The Implications of Membranes Used as Separators in Microbial Fuel Cells
Abstract
1. Introduction
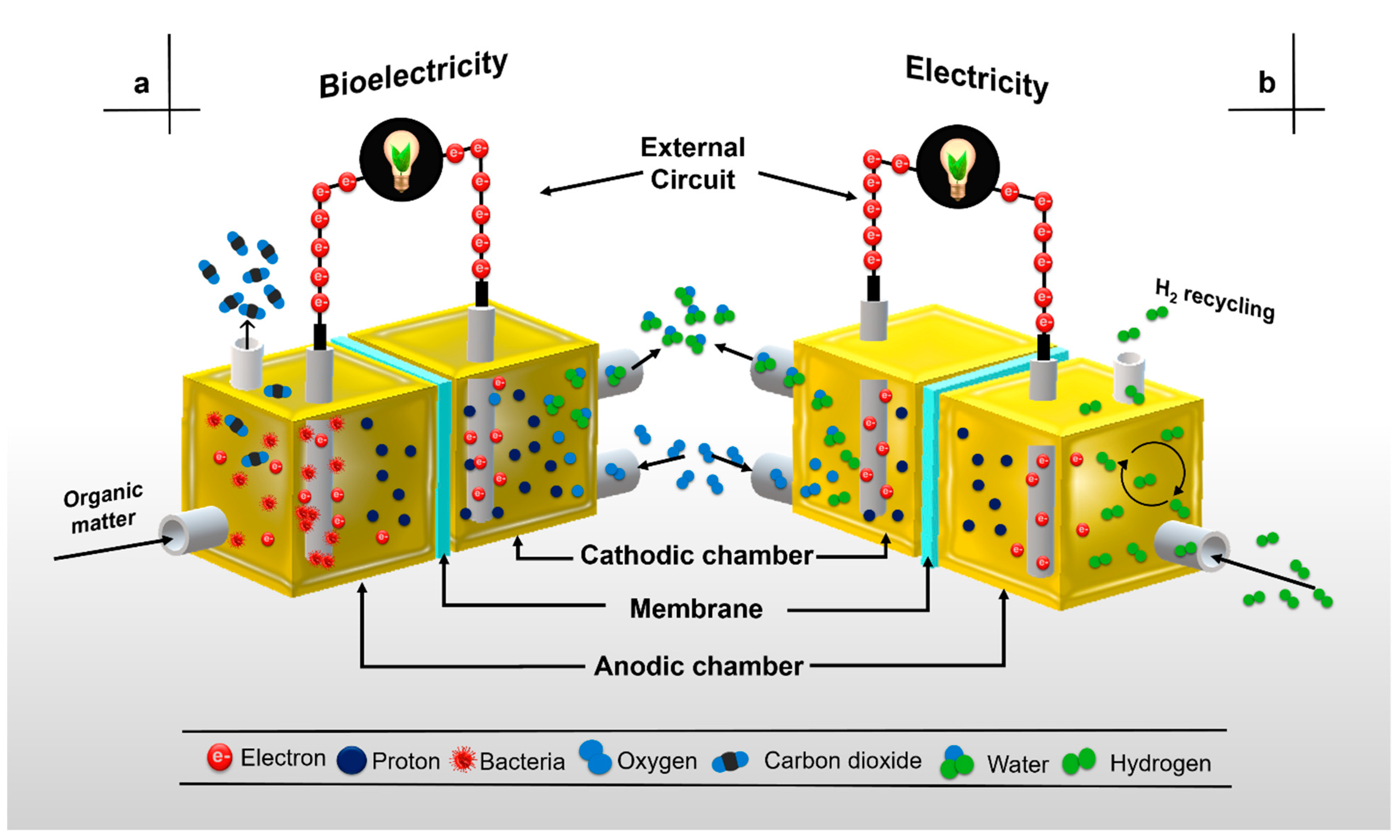
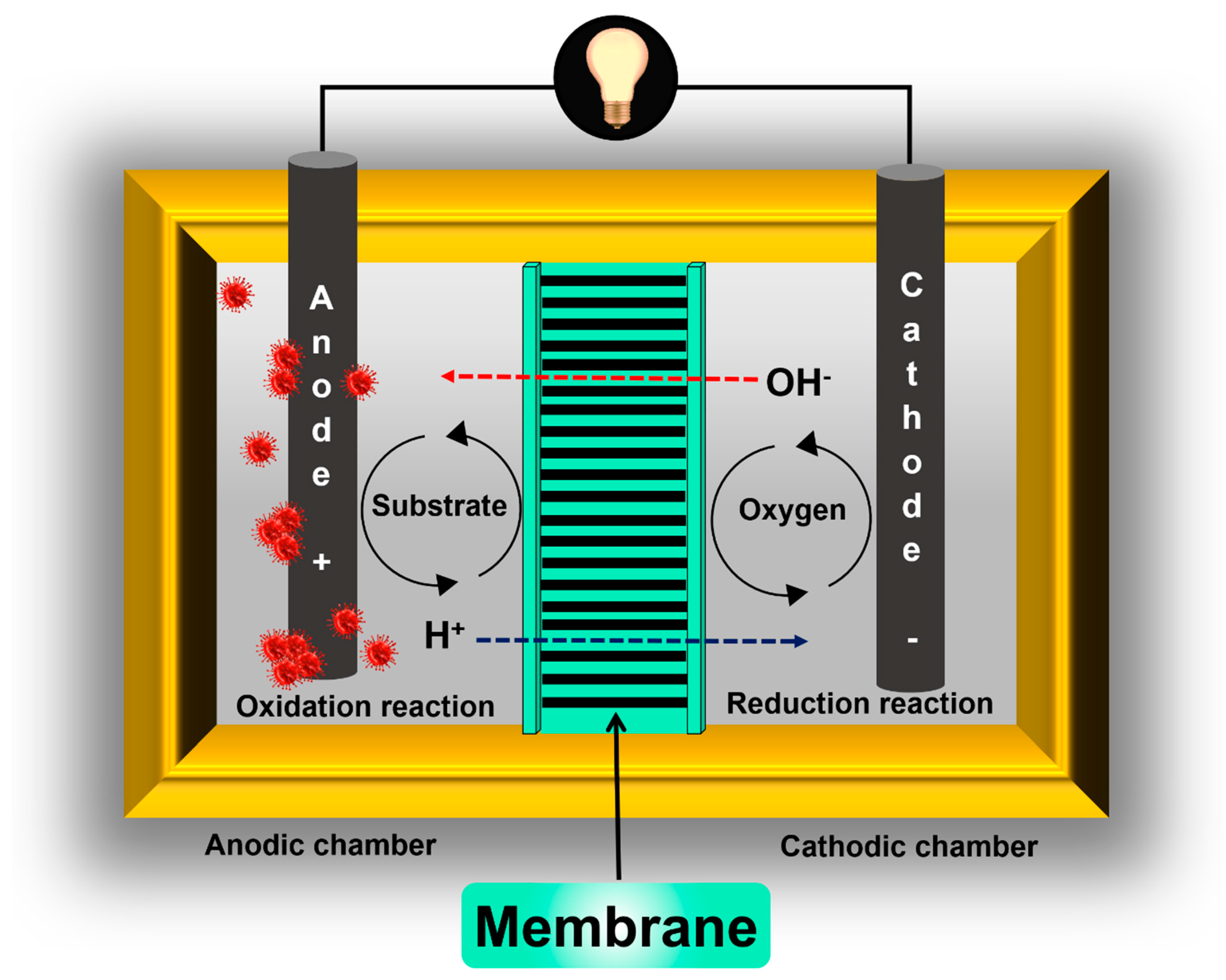
2. Microbial Fuel Cell Components and Basic Functioning
2.1. Electrochemical Membrane Concept
2.2. Membrane Separator Functions in Microbial Fuel Cells
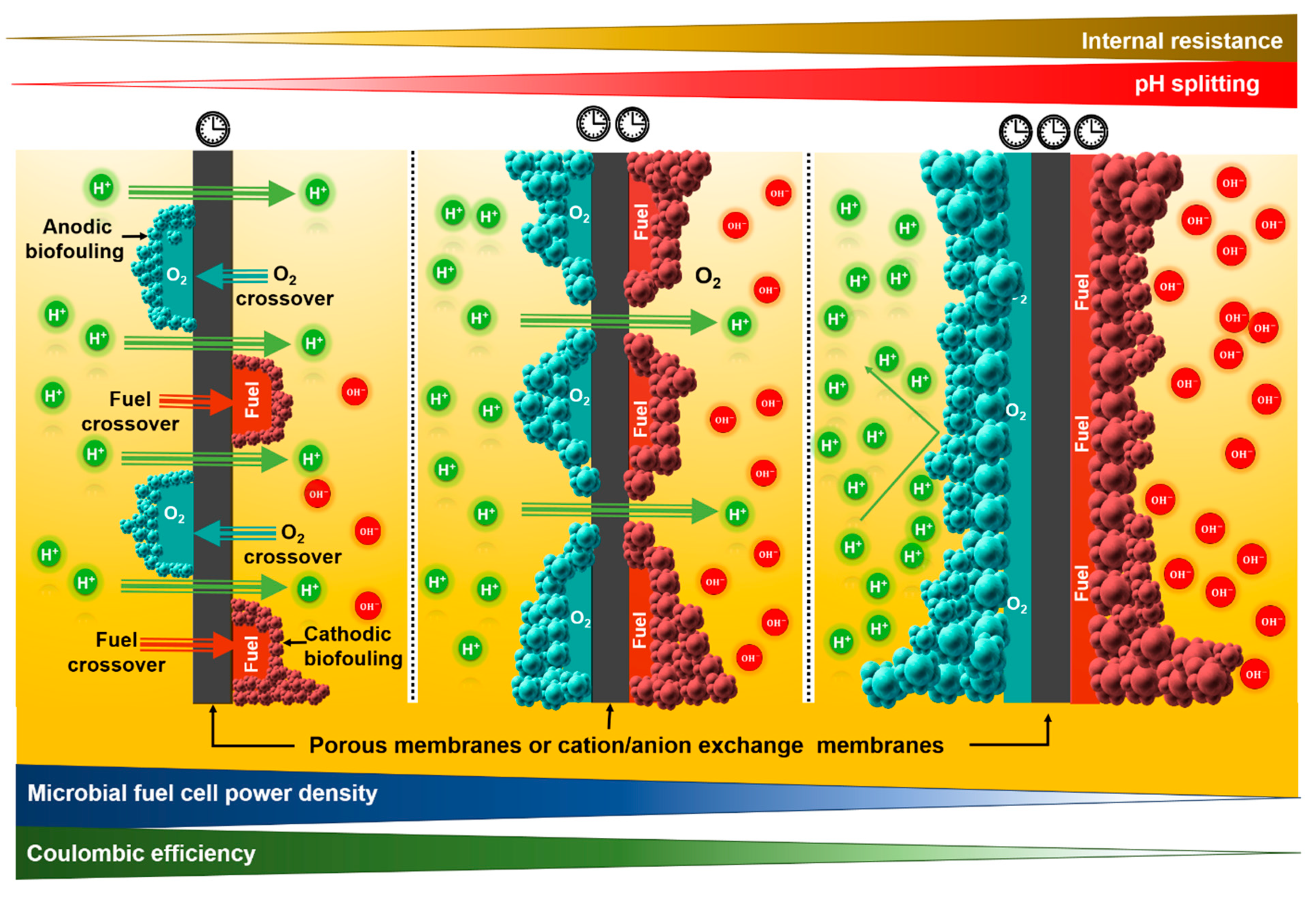
3. Disadvantages of Using Membranes in Microbial Fuel Cells
3.1. Increase in the Total Internal Resistance of the Microbial Fuel Cell
3.2. Oxygen Diffusion
3.3. Substrate Crossover
3.4. Biofouling
3.5. pH Splitting
3.6. Water Loss by Evaporation
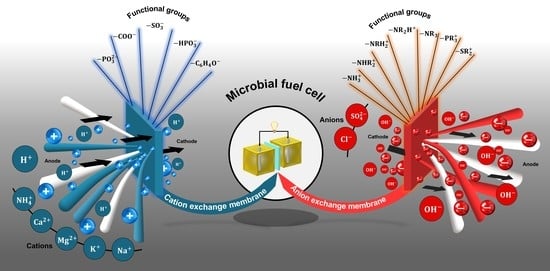
3.7. Undesirable Ions Crossing
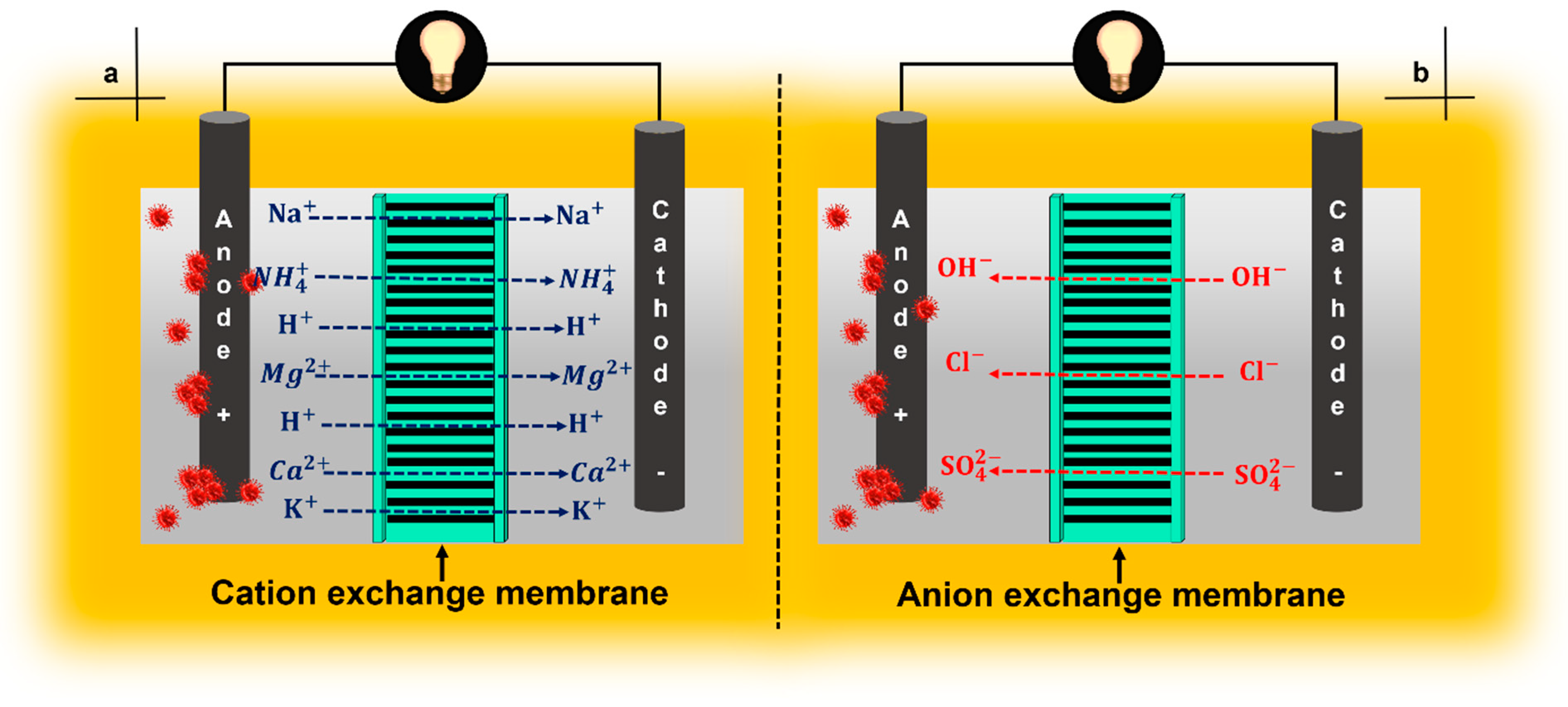
4. Ion-Exchange Membranes
4.1. Cation-Exchange Membranes
Nafion® 117 Membrane Properties
| Membrane Properties | Value | References |
|---|---|---|
| Proton conductivity (mS/cm) | 2.0–9.5 | [5,10,72,78] |
| Thermal stability (°C) | 80–90 | [37,39,79] |
| IEC a (meq/g) | 1.23 | [37,71] |
| Water swelling (%) | 22–25 | [5,37,39] |
| Thickness (µm) | 175–190 | [5,39,72,78] |
| K0 b (cm/s) | 1.6 × 10−5–2.6 × 10−3 | [70,72,74,75,80] |
| c (cm2/s) | 9.95 × 10−7–5.1 × 10−5 | [5,70,72,74,75] |
4.2. Anion-Exchange Membranas
4.3. Bipolar Membranes
5. Porous Membranes
5.1. Ultrafiltration Membranes
5.2. Microfiltration Membrane
5.3. Ceramic Membranes
5.4. Coarse-Pore Filters
6. Membrane-Less Microbial Fuel Cells
7. Salt Bridge
8. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Surface area of the electrode |
| ACS | Actual charge due to the substrate |
| AEM | Anion exchange membrane |
| AMD | Acid mine drainage |
| bCOD | Number of electrons exchanged per mole of oxygen |
| BPM | Bipolar membranes |
| CEM | Cation exchange membrane |
| CI | Current interruption |
| CM | Ceramic membrane |
| COD | Chemical oxygen demand |
| CODf | Final chemical oxygen demand |
| CODi | Initial chemical oxygen demand |
| DC-MFC | Dual-chamber microbial fuel cell |
| DO2 | Oxygen diffusion coefficient |
| EMFC | Voltage generated by the microbial fuel cell |
| F | Faraday’s constant |
| FCs | Fuel cells |
| I | Current |
| IEC | Ion-exchange capacity |
| IEM | Ion-exchange membrane |
| IMFC | Electrical current of the microbial fuel cell |
| KO | Oxygen mass transfer coefficient |
| L | Membrane thickness |
| MCOD | Molecular weight of oxygen |
| MFC | Microbial fuel cells |
| MFM | Microfiltration filtration membrane |
| MWW | Municipal wastewater |
| NF-117 | Nafion® 117 |
| OD | Oxygen diffusion |
| OR | Ohmic resistance |
| ORR | Oxygen reduction reaction |
| P | Power density |
| PC | Proton conductivity |
| PEM | Proton-exchange membrane |
| PEMFC | Proton-exchange membrane fuel cell |
| PFSA | Perfluorinated sulfonic acid |
| PMFC | Power generated by the microbial fuel cell |
| PTFE | Polytetrafluoroethylene |
| R | Membrane resistance |
| Rint | Total internal resistance of the microbial fuel cell |
| SC-MFC | Single chamber microbial fuel cell |
| SPEEK | Sulphonated polyether ether ketone membrane |
| TCS | Theoretical charge due to the substrate |
| UFM | Ultrafiltration membrane |
| V | Volume of liquid in the anode compartment |
| WW | Wastewater |
| ηcoul | Coulombic efficiency |
| ηCOD | Chemical oxygen demand removal efficiency |
| ΔV | Voltage difference |
| ρ | Resistivity |
References
