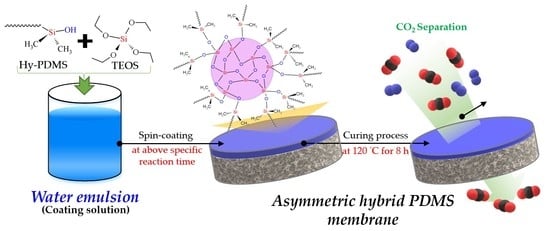
Impacts of Green Synthesis Process on Asymmetric Hybrid PDMS Membrane for Efficient CO2/N2 Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Hybrid PDMS Membranes by Water Emulsion
2.3. Characterizations
2.4. Membrane Performance Evaluation
3. Results and Discussion
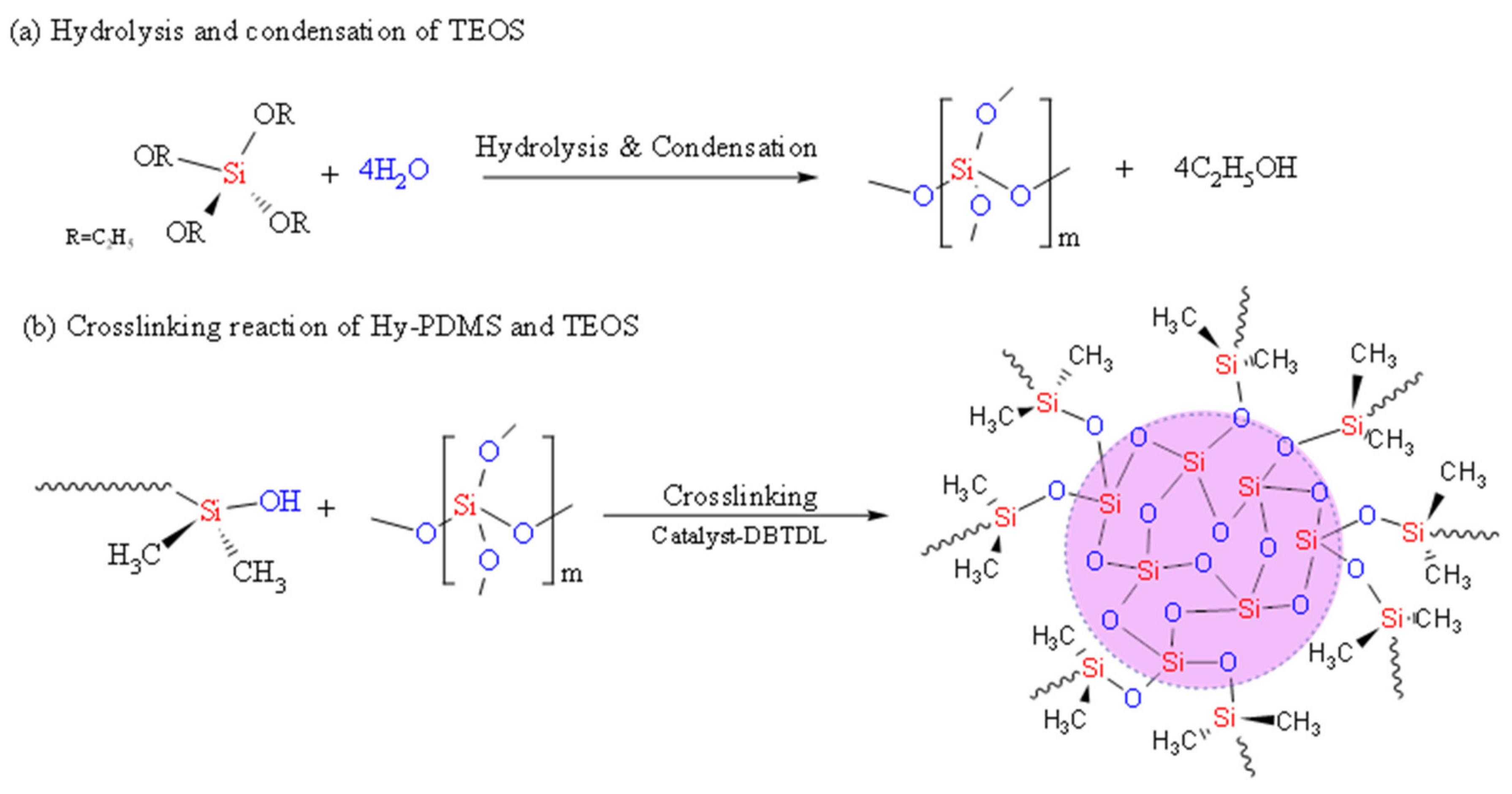
3.1. Effect of TEOS on the Crosslinking Properties of PDMS in Water Emulsion
3.1.1. Viscosity of the Coating Solution
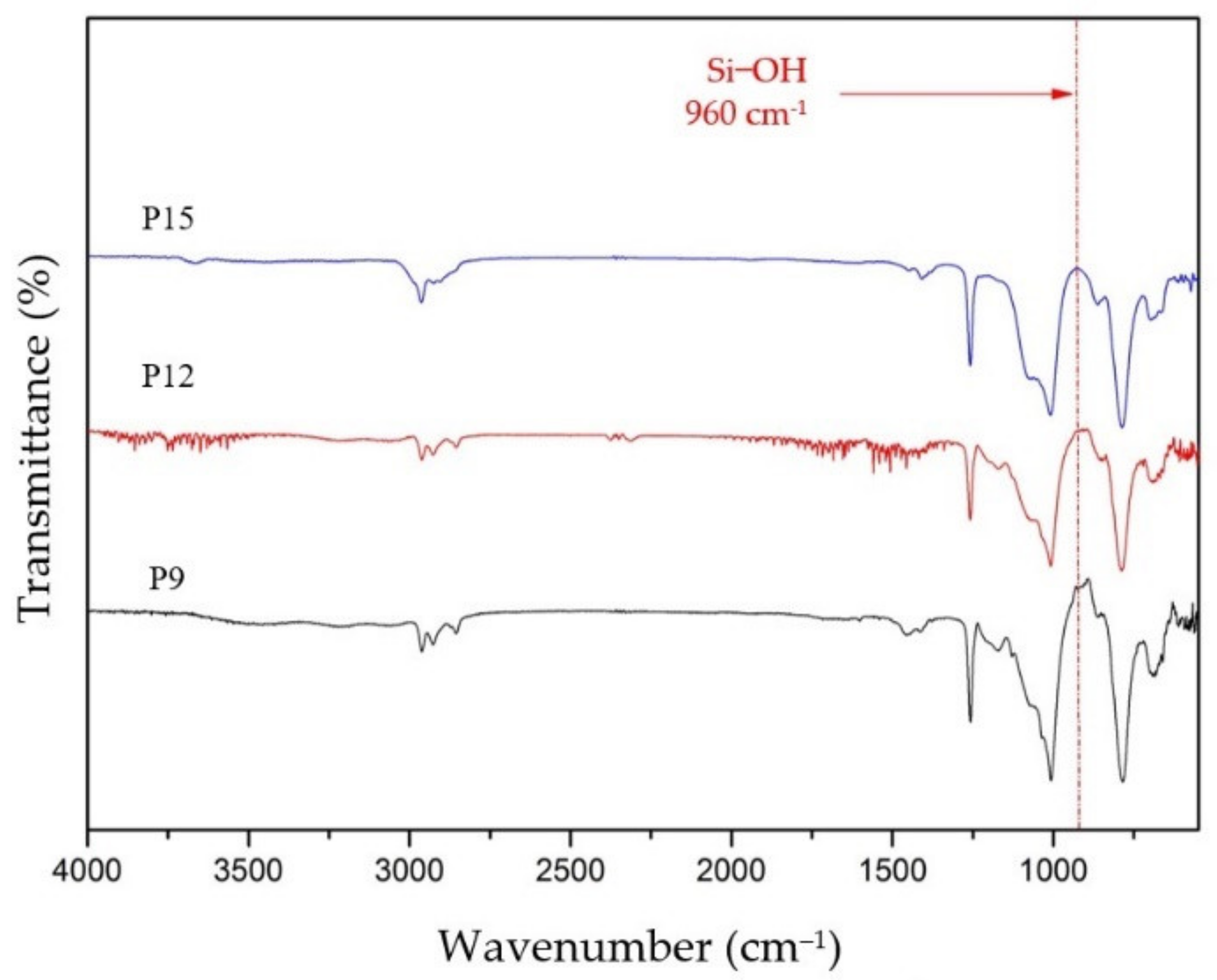
3.1.2. The Crosslinking Properties of the hybrid PDMS membranes
3.2. Effect of the Reaction Time on the Characterization of the Hybrid PDMS Membranes
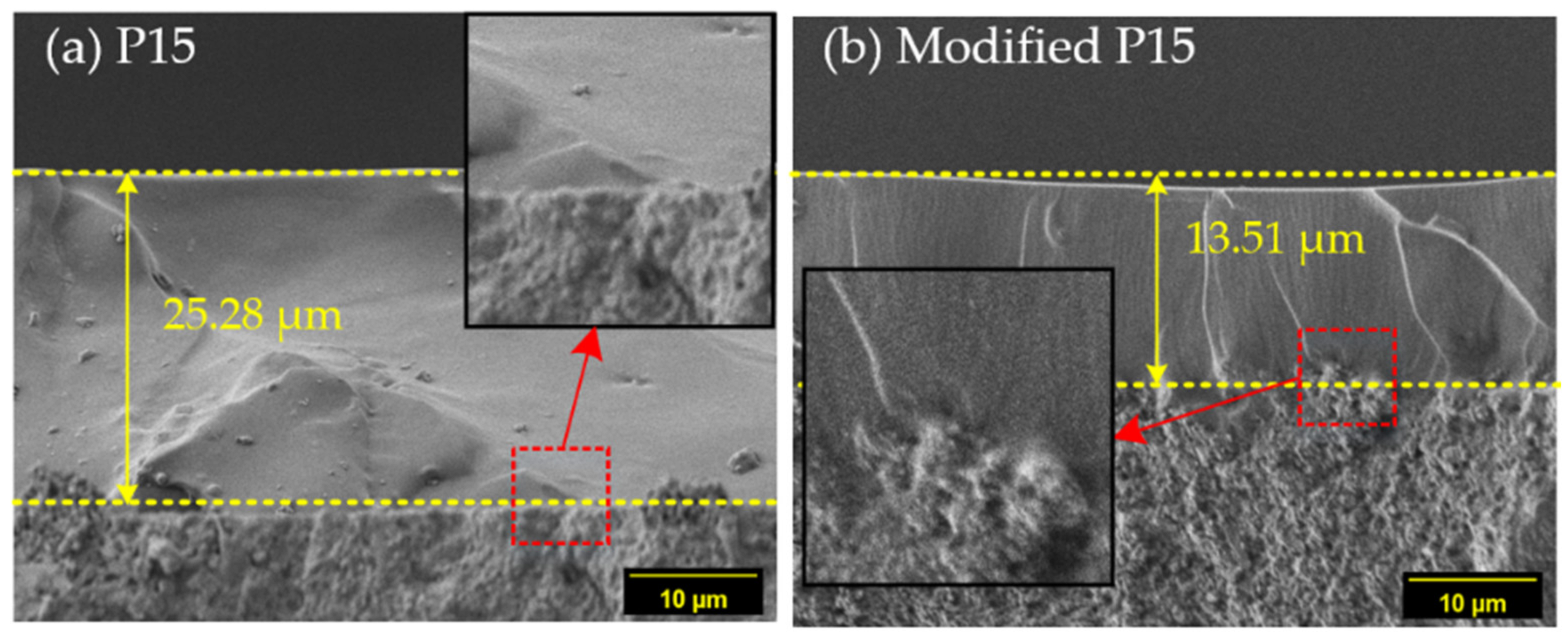
3.2.1. Morphological Characterization
3.2.2. Gas Permeation Properties
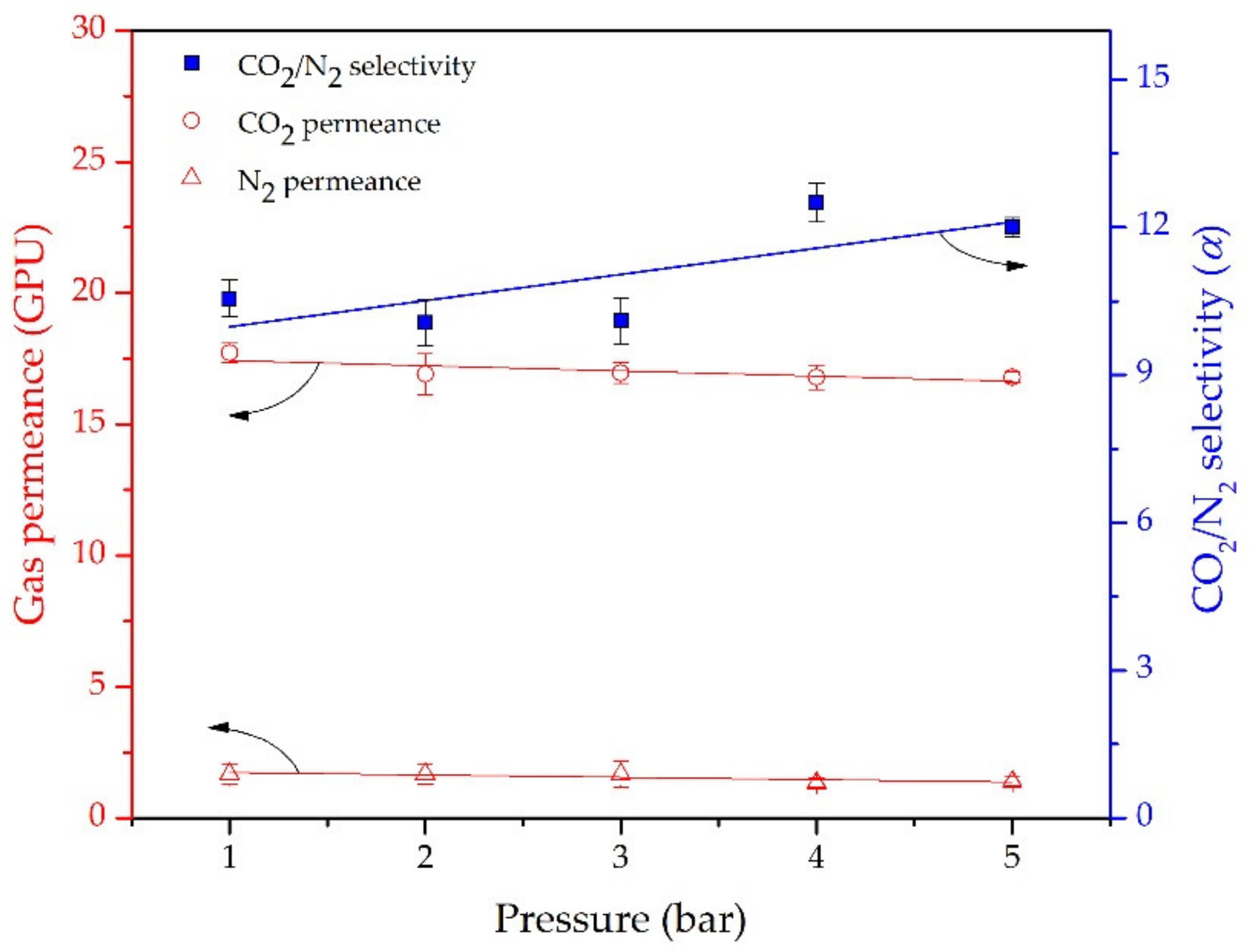
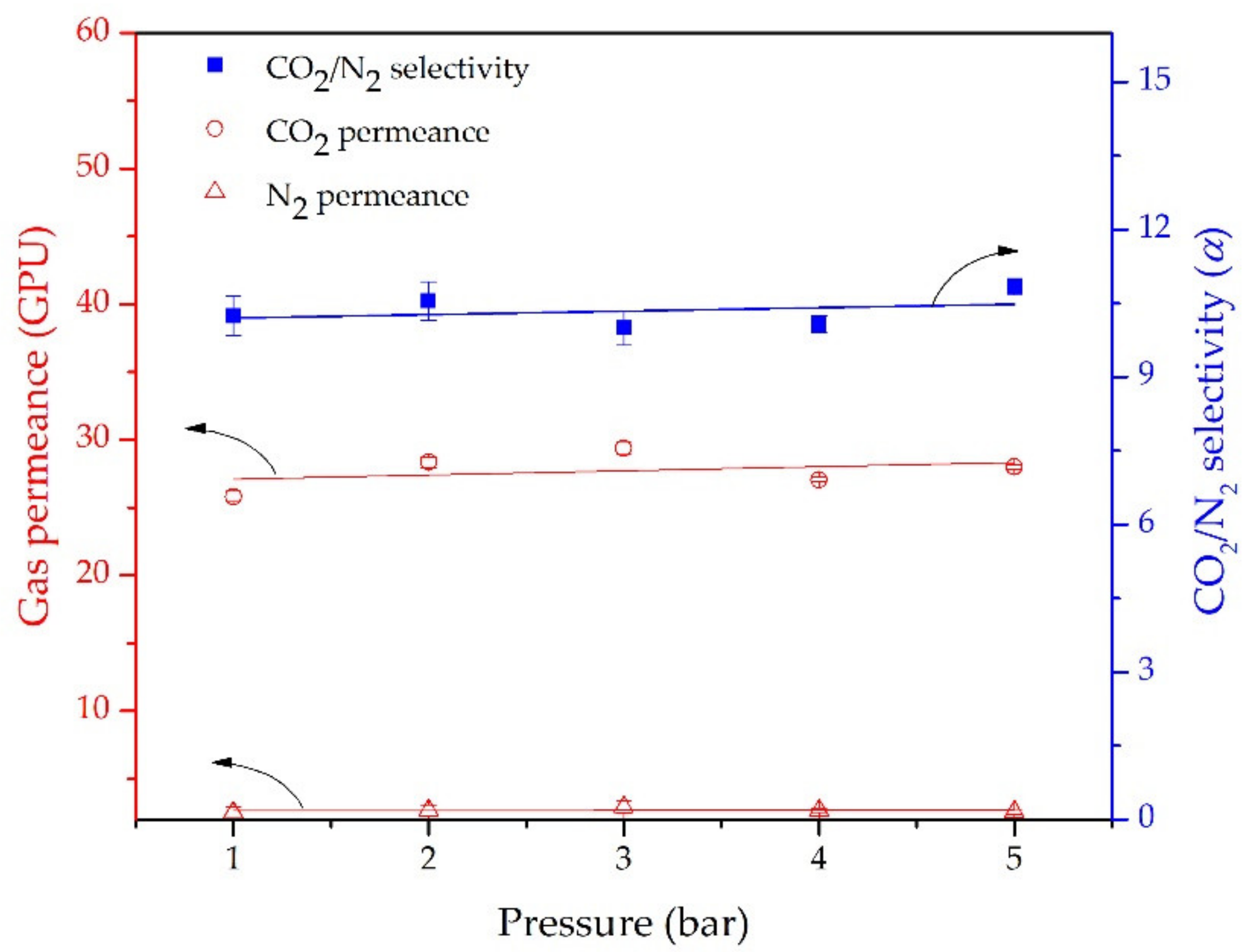
3.3. CO2/N2 Sepaparation of the Hybrid PDMS Membrane
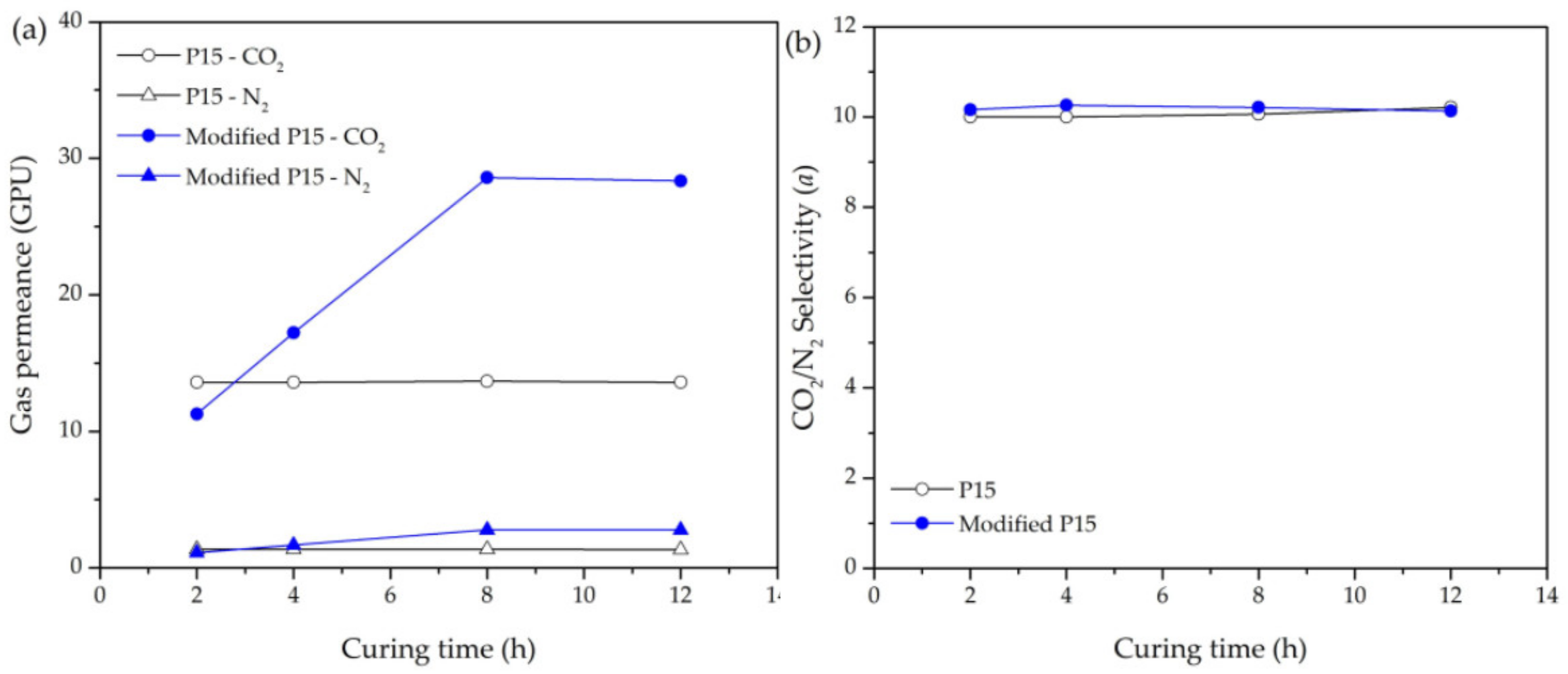
3.4. Enhancing CO2 Permeance Using the Curing Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| PGPU | Gas permeance (GPU, 7.5 × 10−12 m3 (STP)/m2·s·Pa) |
| V | Volume of the downstream chamber (cm3) |
| A | Effective area of the membranes (cm2) |
| T | Temperature (K) |
| dp | Transmembrane pressure (cmHg) |
| dt | Time (s) |
| αi, j | Ideal selectivity of two gases i and j |
| Ra | Surface roughness (nm) |
Abbreviations
| AFM | Atomic Force Microscopy |
| CA | Cellulose Acetate |
| DBSA | 4-Dodecylbenzenesulfonic acid |
| DBTDL | Dibutyltin Dilaurate |
| FTIR | Fourier Transform Infrared |
| GPU | Gas Permeation Unit |
| HMS | Highly Microporous Support |
| Hy-PDMS | Hydroxyl-terminated Polydimethylsiloxane |
| M.W. | Molecular weight |
| PDMS | Polydimethylsiloxane |
| PES | Polyethersulfone |
| SDS | Sodium Dodecyl sulfate |
| SEM | Scanning Electron Microscopy |
| TEOS | Tetraethylorthosilicate |
| TGA | Thermogravimetric |
| TiO2 | Titanium Dioxide |
References
- Norahim, N.; Yaisanga, P.; Faungnawakij, K.; Charinpanitkul, T.; Klaysom, C. Recent Membrane Developments for CO2 Separation and Capture. Chem. Eng. Technol. 2018, 41, 211–223. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley Interscience: New York, NY, USA, 1999. [Google Scholar]
