Preparation of Amino-Functional UiO-66/PIMs Mixed Matrix Membranes with [bmim][Tf2N] as Regulator for Enhanced Gas Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PIM-1
2.3. Synthesis of UiO-66-NH2
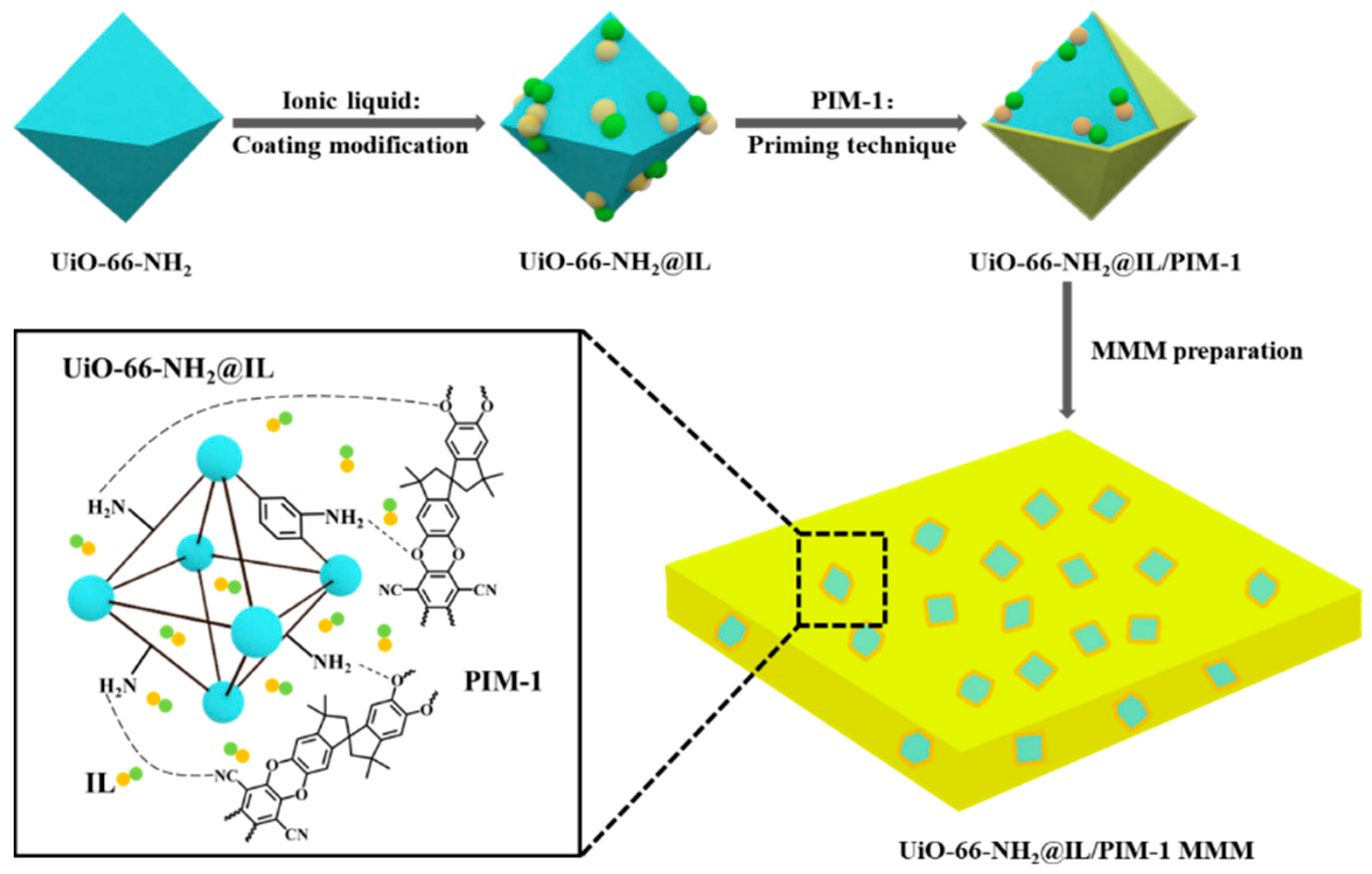
2.4. Synthesis of UiO-66-NH2@IL
2.5. Membrane Preparation
2.6. Characterization Techniques
2.7. Gas Permeation Tests
3. Results and Discussion
3.1. Incorporation of IL in UiO-66-NH2
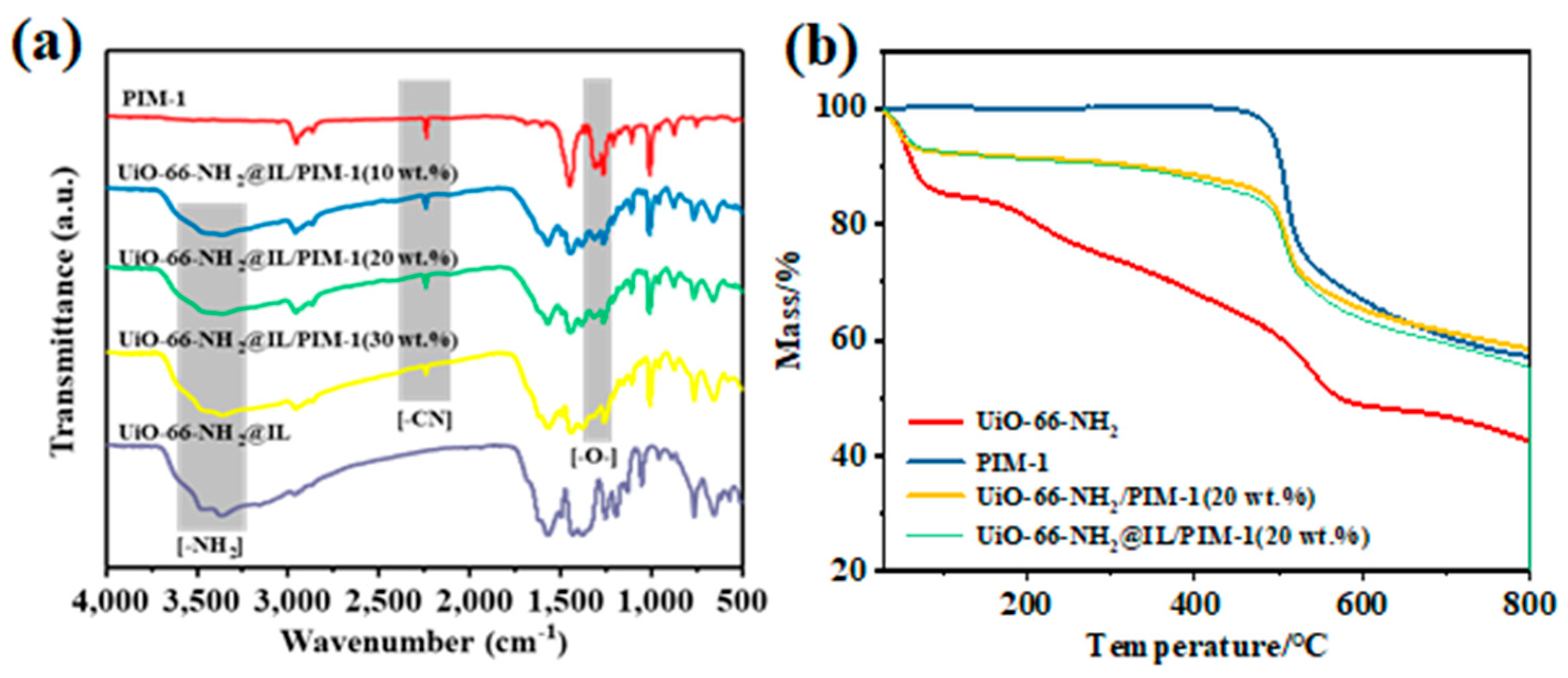
3.2. Characterization of PIM-1
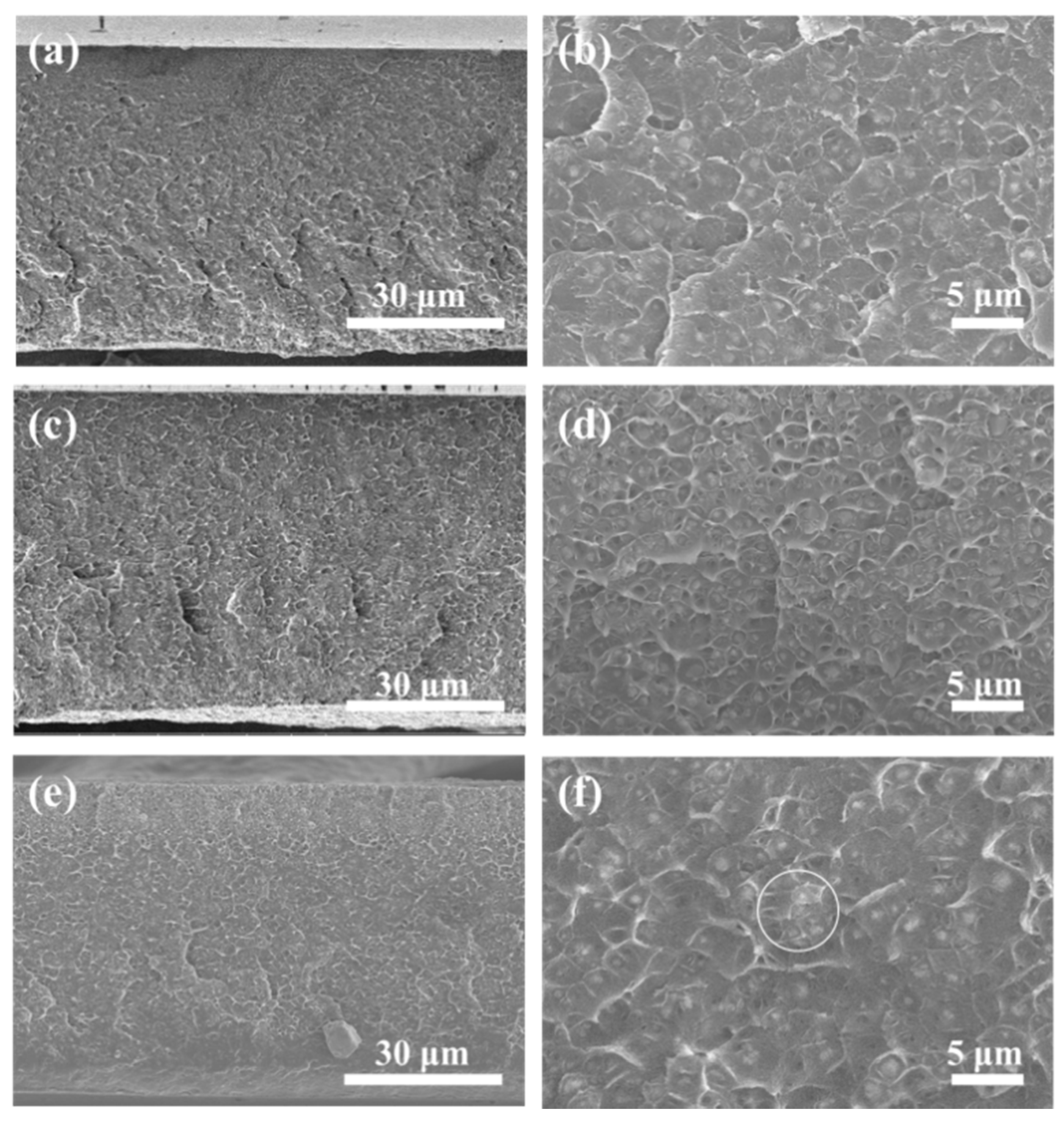
3.3. Characterization of Mixed Matrix Membranes
3.4. Gas Separation Properties
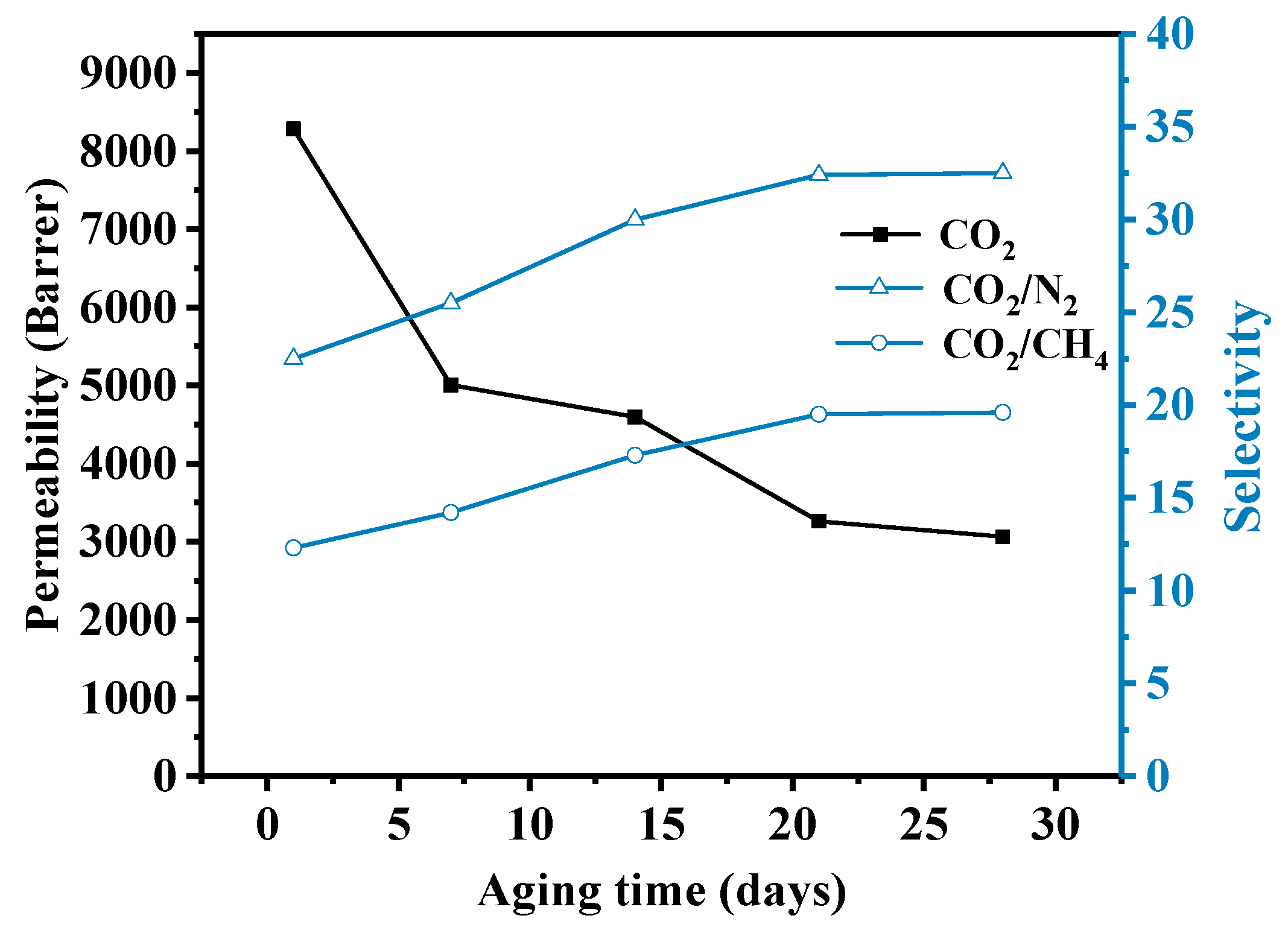
3.5. Aging Test
3.6. Comparison of Membrane Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.F.; Li, X.Q.; Wu, H.; Tian, Z.Z.; Xin, Q.P.; He, G.W.; Peng, D.D.; Chen, S.L.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Dal-Cin, M.M.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ. Sci. 2012, 5, 7306–7322. [Google Scholar] [CrossRef]
- Ma, J.; Ying, Y.P.; Guo, X.Y.; Huang, H.L.; Liu, D.H.; Zhong, C.L. Fabrication of mixed-matrix membrane containing metal–organic framework composite with task-specific ionic liquid for efficient CO2 separation. J. Mater. Chem. A 2016, 4, 7281–7288. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Yin, Y.; Guiver, M.D. Microporous polymers: Ultrapermeable membranes. Nat. Mater. 2017, 16, 880–881. [Google Scholar] [CrossRef]