- Logan, B.E.; Regan, J.M. Microbial challenges and harnessing the metabolic activity of bacteria can provide energy for a variety of applications, once technical and cost obstacles are overcome. Environ. Sci. Technol. 2006, 5172–5180. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, G.; Xu, M. Microbial fuel cells come of age. J. Chem. Technol. Biotechnol. 2010, 86, 625–632. [Google Scholar] [CrossRef]
- Cheng-Dar, Y.; Chung-Ming, L.; Liou, E.M.L. A transition toward a sustainable energy future: Feasibility assessment and development strategies of wind power in Taiwan. Energy Policy 2001, 29, 951–963. [Google Scholar] [CrossRef]
- Tye, Y.Y.; Lee, K.T.; Abdullah, W.N.W.; Leh, C.P. Second-generation bioethanol as a sustainable energy source in Malaysia transportation sector: Status, potential and future prospects. Renew. Sustain. Energy Rev. 2011, 15, 4521–4536. [Google Scholar] [CrossRef]
- Venkatesan, P.N.; Sangeetha, D. Characterization and performance study of sulfonated poly ether ether ketone/Fe3O4nano composite membrane as electrolyte for microbial fuel cell. Chem. Eng. J. 2014, 243, 564–571. [Google Scholar] [CrossRef]
- Lyu, P.; Liu, X.; Qu, J.; Zhao, J.; Huo, Y.; Qu, Z.; Rao, Z. Recent advances of thermal safety of lithium ion battery for energy storage. Energy Stor. Mater. 2020, 31, 195–220. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Zhu, F.; Schmidt, O.G. Recent Progress in Micro-Supercapacitor Design, Integration, and Functionalization. Small Methods 2019, 3, 1800367. [Google Scholar] [CrossRef]
- Krauskopf, K.B.; Bird, D.K. Introduction to Geochemistry, 3rd ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Sangeetha, D.; Kugarajah, V.; Sugumar, M. Membranes for Microbial Fuel Cells. In Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation, Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–194. [Google Scholar] [CrossRef]
- Bakonyi, P.; Koók, L.; Rózsenberszki, T.; Tóth, G.; Bélafi-Bakó, K.; Nemestóthy, N. Development and application of supported ionic liquid membranes in microbial fuel cell technology: A concise overview. Membranes 2020, 10, 16. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. Ser. B 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Schröder, U. Anodic electron transfer mechanisms in microbial fuel cells and their energy efficiency. Phys. Chem. Chem. Phys. 2007, 9, 2619–2629. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Cheng, S.; Logan, B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005, 39, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Kargi, F.; Eker, S. Electricity generation with simultaneous wastewater treatment by a microbial fuel cell (MFC) with Cu and Cu-Au electrodes. J. Chem. Technol. Biotechnol. 2007, 82, 658–662. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley-Interscience: Hoboken, NJ, USA, 2008. [Google Scholar]
- Vazquez-Larios, A.L.; Solorza-Feria, O.; Vazquez-Huerta, G.; Esparza-Garcia, F.J.; Rios-Leal, E.; Rinderknecht-Seijas, N.; Poggi-Varaldo, H.M. A new design improves performance of a single chamber microbial fuel cell. J. New Mater. Electrochem. Syst. 2010, 13, 219–226. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Urine utilisation by microbial fuel cells; energy fuel for the future. Phys. Chem. Chem. Phys. 2012, 14, 94–98. [Google Scholar] [CrossRef]
