Incorporation of Au Nanoparticles on ZnO/ZnS Core Shell Nanostructures for UV Light/Hydrogen Gas Dual Sensing Enhancement
Abstract
:1. Introduction
2. Materials and Methods
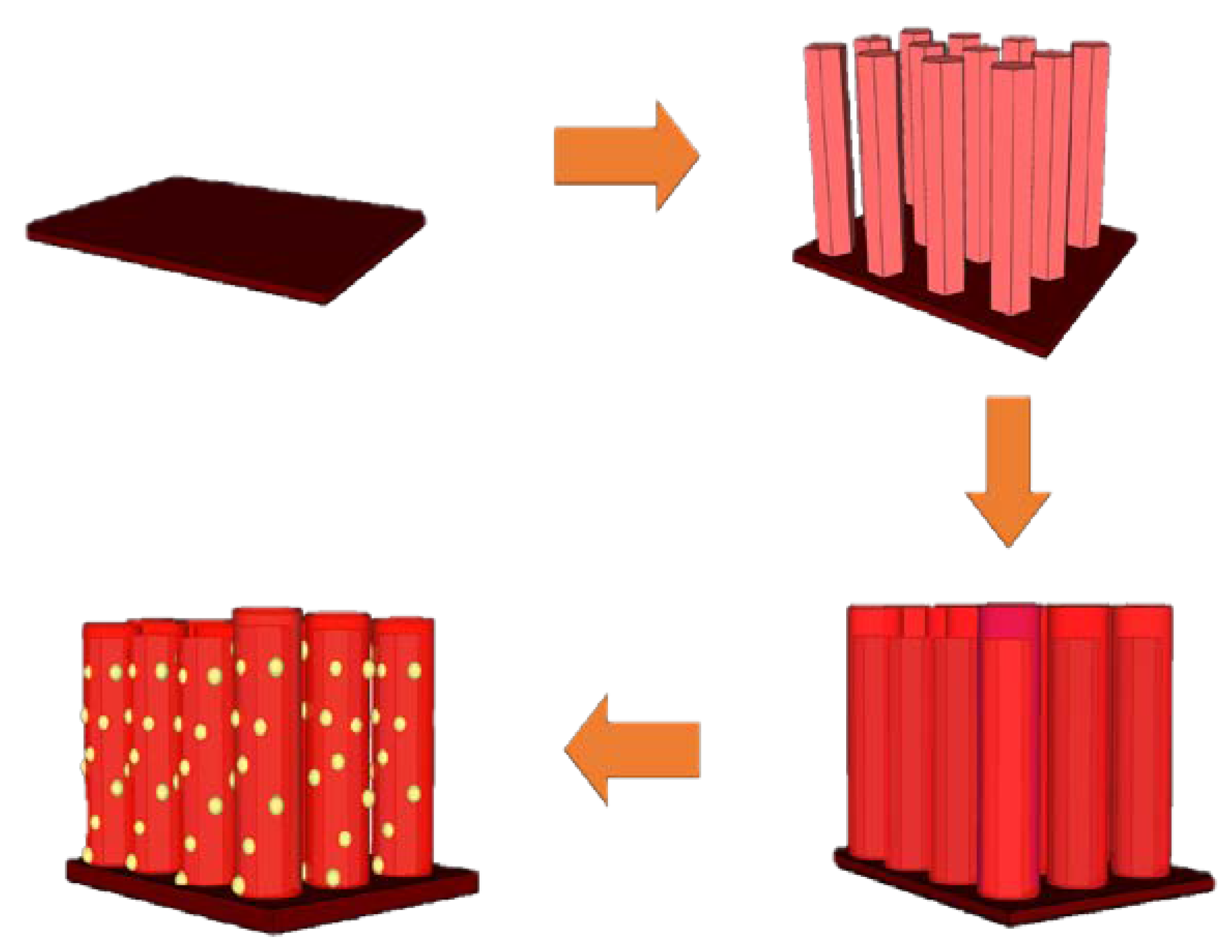
2.1. Preparation of Sensor Chip Substrate
2.2. Preparation of the ZnO Seed Layer
2.3. Preparation of ZnO NRs
2.4. Synthesis of ZnS Shells on the Surface of ZnO NRs