- Cheng, Y.D.; Wang, X.R.; Jia, C.K.; Wang, Y.X.; Zhai, L.Z.; Wang, Q.; Zhao, D. Ultrathin mixed matrix membranes containing two-dimensional metal-organic framework nanosheets for efficient CO2/CH4 separation. J. Membr. Sci. 2017, 539, 213–223. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, H.F.; Su, P.C.; Zhang, X.; Zheng, J.H.; Lu, Y.H.; Liu, Q. PIM-1/PDMS hybrid pervaporation membrane for high-efficiency separation of n-butanol-water mixture under low concentration. Sep. Purif. Technol. 2019, 216, 83–91. [Google Scholar] [CrossRef]
- Mason, C.R.; Maynard-Atem, L.; Heard, K.W.; Satilmis, B.; Budd, P.M.; Friess, K.; Lanc, M.; Bernardo, P.; Clarizia, G.; Jansen, J.C. Enhancement of CO2 affinity in a polymer of intrinsic microporosity by amine modification. Macromolecules 2014, 47, 1021–1029. [Google Scholar] [CrossRef]
- Wu, X.Y.; Liu, W.; Wu, H.; Zong, X.; Yang, L.X.; Wu, Y.Z.; Ren, Y.X.; Shi, C.Y.; Wang, S.F.; Jiang, Z.Y. Nanoporous ZIF-67 embedded polymers of intrinsic microporosity membranes with enhanced gas separation performance. J. Membr. Sci. 2018, 548, 309–318. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.L.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Su, P.C.; Zhang, X.; Li, Y.; Chen, H.F.; Meng, Q.; Zhang, G. Distillation of alcohol/water solution in hybrid metal-organic framework hollow fibers. AIChE J. 2019, 65, e16693. [Google Scholar] [CrossRef]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wang, D.; Zhang, S.X.; Hu, L.; Jin, J. Interfacial design of mixed matrix membranes for improved gas separation performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-matrix membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Soyekwo, F.; Zhang, Q.G.; Hu, C.; Zhu, A.M.; Liu, Q.L. Graphene oxide nanosheets to improve permeability and selectivity of PIM-1 membrane for carbon dioxide separation. J. Ind. Eng. Chem. 2018, 63, 296–302. [Google Scholar] [CrossRef]