- Yeom, C.K.; Lee, S.H.; Lee, J.M. Study of transport of pure and mixed CO2/N2 gases through polymeric membranes. J. Appl. Polym. Sci. 2000, 78, 179–189. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Badieh, M.M.S.; Vatanpour, V.; Ghaemi, N. Effect of titanium dioxide nanoparticles on polydimethylsiloxane/polyethersulfone composite membranes for gas separation. Polym. Eng. Sci. 2012, 52, 2664–2674. [Google Scholar] [CrossRef]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas sorption, diffusion, and permeation in poly(dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Wu, F.; Li, L.; Xu, Z.; Tan, S.; Zhang, Z. Transport study of pure and mixed gases through PDMS membrane. Chem. Eng. J. 2006, 117, 51–59. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Shahidi, K.; Mohammadi, T. Synthesis and gas permeation properties of a single layer PDMS membrane. J. Appl. Polym. Sci. 2010, 117, 33–48. [Google Scholar] [CrossRef]
- Orooji, Y.; Movahedi, A.; Liu, Z.; Asadnia, M.; Ghasali, E.; Ganjkhanlou, Y.; Razmjou, A.; Karimi-Maleh, H.; Kiadeh, N.T.H. Luminescent film: Biofouling investigation of tetraphenylethylene blended polyethersulfone ultrafiltration membrane. Chemosphere 2020. [Google Scholar] [CrossRef]
- Orooji, Y.; Jaleh, B.; Homayouni, F.; Fakhri, P.; Kashfi, M.; Torkamany, M.J.; Yousefi, A.A. Laser Ablation-Assisted Synthesis of Poly (Vinylidene Fluoride)/Au Nanocomposites: Crystalline Phase and Micromechanical Finite Element Analysis. Polymers 2020, 12, 2630. [Google Scholar] [CrossRef]
- Roslan, R.A.; Lau, W.J.; Lai, G.S.; Zulhairun, A.K.; Yeong, Y.F.; Ismail, A.F.; Matsuura, T. Impacts of Multilayer Hybrid Coating on PSF Hollow Fiber Membrane for Enhanced Gas Separation. Membranes 2020, 10, 335. [Google Scholar] [CrossRef]
- Yang, Y.; Han, Y.; Pang, R.; Ho, W.S.W. Amine-Containing Membranes with Functionalized Multi-Walled Carbon Nanotubes for CO2/H2 Separation. Membranes 2020, 10, 333. [Google Scholar] [CrossRef]
