Characterization of Cryopreserved Canine Amniotic Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Canine Amniotic Membranes
2.2. Media
2.3. Sample Collection
2.4. Cryopreservation
2.5. Tensile Strength Test
2.6. Transparency Test
2.7. Cell Viability Test
2.8. Data Analysis
3. Results
3.1. Tensile Strength Test
3.2. Transparency Test
3.3. Cell Viability Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maggs, D.; Miller, P.; Ofri, R. Slatter’s Fundamentals of Veterinary Ophthalmology, 5th ed.; Elsevier Health Sciences: St. Louis, MO, USA, 2017. [Google Scholar]
- Bussieres, M.; Krohne, S.G.; Stiles, J.; Townsend, W. The use of porcine small intestinal submucosa for the repair of full-thickness corneal defects in dogs, cats and horses. Vet. Ophthalmol. 2004, 7, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Riggs, C.M.; Chow, D.W.Y. The use of porcine urinary bladder matrix (UBM) to repair a perforated corneal ulcer with iris prolapse in a horse. Equine Vet. Educ. 2017, 31, 172–178. [Google Scholar] [CrossRef]
- Dulaurent, T.; Azoulay, T.; Goulle, F.; Dulaurent, A.; Mentek, M.; Peiffer, R.L.; Isard, P.-F. Use of bovine pericardium (Tutopatch®) graft for surgical repair of deep melting corneal ulcers in dogs and corneal sequestra in cats. Vet. Ophthalmol. 2013, 17, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Brikshavana, P.; Kanchanachaya, T. Multilayer Human Amniotic Membrane Transplantation for Reconstruction of Corneal Perforation in Dogs. In Proceedings of the 9th Chulalongkorn University Veterinary Annual Conference, Bangkok, Thailand, 1 April 2010; Chulalongkorn University Press: Bangkok, Thailand, 2010. A38. p. 132. [Google Scholar]