- Tian, Z.Z.; Wang, S.F.; Wang, Y.T.; Ma, X.R.; Cao, K.T.; Peng, D.D.; Wu, X.Y.; Wu, H.; Jiang, Z.Y. Enhanced gas separation performance of mixed matrix membranes from graphitic carbon nitride nanosheets and polymers of intrinsic microporosity. J. Membr. Sci. 2016, 514, 15–24. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Wakimoto, K.; Gibbons, A.H.; Isfahani, A.P.; Kusuda, H.; Sivaniah, E.; Ghalei, B. Enhanced PIM-1 membrane gas separation selectivity through efficient dispersion of functionalized POSS fillers. J. Membr. Sci. 2017, 539, 178–186. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Zhang, C.; Meng, Q.; Xu, Z.; Su, P.; Li, Q.; Shen, C.; Fan, Z.; Qin, L.; et al. Transformation of metal-organic frameworks for molecular sieving membranes. Nat. Commun. 2016, 7, 11315. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Chen, X.; Tang, K.; Meng, Q.; Shen, C.; Zhang, G. Zeolite imidazolate framework membranes on polymeric substrates modified with Poly(vinyl alcohol) and alginate composite hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 12605–12612. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Hao, L.; Liao, K.S.; Chung, T.S. Photo-oxidative PIM-1 based mixed matrix membranes with superior gas separation performance. J. Mater. Chem. A 2015, 3, 17273–17281. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Hoang, V.T.; Chen, X.Y.; Rodrigue, D.; Kaliaguine, S. Polymer functionalization to enhance interface quality of mixed matrix membranes for high CO2/CH4 gas separation. J. Mater. Chem. A 2015, 3, 15202–15213. [Google Scholar]