2.5. 20 nm Au NP Dropping
2.6. Gas Sensitivity
2.7. Light Sensitivity
2.8. Material Characterizations
- (1)
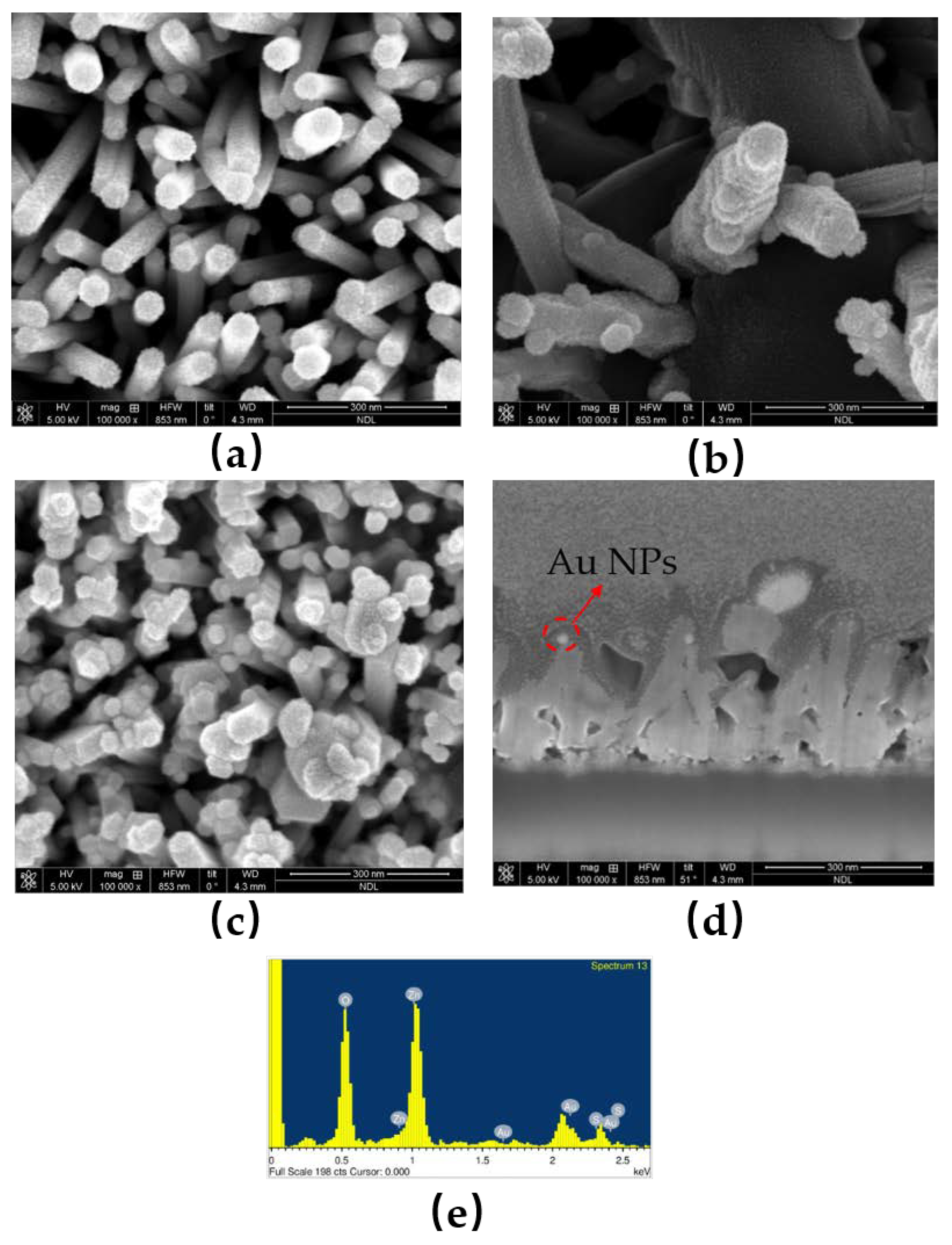
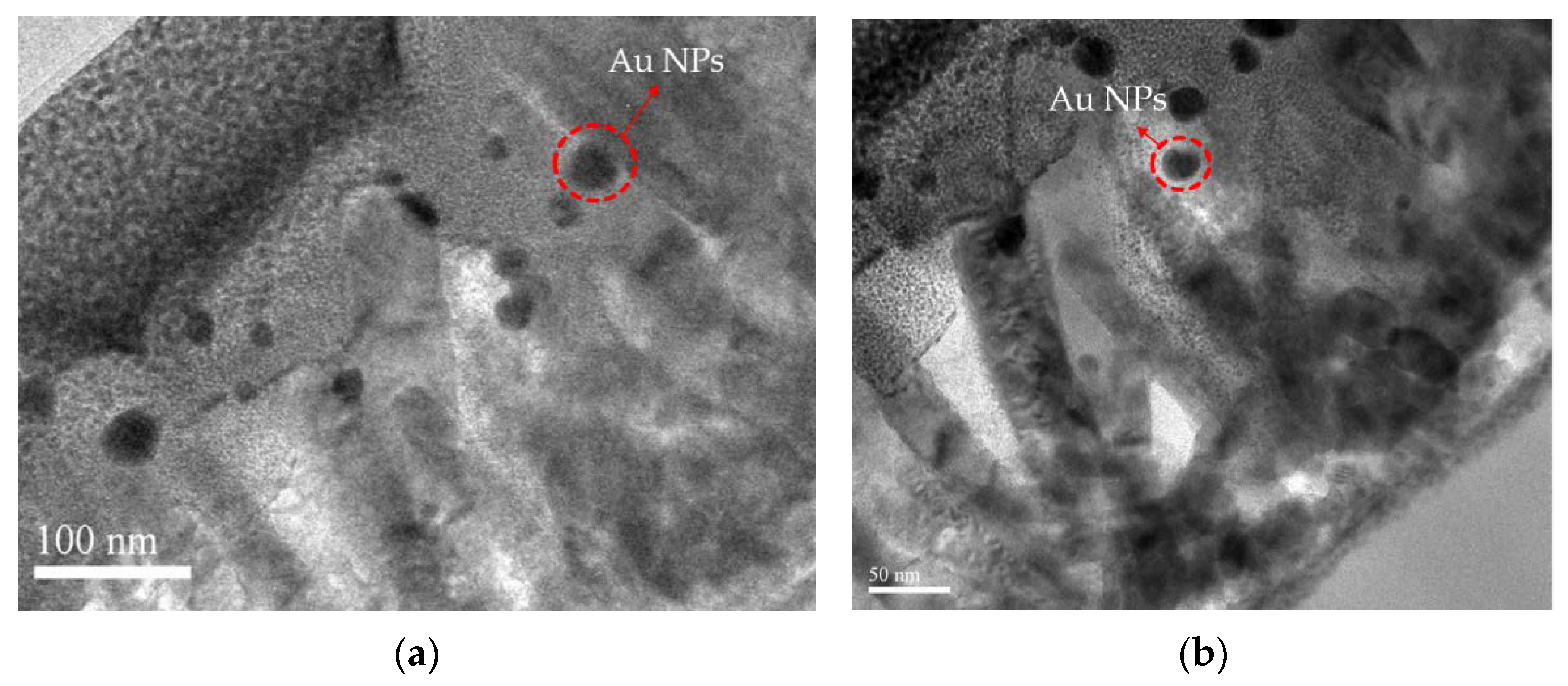
- FESEM and TEM: The FESEM and TEM images were obtained using JEOL JSM-7500F and JEOL JEM 2100 PLUS instruments, respectively. The operating voltages for SEM and TEM were 15 kV and 200 KV, respectively.
- (2)
- XPS: The XPS instrument was a VG Scientific ESCALAB 250 spectrometer. The light source was a twin anode X-ray gun with a maximum energy of 15 kV. The XR5 mono-chromated X-ray gun, with a maximum energy of 15 kV, 200 W, had an aluminum target with a beam size of 650–120 um.