- Monteiro, B.; Nabais, A.R.; Casimiro, M.H.; Martins, A.P.S.; Francisco, R.O.; Neves, L.A.; Pereira, C.C.L. Impact on CO2/N2 and CO2/CH4 Separation Performance Using Cu-BTC with Supported Ionic Liquids-Based Mixed Matrix Membranes. Membranes 2018, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Orooji, Y.; Ghasali, E.; Emami, N.; Noorisafa, F.; Razmjou, A. ANOVA Design for the Optimization of TiO2 Coating on Polyether Sulfone Membranes. Molecules 2019, 24, 2924. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Orooji, Y.; Razmjou, A. Applying Membrane Distillation for the Recovery of Nitrate from Saline Water Using PVDF Membranes Modified as Superhydrophobic Membranes. Polymers 2020, 12, 2774. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.; Bayrakçeken Yurtcan, A. Investigation of synergetic effect of PDMS polymer hydrophobicity and polystyrene-silica particles roughness in the content of microporous layer on water management in PEM fuel cell. Appl. Surf. Sci. 2020, 511, 145415. [Google Scholar] [CrossRef]
- He, X.; Wang, T.; Huang, J.; Chen, J.; Li, J. Fabrication and characterization of superhydrophobic PDMS composite membranes for efficient ethanol recovery via pervaporation. Sep. Purif. Technol. 2020, 241, 116675. [Google Scholar] [CrossRef]
- Ataeivarjovi, E.; Tang, Z.; Chen, J. Study on CO2 Desorption Behavior of a PDMS–SiO2 Hybrid Membrane Applied in a Novel CO2 Capture Process. Acs Appl. Mater. Interfaces 2018, 10, 28992–29002. [Google Scholar] [CrossRef]
- Rosli, A.; Ahmad, A.L.; Low, S.C. Enhancing membrane hydrophobicity using silica end-capped with organosilicon for CO2 absorption in membrane contactor. Sep. Purif. Technol. 2020, 251, 117429. [Google Scholar] [CrossRef]
- Chiun-Jye, Y.; Wei-Jen, H. Application of TEOS/PDMS ormosil in the fabrication of amperometric biosensor. In Proceedings of the IEEE International Workshop on Biomedical Circuits and Systems, Singapore, 27 June 2005; pp. 1–4. [Google Scholar]
- Rao, H.; Zhang, Z.; Song, C.; Qiao, T.; Xu, S. Gas separation properties of siloxane/polydimethylsiloxane hybrid membrane containing fluorine. Sep. Purif. Technol. 2011, 78, 132–137. [Google Scholar] [CrossRef]
- Li, S.; Qin, F.; Qin, P.; Karim, M.N.; Tan, T. Preparation of PDMS membrane using water as solvent for pervaporation separation of butanol-water mixture. Green Chem. 2013, 15, 2180–2190. [Google Scholar] [CrossRef]
