Comparison between a Conventional Anti-Biofouling Compound and a Novel Modified Low-Fouling Polyethersulfone Ultrafiltration Membrane: Bacterial Anti-Attachment, Water Quality and Productivity
Abstract
1. Introduction
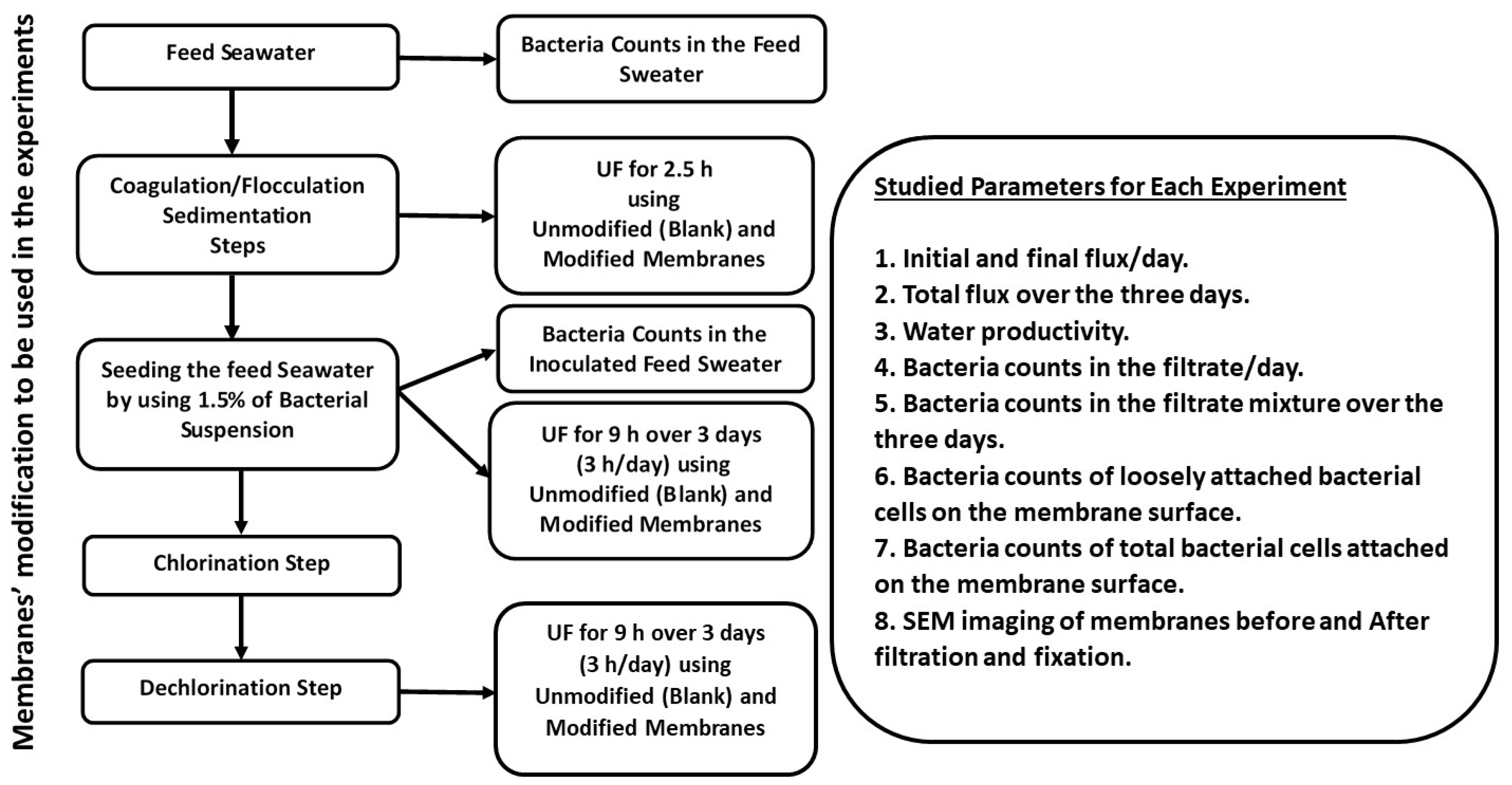
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Laccase Activity