- Modin, O.; Gustavsson, D. Opportunities for microbial electrochemistry in municipal wastewater treatment—An overview. Water Sci. Technol. 2014, 69, 1359–1372. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Santoro, C.; Salar Garcia, M.J.; Walter, X.A.; You, J.; Theodosiou, P.; Gajda, I.; Ieropoulos, I. Urine in bioelectrochemical systems: An overall review. ChemElectroChem 2020, 7, 1312–1331. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O.; Romero-Castañón, T.; Ríos-Leal, E.; Galíndez-Mayer, J.; Esparza-García, F. Batch operation of a microbial fuel cell equipped with alternative proton exchange membrane. Int. J. Hydrogen Energy 2015, 40, 17323–17331. [Google Scholar] [CrossRef]
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Yu, H.-Q. Recent advances in the separators for microbial fuel cells. Bioresour. Technol. 2011, 102, 244–252. [Google Scholar] [CrossRef]
- Pandit, S.; Sengupta, A.; Kale, S.; Das, D. Performance of electron acceptors in catholyte of a two-chambered microbial fuel cell using anion exchange membrane. Bioresour. Technol. 2011, 102, 2736–2744. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Beyenal, H.; Lewandowski, Z. Scaling up Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 7643–7648. [Google Scholar] [CrossRef]
- Hsu, L.; Chadwick, B.; Kagan, J.; Thacher, R.; Wotawa-Bergen, A.; Richter, K. Scale up considerations for sediment microbial fuel Cells. RSC Adv. 2013, 3, 15947–15954. [Google Scholar] [CrossRef]
- Zhou, M.; Chi, M.; Luo, J.; He, H.; Jin, T. An overview of electrode materials in microbial fuel cells. J. Power Sources 2011, 196, 4427–4435. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent progress in electrodes for microbial fuel cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef]
- Cho, K.-Y.; Jung, H.-Y.; Sung, K.A.; Kim, W.-K.; Sung, S.-J.; Park, J.-K.; Choi, J.-H.; Sung, Y.-E. Preparation and characteristics of Nafion membrane coated with a PVdF copolymer/recast Nafion blend for direct methanol fuel cell. J. Power Sources 2006, 159, 524–528. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Ghasemi, M.; Wan, D.W.R.; Yaakob, Z.; Sedighi, M.; Alam, J.; Ismail, A.F. Performance enhancement of microbial fuel cell by PVDF/Nafion nanofibre composite proton exchange membrane. Fuel Process. Technol. 2014, 124, 290–295. [Google Scholar] [CrossRef]
- Harnisch, F.; Schröder, U. Selectivity versus mobility: Separation of anode and cathode in Microbial bioelectrochemical systems. ChemSusChem 2009, 2, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Daud, W.R.W.; Ismail, A.F.; Jafari, Y.; Ismail, M.; Mayahi, A.; Othman, J. Simultaneous wastewater treatment and electricity generation by microbial fuel cell: Performance comparison and cost investigation of using Nafion 117 and SPEEK as separators. Desalination 2013, 325, 1–6. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Sivasankaran, A.; Sangeetha, D. Development of MFC using sulphonated polyether ether ketone (SPEEK) membrane for electricity generation from waste water. Bioresour. Technol. 2011, 102, 11167–11171. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Andrio, A.; Compañ, V.; Solorza-Feria, O.; Poggi-Varaldo, H.M. Synthesis and characterizacion of organic agar-based membranes for microbial fuel cells. J. Power Sources 2019, 435, 226772. [Google Scholar] [CrossRef]
- Scott, K. Membranes and Separators for Microbial Fuel Cells. In Microbial Electrochemical and Fuel Cells: Fundamentals and Applications; Elsevier Ltd.: Newcastle upon Tyne, UK, 2016; pp. 153–178. [Google Scholar] [CrossRef]
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Liew, K.B.; Ismail, M. Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: A comprehensive review. Renew. Sustain. Energy Rev. 2013, 28, 575–587. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Grzebyk, M.; Poźniak, G. Microbial fuel cells (MFCs) with interpolymer cation exchange membranes. Sep. Purif. Technol. 2005, 41, 321–328. [Google Scholar] [CrossRef]