- Bai, Y.; Dong, L.; Zhang, C.; Gu, J.; Sun, Y.; Zhang, L.; Chen, H. ZIF-8 Filled Polydimethylsiloxane Membranes for Pervaporative Separation of n-Butanol from Aqueous Solution. Sep. Sci. Technol. 2013, 48, 2531–2539. [Google Scholar] [CrossRef]
- Riesco, R.; Boyer, L.; Blosse, S.; Lefebvre, P.M.; Assemat, P.; Leichle, T.; Accardo, A.; Malaquin, L. Water-in-PDMS Emulsion Templating of Highly Interconnected Porous Architectures for 3D Cell Culture. ACS Appl. Mater. Interfaces 2019, 11, 28631–28640. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.; Shan, H.; Hu, S.; Cai, D.; Qin, P. Recovery of ethanol via vapor phase by polydimethylsiloxane membrane with excellent performance. Chem. Eng. Res. Des. 2018, 136, 324–333. [Google Scholar] [CrossRef]
- Jiesheng, L.; Ke, L.; Lian, X.; Xiang, H. Synthesis of Polysiloxanes In Microemulsion Via ring opening of D4. Nanochem. Res. 2016, 1, 229–236. [Google Scholar]
- Deka, B.J.; Lee, E.-J.; Guo, J.; Kharraz, J.; An, A.K. Electrospun Nanofiber Membranes Incorporating PDMS-Aerogel Superhydrophobic Coating with Enhanced Flux and Improved Antiwettability in Membrane Distillation. Environ. Sci. Technol. 2019, 53, 4948–4958. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Palmisano, G. Sol-Gel for Environmentally Green Products. In The Sol-Gel Handbook—Synthesis, Characterization and Application; Levy, D., Zayat, M., Eds.; Wiley-VCH: Weinheim, Germany, 2015; pp. 1055–1070. [Google Scholar]
- Ge, M.; Cao, C.; Liang, F.; Liu, R.; Zhang, Y.; Zhang, W.; Zhu, T.; Yi, B.; Tang, Y.; Lai, Y. A “PDMS-in-water” emulsion enables mechanochemically robust superhydrophobic surfaces with self-healing nature. Nanoscale Horiz. 2020, 5, 65–73. [Google Scholar] [CrossRef]
- Zhang, W.; Su, X.; Shi, B.; Wang, L.; Wang, Q.; Li, S. Influence of molecular weight of polydimethylsiloxane precursors and crosslinking content on degree of ethanol swelling of crosslinked networks. React. Funct. Polym. 2015, 86, 264–268. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.S.; Lee, J.-H. High performance and thermally stable PDMS pervaporation membranes prepared using a phenyl-containing tri-functional crosslinker for n-butanol recovery. Sep. Purif. Technol. 2020, 235, 116142. [Google Scholar] [CrossRef]