2.2.2. Modification of PES Membrane Surfaces
2.2.3. Seawater Sampling
2.2.4. Preparation of the Feed Seawater
Pretreatment of the Feed Seawater by Coagulation
Seeding of Seawater with Bacterial Load
Disinfection of the Feed Seawater
Dechlorination Process
2.2.5. Ultrafiltration (UF)
Water Flux
Water Productivity
2.2.6. Bacterial Count
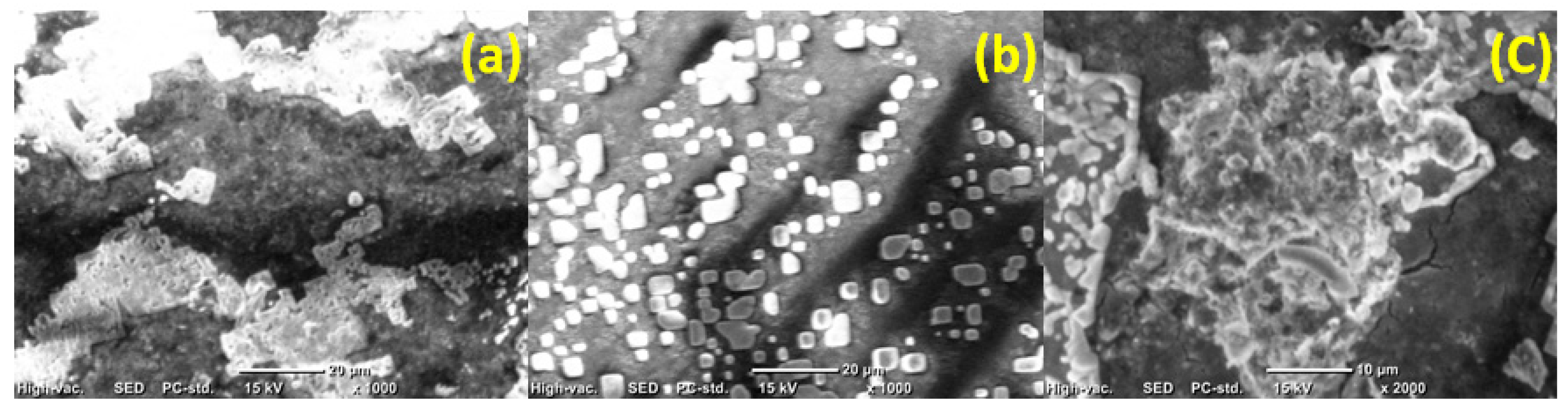
