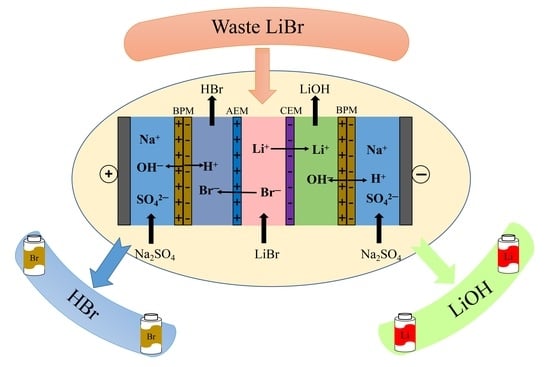
Recycling Lithium from Waste Lithium Bromide to Produce Lithium Hydroxide
Abstract
:1. Introduction
2. Experiments
2.1. Materials
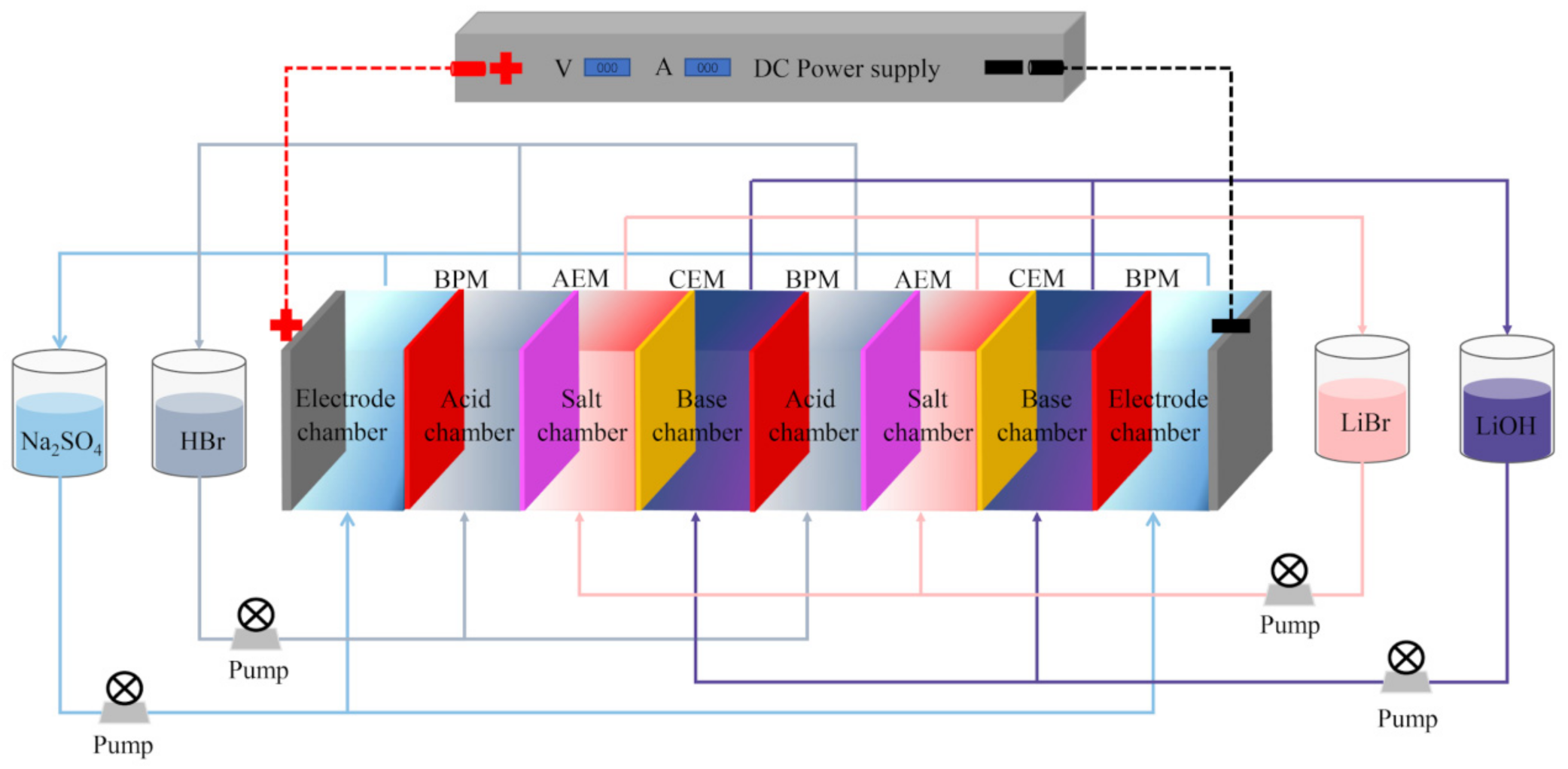
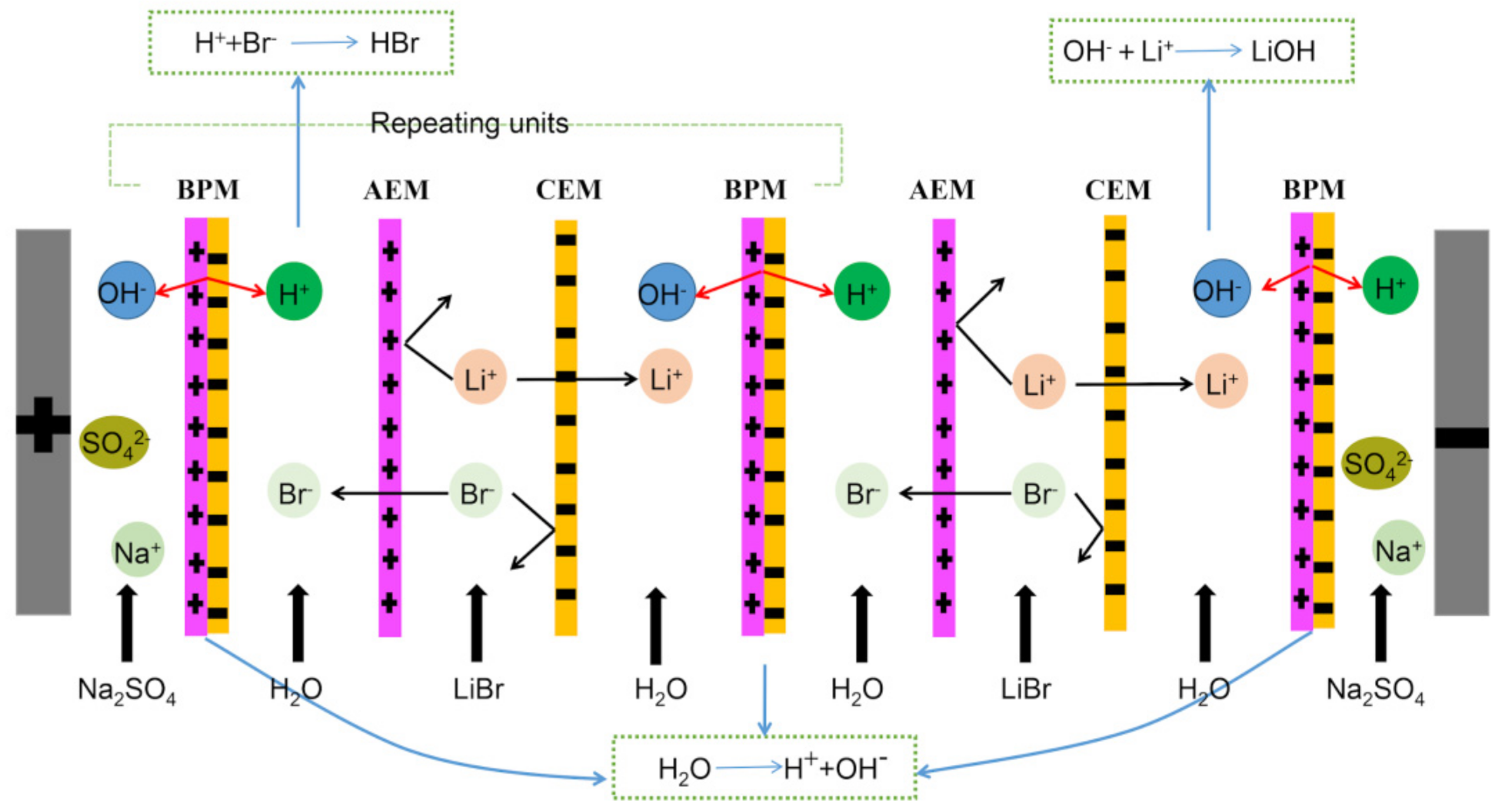
2.2. Experimental Set-Up
2.3. Analysis and Calculation
3. Results and Discussion
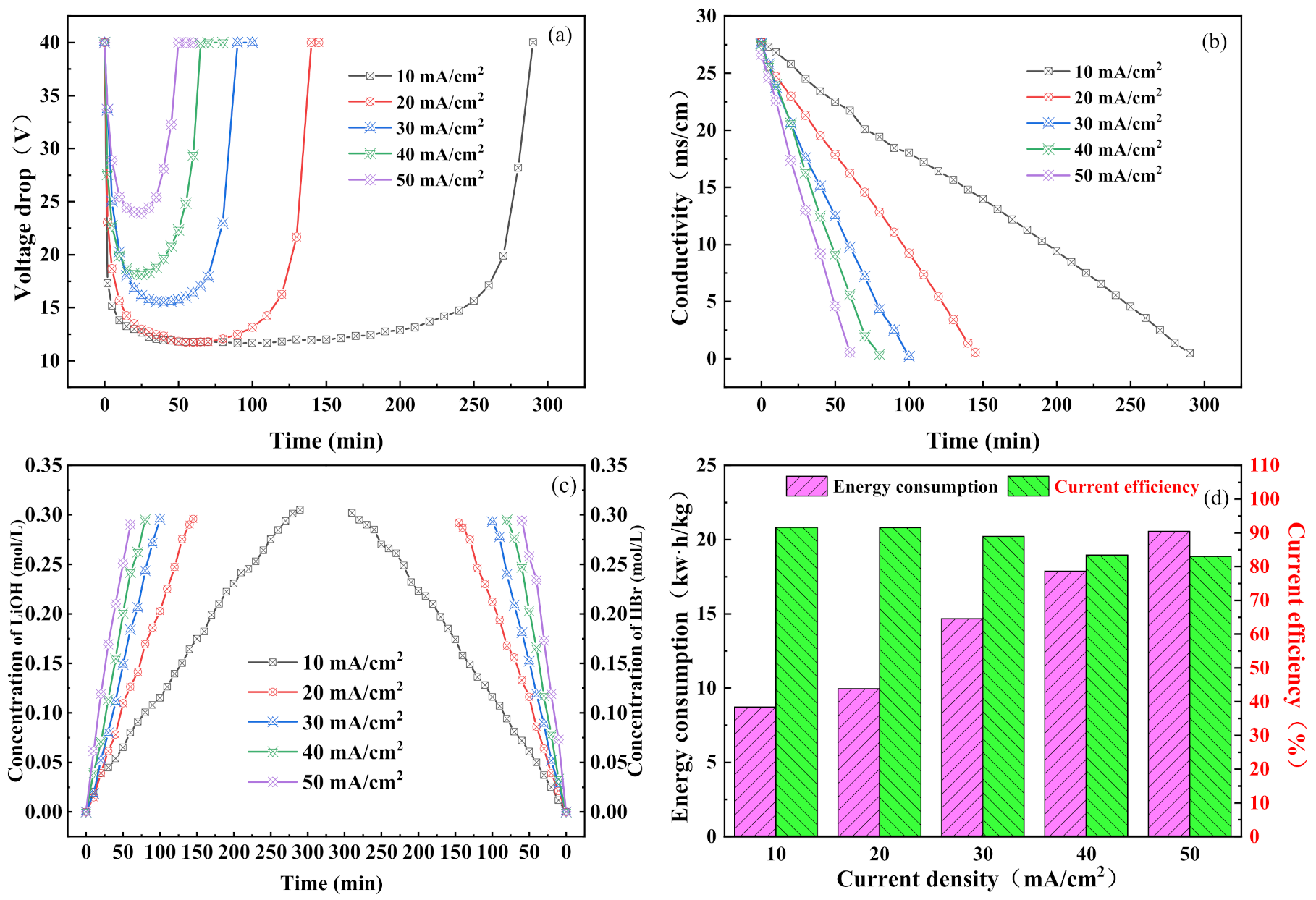
3.1. Effect of Current Density
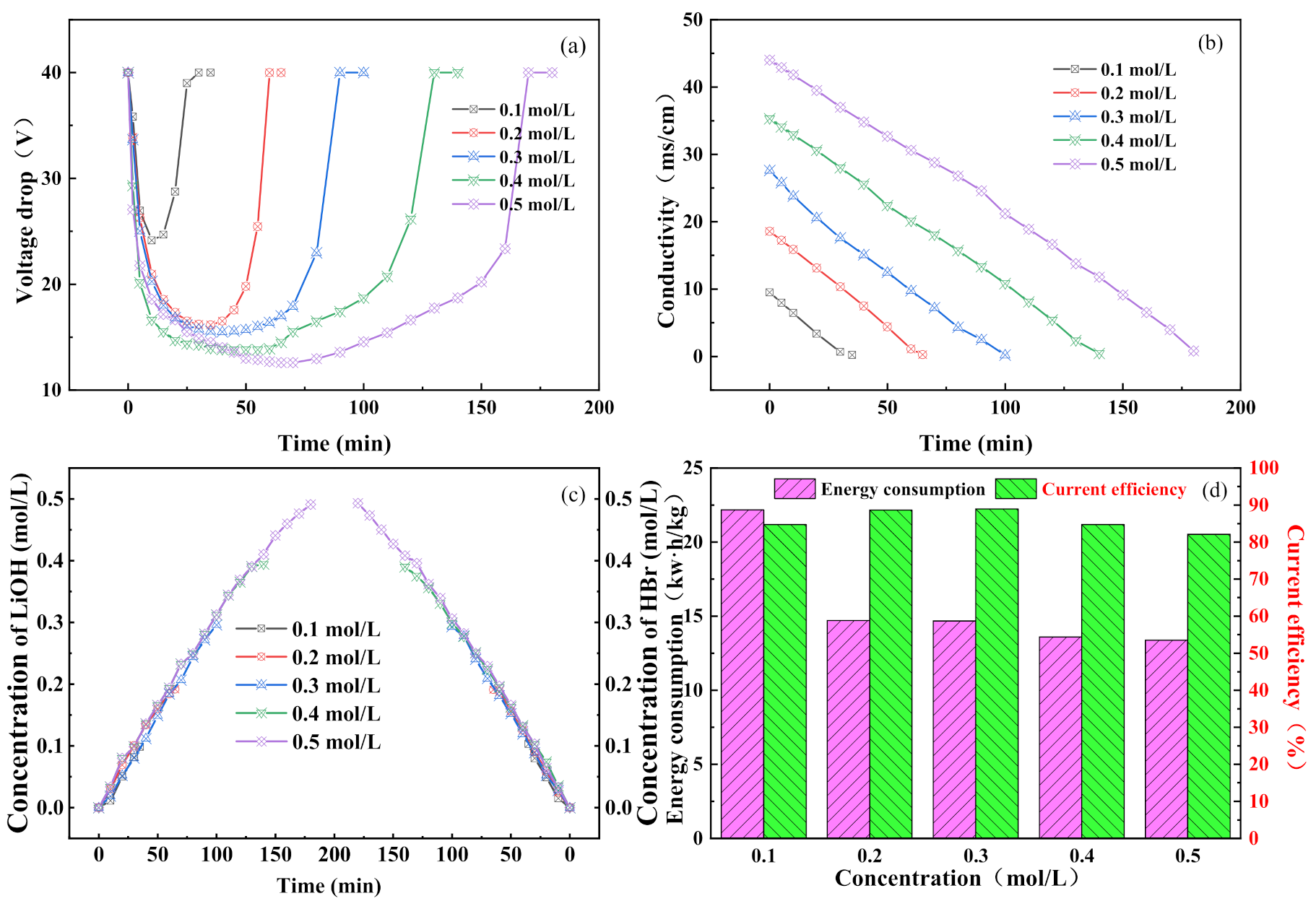
3.2. Effect of Feed Concentration
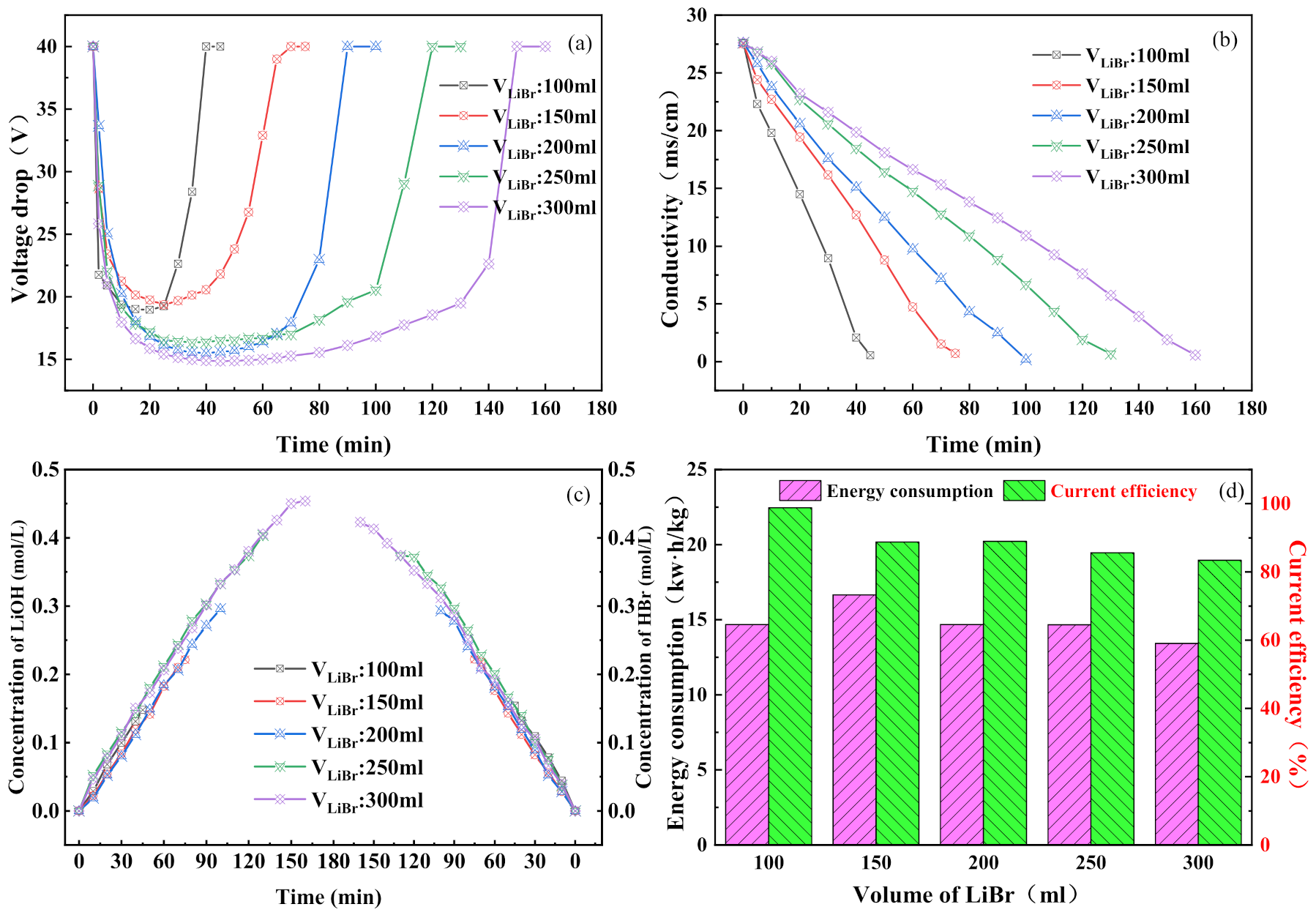
3.3. Effect of Initial Salt Chamber Volume
3.4. Economic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lang, J.; Jin, Y.; Liu, K.; Long, Y.; Zhang, H.; Qi, L.; Wu, H.; Cui, Y. High-purity electrolytic lithium obtained from low-purity sources using solid electrolyte. Nat. Sustain. 2020, 3, 386–390. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Sanders, M. Lithium-ion battery raw material supply and demand 2016–2025. In Proceedings of the Advanced Automotive Battery Conference, San Francisco, CA, USA, 19–22 June 2017; pp. 162–181. [Google Scholar]
- Razmjou, A.; Asadnia, M.; Hosseini, E.; Korayem, A.H.; Chen, V. Design principles of ion selective nanostructured membranes for the extraction of lithium ions. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, B.; Sofen, L.E.; Gisch, D.J.; Kern, B.; Rickard, M.A.; Francis, M.B. Lithium-Chelating Resins Functionalized with Oligoethylene Glycols toward Lithium-Ion Battery Recycling. Adv. Sustain. Syst. 2021, 5, 2000230. [Google Scholar] [CrossRef]
