A Review of Human Circulatory System Simulation: Bridging the Gap between Engineering and Medicine
Abstract
1. Introduction
2. Anatomical Review
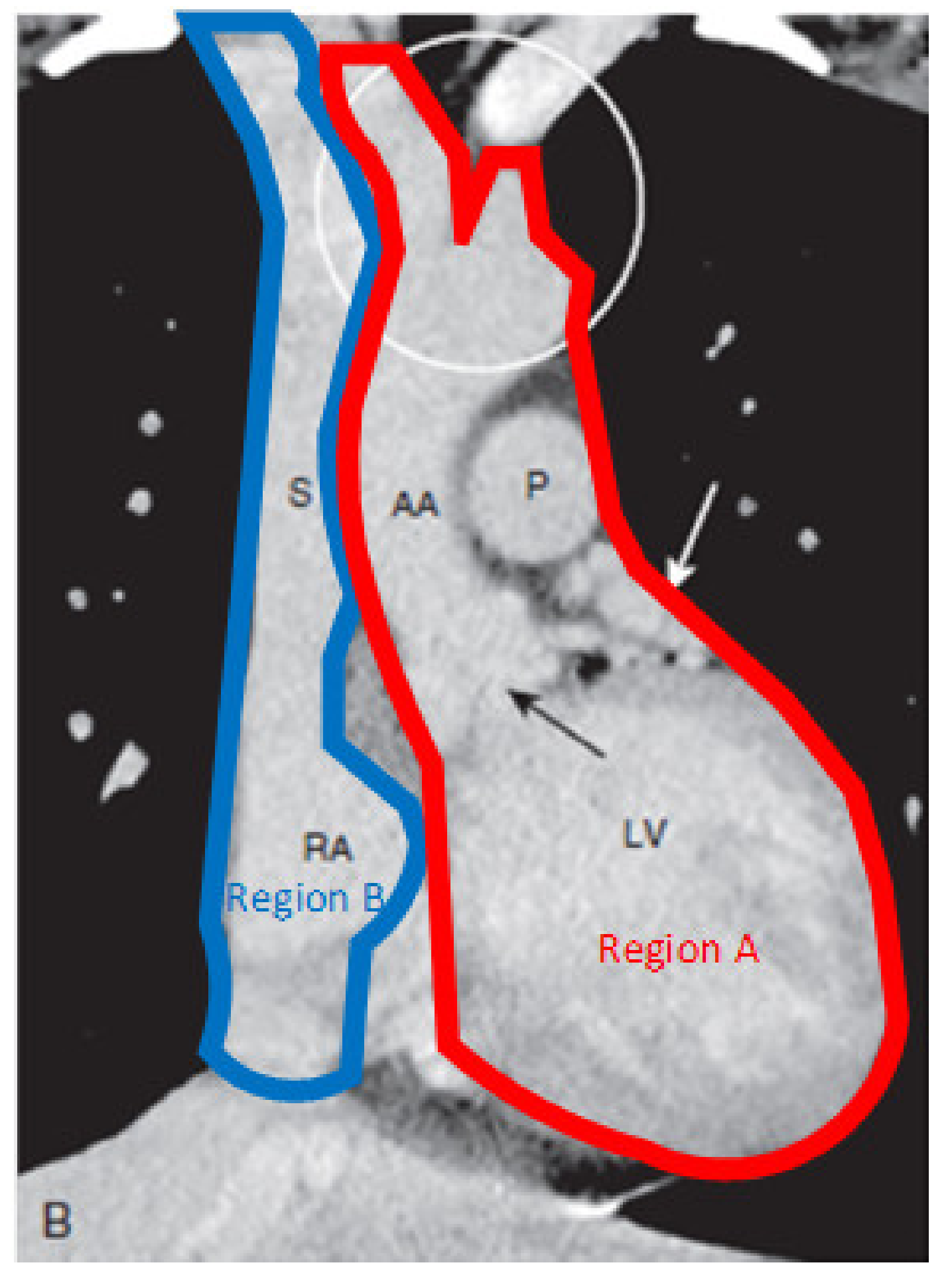
2.1. Heart
2.2. Blood Vessels
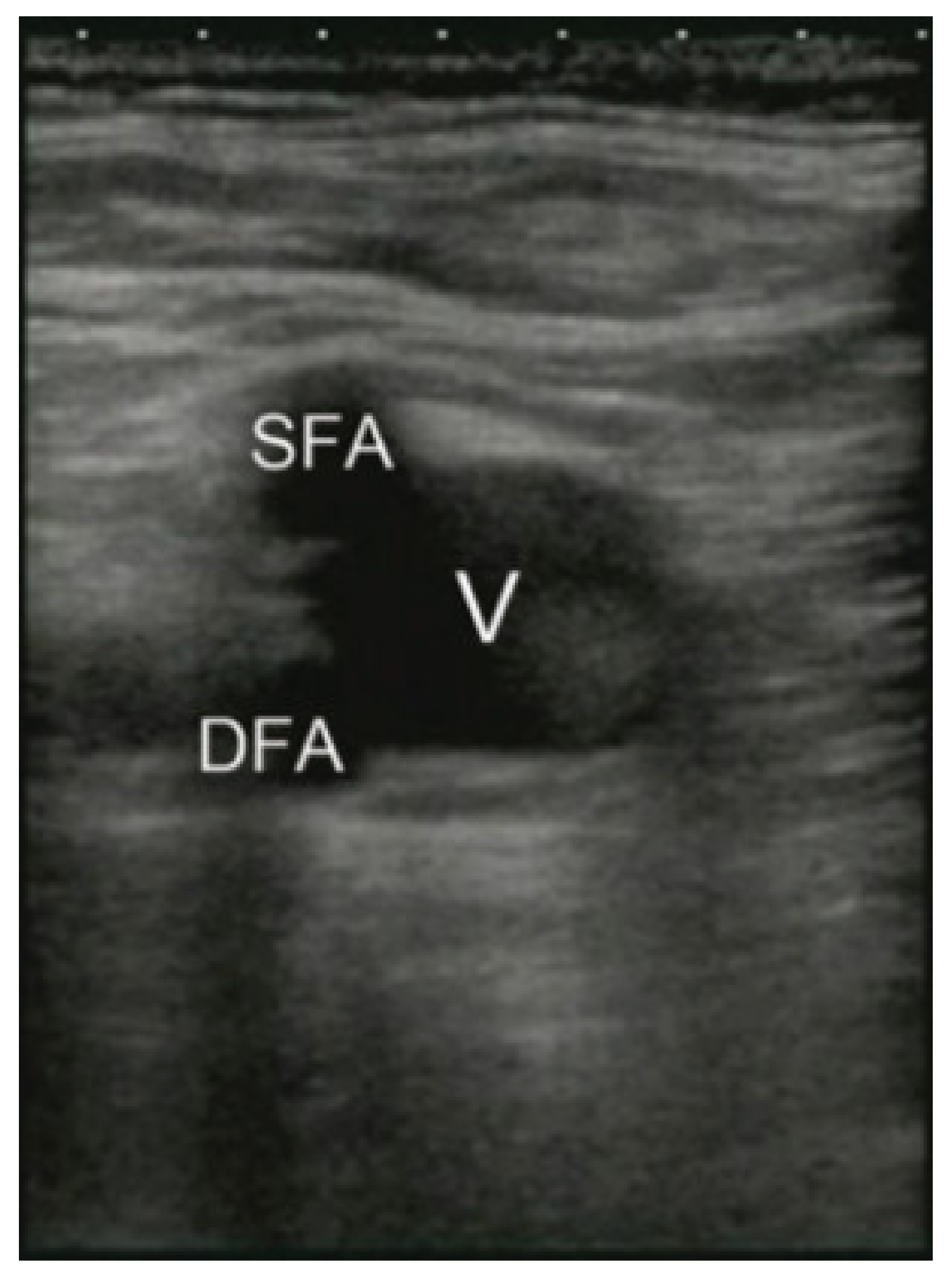
2.3. Access Points
3. Existing Simulators
3.1. Methods
3.2. ECMO Cannulation Systems
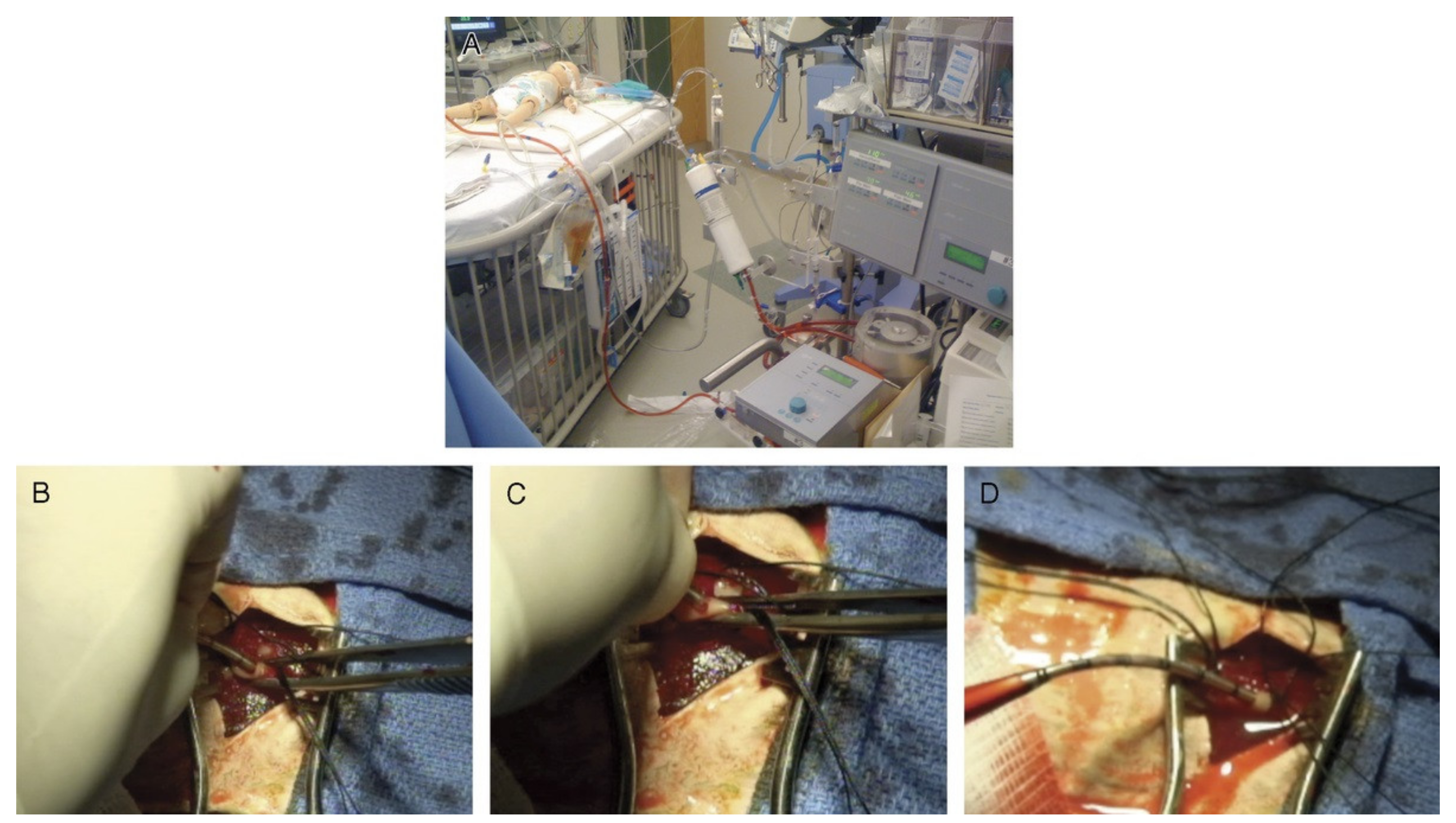
3.2.1. Cadaveric ECMO Cannulation Simulator
3.2.2. A High-Fidelity Surgical Model and Perfusion Simulator
3.2.3. ECMO Professional Simulator
3.2.4. An Extracorporeal Membrane Oxygenation Cannulation Curriculum
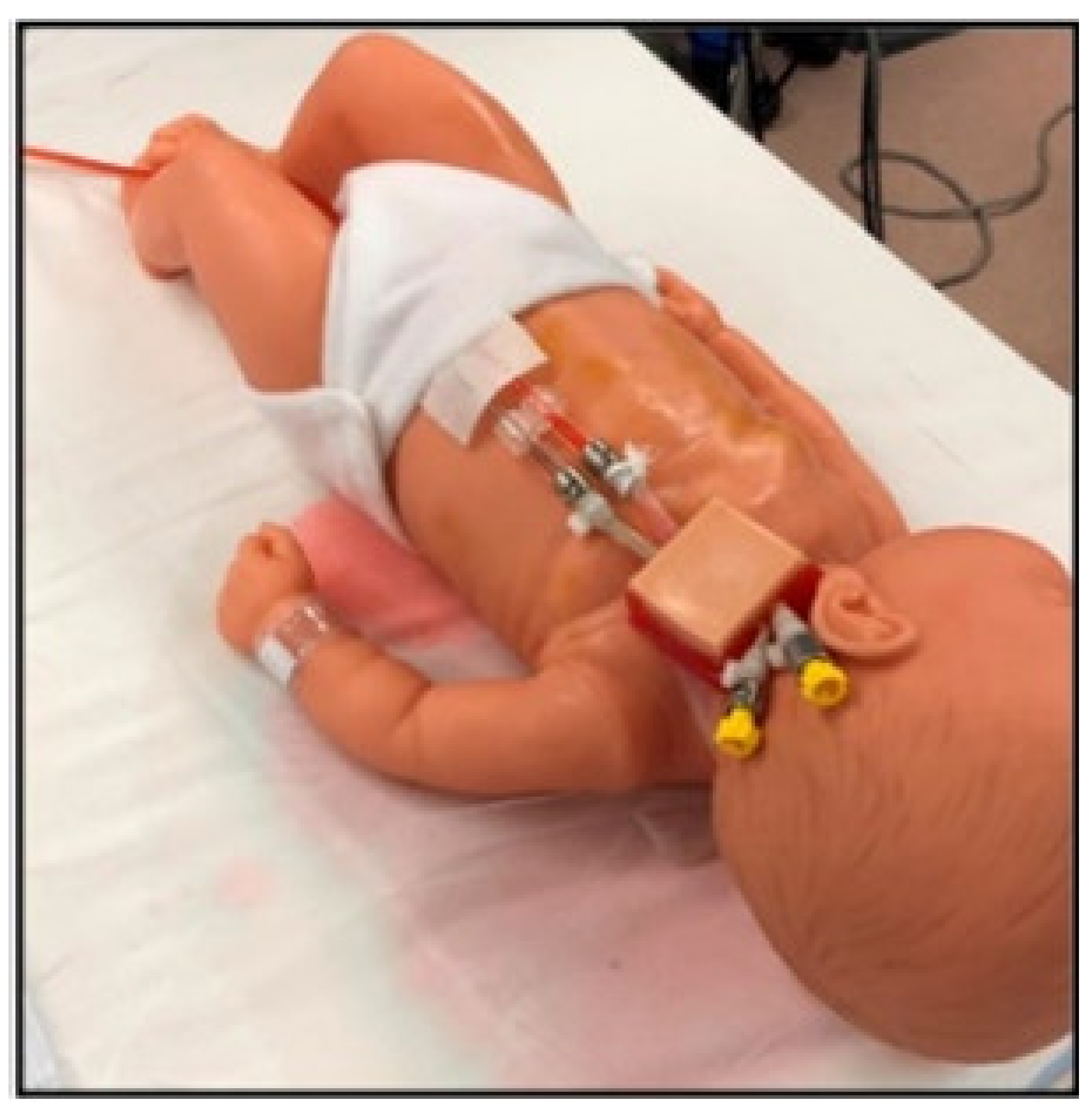
3.2.5. Neonatal Cannulation Simulator
3.2.6. Adult Cannulation Simulator
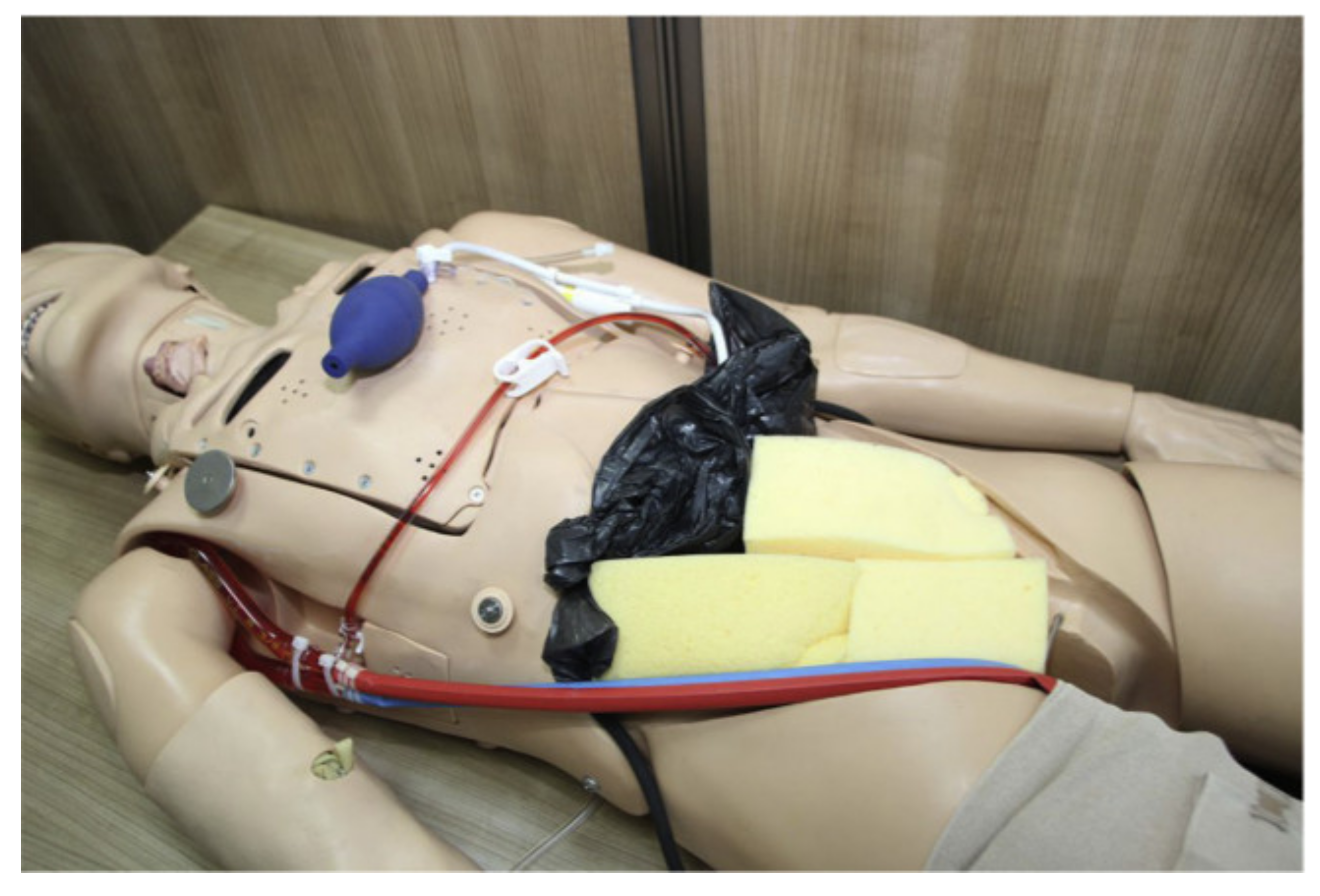
3.2.7. ECPR Simulation Training Mannequin
3.2.8. ECMO Surgical Cannulation Simulators
3.2.9. Training the Component Steps of an ECMO Cannulation
3.2.10. Next-Generation Cannulation Simulator
3.2.11. S2225 Pediatric HAL
3.3. Cardiac Catherization Systems
3.3.1. Catheterization and Cardiovascular Interventions
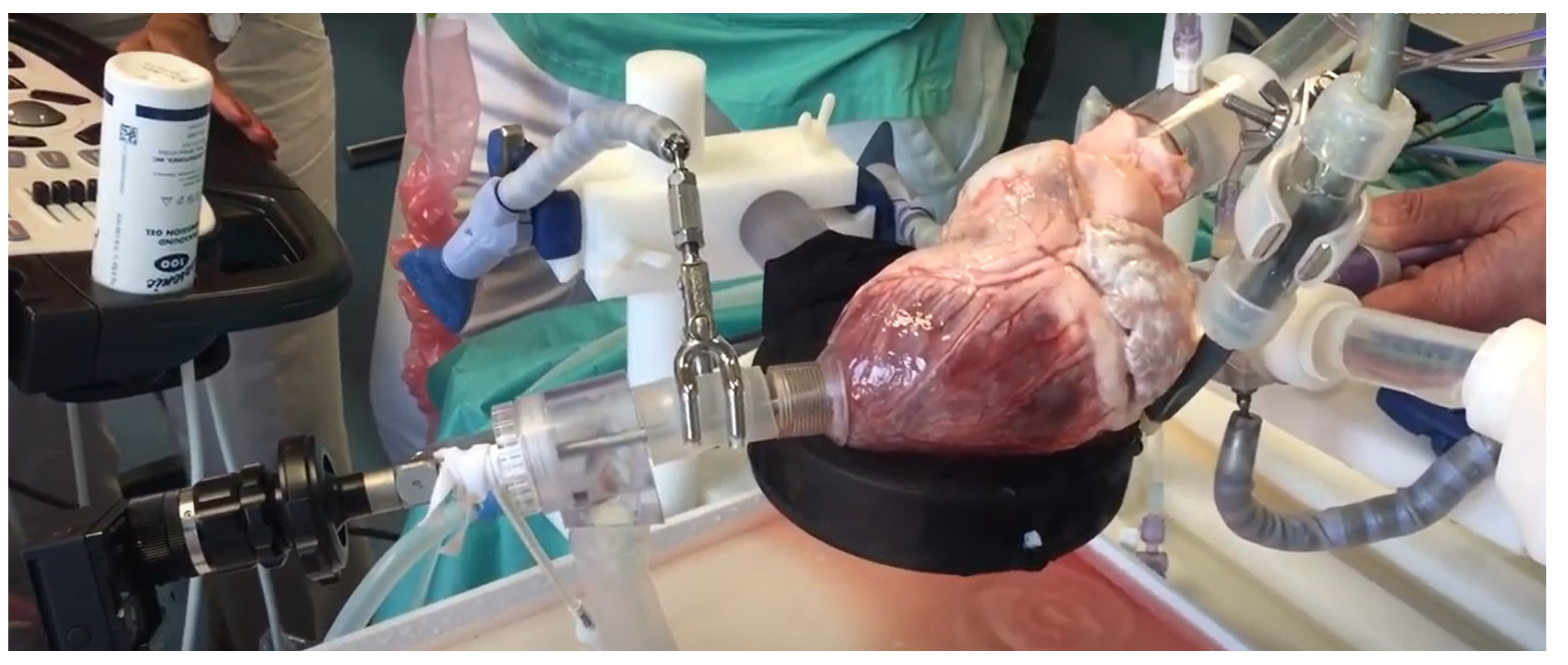