- (3)
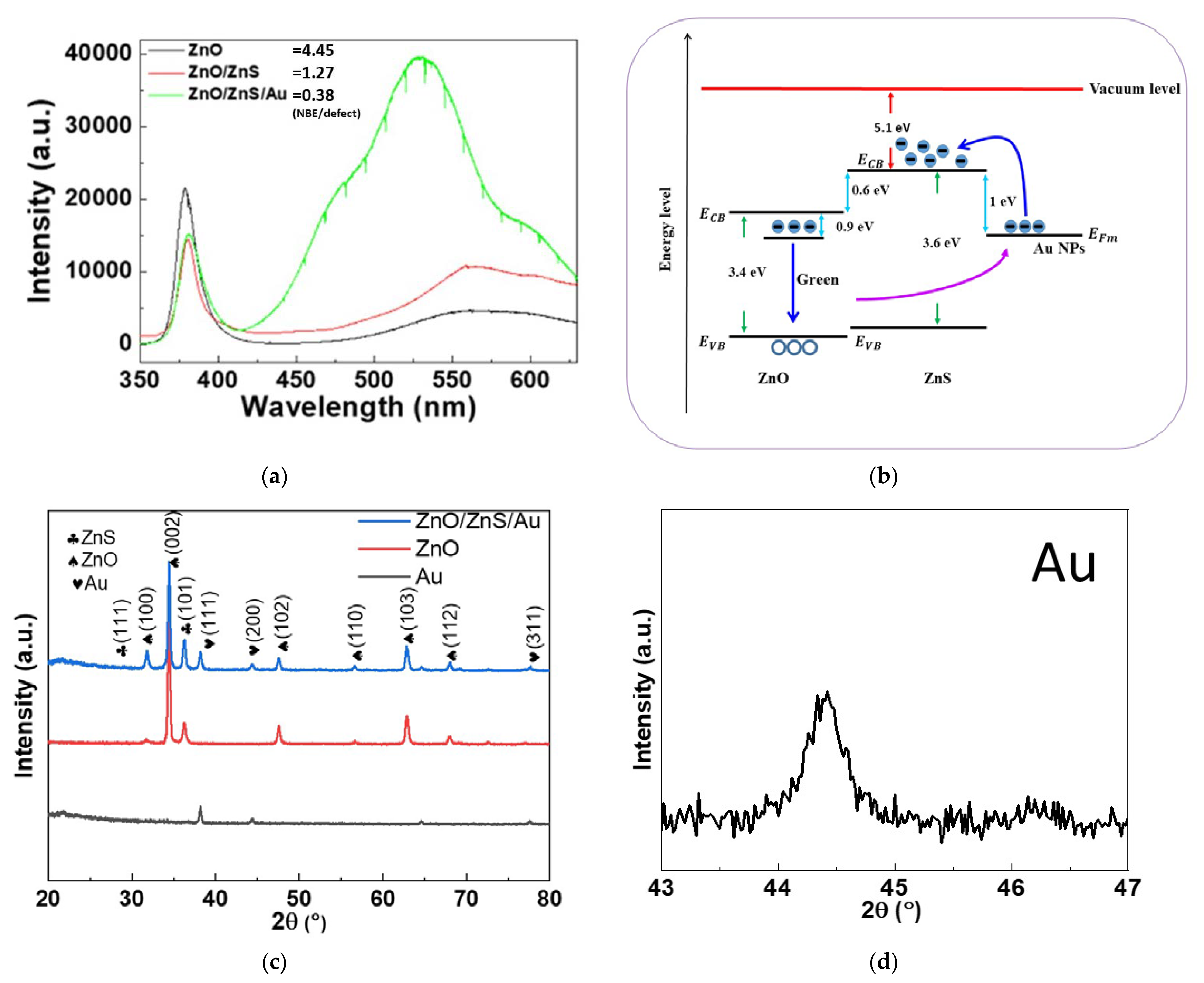
- PL: The PL spectra were obtained using a HI-TACHI F-4500 fluorescence spectrophotometer. The excitation laser wavelength was 325 nm with a laser spot diameter of 1 μm. The PL spectral range was 330–1000 nm (CCD sensor) and 1000–1500 nm (In-GaAs sensor).
- (4)
- XRD: The XRD patterns were acquired using a Bruker D8 Discover microdiffractometer. For the XRD analysis of the samples, a grazing incidence of X-ray beam CuKa (k = 1.542 Å) radiation was used with an incidence angle step of 0.5° in the diffraction angle range (2θ) from 20° to 60°.