- Boryta, D.A. Solubility of lithium bromide in water between -50.deg. and +100.deg. (45 to 70% lithium bromide). J. Chem. Eng. Data 1970, 15, 142–144. [Google Scholar] [CrossRef]
- Muñoz, A.I.; Antón, J.G.; Guiñón, J.; Pérez-Herranz, V. The effect of chromate in the corrosion behavior of duplex stainless steel in LiBr solutions. Corros. Sci. 2006, 48, 4127–4151. [Google Scholar] [CrossRef]
- Pei, W.; Cheng, Q.; Jiao, S.; Liu, L. Performance evaluation of the electrodialysis regenerator for the lithium bromide solution with high concentration in the liquid desiccant air-conditioning system. Energy 2019, 187, 115928. [Google Scholar] [CrossRef]
- Peiró, L.T.; Méndez, G.V.; Ayres, R.U. Lithium: Sources, Production, Uses, and Recovery Outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Somers, C.; Mortazavi, A.; Hwang, Y.; Radermacher, R.; Rodgers, P.; Al-Hashimi, S. Modeling water/lithium bromide absorption chillers in ASPEN Plus. Appl. Energy 2011, 88, 4197–4205. [Google Scholar] [CrossRef]
- Kuang, G.; Liu, Y.; Li, H.; Xing, S.; Li, F.; Guo, H. Extraction of lithium from β-spodumene using sodium sulfate solution. Hydrometallurgy 2018, 177, 49–56. [Google Scholar] [CrossRef]
- Lajoie-Leroux, F.; Dessemond, C.; Soucy, G.; Laroche, N.; Magnan, J.-F. Impact of the impurities on lithium extraction from β-spodumene in the sulfuric acid process. Miner. Eng. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Battaglia, G.; Gurreri, L.; Farulla, G.A.; Cipollina, A.; Pirrotta, A.; Micale, G.; Ciofalo, M. Membrane Deformation and Its Effects on Flow and Mass Transfer in the Electromembrane Processes. Int. J. Mol. Sci. 2019, 20, 1840. [Google Scholar] [CrossRef] [Green Version]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Scarazzato, T.; Panossian, Z.; Ten’orio, J.A.S.; Pérez-Herranz, V.; Espinosa, D.C.R. A review of cleaner production in electroplating industries using electrodialysis. J. Clean. Prod. 2017, 168, 1590–1602. [Google Scholar] [CrossRef]
- Lin, J.; Lin, F.; Chen, X.; Ye, W.; Li, X.; Zeng, H.; Van Der Bruggen, B. Sustainable Management of Textile Wastewater: A Hybrid Tight Ultrafiltration/Bipolar-Membrane Electrodialysis Process for Resource Recovery and Zero Liquid Discharge. Ind. Eng. Chem. Res. 2019, 58, 11003–11012. [Google Scholar] [CrossRef]
- Xu, T.; Huang, C. Electrodialysis-based separation technologies: A critical review. AIChE J. 2008, 54, 3147–3159. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Jaroszek, H.; Dydo, P. Ion-exchange membranes in chemical synthesis—A review. Open Chem. 2016, 14, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ghyselbrecht, K.; Silva, A.; Van der Bruggen, B.; Boussu, K.; Meesschaert, B.; Pinoy, L. Desalination feasibility study of an industrial NaCl stream by bipolar membrane electrodialysis. J. Environ. Manag. 2014, 140, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Qiu, Y.; Zhao, Y.; Tang, C.; Shen, J. A continuous mode operation of bipolar membrane electrodialysis (BMED) for the production of high-pure choline hydroxide from choline chloride. Sep. Purif. Technol. 2020, 233, 116054. [Google Scholar] [CrossRef]