- Tuntivanich, P.; Tuntivanich, N. The use of human amniotic membrane for the treatment of descemetocoele in a dog. J. Thai Vet. Pract. 2006, 18, 57–66. [Google Scholar]
- Lacerda, R.P.; Gimenez, M.T.P.; Laguna, F.; Costa, D.; Ríos, J.; Leiva, M. Corneal grafting for the treatment of full-thickness corneal defects in dogs: A review of 50 cases. Vet. Ophthalmol. 2016, 20, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, F.J.; Kallberg, M.E.; Plummer, C.E.; Barrie, K.P.; O’Reilly, S.; Taylor, D.P.; Gelatt, K.N.; Brooks, D.E. Amniotic membrane transplantation for corneal surface reconstruction after excision of corneolimbal squamous cell carcinomas in nine horses. Vet. Ophthalmol. 2006, 9, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, Y.M.; Jeong, S.W.; Williams, D.L. Effect of bovine freeze-dried amniotic membrane (Amnisite-BA™) on uncomplicated canine corneal erosion. Vet. Ophthalmol. 2009, 12, 36–42. [Google Scholar] [CrossRef]
- Tsuzuki, K.; Yamashita, K.; Izumisawa, Y.; Kotani, T. Microstructure and glycosaminoglycan ratio of canine cornea after reconstructive transplantation with glycerin-preserved porcine amniotic membranes. Vet. Ophthalmol. 2008, 11, 222–227. [Google Scholar] [CrossRef]
- Barros, P.S.M.; Safatle, A.M.V.; Godoy, C.A.; Souza, M.S.B.; Barros, L.F.M.; Brooks, D.E. Amniotic membrane transplantation for the reconstruction of the ocular surface in three cases. Vet. Ophthalmol. 2005, 8, 189–192. [Google Scholar] [CrossRef]
- Berguiga, M.; Mameletzi, E.; Nicolas, M.; Rivier, D.; Majo, F. Long-Term Follow-Up of Multilayer Amniotic Membrane Transplantation (MLAMT) for Non-Traumatic Corneal Perforations or Deep Ulcers with Descemetocele. Klin. Mon. Augenheilkd. 2013, 230, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Barachetti, L.; Giudice, C.; Mortellaro, C.M. Amniotic membrane transplantation for the treatment of feline corneal sequestrum: Pilot study. Vet. Ophthalmol. 2010, 13, 326–330. [Google Scholar] [CrossRef]