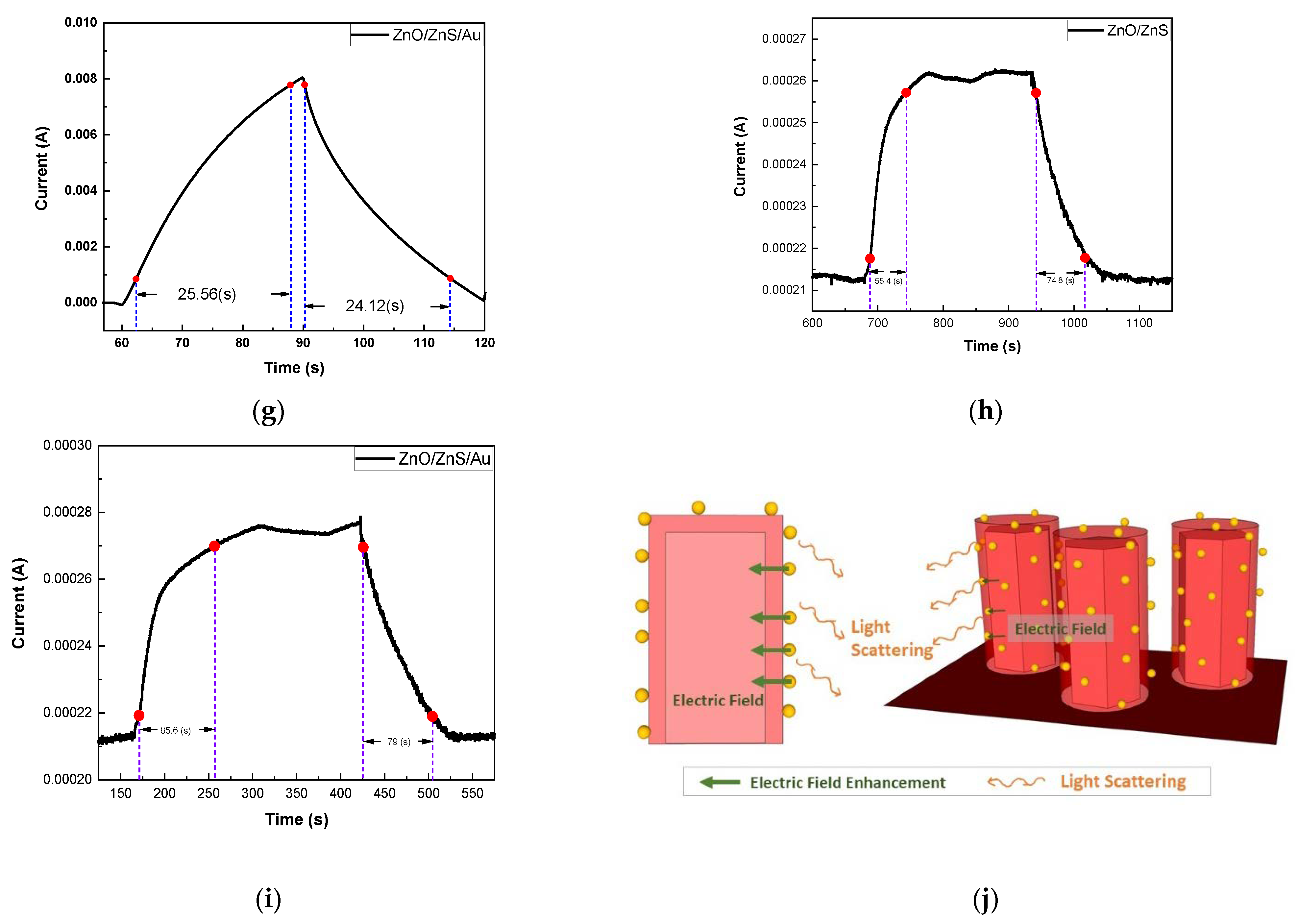
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-surfactant-assisted hydrothermal synthesis of one-dimensional ZnO nanorods for high-performance ethanol gas sensor. Sens. Actuators B Chem. 2019, 286, 501–511. [Google Scholar] [CrossRef]
- Ding, P.; Xu, D.; Dong, N.; Chen, Y.; Xu, P.; Zheng, D.; Li, X. A high-sensitivity H2S gas sensor based on optimized ZnO-ZnS nano-heterojunction sensing material. Chin. Chem. Lett. 2020, 31, 2050–2054. [Google Scholar] [CrossRef]
- Yi, H.; Siwei, X.; Xiang, L.; Jie, J.; Dandan, S.; Shiyong, G. Performance of self-powered UV photodetector based on ZnO/ZnS Heterojunction. Chin. J. Mater. Res. 2019, 33, 523–529. [Google Scholar]
- Khanlary, M.R.; Tarzi, S. Study of structural, optical and morphological properties of ZnO/ZnS hetrostructures deposited by spray pyrolysis method. Opt. Quantum Electron. 2021, 53, 13. [Google Scholar] [CrossRef]
- Deb, S.; Kalita, P. Green synthesis of copper sulfide (CuS) nanostructures for heterojunction diode applications. J. Mater. Sci. Mater. Electron. 2021, 32, 24125–24137. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H.; Li, T.; Huang, B.; Hu, W.; Jiang, Z.; Chen, J.; Niu, Q. Preparation of flower-like ZnO@ZnS core-shell structure enhances photocatalytic hydrogen production. Int. J. Hydrog. Energy 2020, 45, 26967–26978. [Google Scholar] [CrossRef]
- Rahman, G.; Nawab, W.; Zazai, W.; Bilal, S.; Mian, S.A. Exploring the structural and charge storage properties of Ni–ZnS/ZnO composite synthesized by one-pot wet chemical route. Mater. Chem. Phys. 2020, 252, 123203. [Google Scholar] [CrossRef]
- Serrà, A.; Artal, R.; García-Amorós, J.; Sepúlveda, B.; Gómez, E.; Nogués, J.; Philippe, L. Hybrid Ni@ZnO@ZnS-Microalgae for Circular Economy: A Smart Route to the Efficient Integration of Solar Photocatalytic Water Decontamination and Bioethanol Production. Adv. Sci. 2020, 7, 1902447. [Google Scholar] [CrossRef]
- Hassan, M.A.; Johar, M.A.; Waseem, A.; Bagal, I.V.; Ha, J.-S.; Ryu, S.-W. Type-II ZnO/ZnS core-shell nanowires: Earth-abundant photoanode for solar-driven photoelectrochemical water splitting. Opt. Express 2019, 27, A184–A196. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Sharma, S.; Kotnala, R. Tailoring magnetic and photoluminescence properties in ZnS/ZnO core/shell nanostructures through Cr doping. Appl. Surf. Sci. 2013, 284, 33–39. [Google Scholar] [CrossRef]
- Chu, Y.-L.; Ji, L.-W.; Hsiao, Y.-J.; Lu, H.-Y.; Young, S.-J.; Tang, I.-T.; Chu, T.-T.; Chen, X.-J. Fabrication and characterization of Ni-Doped ZnO nanorod arrays for UV photodetector application. J. Electrochem. Soc. 2020, 167, 67506. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.-W.; Sun, D.; Zou, Y.; Cheng, L.; He, C.; Wang, H.; Niu, C.; Wang, L. Au decorated hollow ZnO@ZnS heterostructure for enhanced photocatalytic hydrogen evolution: The insight into the roles of hollow channel and Au nanoparticles. Appl. Catal. B Environ. 2019, 244, 748–757. [Google Scholar] [CrossRef]