- Ye, W.; Huang, J.; Lin, J.; Zhang, X.; Shen, J.; Luis, P.; Van der Bruggen, B. Environmental evaluation of bipolar membrane electrodialysis for NaOH production from wastewater: Conditioning NaOH as a CO2 absorbent. Sep. Purif. Technol. 2015, 144, 206–214. [Google Scholar] [CrossRef]
- Zhao, W.-Y.; Zhou, M.; Yan, B.; Sun, X.; Liu, Y.; Wang, Y.; Xu, T.; Zhang, Y. Waste Conversion and Resource Recovery from Wastewater by Ion Exchange Membranes: State-of-the-Art and Perspective. Ind. Eng. Chem. Res. 2018, 57, 6025–6039. [Google Scholar] [CrossRef]
- Pelletier, S.; Élodie, S.; Mikhaylin, S.; Bazinet, L. Optimization of cranberry juice deacidification by electrodialysis with bipolar membrane: Impact of pulsed electric field conditions. Sep. Purif. Technol. 2017, 186, 106–116. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Yan, H.; Jiang, C.; Xu, T. A sustainable valorization of neopentyl glycol salt waste containing sodium formate via bipolar membrane electrodialysis. Sep. Purif. Technol. 2021, 254, 117563. [Google Scholar] [CrossRef]
- Gao, W.; Fang, Q.; Yan, H.; Wei, X.; Wu, K. Recovery of Acid and Base from Sodium Sulfate Containing Lithium Carbonate Using Bipolar Membrane Electrodialysis. Membranes 2021, 11, 152. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Wang, X.; Wang, Y.; Xu, T. A closed loop production of water insoluble organic acid using bipolar membranes electrodialysis (BMED). J. Membr. Sci. 2016, 520, 345–353. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Wang, Q.; Feng, H.; Xu, T. Production of Lithium Hydroxide from Lake Brines through Electro–Electrodialysis with Bipolar Membranes (EEDBM). Ind. Eng. Chem. Res. 2014, 53, 6103–6112. [Google Scholar] [CrossRef]