- Pant, D.; Bogaert, G.V.; Smet, M.D.; Diels, L.; Vanbroekhoven, K. Use of novel permeable membrane and air cathode in acetate microbial fuel cells. Electrochim. Acta 2010, 55, 7710–7716. [Google Scholar] [CrossRef]
- Lefebvre, O.; Shen, Y.; Tang, Z.; Uzabiaga, A.; Chang, I.S.; Ng, H.Y. A comparison of membranes and enrichment strategies for microbial fuel cells. Bioresour. Technol. 2011, 102, 6291–6294. [Google Scholar] [CrossRef]
- Zhang, F.; Brastad, K.S.; He, Z. Integrating forward osmosis into microbial fuel cells for wastewater treatment, water extraction and bioelectricity generation. Environ. Sci. Technol. 2011, 45, 6690–6696. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.J.; Saito, T.; Hickner, M.A.; Logan, B.E. Polymer coatings as separator layers for microbial fuel cell cathodes. J. Power Sources 2011, 196, 3009–3014. [Google Scholar] [CrossRef]
- Ghasemi, M.; Shahgaldi, S.; Ismail, M.; Yaakob, Z.; Daud, W.R.W. New generation of carbon nanocomposite proton exchange membranes in microbial fuel cell systems. Chem. Eng. J. 2012, 184, 82–89. [Google Scholar] [CrossRef]
- Choi, T.H.; Won, Y.-B.; Lee, J.-W.; Shin, D.W.; Lee, Y.M.; Kim, M.; Park, H.B. Electrochemical performance of microbial fuel cells based on disulfonated poly(arylene ether sulfone) membranes. J. Power Sources 2012, 220, 269–279. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, S.H.; Chang, I.S.; Moon, S.H. Characterization of uncharged and sulfonated porous poly(vinylidene fluoride) membranes and their performance in microbial fuel cells. J. Membr. Sci. 2014, 463, 205–214. [Google Scholar] [CrossRef]
- Harnisch, F.; Warmbier, R.; Schneider, R.; Schröder, U. Modeling the ion transfer and polarization of ion exchange membranes in bioelectrochemical systems. Bioelectrochemistry 2009, 75, 136–141. [Google Scholar] [CrossRef]
- Castellan, G.W. Physical Chemistry, 3rd ed.; Addison-Wesley Publishing Company, Inc.: Boston, MA, USA, 1987. [Google Scholar]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O.; Ponce-Noyola, M.T.; Romero-Castañón, T.; Rinderknecht-Seijas, N.; Galíndez-Mayer, J. Characteristics of a single chamber microbial fuel cell equipped with a low cost membrane. Int. J. Hydrogen Energy 2015, 40, 17380–17387. [Google Scholar] [CrossRef]
- Vélez-Pérez, L.S.; Ramirez-Nava, J.; Hernández-Flores, G.; Talavera-Mendoza, O.; Escamilla-Alvarado, C.; Poggi-Varaldo, H.M.; Solorza-Feria, O.; López-Díaz, J.A. Industrial acid mine drainage and municipal wastewater co-treatment by dual-chamber microbial fuel cells. Int. J. Hydrogen Energy 2020, 45, 13757–13766. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaroe, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 133–138. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O. Comparison of alternative membranes to replace high cost Nafion ones in microbial fuel cells. Int. J. Hydrogen Energy 2016, 41, 23354–23362. [Google Scholar] [CrossRef]
- Catal, T.; Bermek, H.; Liu, H. Removal of selenite from wastewater using microbial fuel cells. Biotechnol. Lett. 2009, 31, 1211–1216. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. Bioelectrochemical metal recovery from wastewater: A review. Water Res. 2014, 66, 219–232. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Yakhmi, J.V. Microbial fuel cells to recover heavy metals. Environ. Chem. Lett. 2014, 12, 483–494. [Google Scholar] [CrossRef]