- Tsai, Y.-S.; Lin, X.; Wu, Y.S.; Chen, H.; Han, J. Dual UV light and CO gas sensing properties of ZnO/ZnS hybrid nanocomposite. IEEE Sens. J. 2021, 21, 11040–11045. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Z.; Huang, H.; Liu, Z.; Chen, D.; Shen, G. Gas sensors, thermistor and photodetector based on ZnS nanowires. J. Mater. Chem. 2012, 22, 6845–6850. [Google Scholar] [CrossRef]
- Lin, C.-W.; Huang, K.-L.; Chang, K.-W.; Chen, J.-H.; Chen, K.-L.; Wu, C.-H. Ultraviolet photodetector and gas sensor based on amorphous In-Ga-Zn-O film. Thin Solid Film. 2016, 618, 73–76. [Google Scholar] [CrossRef]
- Tsai, Y.-S.; Tsai, S.C.; Kuo, C.C.; Chan, W.L.; Lin, W.H.; Wu, Y.S.; Lin, Y.S.; Li, M.H.; Kuo, M.-Y.; Chen, H. Organic/inorganic hybrid nanostructures of polycrystalline perylene diimide decorated ZnO nanorods highly enhanced dual sensing performance of UV light/CO gas sensors. Results Phys. 2021, 24, 104173. [Google Scholar] [CrossRef]
- Horsham, C.; Antrobus, J.; Olsen, C.M.; Ford, H.; Abernethy, D.; Hacker, E. Testing wearable UV sensors to improve sun protection in young adults at an outdoor festival: Field study. JMIR Mhealth Uhealth 2020, 8, e21243. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Kim, J.-Y.; Lee, J.-H.; Kim, J.-H.; Iatsunskyi, I.; Coy, E.; Miele, P.; Bechelany, M.; Kim, S.S. Highly efficient hydrogen sensors based on Pd nanoparticles supported on boron nitride coated ZnO nanowires. J. Mater. Chem. A 2019, 7, 8107–8116. [Google Scholar] [CrossRef]
- Le, H.-J.; Van Dao, D.; Yu, Y.-T. Superfast and efficient hydrogen gas sensor using PdAualloy@ZnO core–shell nanoparticles. J. Mater. Chem. A 2020, 8, 12968–12974. [Google Scholar] [CrossRef]
- Polsongkram, D.; Chamninok, P.; Pukird, S.; Chow, L.; Lupan, O.; Chai, G.; Khallaf, H.; Park, S.; Schulte, A. Effect of synthesis conditions on the growth of ZnO nanorods via hydrothermal method. Phys. B Condens. Matter 2008, 403, 3713–3717. [Google Scholar] [CrossRef]
- Binsbergen, F. Heterogeneous nucleation in the crystallization of polyolefins: Part 1. Chemical and physical nature of nucleating agents. Polymer 1970, 11, 253–267. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Cho, C.-F.; Chiu, J.L.; Tsai, C.-T.; Chen, H. Hydrothermal fabrication and characterization of ZnO/ZnS core-shell structures on white reflective films. Results Phys. 2018, 10, 449–457. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO plasmonic nanostructures for enhanced UV photodetector and room temperature NO sensing devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Guo, Q.; Lin, S.; Chen, B.; Zheng, D. Growth and properties of ZnO/ZnS core/shell nanostructures. J. Phys. Conf. Ser. 2009, 152, 012018. [Google Scholar] [CrossRef]