3.3.2. Beating Heart Porcine High-Fidelity Simulator
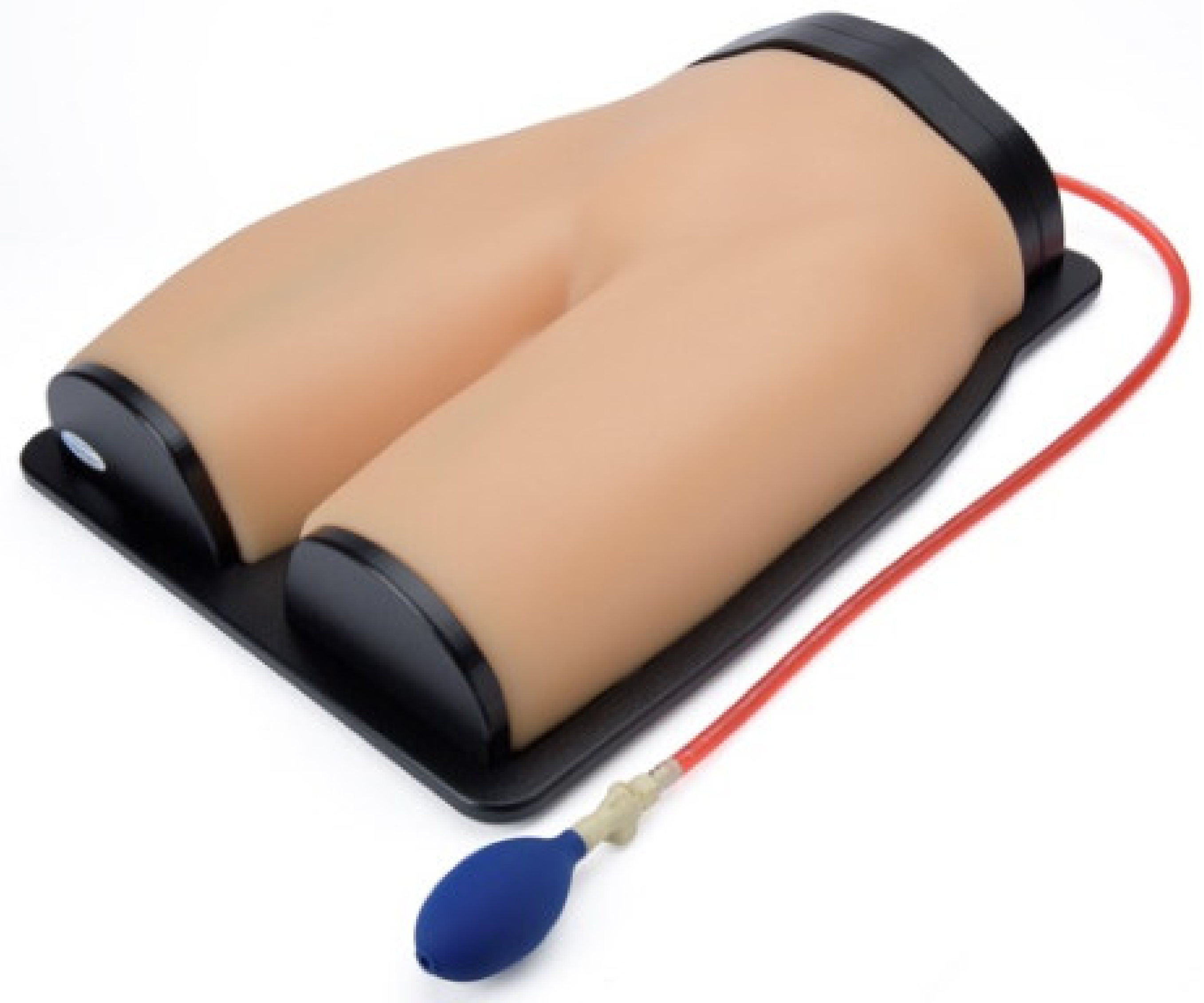
3.3.3. Gen II Femoral Vascular Access and Regional Anastasia Ultrasound Training Model
3.4. Auxiliary Devices
3.4.1. ECMO Therapy Simulator for Extracorporeal Life Support
3.4.2. Design and Development of a Mechatronic Training Simulator for Adult ECMO
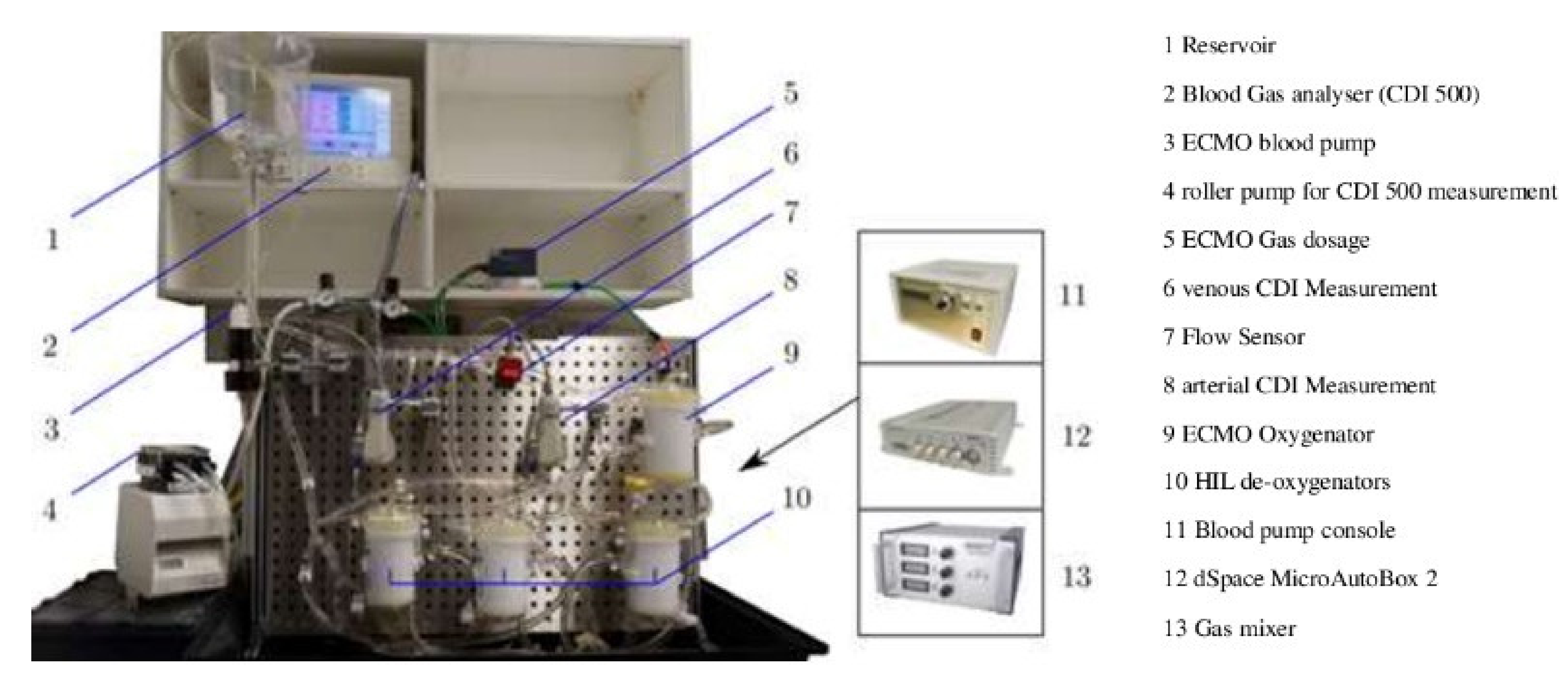
3.4.3. Hardware-in-the-Loop Test Bench for Artificial Lungs
3.4.4. A Hybrid Cardiopulmonary Simulation Platform
3.4.5. Simulation Training for Extracorporeal Membrane Oxygenation
3.4.6. Dynamic Extracorporeal Membrane Oxygenation Simulation
3.4.7. Optical Skill-Assist Device for Ultrasound-Guided Vascular Access
3.4.8. ECMO Simulation with Affordable Yet High-Fidelity Technology
4. Recommendations for an Ideal Simulator
4.1. Heart Emulation
4.2. Blood Vessels
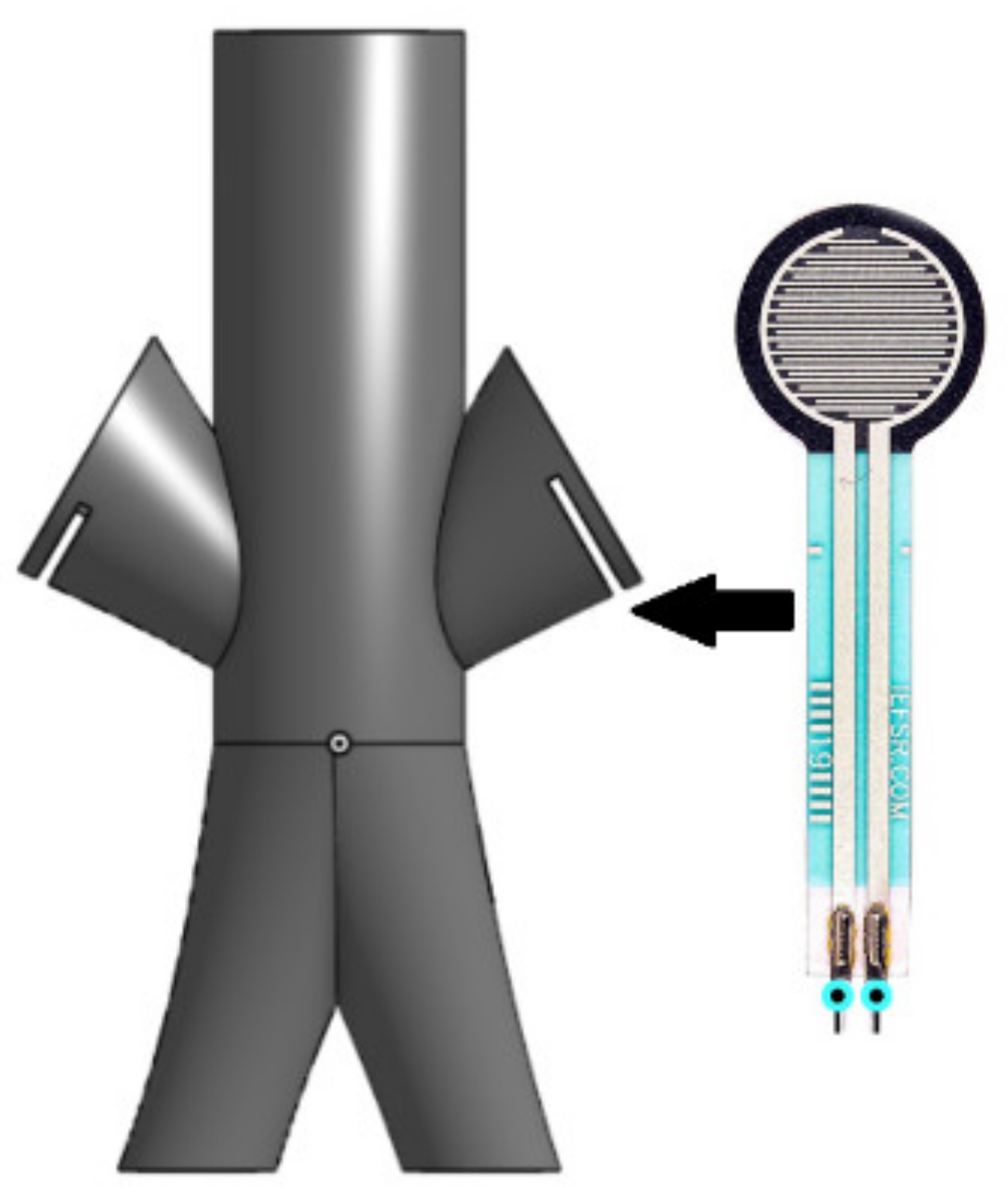
4.3. Access Points
4.4. Embedded System
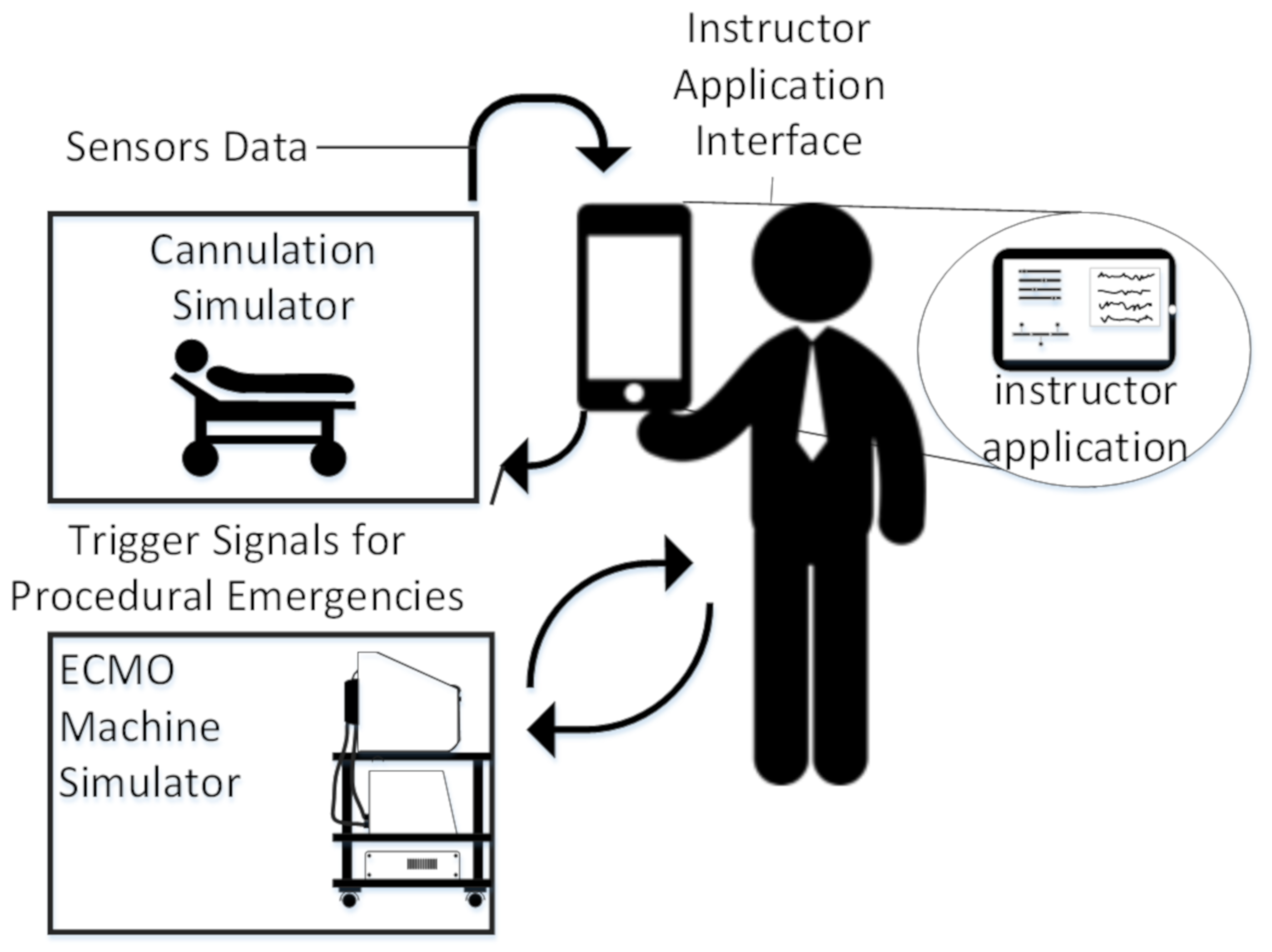
4.5. Instructor Application
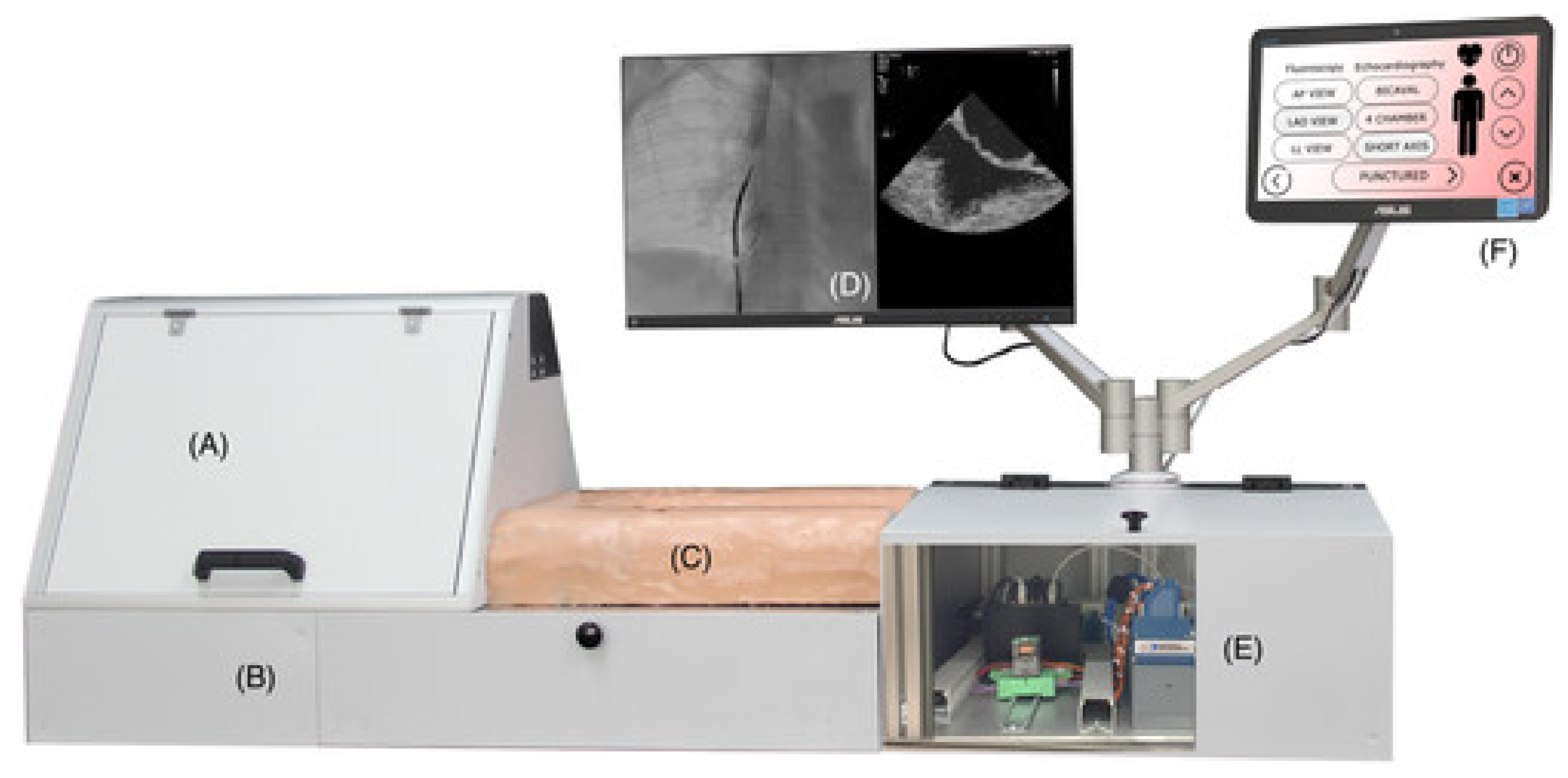
5. Case Study
5.1. Closed Loop