- Wichayacoop, T.; Wongpithayadisai, K.; Chaiprakit, K.; Briksawan, P.; Sunthronvipat, K.; Tuntivanich, P.; Puangsricharern, V. The use of human amniotic membrane for deep corneal ulcer repair in dogs. Thai J. Vet. Med. 2005, 35, 97–102. [Google Scholar]
- Plummer, C.E. The use of amniotic membrane transplantation for ocular surface reconstruction: A review and series of 58 equine clinical cases (2002–2008). Vet. Ophthalmol. 2009, 12, 17–24. [Google Scholar] [CrossRef]
- Laurent, R.; Nallet, A.; Obert, L.; Nicod, L.; Gindraux, F. Storage and qualification of viable intact human amniotic graft and technology transfer to a tissue bank. Cell Tissue Bank. 2014, 15, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Walter, P.; Salla, S.; Johnen, S.; Plange, N.; Rütten, S.; Goecke, T.W.; Fuest, M. Cryopreservation of amniotic membrane with and without glycerol additive. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1117–1126. [Google Scholar] [CrossRef]
- Laranjo, M. Preservation of Amniotic Membrane. In Amniotic Membrane; Mamede, A., Botelho, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 209–230. [Google Scholar]
- Riau, A.; Beuerman, R.W.; Lim, L.S.; Mehta, J.S. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 2010, 31, 216–225. [Google Scholar] [CrossRef]
- Tseng, S.C.G. Evolution of amniotic membrane transplantation. Clin. Exp. Ophthalmol. 2007, 35, 109–110. [Google Scholar] [CrossRef]