- Bunani, S.; Arda, M.; Kabay, N.; Yoshizuka, K.; Nishihama, S. Effect of process conditions on recovery of lithium and boron from water using bipolar membrane electrodialysis (BMED). Desalination 2017, 416, 10–15. [Google Scholar] [CrossRef]
- Gutiérrez, L.-F.; Bazinet, L.; Hamoudi, S.; Belkacemi, K. Production of lactobionic acid by means of a process comprising the catalytic oxidation of lactose and bipolar membrane electrodialysis. Sep. Purif. Technol. 2013, 109, 23–32. [Google Scholar] [CrossRef]
- Strathmann, H.; Krol, J.; Rapp, H.-J.; Eigenberger, G. Limiting current density and water dissociation in bipolar membranes. J. Membr. Sci. 1997, 125, 123–142. [Google Scholar] [CrossRef] [Green Version]
- Beck, A.; Ernst, M. Kinetic modeling and selectivity of anion exchange in Donnan dialysis. J. Membr. Sci. 2015, 479, 132–140. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, C.; Dominguez-Ramos, A.; Ibañez, R.; Chen, Y.; Irabien, A. Valorization of desalination brines by electrodialysis with bipolar membranes using nanocomposite anion exchange membranes. Desalination 2017, 406, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ke, X.; Wu, X.; Ke, C.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y.; Van Der Bruggen, B. Simultaneous Removal of Trivalent Chromium and Hexavalent Chromium from Soil Using a Modified Bipolar Membrane Electrodialysis System. Environ. Sci. Technol. 2020, 54, 13304–13313. [Google Scholar] [CrossRef]