- Wang, K.; Chen, J.; Zeng, Z.; Tarr, J.; Zhou, W.; Zhang, Y.; Yan, Y.; Jiang, C.; Pern, J.; Mascarenhas, A. Synthesis and photovoltaic effect of vertically aligned ZnO/ZnS core/shell nanowire arrays. Appl. Phys. Lett. 2010, 96, 123105. [Google Scholar] [CrossRef] [Green Version]
- Tsai, Y.-S.; Hung, C.H.; Chan, W.L.; Tsai, S.J.; Lee, Y.S.; Huang, W.C.; Wu, Y.S.; Chen, H. Morphological and crystalline analysis of ZnO/ZnS nanostructures on porous silicon substrate. Vacuum 2020, 178, 109454. [Google Scholar] [CrossRef]
- Huang, S.-C.; Lu, C.-C.; Su, W.-M.; Weng, C.-Y.; Chen, Y.-C.; Wang, S.-C.; Lu, T.-C.; Chen, C.-P.; Chen, H. Characterization of spatial manipulation on ZnO nanocomposites consisting of Au nanoparticles, a graphene layer, and ZnO nanorods. Appl. Phys. A 2018, 124, 69. [Google Scholar] [CrossRef]
- Hu, Y.; Qian, H.; Liu, Y.; Du, G.; Zhang, F.; Wang, L.; Hu, X. A microwave-assisted rapid route to synthesize ZnO/ZnS core–shell nanostructures via controllable surface sulfidation of ZnO nanorods. CrystEngComm 2011, 13, 3438–3443. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50 nm. J. Am. Chem. Soc. 2003, 125, 4430–4431. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.C.; Wang, K.; Ding, Y.; Marmon, J.K.; Bhatt, M.; Zhang, Y.; Zhou, W.; Wang, Z.L. Piezo-phototronic effect enhanced UV/visible photodetector based on fully wide band gap type-II ZnO/ZnS core/shell nanowire array. ACS Nano 2015, 9, 6419–6427. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-C.; Weng, W.C.; Lin, H.I.; Chiu, J.L.; Jhao, H.-Y.; Liao, Y.T.A.; Yu, C.T.R.; Chen, H. Fabrication and characterization of distinctive ZnO/ZnS core–shell structures on silicon substrates via a hydrothermal method. RSC Adv. 2018, 8, 26341–26348. [Google Scholar] [CrossRef] [Green Version]
- Sookhakian, M.; Amin, Y.M.; Basirun, W.J.; Tajabadi, M.; Kamarulzaman, N. Synthesis, structural, and optical properties of type-II ZnO–ZnS core–shell nanostructure. J. Lumin. 2014, 145, 244–252. [Google Scholar] [CrossRef]
- Tsai, Y.-S.; Chou, T.-W.; Xu, C.Y.; Huang, W.C.; Lin, C.F.; Wu, Y.S.; Lin, Y.-S.; Chen, H. ZnO/ZnS core-shell nanostructures for hydrogen gas sensing performances. Ceram. Int. 2019, 45, 17751–17757. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, S.; Gao, F.; Wang, L.; Xie, Z.; Yang, W. Enhanced field emission of Au nanoparticle-decorated SiC nanowires. J. Mater. Chem. C 2016, 4, 1363–1368. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, C.; Yang, M.-S.; Jeong, S.; Kim, D.; Kang, T. Two-dimensional hyper-branched gold nanoparticles synthesized on a two-dimensional oil/water interface. Sci. Rep. 2014, 4, 6119. [Google Scholar] [CrossRef]