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank. 2017, 18, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Thomasen, H.; Pauklin, M.; Noelle, B.; Geerling, G.; Vetter, J.; Steven, P.; Steuhl, K.-P.; Meller, D. The Effect of Long-Term Storage on the Biological and Histological Properties of Cryopreserved Amniotic Membrane. Curr. Eye Res. 2010, 36, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Miglino, M.A.; Ambrósio, C.E.; Martins, D.D.S.; Wenceslau, C.V.; Pfarrer, C.; Leiser, R. The carnivore pregnancy: The development of the embryo and fetal membranes. Theriogenology 2006, 66, 1699–1702. [Google Scholar] [CrossRef]
- Kalpravidh, M.; Tuntivanich, P.; Vongsakul, S.; Sirivaidyapong, S. Canine amniotic membrane transplantation for corneal reconstruction after the excision of dermoids in dogs. Vet. Res. Commun. 2009, 33, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Vongsakul, S.; Tuntivanich, P.; Sirivaidyapong, S.; Kalpravidh, M. Canine amniotic membrane transplantation for ocular surface reconstruction of created deep corneal ulcers in dogs. Thai J. Vet. Med. 2009, 39, 135–144. [Google Scholar]
- Nam, E.; Takahashi, A.; Fujita, N.; Tsuzuki, K.; Nishimura, R. Cultivation of corneal epithelial cell sheets on canine amniotic membrane. Vet. Ophthalmol. 2012, 16, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Chuck, R.S.; Graff, J.M.; Bryant, M.R.; Sweet, P.M. Biomechanical Characterization of Human Amniotic Membrane Preparations for Ocular Surface Reconstruction. Ophthalmic Res. 2004, 36, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hariya, T.; Tanaka, Y.; Yokokura, S.; Nakazawa, T. Transparent, resilient human amniotic membrane laminates for corneal transplantation. Biomaterials 2016, 101, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Von Versen-Hoeynck, F.; Steinfeld, A.P.; Becker, J.; Hermel, M.; Rath, W.; Hesselbarth, U. Sterilization