- Xiangli, F.; Chen, Y.; Jin, W.; Xu, N. Polydimethylsiloxane (PDMS)/Ceramic Composite Membrane with High Flux for Pervaporation of Ethanol−Water Mixtures. Ind. Eng. Chem. Res. 2007, 46, 2224–2230. [Google Scholar] [CrossRef]
- Stafie, N.; Stamatialis, D.F.; Wessling, M. Effect of PDMS Crosslinking degree on the permeation performance of PAN/PDMS composite nanofiltration membranes. Sep. Purif. Technol. 2005, 45, 220–231. [Google Scholar] [CrossRef]
- Berean, K.; Ou, J.Z.; Nour, M.; Latham, K.; McSweeney, C.; Paull, D.; Halim, A.; Kentish, S.; Doherty, C.M.; Hill, A.J.; et al. The effect of crosslinking temperature on the permeability of PDMS membranes: Evidence of extraordinary CO2 and CH4 gas permeation. Sep. Purif. Technol. 2014, 122, 96–104. [Google Scholar] [CrossRef]
- Téllez, L.; Rubio, J.; Rubio, F.; Morales, E.; Oteo, J.L. Synthesis of inorganic-organic hybrid materials from TEOS, TBT and PDMS. J. Mater. Sci. 2003, 38, 1773–1780. [Google Scholar] [CrossRef]
- Johnson, L.M.; Gao, L.; Shields Iv, C.W.; Smith, M.; Efimenko, K.; Cushing, K.; Genzer, J.; López, G.P. Elastomeric microparticles for acoustic mediated bioseparations. J. Nanobiotechnol. 2013, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, S.S.; Khan, A.A.; Zafar, M.N.; Alhaji, M.H.; Sanaullah, K.; Shafqat, S.R.; Murtaza, S.; Pang, S.C. Development of amino-functionalized silica nanoparticles for efficient and rapid removal of COD from pre-treated palm oil effluent. J. Mater. Res. Technol. 2019, 8, 385–395. [Google Scholar] [CrossRef]
- Groza, A.; Surmeian, A. Characterization of the Oxides Present in a Polydimethylsiloxane Layer Obtained by Polymerisation of Its Liquid Precursor in Corona Discharge. J. Nanomater. 2015, 2015, 204296. [Google Scholar] [CrossRef]
- Kuo, A.C.M. Polymer Data Handbook; Oxford University Press: Oxford, UK, 1999; pp. 411–435. [Google Scholar]
- Rao, H.-X.; Liu, F.-N.; Zhang, Z.-Y. Preparation and oxygen/nitrogen permeability of PDMS crosslinked membrane and PDMS/tetraethoxysilicone hybrid membrane. J. Membr. Sci. 2007, 303, 132–139. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, G.; Liu, S.; Liu, Z.; Jin, W. High performance ceramic hollow fiber supported PDMS composite pervaporation membrane for bio-butanol recovery. J. Membr. Sci. 2014, 450, 38–47. [Google Scholar] [CrossRef]