- Shen, J.; Huang, J.; Liu, L.; Ye, W.; Lin, J.; Van der Bruggen, B. The use of BMED for glyphosate recovery from glyphosate neutralization liquor in view of zero discharge. J. Hazard. Mater. 2013, 260, 660–667. [Google Scholar] [CrossRef]
- Eisaman, M.D.; Alvarado, L.; Larner, D.; Wang, P.; Garg, B.; Littau, K.A. CO2 separation using bipolar membrane electrodialysis. Energy Environ. Sci. 2011, 4, 1319–1328. [Google Scholar] [CrossRef]
- Donnan, F.G. The Theory of Membrane Equilibria. Chem. Rev. 1924, 1, 73–90. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Berkessa, Y.W.; Lang, Q.; Yan, B.; Kuang, S.; Mao, D.; Shu, L.; Zhang, Y. Anion exchange membrane organic fouling and mitigation in salt valorization process from high salinity textile wastewater by bipolar membrane electrodialysis. Desalination 2019, 465, 94–103. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of nanofiltration and bipolar electrodialysis for valorization of seawater desalination brines: Production of drinking and waste water treatment chemicals. Desalination 2016, 382, 13–20. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.Q.; Wang, Q.; Xu, T.; Kentish, S.E. Transforming salty whey into cleaning chemicals using electrodialysis with bipolar membranes. Desalination 2020, 492, 114598. [Google Scholar] [CrossRef]
