSBR/OMMT12 | 1.24 |
| CSBR/OMMT15 | 3.22 |
| Membrane | Total Flux (g m−2 h−1) | Separation Factor | PSI (g m−2 h−1) |
|---|---|---|---|
| CSBR/OMMT3 | 126.31 | 1.03 | 04.67 |
| CSBR/OMMT6 | 182.56 | 1.08 | 14.78 |
| CSBR/OMMT9 | 247.92 | 1.20 | 51.62 |
| CSBR/OMMT12 | 260.67 | 1.43 | 113.81 |
| CSBR/OMMT15 | 182.2 | 1.09 | 16.66 |
| Membrane | Toluene in the Feed (wt%) | T (°C) | J (g·m−2 h−1) | PSI (g·m−2 h−1) | Ref. | |
|---|---|---|---|---|---|---|
| CSBR/OMMT12 | 32% (azeotropic composition) | 30 | 260.67 | 1.43 | 113.81 | This study |
| SBR-1 a | 0.55 | 30 | 12 | 162 | 1932 | [34] |
| T5IL1 b | 27.2 | 24 | 75 | 128 | 9525 | [39] |
| NR-3 c | 10.5 | 30 | 2.3 | 25 | 55.2 | [34] |
| NR-20 d | 0.55 | 30 | 10 | 286 | 2850 | [68] |
| NRZ10 e | 32 | 30 | 286.87 | 45.9 | 12,880.46 | [33] |
| NRC10 f | 32 | 30 | 279.37 | 44.7 | 12,208.46 | [33] |
| NRCB10 g | 32 | 30 | 256.87 | 41.1 | 10,300.48 | [33] |
| NRZ | 10.5 | 30 | 66.25 | 113.7 | 7466.37 | [33] |
| NRC | 10.5 | 30 | 73.75 | 105.9 | 7736.37 | [33] |
| NRCB | 10.5 | 30 | 78.12 | 102.7 | 7944.80 | [33] |
| PDMS h | 32 | 25 | 95.36 | 2.67 | 159.25 | [38] |
| (PU-PDMS) i | 32 | 25 | 113.14 | 3.66 | 300.95 | [38] |
| Membrane | Di × 10−9 m2·s−1 | |
|---|---|---|
| Methanol | Toluene | |
| CSBR/OMMT3 | 0.11 | 0.30 |
| CSBR/OMMT6 | 0.57 | 1.53 |
| CSBR/OMMT9 | 1.30 | 3.58 |
| CSBR/OMMT12 | 0.56 | 1.55 |
| CSBR/OMMT15 | 0.81 | 1.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allel, A.; Benguergoura, H.; Naceur, M.W.; Ledoux, A.; Saeed, W.S.; Aouak, T. Poly(styrene-co-butadiene)/Maghnia-Organo-Montmorillonite Clay Nanocomposite. Preparation, Properties and Application as Membrane in the Separation of Methanol/Toluene Azeotropic Mixture by Pervaporation. Membranes 2021, 11, 921. https://doi.org/10.3390/membranes11120921
Allel A, Benguergoura H, Naceur MW, Ledoux A, Saeed WS, Aouak T. Poly(styrene-co-butadiene)/Maghnia-Organo-Montmorillonite Clay Nanocomposite. Preparation, Properties and Application as Membrane in the Separation of Methanol/Toluene Azeotropic Mixture by Pervaporation. Membranes. 2021; 11(12):921. https://doi.org/10.3390/membranes11120921
Chicago/Turabian StyleAllel, Amina, Hassiba Benguergoura, Mohamed Wahib Naceur, Alain Ledoux, Waseem Sharaf Saeed, and Taïeb Aouak. 2021. "Poly(styrene-co-butadiene)/Maghnia-Organo-Montmorillonite Clay Nanocomposite. Preparation, Properties and Application as Membrane in the Separation of Methanol/Toluene Azeotropic Mixture by Pervaporation" Membranes 11, no. 12: 921. https://doi.org/10.3390/membranes11120921
APA StyleAllel, A., Benguergoura, H., Naceur, M. W., Ledoux, A., Saeed, W. S., & Aouak, T. (2021). Poly(styrene-co-butadiene)/Maghnia-Organo-Montmorillonite Clay Nanocomposite. Preparation, Properties and Application as Membrane in the Separation of Methanol/Toluene Azeotropic Mixture by Pervaporation. Membranes, 11(12), 921. https://doi.org/10.3390/membranes11120921