- Calleja, A.; Ricart, S.; Aklalouch, M.; Mestres, N.; Puig, T.; Obradors, X. Thickness–concentration–viscosity relationships in spin-coated metalorganic ceria films containing polyvinylpyrrolidone. J. Sol Gel Sci. Technol. 2014, 72, 21–29. [Google Scholar] [CrossRef]
- Li, Z.; Han, W.; Kozodaev, D.; Brokken-Zijp, J.C.M.; de With, G.; Thüne, P.C. Surface properties of poly(dimethylsiloxane)-based inorganic/organic hybrid materials. Polymer 2006, 47, 1150–1158. [Google Scholar] [CrossRef]
- Javaid, A. Membranes for solubility-based gas separation applications. Chem. Eng. J. 2005, 112, 219–226. [Google Scholar] [CrossRef]
- Shao, L.; Low, B.T.; Chung, T.-S.; Greenberg, A.R. Polymeric membranes for the hydrogen economy: Contemporary approaches and prospects for the future. J. Membr. Sci. 2009, 327, 18–31. [Google Scholar] [CrossRef]
- Oyama, S.T.; Yamada, M.; Sugawara, T.; Takagaki, A.; Kikuchi, R. Review on Mechanisms of Gas Permeation through Inorganic Membranes. J. Jpn. Pet. Inst. 2011, 54, 298–309. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Fundamentals of Gas Permeation Through Membranes. In Gas Separation Membranes: Polymeric and Inorganic; Springer: Berlin/Heidelberg, Germany, 2015; pp. 11–35. [Google Scholar]
- Kim, D.; Hossain, I.; Kim, Y.; Choi, O.; Kim, T.-H. PEG/PPG-PDMS-Adamantane-based Crosslinked Terpolymer Using the ROMP Technique to Prepare a Highly Permeable and CO(2)-Selective Polymer Membrane. Polymers 2020, 12, 1674. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.R.; Pourafshari Chenar, M.; Noie, S.H. Using PDMS coated TFC-RO membranes for CO2/N2 gas separation: Experimental study, modeling and optimization. Polym. Test. 2016, 56, 287–298. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Badieh, M.M.S.; Vatanpour, V. Effect of coating method on gas separation by PDMS/PES membrane. Polym. Eng. Sci. 2013, 53, 1878–1885. [Google Scholar] [CrossRef]
- Brunchi, C.-E.; Bercea, M.; Morariu, S.; Dascalu, M. Some properties of xanthan gum in aqueous solutions: Effect of temperature and pH. J. Polym. Res. 2016, 23, 123. [Google Scholar] [CrossRef]