and preservation influence the biophysical properties of human amnion grafts. Biologicals 2008, 36, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Borazjani, A.; Weed, B.C.; Patnaik, S.S.; Feugang, J.M.; Christiansen, D.; Elder, S.H.; Ryan, P.L.; Liao, J. A comparative biomechanical analysis of term fetal membranes in human and domestic species. Am. J. Obstet. Gynecol. 2011, 204, 365.e25–365.e36. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, G.A. Biomechanics of soft tissue. In The Handbook of Materials Behavior Models; Biomech Preprint; Academic Press: San Diego, CA, USA, 2001; Volume 3, pp. 1049–1063. [Google Scholar]
- Connon, C.J.; Doutch, J.; Chen, B.; Hopkinson, A.; Mehta, J.S.; Nakamura, T.; Kinoshita, S.; Meek, K.M. The variation in transparency of amniotic membrane used in ocular surface regeneration. Br. J. Ophthalmol. 2009, 94, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Hennerbichler, S.; Reichl, B.; Pleiner, D.; Gabriel, C.; Eibl, J.; Redl, H. The influence of various storage conditions on cell viability in amniotic membrane. Cell Tissue Bank. 2006, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Feng, Z.; Kosawada, T.; Sato, D.; Nakamura, T.; Umezu, M. Stress relaxation and stress-strain characteristics of porcine amniotic membrane. Bio-Med. Mater. Eng. 2017, 27, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Schenke-Layland, K.; Madershahian, N.; Riemann, I.; Starcher, B.; Halbhuber, K.-J.; König, K.; Stock, U.A. Impact of Cryopreservation on Extracellular Matrix Structures of Heart Valve Leaflets. Ann. Thorac. Surg. 2006, 81, 918–926. [Google Scholar] [CrossRef]