- Liu, Y.; Ke, X.; Zhu, H.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y.; Van der Bruggen, B. Treatment of raffinate generated via copper ore hydrometallurgical processing using a bipolar membrane electrodialysis system. Chem. Eng. J. 2020, 382, 122956. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Jiang, C.; Xu, T. Electrodialysis Process for the Recycling and Concentrating of Tetramethylammonium Hydroxide (TMAH) from Photoresist Developer Wastewater. Ind. Eng. Chem. Res. 2013, 52, 18356–18361. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Yan, H.; Wu, K.; Xu, T. Purification of Methylsulfonylmethane from Mixtures Containing Salt by Conventional Electrodialysis. Membranes 2020, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Karrech, A.; Azadi, M.; Elchalakani, M.; Shahin, M.; Seibi, A. A review on methods for liberating lithium from pegmatities. Miner. Eng. 2020, 145, 106085. [Google Scholar] [CrossRef]
| Membrane Characteristics | AMX | CMX | BP-1 |
|---|---|---|---|
| IEC (meq·g−1) | 1.4–1.7 | 1.5–1.8 | - |
| Thickness (µm) | 120–180 | 220–260 | 200–350 |
| Area resistance (Ω·cm2) | 2.0–3.5 | 2.0–3.5 | - |
| Voltage drop (V) | - | - | 1.2–2.2 |
| Current efficiency (%) | - | - | >98 |
| Transport number (%) | 91 | 98 | >98 |
| Parameters | BMED Process |
|---|---|
| Feed volume (L) | 0.2 |
| Feed salt concentration (g·L−1) | 26.055 |
| Current density (mA·m−2) | 30 |
| Batch experiment time (h) | 1.67 |
| Effective each membrane area (cm2) | 18 |
| Energy consumption (kWh·kg−1 LiOH) | 14.672 |
| Treatment capacity (kg LiOH·year−1) | 13.6 |
| Price of bipolar membrane (USD·m−2) | 800 |