- Qu, Y.; Feng, Y.; Wang, X.; Logan, B.E. Use of a coculture to enable current production by Geobacter sulfurreducens. Appl. Environ. Microbiol. 2012, 78, 3484–3487. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Vazquez-Larios, A.; Solorza-Feria, O. Microbial Fuel Cells. In Fuel Cells; Rodríguez-Varela, F.J., Solorza-Feria, O., Hernández-Pacheco, E., Eds.; Book Livres: Montréal, QC, Canada, 2010; pp. 124–161. [Google Scholar]
- Noyola, A.; Morgan-Sagastume, J.M.; Guereca, L.P. Selección de Tecnologías para el Tratamiento de Aguas Residuales Municipales. In Guía de Apoyo para Ciudades Pequeñas y Medianas; Instituto de Ingeniería UNAM: Mexico City, Mexico, 2013; ISBN 978-607-02-4822-1. [Google Scholar]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Romero-Castañón, T.; Solorza-Feria, O.; Rinderknecht-Seijas, N. Harvesting energy from leachates in microbial fuel cells using an anion exchange membrane. Int. J. Hydrogen Energy 2017, 42, 30374–30382. [Google Scholar] [CrossRef]
- Kazemi, S.; Fatih, K.; Mohseni, M.; Wang, H. Investigating Separators to Improve Performance of Flat-Plate Microbial Fuel Cells. In Meeting Abstracts: The Electrochemical Society; The Electrochemical Society: Pennington, NJ, USA, 2012; 3593p. [Google Scholar] [CrossRef]
- Pandit, S.; Das, D. (Eds.) Principles of Microbial Fuel Cell for the Power Generation; Capital Publishing Company: New Delhi, India, 2018. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.V.; Guisman, C.J.N. Effects of membrane cation transport on pH and microbial fuel cell performance. Environ. Sci. Technol. 2006, 40, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
- Negro, E.; Vittadello, M.; Vezzùd, K.; Paddison, S.J.; Di Noto, V. The influence of the cationic form and degree of hydration on the structure of Nafion™. Solid State Ion. 2013, 252, 84–92. [Google Scholar] [CrossRef]
- Dicks, A.; Rand, D. Proton-Exchange Membrane Fuel Cells. In Fuel Cell Systems Explained, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 69–133. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Chae, K.J.; Choi, M.; Ajayi, F.F.; Park, W.; Chang, I.S.; Kim, I.S. Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energy Fuels 2008, 22, 169–176. [Google Scholar] [CrossRef]
- Rudra, R.; Kumar, V.; Nandy, A.; Kundu, P.P. Performances of separator and Membraneless Microbial Fuel Cell. In Microbial Fuel Cell a Bioelectrochemical System that Converts Waste to Watts; Das, D., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 125–140. [Google Scholar] [CrossRef]
- Christgen, B.; Scott, K.; Dolfing, J.; Head, I.M.; Curtis, T.P. An evaluation of the performance and economics of membranes and separators in single chamber microbial fuel cells treating domestic wastewater. PLoS ONE 2015, 10, e0136108. [Google Scholar] [CrossRef]
- Stenina, I.A.; Sistat, P.; Rebrov, A.I.; Pourcelly, G.; Yaroslavtsev, A.B. Ion mobility in Nafion-117 membranes. Desalination 2004, 170, 49–57. [Google Scholar] [CrossRef]
- Zhang, S.; Hui, Y.; Han, B. Effects of Three Types of Separator Membranes on the Microbial Fuel Cells Performance; Atlantis Press: Paris, France, 2015; pp. 1592–1596. [Google Scholar] [CrossRef][Green Version]