- Guo, X.Y.; Huang, H.L.; Ban, Y.J.; Yang, Q.Y.; Xiao, Y.L.; Li, Y.S.; Yang, W.S.; Zhong, C.L. Mixed matrix membranes incorporated with amine-functionalized titanium-based metal-organic framework for CO2/CH4 separation. J. Membr. Sci. 2015, 478, 130–139. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Rodrigue, D.; Kaliaguine, S. In-situ cross interface linking of PIM-1 polymer and UiO-66-NH2 for outstanding gas separation and physical aging control. J. Membr. Sci. 2018, 548, 429–438. [Google Scholar]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Perez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Denny, M.D.; Cohen, S.M. In situ modification of metal–organic frameworks in mixedmatrix membranes. Angew. Chem. Int. Ed. 2015, 54, 9029–9032. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K.; Nehache, S.; Vankelecom, I.; Deratani, A.; Quemener, D. MOF-mixed matrix membranes: Precise dispersion of MOF particles with better compatibility via a particle fusion approach for enhanced gas separation properties. J. Membr. Sci. 2015, 492, 21–31. [Google Scholar] [CrossRef]
- Ban, Y.J.; Li, Z.J.; Li, Y.S.; Peng, Y.; Jin, H.; Jiao, W.M.; Guo, A.; Wang, P.; Yang, Q.Y.; Zhong, C.L.; et al. Confinement of ionic liquids in nanocages: Tailoring the molecular sieving properties of ZIF-8 for membrane-based CO2 capture. Angew. Chem. Int. Ed. 2015, 54, 15483–15487. [Google Scholar] [CrossRef]