- Selyanchyn, R.; Ariyoshi, M.; Fujikawa, S. Thickness Effect on CO2/N2 Separation in Double Layer Pebax-1657®/PDMS Membranes. Membranes 2018, 8, 121. [Google Scholar] [CrossRef]
- Dong, Z.; Zhu, H.; Hang, Y.; Liu, G.; Jin, W. Polydimethylsiloxane (PDMS) Composite Membrane Fabricated on the Inner Surface of a Ceramic Hollow Fiber: From Single-Channel to Multi-Channel. Engineering 2020, 6, 89–99. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Thin film composite and/or thin film nanocomposite hollow fiber membrane for water treatment, pervaporation, and gas/vapor separation. Polymer 2018, 10, 1051. [Google Scholar] [CrossRef]
- Chen, H.Z.; Thong, Z.; Li, P.; Chung, T.-S. High performance composite hollow fiber membranes for CO2/H2 and CO2/N2 separation. Int. J. Hydrog. Energy 2014, 39, 5043–5053. [Google Scholar] [CrossRef]
- Gurr, P.A.; Scofield, J.M.P.; Kim, J.; Fu, Q.; Kentish, S.E.; Qiao, G.G. Polyimide polydimethylsiloxane triblock copolymers for thin film composite gas separation membranes. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 3372–3382. [Google Scholar] [CrossRef]
- Cao, P.-F.; Li, B.; Hong, T.; Xing, K.; Voylov, D.N.; Cheng, S.; Yin, P.; Kisliuk, A.; Mahurin, S.M.; Sokolov, A.P.; et al. Robust and Elastic Polymer Membranes with Tunable Properties for Gas Separation. Acs Appl. Mater. Interfaces 2017, 9, 26483–26491. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Kanezashi, M.; Nagasawa, H.; Yu, L.; Yamamoto, K.; Gunji, T.; Ohshita, J.; Tsuru, T. Tailoring the microstructure and permeation properties of bridged organosilica membranes via control of the bond angles. J. Membr. Sci. 2019, 584, 56–65. [Google Scholar] [CrossRef]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. The effect of hydrogen sulfide, carbon monoxide and water on the performance of a PDMS membrane in carbon dioxide/nitrogen separation. J. Membr. Sci. 2010, 350, 189–199. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Wei, W.; Xiangli, F.; Jin, W. Ceramic Supported PDMS and PEGDA Composite Membranes for CO2 Separation. Chin. J. Chem. Eng. 2013, 21, 348–356. [Google Scholar] [CrossRef]
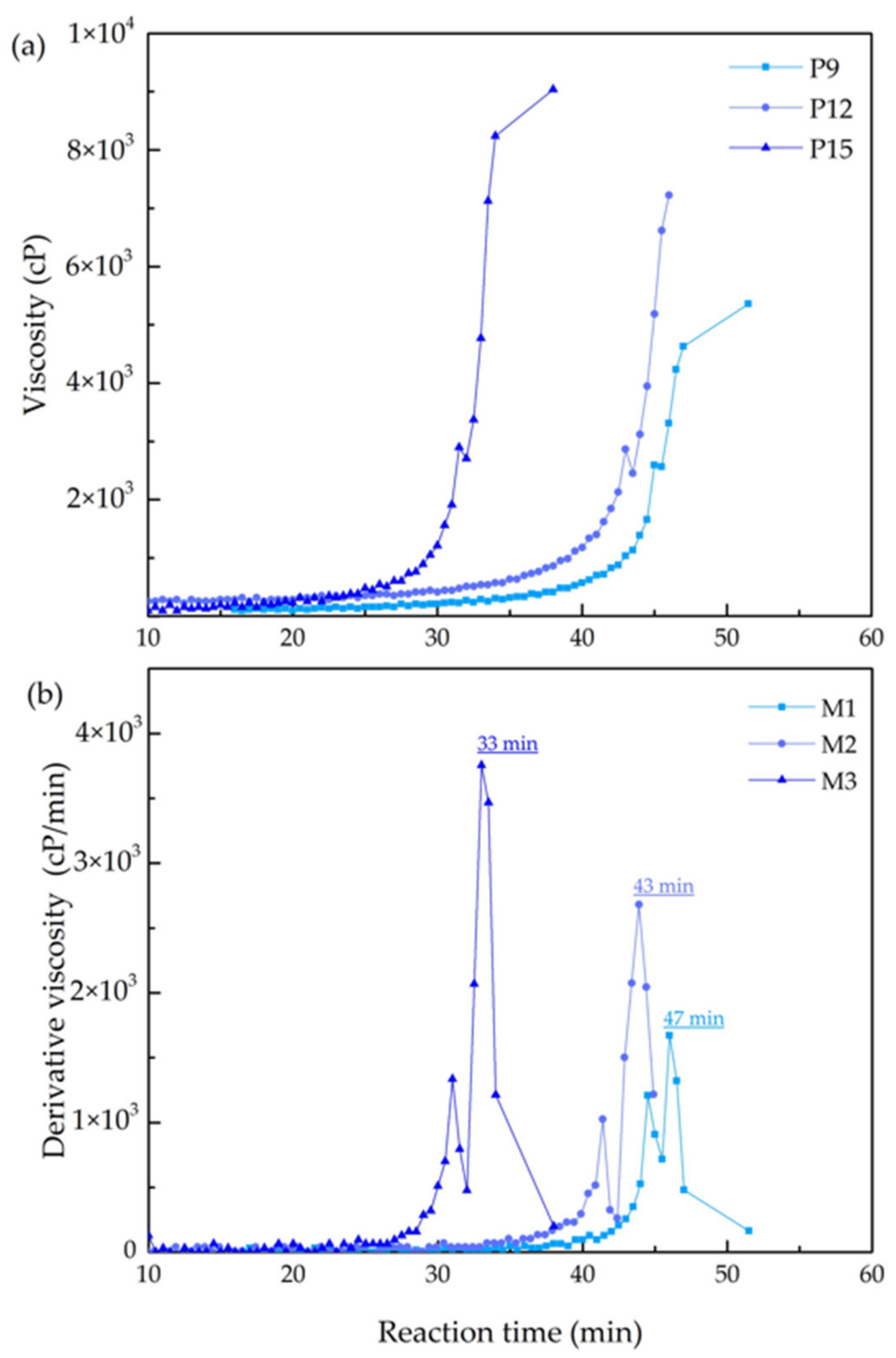
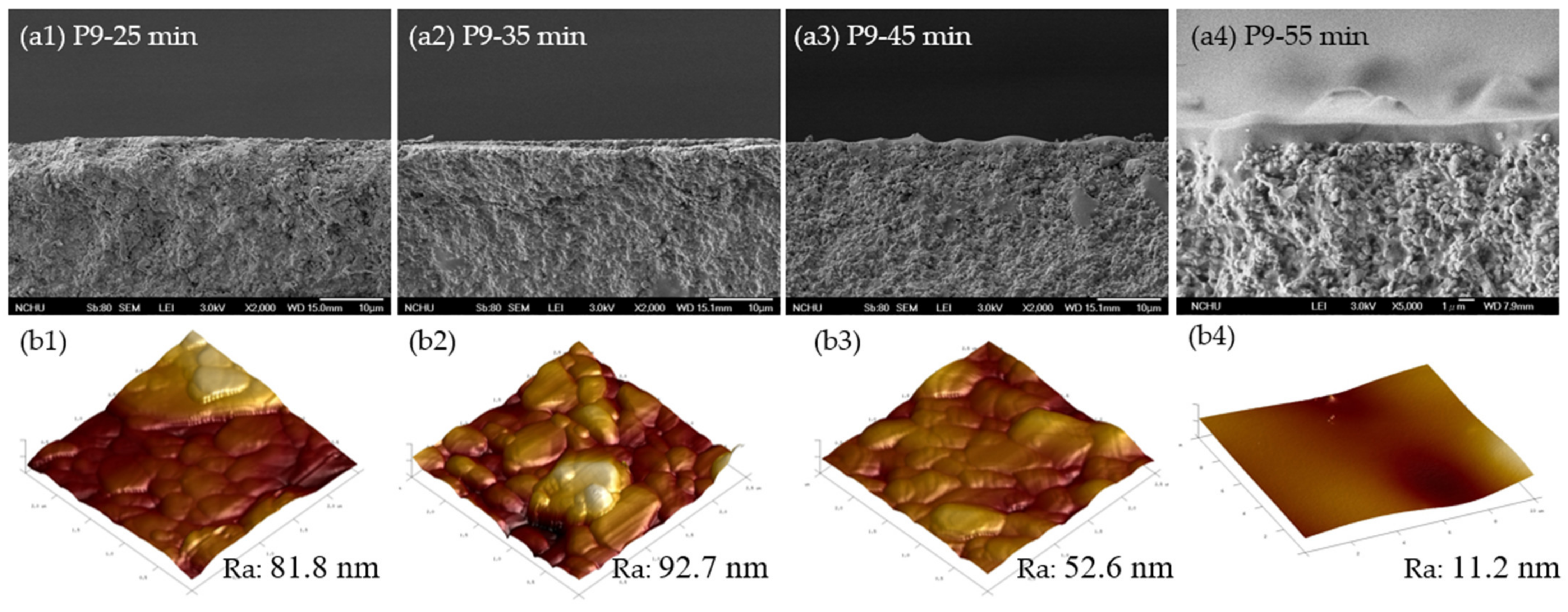
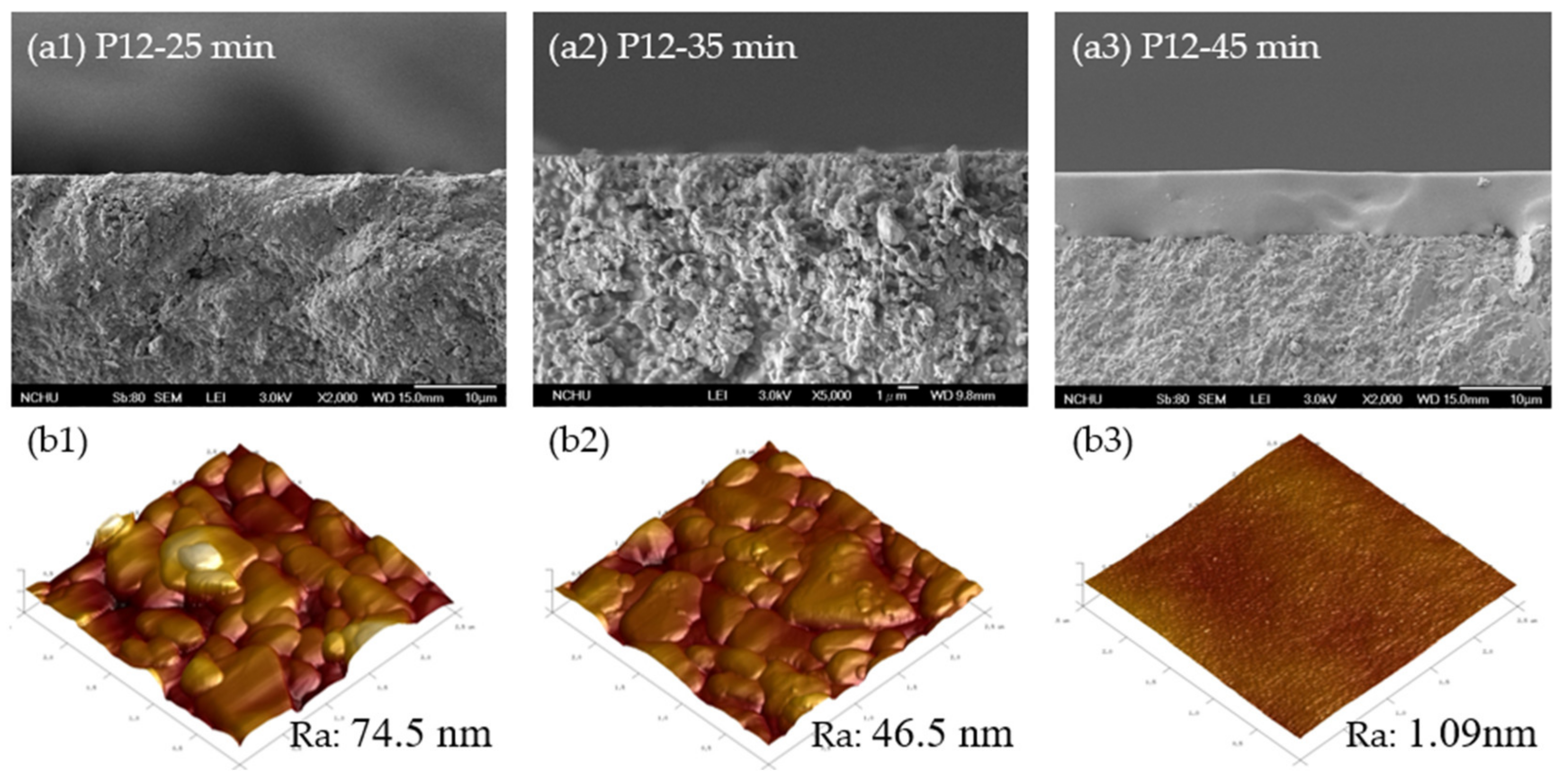

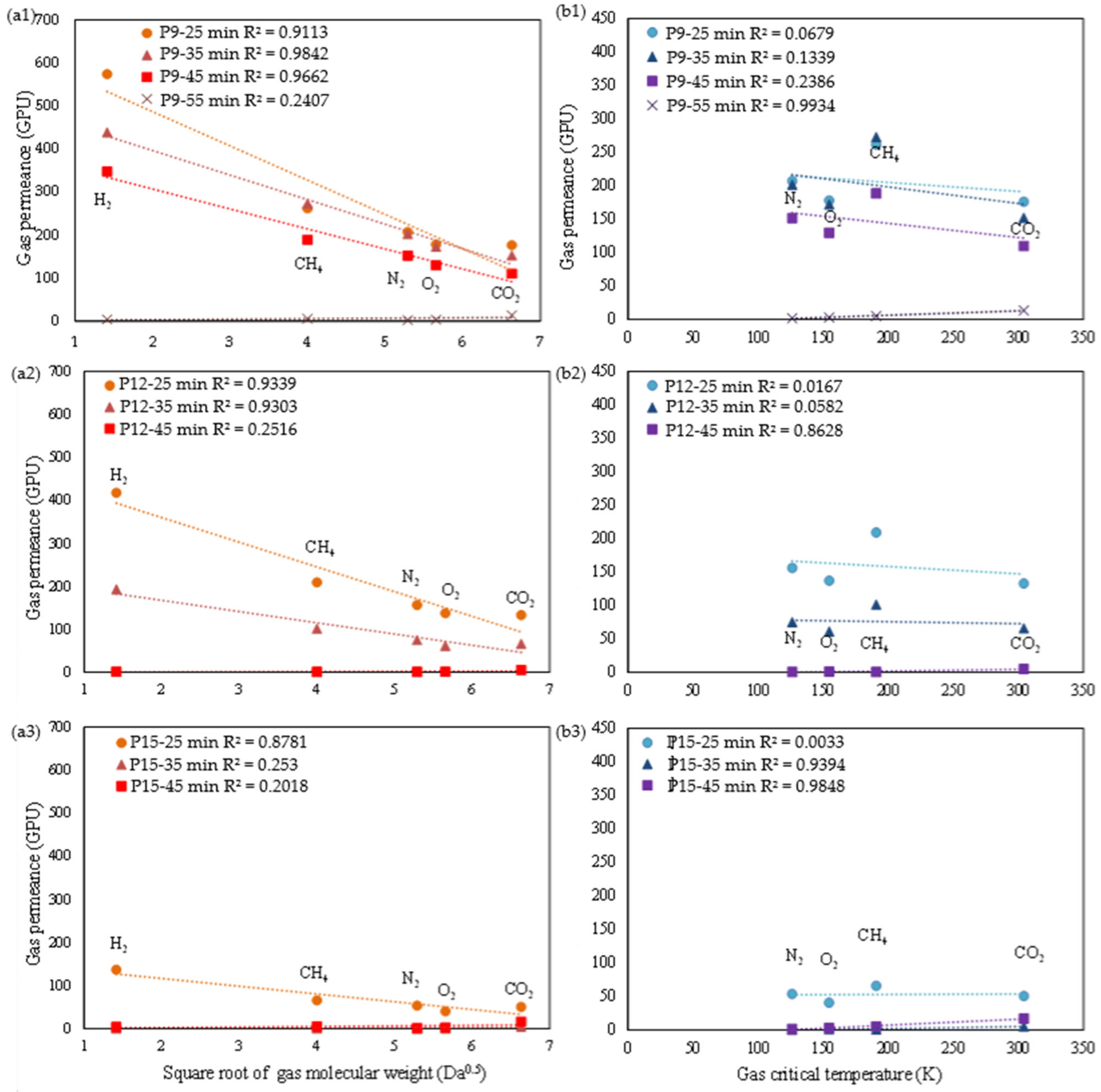