- Kim, J.R.; Cheng, S.; Oh, S.-E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Comprehensive study on ceramic membranes for low-cost microbial fuel cells. ChemSusChem 2016, 9, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Guo, K.; Li, H.; Du, Z.; Tian, J. Microfiltration membrane performance in two-chamber microbial fuel cells. Biochem. Eng. J. 2010, 52, 194–198. [Google Scholar] [CrossRef]
- Savadogo, O. Emerging membranes for electrochemical systems: Part II. High temperature composite membranes for polymer electrolyte fuel cell (PEFC) applications. J. Power Sources 2004, 127, 135–161. [Google Scholar] [CrossRef]
- Yee, R.S.L.; Rozendal, R.A.; Zhang, K.; Ladewig, B.P. Cost effective cation exchange membranes: A review. Chem. Eng. Res. Des. 2012, 90, 950–959. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Lee, J.; Kakarla, R.; Kim, H.S.; Min, B. Low-cost separators for enhanced power production and field application of microbial fuel cells (MFCs). Electrochim. Acta 2014, 132, 434–440. [Google Scholar] [CrossRef]
- Biffinger, J.; Ringeisen, B. Engineering microbial fuels cells: Recent patents and new directions. Recent Pat. Biotechnol. 2008, 2, 150–155. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Hassan, S.H.A.; Oh, S.-E.; Ismail, M.; Rahimnejad, M.; Jahim, J.M. Nano-structured carbon as electrode material in microbial fuel cells: A comprehensive review. J. Alloys Compd. 2013, 580, 245–255. [Google Scholar] [CrossRef]
- Tiwari, B.R.; Noori, M.T.; Ghangrekar, M.M. A novel low cost polyvinyl alcohol-Nafion-borosilicate membrane separator for microbial fuel cell. Mater. Chem. Phys. 2016, 182, 86–93. [Google Scholar] [CrossRef]
- Fornero, J.J.; Rosenbaum, M.; Cotta, M.A.; Angenent, L.T. Microbial fuel cell performance with a pressurized cathode chamber. Environ. Sci. Technol. 2008, 42, 8578–8584. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Ge, X.; He, Y.; Guiver, M.D.; Wu, L.; Ran, J.; Yang, Z.; Xu, T. Alkaline anion-exchange membranes containing mobile ion shuttles. Adv. Mater. 2016, 28, 3467–3472. [Google Scholar] [CrossRef]
- Mahendiravarman, E.; Sangeetha, D. Application of polysulphone based anion exchange membrane electrolyte for improved electricity generation in microbial fuel cell. Mater. Chem. Phys. 2017, 199, 528–536. [Google Scholar] [CrossRef]
- Rossi, R.; Wang, X.; Logan, B.E. High performance flow through microbial fuel cells with anion exchange membrane. J. Power Sources 2020, 475, 228–633. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Cheng, S.A.; Huang, X.; Logan, B.E. The use of nylon and glass fiber filter separators with different pore sizes in air-cathode single-chamber microbial fuel cells. Energy Environ. Sci. 2010, 3, 659–664. [Google Scholar] [CrossRef]
- Guo, D.; Zhuo, Y.Z.; Lai, A.N.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Interpenetrating anion exchange membranes using poly (1-vinylimidazole) as bifunctional crosslinker for fuel cells. J. Membr. Sci. 2016, 518, 295–304. [Google Scholar] [CrossRef]
- Mahendiravarman, E.; Sangeetha, D. Increased microbial fuel cell performance using quaternized poly ether ether ketone anionic membrane electrolyte for electricity generation. Int. J. Hydrogen Energy 2013, 38, 2471–2479. [Google Scholar] [CrossRef]
- Heijne, A.T.; Hamelers, H.V.M.; De Wilde, V.; Rozendal, R.A.; Buisman, C.J. A Bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5200–5205. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, C.R.; Song, Y.E.; Heo, J.; Choi, S.M.; Lim, D.-H.; Cho, J.; Park, C.; Jang, M.; Kim, J.R. Hexavalent chromium as a cathodic electron acceptor in a bipolar membrane microbial fuel cell with the simultaneous treatment of electroplating wastewater. Chem. Eng. J. 2017, 328, 703–707. [Google Scholar] [CrossRef]