- Lin, R.J.; Ge, L.; Diao, H.; Rudolph, V.; Zhu, Z.H. Ionic liquids as the MOFs/polymer interfacial binder for efficient membrane separation. ACS Appl. Mater. Interfaces 2016, 8, 32041–32049. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Leo, C.P.; Mohammad, A.W.; Ahmad, A.L. Interfacial sealing and functionalization of polysulfone/SAPO-34 mixed matrix membrane using acetate-based ionic liquid in post-impregnation for CO2 capture. Sep. Purif. Technol. 2018, 197, 439–448. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Zornoza, B.; Téllez, C.; Coronas, J.; Irabien, Á. Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2 separation. RSC Adv. 2015, 5, 102350–102361. [Google Scholar] [CrossRef]
- Estahbanati, E.G.; Omidkhah, M.; Amooghin, A.E. Interfacial design of ternary mixed matrix membranes containing Pebax 1657/Silver-nanopowder/[BMIM][BF4] for improved CO2 separation performance. ACS Appl. Mater. Interfaces 2017, 9, 10094–10105. [Google Scholar] [CrossRef] [PubMed]
- Du, N.Y.; Robertson, G.P.; Song, J.S.; Pinnau, I.; Thomas, S.; Guiver, M.D. Polymers of Intrinsic Microporosity containing trifluoromethyl and phenylsulfone groups as materials for membrane gas separation. Macromolecules 2008, 41, 9656–9662. [Google Scholar] [CrossRef]
- Wang, Z.G.; Ren, H.T.; Zhang, S.X.; Zhang, F.; Jin, J. Polymers of intrinsic microporosity/metal-organic framework hybrid membranes with improved interfacial interaction for high-performance CO2 separation. J. Mater. Chem. A 2017, 5, 10968–10977. [Google Scholar] [CrossRef]
- Li, H.; Tuo, L.H.; Yang, K.; Jeong, H.-K.; Dai, Y.; He, G.H.; Zhao, W. Simultaneous enhancement of mechanical properties and CO2 selectivity of ZIF-8 mixed matrix membranes: Interfacial toughening effect of ionic liquid. J. Membr. Sci. 2016, 511, 130–142. [Google Scholar] [CrossRef]