| Membrane | Specific Reaction Time by Viscosity (min) | Time Difference (∆t, min) 1 | Correlation between Gas Permeance and Gas Properties (R2) | |
|---|---|---|---|---|
| Molecular Weight | Critical Temperature | |||
| P9-25 min | 47 | −22 | 0.91 | 0.06 |
| P9-35 min | −12 | 0.98 | 0.13 | |
| P9-45 min | −2 | 0.97 | 0.23 | |
| P9-55 min | +8 | 0.24 | 0.99 | |
| P12-25 min | 43 | −18 | 0.93 | 0.52 |
| P12-35 min | −8 | 0.93 | 0.17 | |
| P12-45 min | +2 | 0.25 | 0.86 | |
| P15-25 min | 33 | −8 | 0.88 | 0.01 |
| P15-35 min | +2 | 0.25 | 0.94 | |
| P15-45 min | +12 | 0.20 | 0.98 | |
| Membrane | Substrate 1 | Solvent | Permeance (GPU) | CO2/N2 (α) | M.W. of PDMS (Da) 2 | Ref. | |
|---|---|---|---|---|---|---|---|
| CO2 | N2 | ||||||
| PDMS/~100 μm | - | toluene | 47.01 ± 2 | 6.3 ± 0.2 | 7.5 | - | [7] |
| PDMS/25 μm | HMS | n-heptane | 108.76 ± 2 | 11.5 ± 0.3 | 9.5 | - | [5] |
| PDMS/14 μm | CA | n-heptane | 117.9 | 14.4 | 8.2 | 5000 | [6] |
| PERVAP 4060/~10 μm | HMS | - | 475.7 ± 63 | 56.4 ± 20 | 8.4 | - | [58] |
| PDMS/8–9 μm | ZrO2/Al2O3 | n-heptane | 410.9 | 47.8 | 8.6 | 60,000 | [59] |
| PDMS/6 μm | TiO2/PES | n-hexane | 80~95 | - | 5.5~6.3 | [4] | |
| P15/13.5 μm | Al2O3 disc | water | 27.7 ± 1.3 | 2.7 ± 0.2 | 10.3 ± 0.3 | 4200 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, G.-L.; Wu, C.-F.; Wey, M.-Y.; Tseng, H.-H. Impacts of Green Synthesis Process on Asymmetric Hybrid PDMS Membrane for Efficient CO2/N2 Separation. Membranes 2021, 11, 59. https://doi.org/10.3390/membranes11010059
Zhuang G-L, Wu C-F, Wey M-Y, Tseng H-H. Impacts of Green Synthesis Process on Asymmetric Hybrid PDMS Membrane for Efficient CO2/N2 Separation. Membranes. 2021; 11(1):59. https://doi.org/10.3390/membranes11010059
Chicago/Turabian StyleZhuang, Guo-Liang, Chao-Fong Wu, Ming-Yen Wey, and Hui-Hsin Tseng. 2021. "Impacts of Green Synthesis Process on Asymmetric Hybrid PDMS Membrane for Efficient CO2/N2 Separation" Membranes 11, no. 1: 59. https://doi.org/10.3390/membranes11010059
APA StyleZhuang, G.-L., Wu, C.-F., Wey, M.-Y., & Tseng, H.-H. (2021). Impacts of Green Synthesis Process on Asymmetric Hybrid PDMS Membrane for Efficient CO2/N2 Separation. Membranes, 11(1), 59. https://doi.org/10.3390/membranes11010059