- Behera, M.; Jana, P.S.; Ghangrekar, M.M. Performance evaluation of low cost microbial fuel cell fabricated using earthen pot with biotic and abiotic cathode. Bioresour. Technol. 2010, 101, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Zeikus, J.G. Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol. Bioeng. 2003, 81, 348–355. [Google Scholar] [CrossRef]
- Behera, M.; Ghangrekar, M.M. Electricity generation in low cost microbial fuel cell made up of earthenware of different thickness. Water Sci. Technol. 2010, 64, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.; Jana, P.S.; More, T.T.; Ghangrekar, M.M. Rice mill wastewater treatment in microbial fuel cells fabricated using proton exchange membrane and earthen pot at different pH. Bioelectrochemistry 2010, 79, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Winfield, J.; Greenman, J.; Huson, D.; Ieropoulos, I. Comparing terracotta and earthenware for multiple functionalities in microbial fuel cells. Bioproc. Biosyst. Eng. 2013, 36, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Winfield, J.; Chambers, L.D.; Rossiter, J.; Ieropoulos, I. Comparing the short and long term stability of biodegradable, ceramic and cation exchange membranes in microbial fuel cells. Bioresour. Technol. 2013, 148, 480–486. [Google Scholar] [CrossRef]
- Winfield, J.; Gajda, I.; Greenman, J.; Ieropoulos, I. A review into the use of ceramics in microbial fuel cells. Bioresour. Technol. 2016, 215, 296–303. [Google Scholar] [CrossRef]
- Merino-Jimenez, I.; Greenman, J.; Ieropoulos, I. Electricity and catholyte production from ceramic MFCs treating urine. Int. J. Hydrogen Energy 2017, 42, 1791–1799. [Google Scholar] [CrossRef]
- Merino-Jimenez, I.; Gonzalez-Juarez, F.; Greenman, J.; Ieropoulos, I. Effect of the ceramic membrane properties on the microbial fuel cell power output and catholyte generation. J. Power Sources 2019, 429, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J.R.; Cha, J.; Kim, Y.; Premier, G.C.; Kim, C. Enhanced power production of a membrane electrode assembly microbial fuel cell (MFC) using a cost effective poly [2,5-benzimidazole] (ABPBI) impregnated non-woven fabric filter. Bioresour. Technol. 2013, 128, 14–21. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Wang, X.; Huang, X.; Logan, B.E. Separator characteristics for increasing performance of microbial fuel cells. Environ. Sci. Technol. 2009, 43, 8456–8641. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, H.; Liu, H. Enhanced coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J. Power Sources 2007, 171, 348–354. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhou, S.; Wang, Y.; Liu, C.; Geng, S. Membrane-less cloth cathode assembly (CCA) for scalable microbial fuel cells. Biosens. Bioelectron. 2009, 24, 3652–3656. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, Q.; Tong, M.; Li, S.; Li, H. Electricity generation using membrane-less microbial fuel cell during wastewater treatment. Chin. J. Chem. Eng. 2008, 16, 772–777. [Google Scholar] [CrossRef]
- Du, F.; Xie, B.; Dong, W.; Jia, B.; Dong, K.; Liu, H. Continuous flowing membraneless microbial fuel cells with separated electrode chambers. Bioresour. Technol. 2011, 102, 8914–8920. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Huang, X.; Logan, B.E. Improved performance of single-chamber microbial fuel cells through control of membrane deformation. Biosens. Bioelectron. 2009, 25, 1825–1828. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Shinde, V.B. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour. Technol. 2007, 98, 2879–2885. [Google Scholar] [CrossRef]