- Su, N.C.; Sun, D.T.; Beavers, C.M.; Britt, D.K.; Queen, W.L.; Urban, J.J. Enhanced permeation arising from dual transport pathways in hybrid polymer-MOF membranes. Energy Environ. Sci. 2016, 9, 922–931. [Google Scholar] [CrossRef]
- Mitra, T.; Bhavsar, R.S.; Adams, D.J.; Budd, P.M.; Cooper, A.I. PIM-1 mixed matrix membranes for gas separations using cost-effective hypercrosslinked nanoparticle fillers. Chem. Commun. 2016, 52, 5581–5584. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Mitra, T.; Adams, D.J.; Cooper, A.I.; Budd, P.M. Ultrahigh-permeance PIM-1 based thin film nanocomposite membranes on PAN supports for CO2 separation. J. Membr. Sci. 2018, 564, 878–886. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Navarro, M.; Lhotka, M.; Zornoza, B.; Tellez, C.; de Vos, W.M.; Benes, N.E.; Konnertz, N.M.; Visser, T.; Semino, R.; et al. Enhanced gas separation performance of 6FDA-DAM based mixed matrix membranes by incorporating MOF UiO-66 and its derivatives. J. Membr. Sci. 2018, 558, 64–77. [Google Scholar] [CrossRef]
- Knebel, A.; Bavykina, A.; Datta, S.J.; Sundermann, L.; Garzon-Tovar, L.; Lebedev, Y.; Durini, S.; Ahmad, R.; Kozlov, S.M.; Shterk, G.; et al. Solution processable metal-organic frameworks for mixed matrix membranes using porous liquids. Nat. Mater. 2020, 19, 1346–1353. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Castro-Munoz, R.; Budd, P.M. Boosting gas separation performance and suppressing the physical aging of polymers of intrinsic microporosity (PIM-1) by nanomaterial blending. Nanoscale 2020, 12, 23333–23370. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zou, X.; Sun, L.; Liu, B.; Wang, Z.; Zhang, P.; Zhu, G. Constructing connected paths between UiO-66 and PIM-1 to improve membrane CO2 separation with crystal-like gas selectivity. Adv. Mater. 2019, 31, 1806853. [Google Scholar] [CrossRef] [PubMed]
- Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; Rose, I.; Ferrari, M.C.; Esposito, E.; Fuoco, A.; Jansen, J.C.; McKeown, N.B. Redefining the Robeson upper bounds for CO2/CH4 and CO2/N2 separations using a series of ultrapermeable benzotriptycene-based polymers of intrinsic microporosity. Energy Environ. Sci. 2019, 12, 2733–2740. [Google Scholar] [CrossRef]
- Bushell, A.F.; Attfield, M.P.; Mason, C.R.; Budd, P.M.; Yampolskii, Y.; Starannikova, L.; Rebrov, A.; Bazzarelli, F.; Bernardo, P.; Carolus Jansen, J.; et al. Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8. J. Membr. Sci. 2013, 427, 48–62. [Google Scholar] [CrossRef]
- Smith, S.J.; Ladewig, B.P.; Hill, A.J.; Lau, C.H.; Hill, M.R. Post-synthetic Ti exchanged UiO-66 metal-organic frameworks that deliver exceptional gas permeability in mixed matrix membranes. Sci. Rep. 2015, 5, 7823. [Google Scholar] [CrossRef]
- Prasetya, N.; Ladewig, B.P. New azo-DMOF-1 MOF as a photoresponsive low-energy CO2 adsorbent and its exceptional CO2/N2 separation performance in mixed matrix membranes. ACS Appl. Mater. Interfaces 2018, 10, 34291–34301. [Google Scholar] [CrossRef]
- Wu, X.M.; Zhang, Q.G.; Lin, P.J.; Qu, Y.; Zhu, A.M.; Liu, Q.L. Towards enhanced CO2 selectivity of the PIM-1 membrane by blending with polyethylene glycol. J. Membr. Sci. 2015, 493, 147–155. [Google Scholar]