5.2. Cannulation Access Point
5.3. Embedded System
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunkler, K. The role of medical simulation: An overview. Int. J. Med. Robot. Comput. Assist. Surg. 2006, 2, 203–210. [Google Scholar] [CrossRef]
- McGaghie, W.C.; Issenberg, S.B.; Cohen, E.R.; Barsuk, J.H.; Wayne, D.B. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad. Med. 2011, 86, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Alinier, G.; Platt, A. International overview of high-level simulation education initiatives in relation to critical care. Nurs. Crit. Care 2014, 19, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hutin, A.; Abu-Habsa, M.; Burns, B.; Bernard, S.; Bellezzo, J.; Shinar, Z.; Torres, E.C.; Gueugniaud, P.Y.; Carli, P.; Lamhaut, L. Early ECPR for out-of-hospital cardiac arrest: Best practice in 2018. Resuscitation 2018, 130, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Herring, W. Learning Radiology: Recognizing the Basics; Elsevier: Philadelphia, PA, USA, 2019; ISBN 978-0323567299. [Google Scholar]
- Moore, K.L.; Dalley, A.F.; Agur, A.M. Clinically Oriented Anatomy, 7th ed.; Lippincott: Baltimore, MD, USA, 2014; ISBN 9781451119459. [Google Scholar] [CrossRef]
- Burns, K.; Fox, J.C. Venous ultrasound. In Atlas of Emergency Ultrasound; Cambridge University Press: Irvine, CA, USA, 2011. [Google Scholar] [CrossRef]
- Biolo, M.; Salton, F.; Ruaro, B.; Busca, A.; Santagiuliana, M.; Fontanesi, L.; Gabrielli, M.; Baratella, E.; Confalonieri, M. Emergency laser treatment of a tracheobronchial carcinoid during ECMO. Med. Res. Arch. 2020, 8. [Google Scholar] [CrossRef]
- Pavlushkov, E.; Berman, M.; Valchanov, K. Cannulation techniques for extracorporeal life support. Ann. Transl. Med. 2017, 5, 70. [Google Scholar] [CrossRef]
- Ruaro, B.; Confalonieri, M.; Salton, F.; Wade, B.; Baratella, E.; Geri, P.; Confalonieri, P.; Kodric, M.; Biolo, M.; Bruni, C. The relationship between pulmonary damage and peripheral vascular manifestations in systemic sclerosis patients. Pharmaceuticals 2021, 14, 403. [Google Scholar] [CrossRef]