- Jang, J.K.; Pham, T.H.; Chang, I.S.; Kang, K.H.; Moon, H.; Cho, K.S.; Kim, B.H. Construction and operation of a novel mediator- and membrane-less microbial fuel cell. Process. Biochem. 2004, 39, 1007–1012. [Google Scholar] [CrossRef]
- Walter, X.A.; Greenman, J.; Ieropoulos, I. Microbial fuel cells directly powering a microcomputer. J. Power Sources 2020, 446, 227–328. [Google Scholar] [CrossRef]
- Winfield, J.; Chambers, L.D.; Rossiter, J.; Greenman, J.; Ieropoulos, I. Urine-activated origami microbial fuel cells to signal proof of life. J. Mater. Chem. A 2015, 3, 7058–7065. [Google Scholar] [CrossRef]
- Winfield, J.; Chambers, L.D.; Rossiter, J.; Stinchcombe, A.; Walter, X.A.; Greenman, J.; Ieropoulos, I. Fade to Green: A Biodegradable Stack of Microbial Fuel Cells. ChemSusChem 2015, 8, 2705–2712. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Huang, L.; Logan, B.E. Scale-up of membrane-free single-chamber microbial fuel cells. J. Power Sources 2008, 179, 274–279. [Google Scholar] [CrossRef]
- Santoro, C.; Ieropoulos, I.; Greenman, J.; Cristiani, P.; Vadas, T.; Mackay, A.; Li, B. Power generation and contaminant removal in single chamber microbial fuel cells (SCMFCs) treating human urine. Int. J. Hydrogen Energy 2013, 38, 11543–11551. [Google Scholar] [CrossRef]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biot. 2009, 85, 1665–1671. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef]
- Tartakovsky, B.; Guiot, S.R. A comparison of air and hydrogen peroxide oxygenated microbial fuel cell reactors. Biotechnol. Prog. 2006, 22, 241–246. [Google Scholar] [CrossRef]
- Karube, I.; Matsunaga, T.; Tsuru, S.; Suzuki, S. Biochemical fuel cell utilizing immobilized cells of clostridium butyricum. Biotechnol. Bioeng. 1977, 19, 1727–1733. [Google Scholar] [CrossRef]
- Mohan, Y.; Manojmuthukumar, S.; Das, D. Electricity generation using microbial fuel cells. Int. J. Hydrogen Energy 2008, 33, 423–426. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Nava, J.; Martínez-Castrejón, M.; García-Mesino, R.L.; López-Díaz, J.A.; Talavera-Mendoza, O.; Sarmiento-Villagrana, A.; Rojano, F.; Hernández-Flores, G. The Implications of Membranes Used as Separators in Microbial Fuel Cells. Membranes 2021, 11, 738. https://doi.org/10.3390/membranes11100738
Ramirez-Nava J, Martínez-Castrejón M, García-Mesino RL, López-Díaz JA, Talavera-Mendoza O, Sarmiento-Villagrana A, Rojano F, Hernández-Flores G. The Implications of Membranes Used as Separators in Microbial Fuel Cells. Membranes. 2021; 11(10):738. https://doi.org/10.3390/membranes11100738
Chicago/Turabian StyleRamirez-Nava, Jonathan, Mariana Martínez-Castrejón, Rocío Lley García-Mesino, Jazmin Alaide López-Díaz, Oscar Talavera-Mendoza, Alicia Sarmiento-Villagrana, Fernando Rojano, and Giovanni Hernández-Flores. 2021. "The Implications of Membranes Used as Separators in Microbial Fuel Cells" Membranes 11, no. 10: 738. https://doi.org/10.3390/membranes11100738
APA StyleRamirez-Nava, J., Martínez-Castrejón, M., García-Mesino, R. L., López-Díaz, J. A., Talavera-Mendoza, O., Sarmiento-Villagrana, A., Rojano, F., & Hernández-Flores, G. (2021). The Implications of Membranes Used as Separators in Microbial Fuel Cells. Membranes, 11(10), 738. https://doi.org/10.3390/membranes11100738