- Dong, G.; Zhang, J.; Wang, Z.; Wang, J.; Zhao, P.; Cao, X.; Zhang, Y. Interfacial property modulation of PIM-1 through polydopamine-derived submicrospheres for enhanced CO2/N2 separation performance. ACS Appl. Mater. Interfaces 2019, 11, 19613–19622. [Google Scholar] [CrossRef]
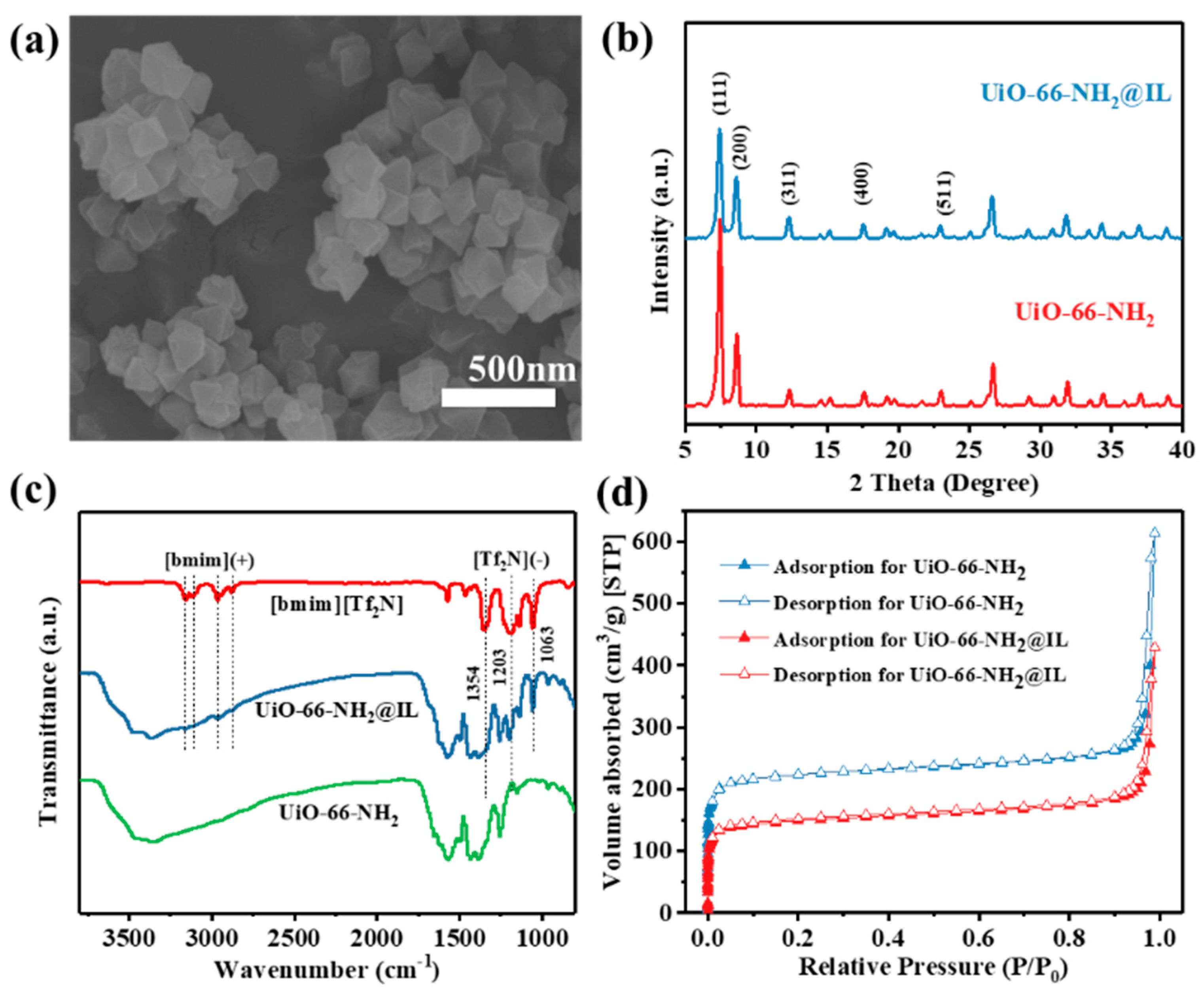



| Sample | Surface Area (m2 g−1) [a] | Surface Area (m2 g−1) [b] | Pore Volume (mL g−1) [b] | Pore Width (nm) [b] |
|---|---|---|---|---|
| UiO-66-NH2 | 887.667 | 936.027 | 0.428 | 0.567 |
| UiO-66-NH2@IL | 585.413 | 610.922 | 0.307 | 0.524 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Zhang, X.; Xu, L.; Zhang, G.; Zheng, J.; Tong, Z.; Shen, C.; Meng, Q. Preparation of Amino-Functional UiO-66/PIMs Mixed Matrix Membranes with [bmim][Tf2N] as Regulator for Enhanced Gas Separation. Membranes 2021, 11, 35. https://doi.org/10.3390/membranes11010035
Lu J, Zhang X, Xu L, Zhang G, Zheng J, Tong Z, Shen C, Meng Q. Preparation of Amino-Functional UiO-66/PIMs Mixed Matrix Membranes with [bmim][Tf2N] as Regulator for Enhanced Gas Separation. Membranes. 2021; 11(1):35. https://doi.org/10.3390/membranes11010035
Chicago/Turabian StyleLu, Jiangfeng, Xu Zhang, Lusheng Xu, Guoliang Zhang, Jiuhan Zheng, Zhaowei Tong, Chong Shen, and Qin Meng. 2021. "Preparation of Amino-Functional UiO-66/PIMs Mixed Matrix Membranes with [bmim][Tf2N] as Regulator for Enhanced Gas Separation" Membranes 11, no. 1: 35. https://doi.org/10.3390/membranes11010035
APA StyleLu, J., Zhang, X., Xu, L., Zhang, G., Zheng, J., Tong, Z., Shen, C., & Meng, Q. (2021). Preparation of Amino-Functional UiO-66/PIMs Mixed Matrix Membranes with [bmim][Tf2N] as Regulator for Enhanced Gas Separation. Membranes, 11(1), 35. https://doi.org/10.3390/membranes11010035