- Rudolph, J.W.; Simon, R.; Raemer, D.B. Which reality matters? Questions on the path to high engagement in healthcare simulation. Simul. Healthc. 2007, 2, 161–163. [Google Scholar] [CrossRef]
- Alexander, A.; Brunyé, T.T.; Sidman, J.; Weil, S.A. From Gaming to Training: A Review of Studies on Fidelity, Immersion, Presence, and Buy-in and Their Effects on Transfer in PC-Based Simulations and Games. DARWARS Train. Impact Group 2005, 5, 1–14. [Google Scholar]
- Fagan, A.; Gould, J.; Horne, D.; Morrison, S.; Kovacs, G.; Sandeski, R. Cadaveric Ecmo Cannulation Simulator. Can. J. Cardiol. 2019, 35, S93. [Google Scholar] [CrossRef][Green Version]
- Palmer, D.; Aspenleiter, M.; da Silva, J.; Castro-Medina, M.; Morell, V.; Sharma, M.; Viegas, M. A High-Fidelity Surgical Model and Perfusion Simulator Used to Demonstrate ECMO Cannulation, Initiation, and Stabilization. J. Extra Corpor. Technol. 2019, 51, 94. [Google Scholar]
- Erler Zimmer ECMO Professional Simulator the Most Immersive ECMO Simulator on the Market. Available online: https://www.mentone-educational.com.au/simulation/surgical-simulation/ecmo-professional-simulator (accessed on 10 May 2019).