- Pogozhykh, O.; Hofmann, N.; Gryshkov, O.; Von Von Kaisenberg, C.; Mueller, M.; Glasmacher, B.; Pogozhykh, D.; Börgel, M.; Blasczyk, R.; Figueiredo, C. Repeated Freezing Procedures Preserve Structural and Functional Properties of Amniotic Membrane for Application in Ophthalmology. Int. J. Mol. Sci. 2020, 21, 4029. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kubota, A.; Yokokura, S.; Uematsu, M.; Shi, D.; Yamato, M.; Okano, T.; Quantock, A.J.; Nishida, K. Optical mechanical refinement of human amniotic membrane by dehydration and cross-linking. J. Tissue Eng. Regen. Med. 2012, 6, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Börgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A review on tissue engineering, application, and storage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1198–1215. [Google Scholar] [CrossRef]
- Koh, C.T.; Tonsomboon, K.; Oyen, M.L. Fracture toughness of human amniotic membranes. Interface Focus 2019, 9, 20190012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhasawat, P.; Tesavibul, N.; Komolsuradej, W. Single and multilayer amniotic membrane transplantation for persistent corneal epithelial defect with and without stromal thinning and perforation. Br. J. Ophthalmol. 2001, 85, 1455–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, J.T.G.d.O.; Costa, M.M.; Alves, P.C.S.; Sant’Anna, L.B. Effects of Preservation Methods in the Composition of the Placental and Reflected Regions of the Human Amniotic Membrane. Cells Tissues Organs 2021, 210, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Becktell, L.; Matuska, A.M.; Hon, S.; Delco, M.L.; Cole, B.J.; Begum, L.; Zhang, S.; Fortier, L.A. Proteomic Analysis and Cell Viability of Nine Amnion, Chorion, Umbilical Cord, and Amniotic Fluid–Derived Products. Cartilage 2020, 1947603520976767. [Google Scholar] [CrossRef]