- Pham, X.-H.; Lee, M.; Shim, S.; Jeong, S.; Kim, H.-M.; Hahm, E.; Lee, S.H.; Lee, Y.-S.; Jeong, D.H.; Jun, B.-H. Highly sensitive and reliable SERS probes based on nanogap control of a Au–Ag alloy on silica nanoparticles. RSC Adv. 2017, 7, 7015–7021. [Google Scholar] [CrossRef] [Green Version]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The key role of active sites in the development of selective metal oxide sensor materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, B.; Hajzadeh, I.; Taheri, M.; Karimzadeh, R.; Mohajerzadeh, S.; Mohseni, S. Plasmonic improvement photoresponse of vertical-MoS2 nanostructure photodetector by Au nanoparticles. Appl. Surf. Sci. 2019, 490, 165–171. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Liao, M.; Aono, M. Giant improvement of the performance of ZnO nanowire photodetectors by Au nanoparticles. J. Phys. Chem. C 2010, 114, 19835–19839. [Google Scholar] [CrossRef]


| Item | Materials | Gas/Light | Operating Codition | Sensivity | Ref. |
|---|---|---|---|---|---|
| 1 | ZnS | Gas: Acetone, Ethanol Light: 254 (nm), 365 (nm) | Gas: 320 °C Light: 5 V | Gas: 21.1 a, 13.3 a Light: 12 b, 28 b | [14] |
| 2 | In-Ga-Zn-O | Gas: O3 Light: 365 (nm) | Gas: UV intensity ~945 mW/m2 Light: N/A | Gas 4.74 c Light: 23,924.31 d | [15] |
| 3 | ZnO/ZnS | Gas: CO Light: 395 (nm) | Gas: 200 °C Light: 0.1 V | Gas 1.561 e Light: 65.6 f | [13] |
| 4 | ZnO/ Perylene diimide | Gas: CO Light: 395 (nm) | Gas: 200 °C Light: 2 V | Gas 1.0851 N/A Light: 4.114 (A/W) | [16] |
| 5 | ZnO/ZnS/Au | Gas: H2 Light: 365 (nm) | Gas: 300 °C Light: 5 V | Gas 0.384 c Light: 673.33 d | This work |
| O | S | Zn | Au | |
|---|---|---|---|---|
| Weight% | 32.08 | 13.04 | 50.41 | 4.47 |
| Atomic% | 62.55 | 12.69 | 24.05 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-S.; Wang, D.-Y.; Chang, J.-J.; Liang, K.-T.; Lin, Y.-H.; Kuo, C.-C.; Lu, S.-H.; Wu, Y.S.; Lee, L.J.-H.; Chen, H.; et al. Incorporation of Au Nanoparticles on ZnO/ZnS Core Shell Nanostructures for UV Light/Hydrogen Gas Dual Sensing Enhancement. Membranes 2021, 11, 903. https://doi.org/10.3390/membranes11110903
Tsai Y-S, Wang D-Y, Chang J-J, Liang K-T, Lin Y-H, Kuo C-C, Lu S-H, Wu YS, Lee LJ-H, Chen H, et al. Incorporation of Au Nanoparticles on ZnO/ZnS Core Shell Nanostructures for UV Light/Hydrogen Gas Dual Sensing Enhancement. Membranes. 2021; 11(11):903. https://doi.org/10.3390/membranes11110903
Chicago/Turabian StyleTsai, Yu-Sheng, Deng-Yi Wang, Jia-Jie Chang, Keng-Tien Liang, Ya-Hsuan Lin, Chih-Chen Kuo, Ssu-Han Lu, Yewchung Sermon Wu, Lukas Jyuhn-Hsiarn Lee, Hsiang Chen, and et al. 2021. "Incorporation of Au Nanoparticles on ZnO/ZnS Core Shell Nanostructures for UV Light/Hydrogen Gas Dual Sensing Enhancement" Membranes 11, no. 11: 903. https://doi.org/10.3390/membranes11110903
APA StyleTsai, Y.-S., Wang, D.-Y., Chang, J.-J., Liang, K.-T., Lin, Y.-H., Kuo, C.-C., Lu, S.-H., Wu, Y. S., Lee, L. J.-H., Chen, H., & Wuu, D.-S. (2021). Incorporation of Au Nanoparticles on ZnO/ZnS Core Shell Nanostructures for UV Light/Hydrogen Gas Dual Sensing Enhancement. Membranes, 11(11), 903. https://doi.org/10.3390/membranes11110903