- Allan, C.K.; Pigula, F.; Bacha, E.A.; Emani, S.; Fynn-Thompson, F.; Thiagarajan, R.R.; Imprescia, A.; Hayes, G.; Weinstock, P. An extracorporeal membrane oxygenation cannulation curriculum featuring a novel integrated skills trainer leads to improved performance among pediatric cardiac surgery trainees. Simul. Healthc. 2013, 8, 221–228. [Google Scholar] [CrossRef]
- Thompson, J.L.; Grisham, L.M.; Scott, J.; Mogan, C.; Prescher, H.; Biffar, D.; Jarred, J.; Meyer, R.J.; Hamilton, A.J. Construction of a reusable, high-fidelity model to enhance extracorporeal membrane oxygenation training through simulation. Adv. Neonatal Care 2014, 14, 103–109. [Google Scholar] [CrossRef][Green Version]
- Endo, T.; Kagaya, Y.; Arata, Y.; Imai, H. Long-term efficacy of an extracorporeal membrane oxygenation simulation with a novel, low-cost vascular model “Endo-Circuit”. Acute Med. Surg. 2016, 4, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Pang, G.; Futter, C.; Pincus, J.; Dhanani, J.; Laupland, K.B. Development and testing of a low cost simulation manikin for extracorporeal cardiopulmonary resuscitation (ECPR) using 3-dimensional printing. Resuscitation 2020, 149, 24–29. [Google Scholar] [CrossRef] [PubMed]
- McMullan, D.M. Novel ECMO surgical cannulation simulators. Qatar Med. J. 2017, 2017, 61. [Google Scholar] [CrossRef]
- Botden, S.M.; Bökkerink, G.M.; Leijte, E.; Antonius, T.; de Blaauw, I. Training the component steps of an extra-corporeal membrane oxygenation (ECMO) cannulation outside the clinical setting. J. Artif. Organs 2020, 23, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Hssain, A.A.; Alinier, G.; Hassan, I.; Khurshid, U.; Abducarim, A.; Mahmud, S.; Abdallah, O.; Mohamed, E.; Alsalemi, A.; et al. Preliminary Implementation of the Next Generation Cannulation Simulator. In Proceedings of the 2019 IEEE Student Conference on Research and Development, SCOReD 2019, Bandar Seri Iskandar, Malaysia, 15–17 October 2019. [Google Scholar] [CrossRef]
- Gaumard Scientific S2225 Pediatric HAL|Gaumard Scientific. Available online: https://www.gaumard.com/s2225#:~:text=PediatricHAL®isthe,expressions%2Cmovement%2Candspeech (accessed on 3 September 2020).
- Zimmermann, J.M.; Steffen, O.J.; Vicentini, L.; Schmid Daners, M.; Taramasso, M.; Maisano, F.; Meboldt, M. Novel augmented physical simulator for the training of transcatheter cardiovascular interventions. Catheter. Cardiovasc. Interv. 2019, 95, 1202–1209. [Google Scholar] [CrossRef]
- Gollmann-Tepeköylü, C.; Holfeld, J.; Pölzl, G.; Metzler, B.; Hintringer, F.; Adukauskaite, A.; Stijnen, M.; van Tuijl, S.; Müller, L.; Grimm, M.; et al. Beating heart porcine high-fidelity simulator for the training of edge-to-edge mitral valve repair. Multimed. Man. Cardiothorac. Surg. MMCTS 2018. [Google Scholar] [CrossRef]
- Adam Rouilly Gen II Femoral Vascular Access and Regional Anesthesia Ultrasound Training Model. Available online: https://www.adam-rouilly.co.uk/products/clinical-skills-simulators/blue-phantom-ultrasound-training-models/abp136-gen-ii-femoral-vascular-access-and-regional-anesthesia-ultrasound-training-model (accessed on 3 September 2020).
- Puślecki, M.; Ligowski, M.; Kiel, M.; Dąbrowski, M.; Stefaniak, S.; Maciejewski, A.; Kiel-Puślecka, I.; Telec, W.; Czekajlo, M.; Jemielity, M. ECMO therapy simulator for extracorporeal life support. Am. J. Emerg. Med. 2018, 36, 506–508. [Google Scholar] [CrossRef]
- Mehta, I. Design and Development of a Mechatronic Training Simulator for Adult ECMO, 2019. Master’s Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2019. [Google Scholar]
- Walter, M.; Eisenbrand, S.; Kopp, R.; Leonhardt, S. Hardware-in-the-loop test bench for artificial lungs. AIP Conf. Proc. 2019, 2140, 020078. [Google Scholar] [CrossRef]