| Price of mono membrane (USD·m−2) | 200 |
| Membrane lifetime and amortization of the peripheral equipment (year) | 3 |
| Electricity charge (USD·kWh−1) | 0.0825 |
| Membrane cost (USD) | 5.76 |
| Membrane stack cost (USD) | 8.64 |
| Peripheral equipment cost (USD) | 12.96 |
| Total investment cost (USD) | 27.36 |
| Amortization (USD·year−1) | 9.12 |
| Interest (USD·year−1) | 2.1888 |
| Maintenance (USD·year−1) | 2.736 |
| Total fixed cost (USD·year−1) | 14.0448 |
| Total fixed cost (USD∙kg−1 LiOH) | 1.033 |
| Energy cost (USD·kg−1 LiOH) | 1.21 |
| Total process cost (USD·kg−1 LiOH) | 2.243 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Wei, X.; Chen, J.; Jin, J.; Wu, K.; Meng, W.; Wang, K. Recycling Lithium from Waste Lithium Bromide to Produce Lithium Hydroxide. Membranes 2021, 11, 759. https://doi.org/10.3390/membranes11100759
Gao W, Wei X, Chen J, Jin J, Wu K, Meng W, Wang K. Recycling Lithium from Waste Lithium Bromide to Produce Lithium Hydroxide. Membranes. 2021; 11(10):759. https://doi.org/10.3390/membranes11100759
Chicago/Turabian StyleGao, Wenjie, Xinlai Wei, Jun Chen, Jie Jin, Ke Wu, Wenwen Meng, and Keke Wang. 2021. "Recycling Lithium from Waste Lithium Bromide to Produce Lithium Hydroxide" Membranes 11, no. 10: 759. https://doi.org/10.3390/membranes11100759
APA StyleGao, W., Wei, X., Chen, J., Jin, J., Wu, K., Meng, W., & Wang, K. (2021). Recycling Lithium from Waste Lithium Bromide to Produce Lithium Hydroxide. Membranes, 11(10), 759. https://doi.org/10.3390/membranes11100759