- Qureshi, I.Z.; Fareeha, A.; Khan, W.A. Technique for processing and preservation of human amniotic membrane for ocular surface reconstruction. World Acad. Sci. Eng. Technol. 2010, 69, 763–766. [Google Scholar]
- Favaron, P.; Carvalho, R.; Borghesi, J.; Anunciação, A.; Miglino, M. The amniotic membrane: Development and potential applications—A review. Reprod. Domest. Anim. 2015, 50, 881–892. [Google Scholar] [CrossRef]
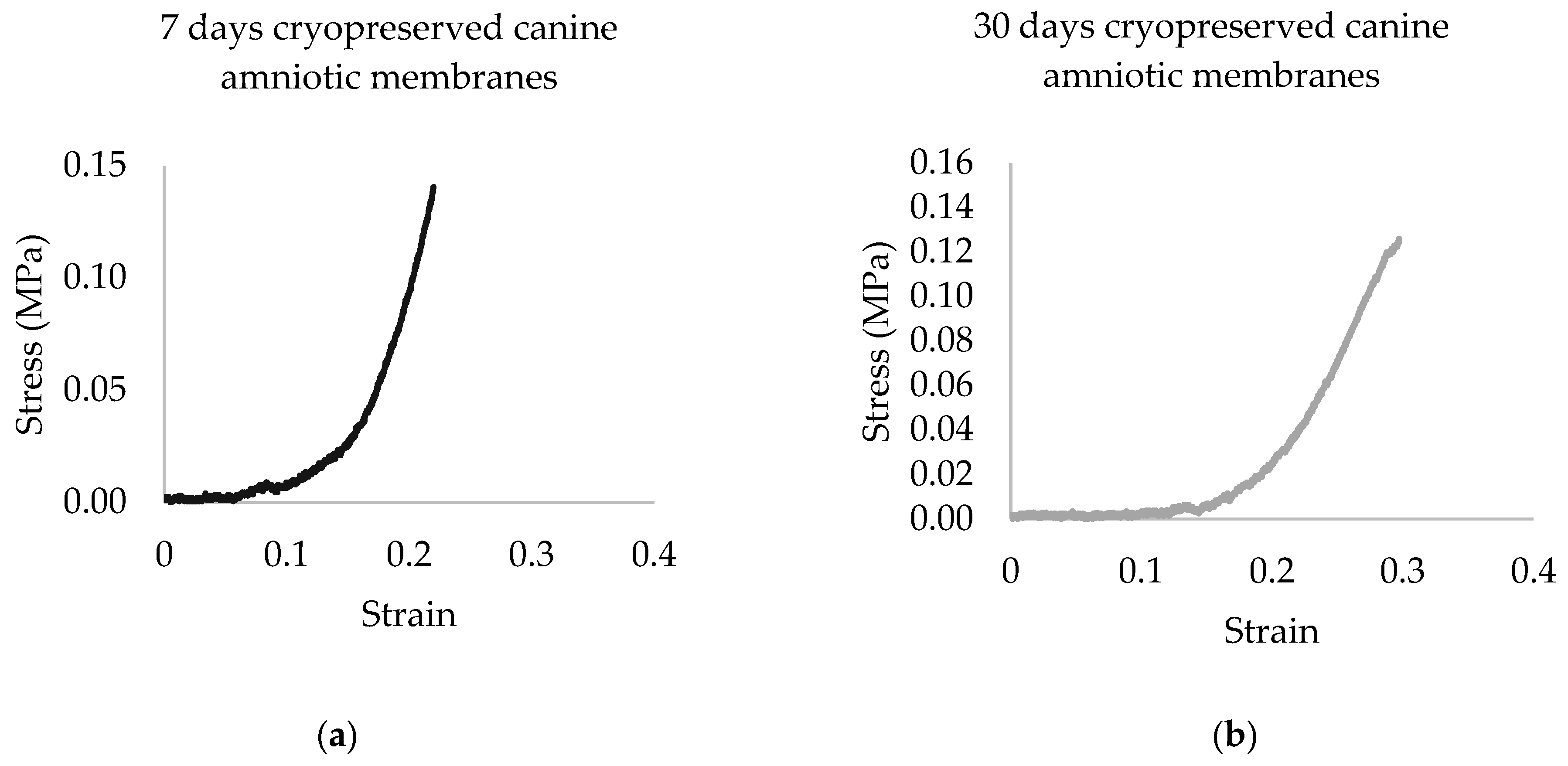
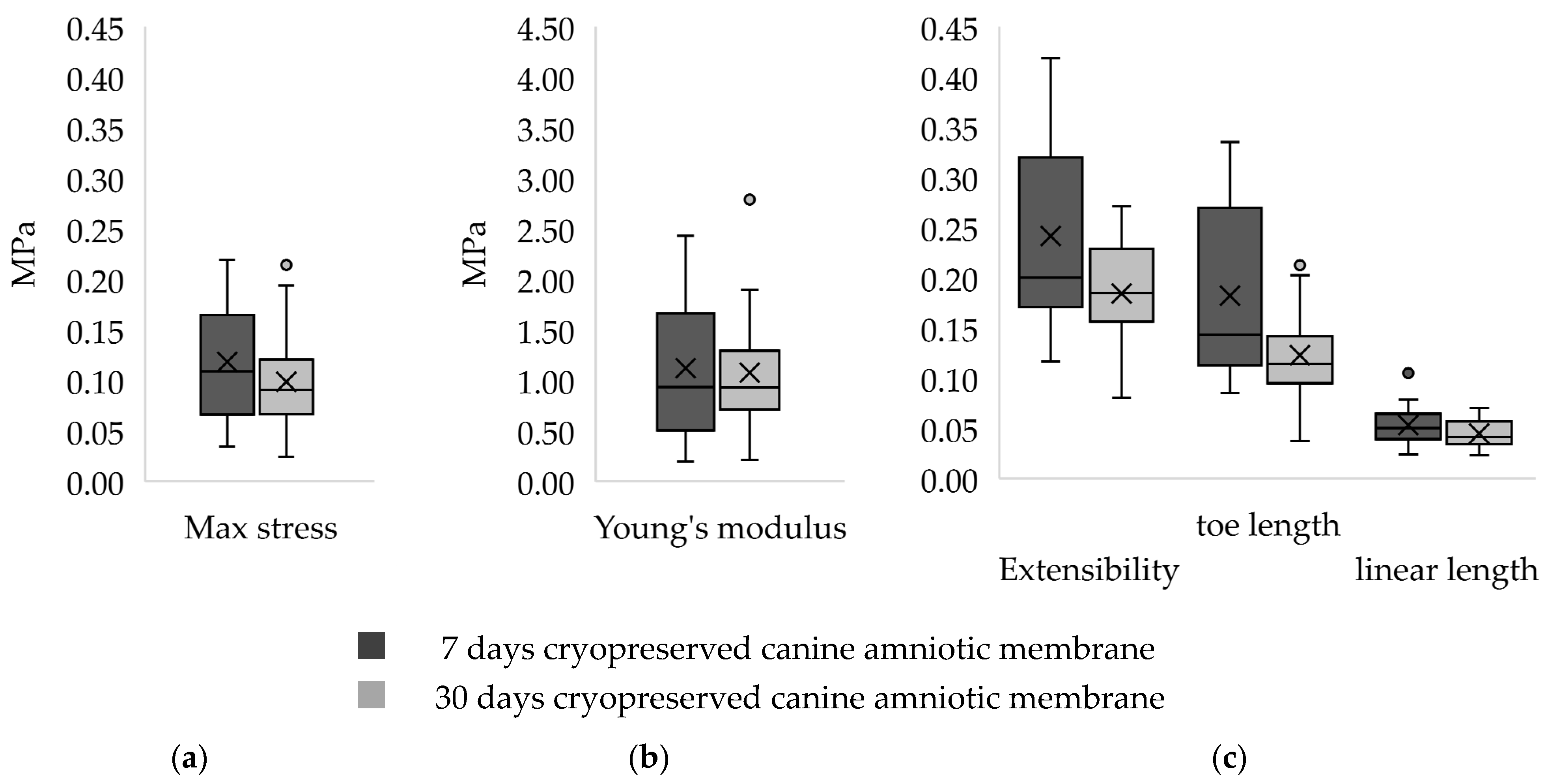
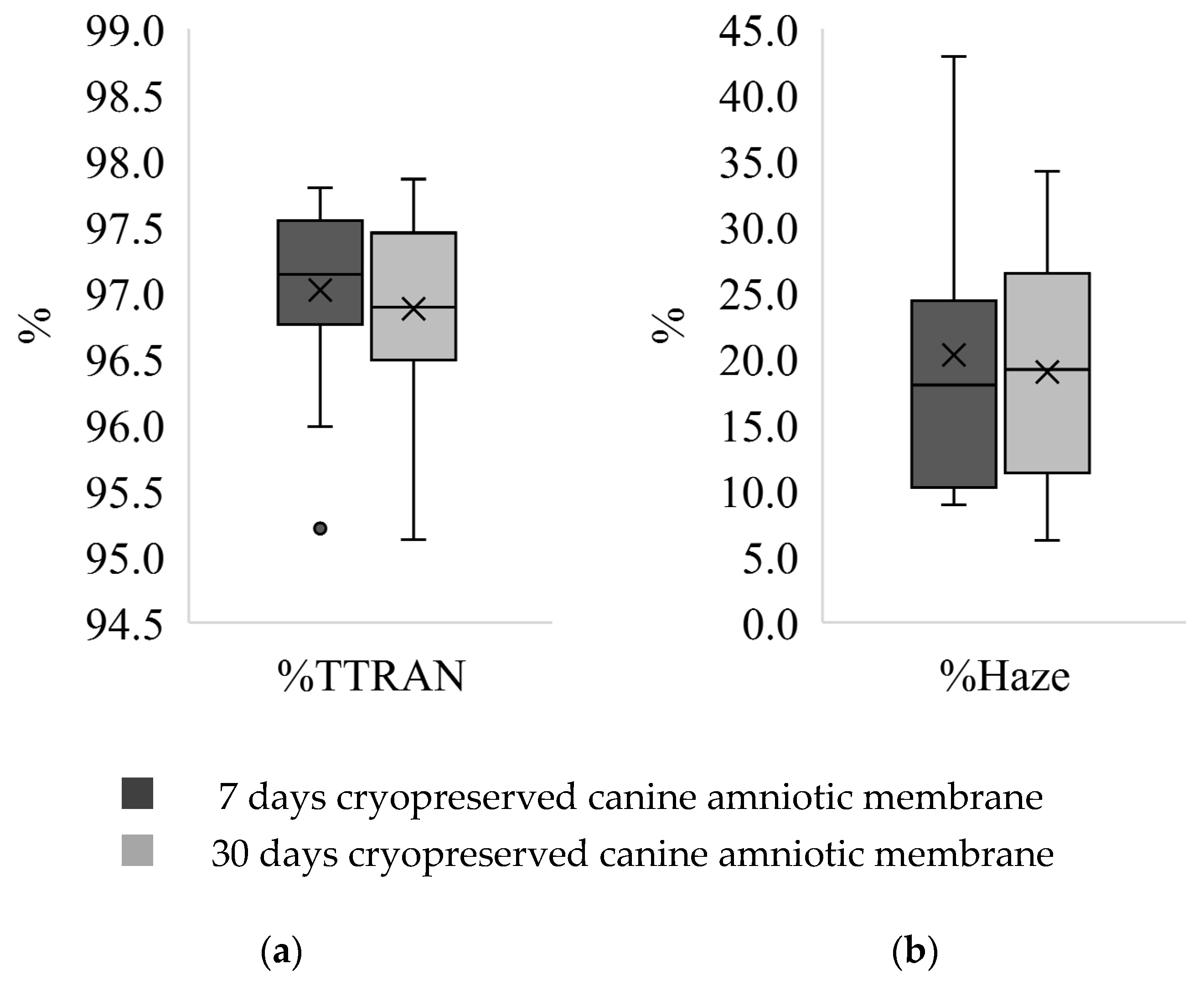
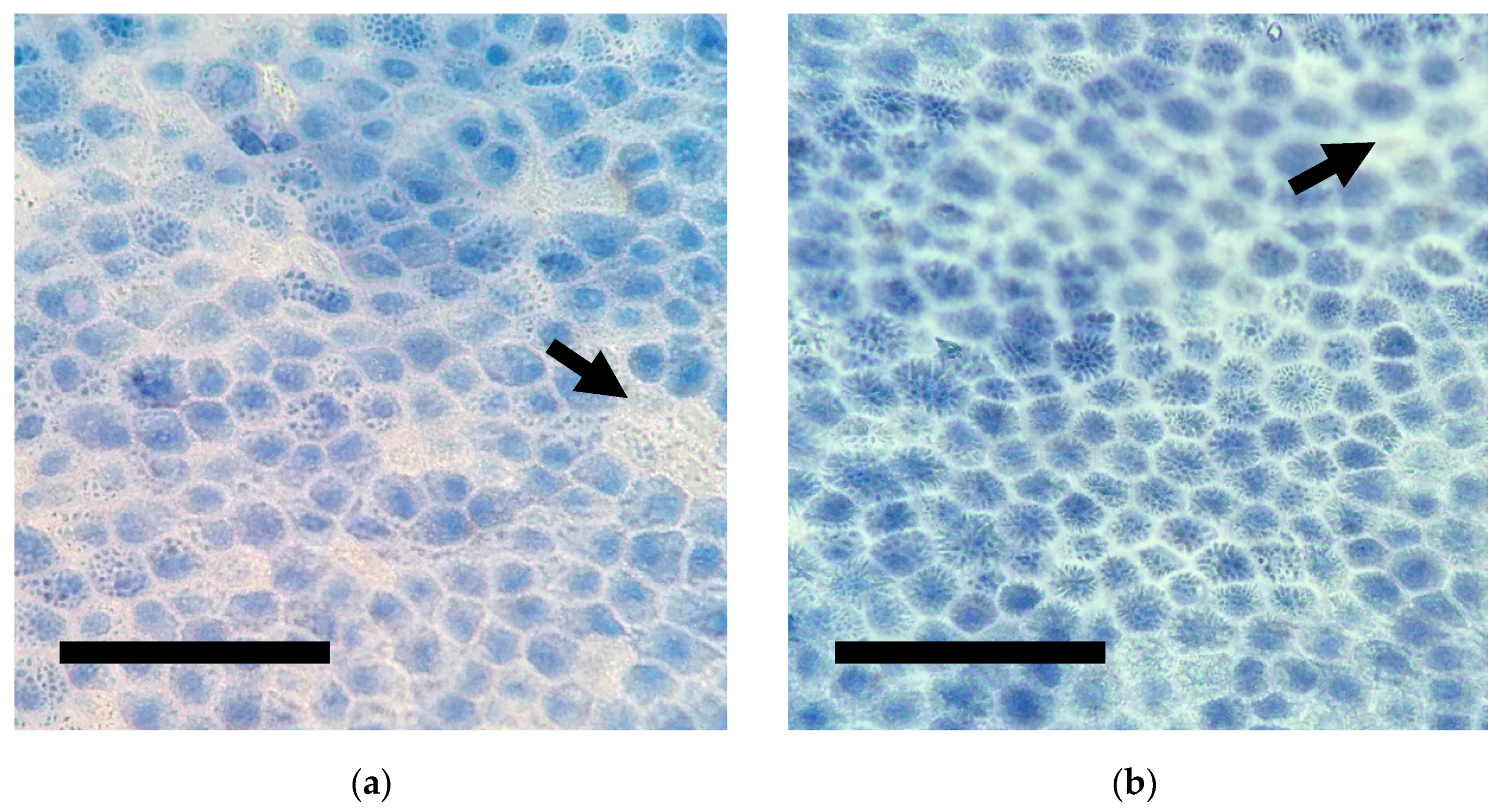
| Parameter/Canine Amniotic Membrane | 7 Days 1 | 30 Days 1 |
|---|---|---|
| Maximum stress (MPa) | 0.11 ± 0.10 | 0.09 ± 0.05 |
| Extensibility | 0.29 ± 0.15 | 0.19 ± 0.07 |
| Young’s Modulus (MPa) | 0.93 ± 1.16 | 0.11 ± 0.05 |
| Toe region length | 0.14 ± 0.16 | 0.11 ± 0.05 |
| Linear region length | 0.05 ± 0.03 | 0.04 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Withavatpongtorn, N.; Tuntivanich, N. Characterization of Cryopreserved Canine Amniotic Membrane. Membranes 2021, 11, 824. https://doi.org/10.3390/membranes11110824
Withavatpongtorn N, Tuntivanich N. Characterization of Cryopreserved Canine Amniotic Membrane. Membranes. 2021; 11(11):824. https://doi.org/10.3390/membranes11110824
Chicago/Turabian StyleWithavatpongtorn, Nathawan, and Nalinee Tuntivanich. 2021. "Characterization of Cryopreserved Canine Amniotic Membrane" Membranes 11, no. 11: 824. https://doi.org/10.3390/membranes11110824
APA StyleWithavatpongtorn, N., & Tuntivanich, N. (2021). Characterization of Cryopreserved Canine Amniotic Membrane. Membranes, 11(11), 824. https://doi.org/10.3390/membranes11110824