- Zieliński, K.; Okrzeja, P.; Stecka, A.; Kozarski, M.; Darowski, M. A hybrid cardio-pulmonary simulation platform—An application for extracorporeal assist devices. In IFMBE Proceedings, Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Coimbra, Portugal, 26–28 September 2019; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Brum, R.; Rajani, R.; Gelandt, E.; Morgan, L.; Raguseelan, N.; Butt, S.; Nelmes, D.; Auzinger, G.; Broughton, S. Simulation training for extracorporeal membrane oxygenation. Ann. Card. Anaesth. 2015, 18, 185. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.S. Devices and Methods for Dynamic Extracorporeal Membrane Oxygenation Simulation. U.S. Patent 10,984,678, 13 April 2016. [Google Scholar]
- Asao, T.; Kikuchi, M.; Tokumine, J.; Matsushima, H.; Andoh, H.; Tanaka, K.; Kanamoto, M.; Ideno, Y. Optical skill-assist device for ultrasound-guided vascular access. Medicine 2019, 98, e16126. [Google Scholar] [CrossRef] [PubMed]
- Al Disi, M.; Alsalemi, A.; Alhomsi, Y.; Bensaali, F.; Amira, A.; Alinier, G. Using thermochromism to simulate blood oxygenation in extracorporeal membrane oxygenation. Perfusion 2018, 34, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Alsalemi, A.; Al Disi, M.; Ahmed, I.; Alhomsi, Y.; Bensaali, F.; Amira, A.; Alinier, G. Developing cost-effective simulators for patient management: A modular approach. In Proceedings of the International Conference on Advances in Biomedical Engineering, ICABME, Beirut, Lebanon, 19–21 October 2017. [Google Scholar] [CrossRef]
- Al Disi, M.; Alsalemi, A.; Alhomsi, Y.; Bensaali, F.; Amira, A.; Alinier, G. Revolutionizing ECMO simulation with affordable yet high-Fidelity technology. Am. J. Emerg. Med. 2018, 36, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.; Alsalemi, A.; Bensaali, F.; Amira, A.; Hssain, A.A.; Alinier, G.; Hassan, I.F. A Leap Towards the Next Generation Cannulation Simulator. In Proceedings of the 30th Annual Elso Conference Abstracts, Austin, TX, USA, 12–15 September 2019; p. 10. [Google Scholar]
- Alhomsi, Y.; Alsalemi, A.; Noorizadeh, M.; Bensaali, F.; Meskin, N.; Hssain, A.A. A Modular Approach for a Patient Unit for Extracorporeal Membrane Oxygenation Simulator. Membranes 2021, 11, 424. [Google Scholar] [CrossRef]
- Alsalemi, A.; Alhomsi, Y.; Bensaali, F.; Hssain, A.A. A High-Realism and Cost-Effective Training Simulator for Extracorporeal Membrane Oxygenation. IEEE Access 2021, 9, 20893–20901. [Google Scholar] [CrossRef]
- Najjari, M.R.; Plesniak, M.W. PID controller design to generate pulsatile flow rate for in vitro experimental studies of physiological flows. Biomed. Eng. Lett. 2017, 7, 339–344. [Google Scholar] [CrossRef]



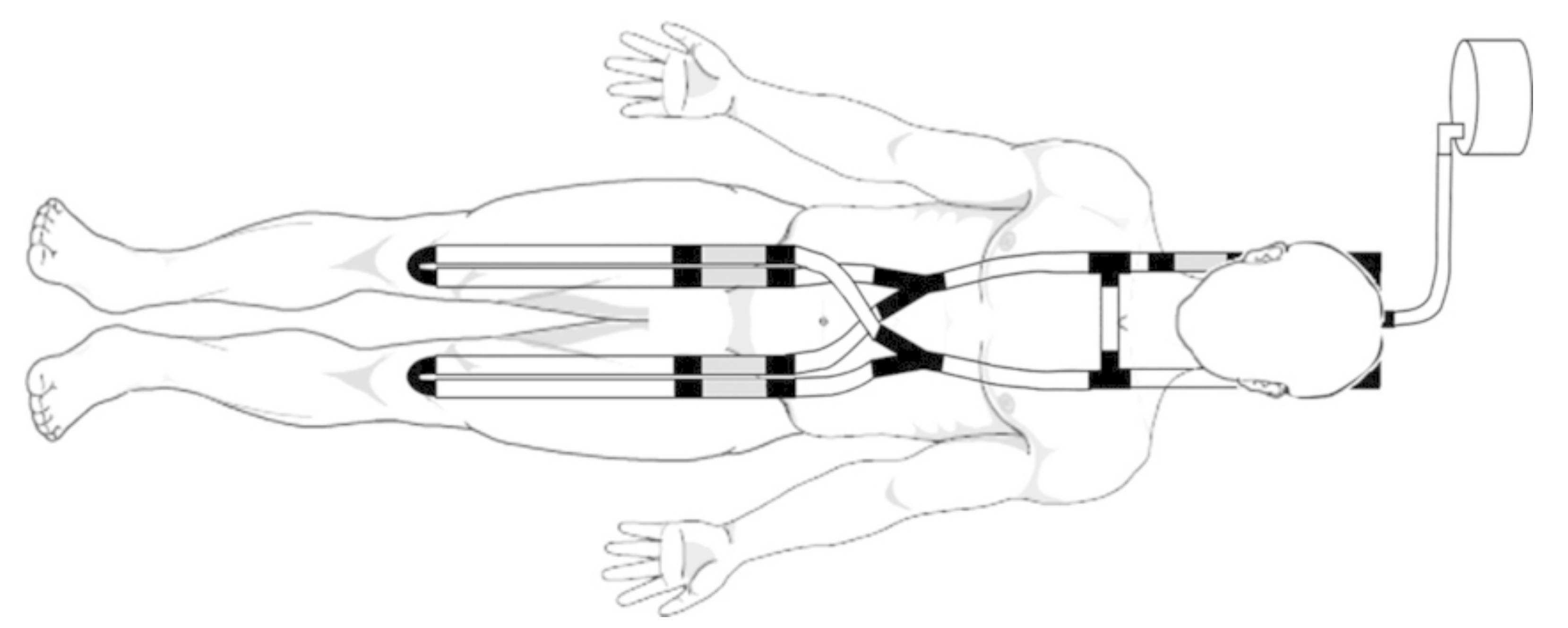
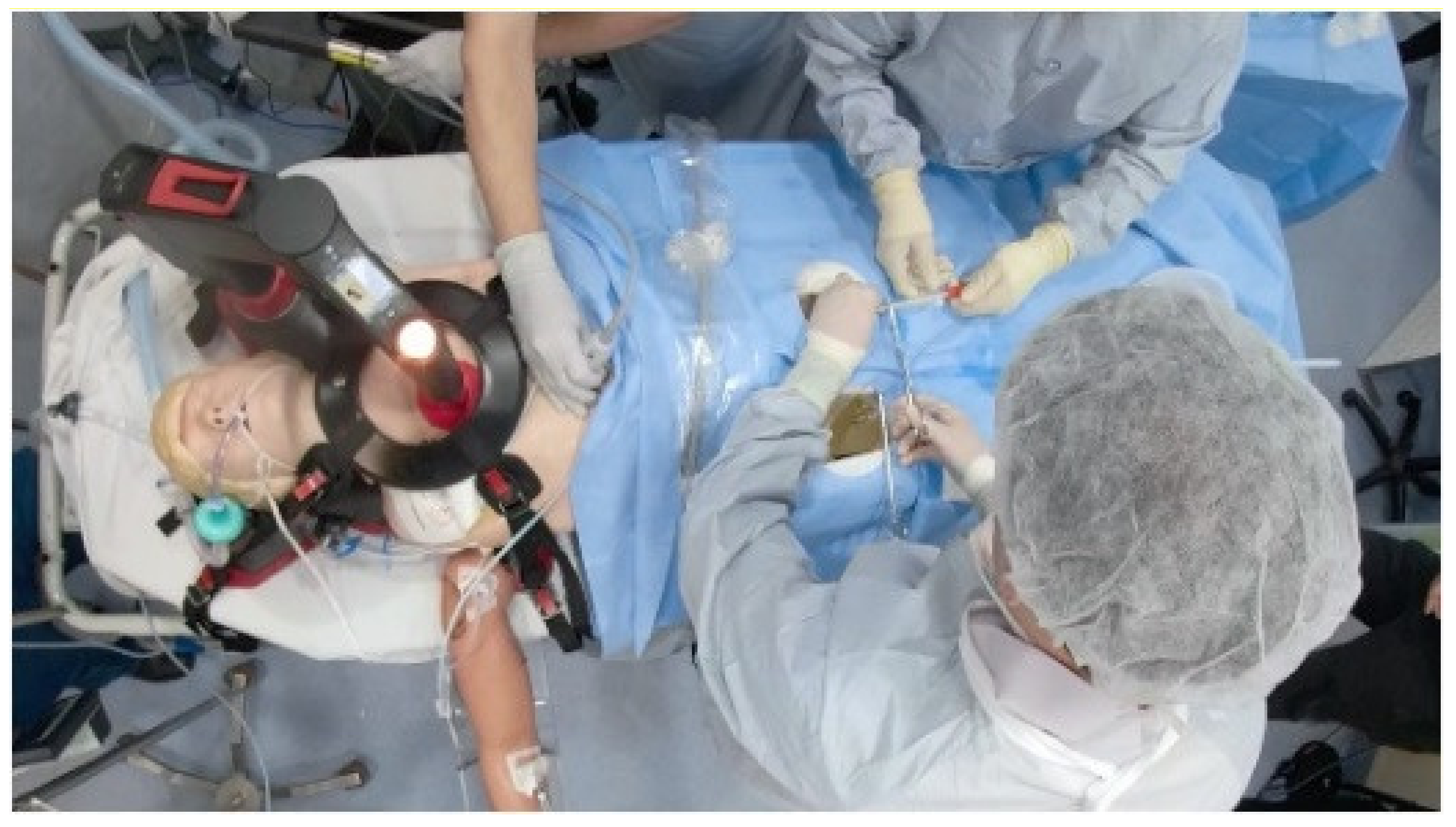
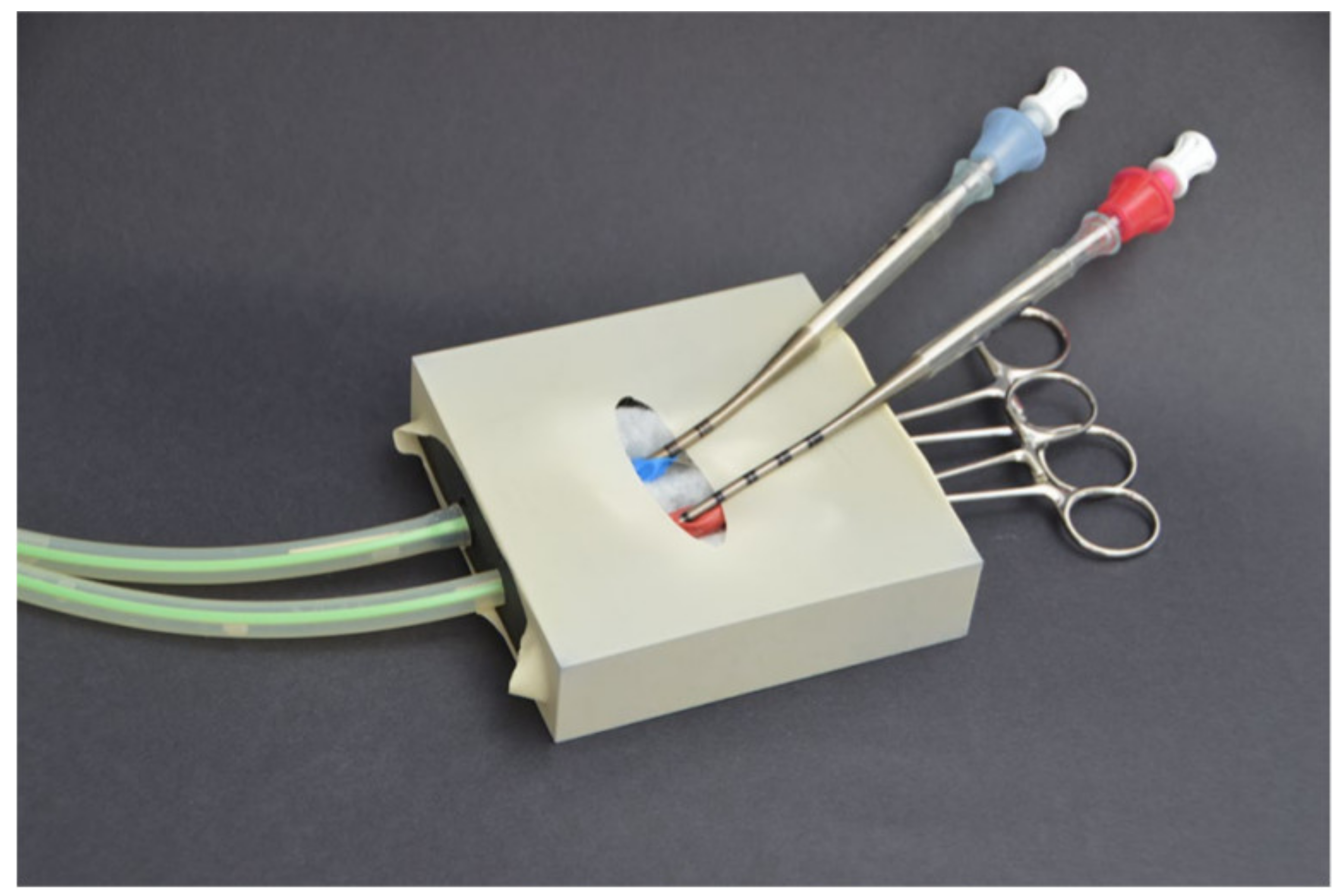
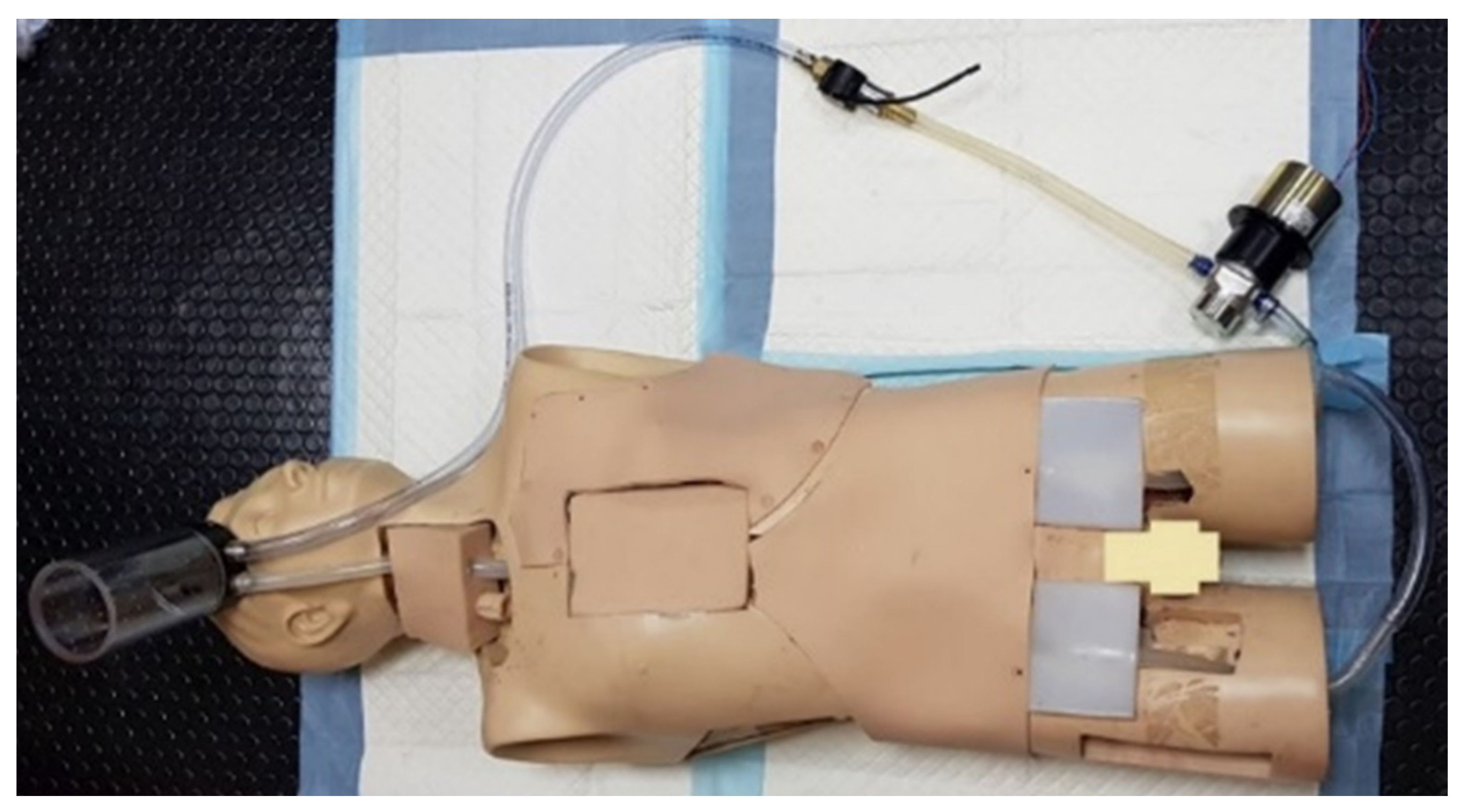





| Simulator | Evaluation Metric | |||
|---|---|---|---|---|
| Overall Fidelity | Cost | Features | Drawbacks | |
| Cadaveric ECMO cannulation simulator | High | High | Training on cadavers | High price, disinfection required, real equipment needed |
| A high-fidelity surgical model and perfusion simulator | Average | Average | Three-layers access point | Inaccurate blood vessel simulation |
| ECMO professional simulator | High | High | Easy maintenance, ECG simulation, arteries, and veins simulated, include pulsatile flow | Expensive, limited anatomical fidelity |
| An extracorporeal membrane oxygenation cannulation curriculum | High | High | Procedural emergencies, realistic, blood flow, tissue simulation | Expensive, veins only |
| Neonatal cannulation simulator | Low | Low | Realistic shape | Veins only, anatomically inaccurate |
| Adult cannulation simulator | Average | Low | Low cost, realistic appearance | Single closed loop, no heart emulation |
| ECPR simulation training mannequin | High | Average | Dual closed loops, realistic appearance | No heart emulation |
| ECMO surgical cannulation simulators | Low | Low | Relatively cheap | Primitive simulator |
| Next-generation cannulation simulator | High | Low | Easy maintenance, dual flow, interactive learning experience, anatomical fidelity | Recalibration of sensors |
| S2225 pediatric HAL | Very high | Very high | Multiple sensory peripherals, realistic biometric signals, and a fully immersive experience | Very expensive |
| Optical skill-assist device for ultrasound-guided vascular access | Average | Average | Ultrasound emulation, realistic ultrasound imaging | Only access point |
| Catheterization and cardiovascular interventions | High | Average | Fully ultrasound-able system, anatomically accurate system | Does not look like a patient |
| Beating heart porcine high-fidelity simulator | High | High | Anatomically accurate heart | Requires disinfection of equipment |
| Gen II femoral vascular access training model | High | High | Anatomically accurate access point | Only access point |
| ECMO therapy simulator for extracorporeal life support | Low | Low | Cheap auxiliary device emulation, easy to maintain | Only demo for controlling the ECMO machine, no surgical training |
| Design and development of a mechatronic training simulator for adult ECMO | NA | NA | Synthetic blood emulation, vital signals generation | Insufficient details |
| Hardware-in-the-loop test bench for artificial lungs | High | High | Blood oxygenation, full ECMO machine control | Requires disinfection, expensive oxygenation devices |
| A hybrid cardiopulmonary simulation platform | Average | High | Blood oxygenation, full ECMO machine control | Requires disinfection, expensive oxygenation devices |
| Simulation training for extracorporeal membrane oxygenation | High | Very high | Blood oxygenation | Requires disinfection, expensive off-the-shelf systems |
| Dynamic extracorporeal membrane oxygenation simulation | High | High | Blood oxygenation, full ECMO machine control | Requires disinfection, expensive oxygenation devices |
| ECMO simulation with affordable yet high-fidelity technology | High | Low | No disinfection required, full ECMO machine control, modular design | Heater scenario interference |
| Part Name | 3D Model | Description |
|---|---|---|
| Body |  |
|
| Arterial Rod |  |
|
| Veins Rod |  |
|
| Overview | 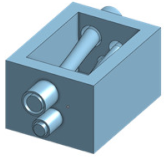 | Just write for example fully designed mold for femoral pad |
| Part Name | 3D Model | Description |
|---|---|---|
| Body |  |
|
| Veins Rod |  |
|
| Arterial Rod |  |
|
| Plug |  |
|
| Overview | 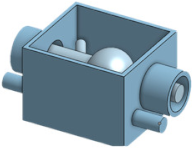 |
|
| Part Name | 3D Model | Description |
|---|---|---|
| Body |  |
|
| Cam |  |
|
| Follower |  |
|
| Overview | 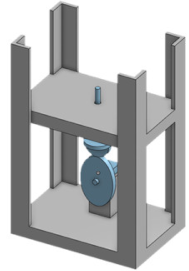 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.; Alsalemi, A.; Bensaali, F.; Hssain, A.A.; Hassan, I. A Review of Human Circulatory System Simulation: Bridging the Gap between Engineering and Medicine. Membranes 2021, 11, 744. https://doi.org/10.3390/membranes11100744
Mahmoud A, Alsalemi A, Bensaali F, Hssain AA, Hassan I. A Review of Human Circulatory System Simulation: Bridging the Gap between Engineering and Medicine. Membranes. 2021; 11(10):744. https://doi.org/10.3390/membranes11100744
Chicago/Turabian StyleMahmoud, Abdulrahman, Abdullah Alsalemi, Faycal Bensaali, Ali Ait Hssain, and Ibrahim Hassan. 2021. "A Review of Human Circulatory System Simulation: Bridging the Gap between Engineering and Medicine" Membranes 11, no. 10: 744. https://doi.org/10.3390/membranes11100744
APA StyleMahmoud, A., Alsalemi, A., Bensaali, F., Hssain, A. A., & Hassan, I. (2021). A Review of Human Circulatory System Simulation: Bridging the Gap between Engineering and Medicine. Membranes, 11(10), 744. https://doi.org/10.3390/membranes11100744