2.2.7. Scanning Electron Microscope Imaging
2.2.8. Atomic Absorption Spectroscopy Analysis
3. Results and Discussion
3.1. Chemical Analysis of the Used Seawater
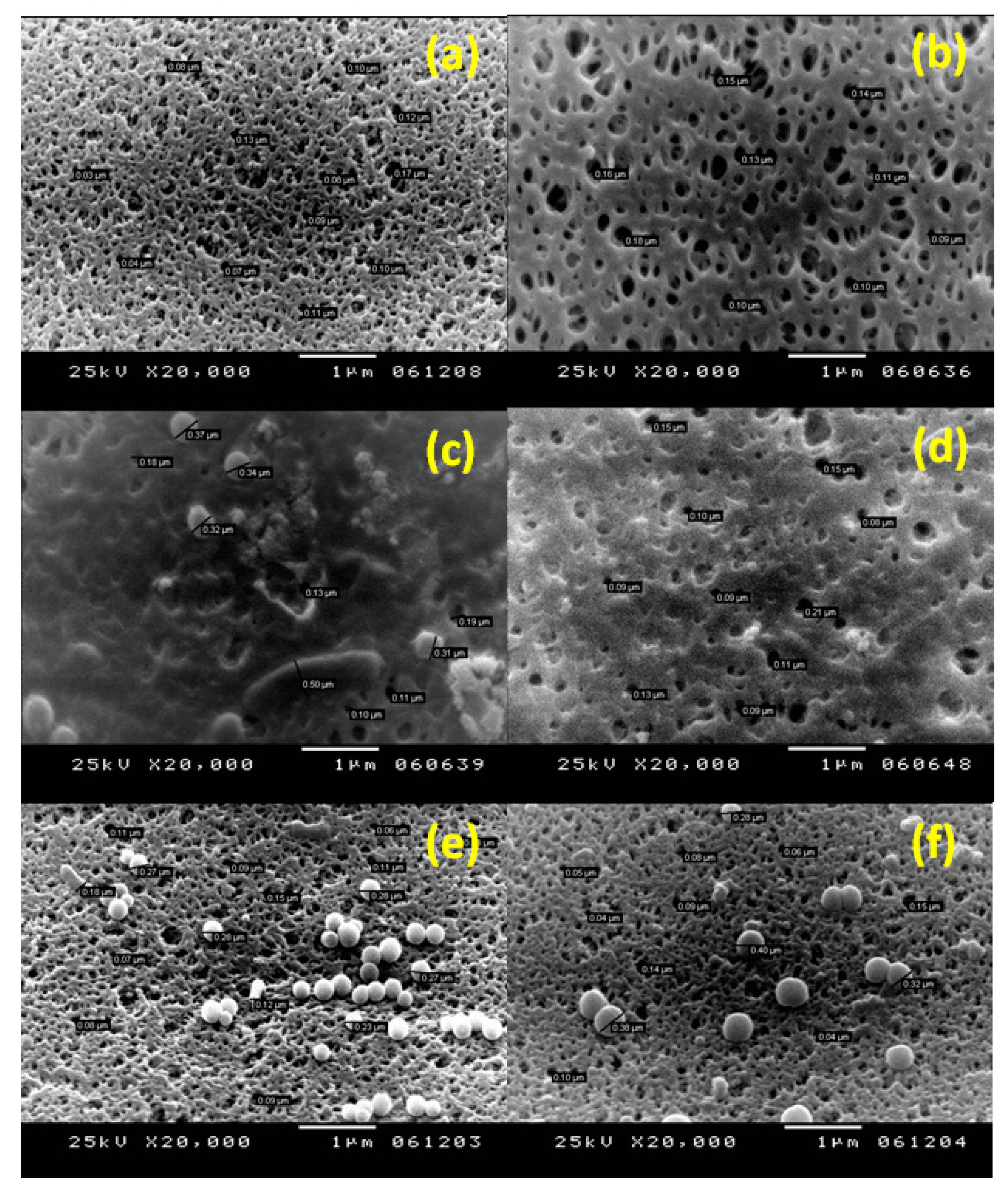
3.2. Membrane Characterization
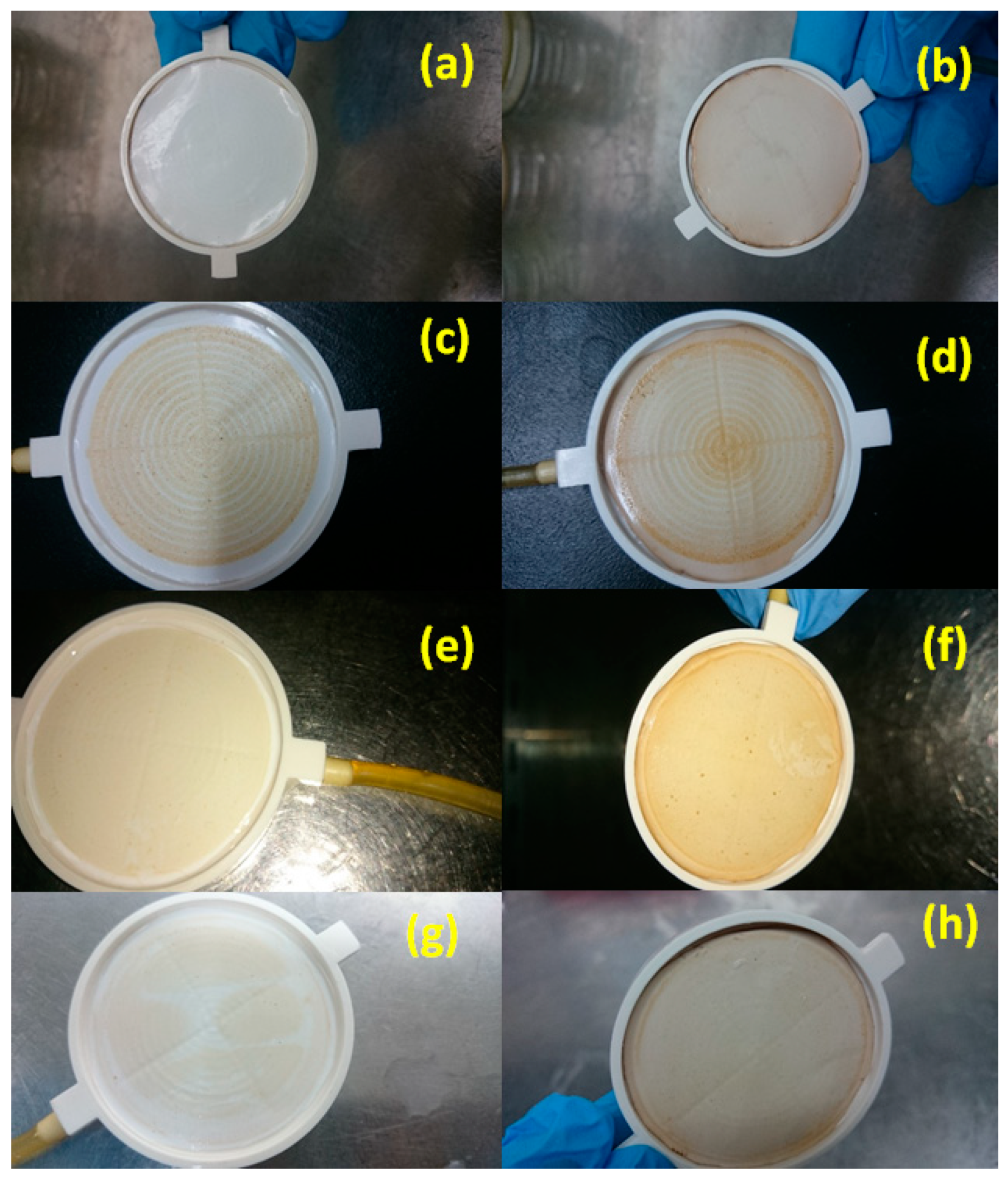
3.3. Pretreatment of the Feed Seawater (Coagulation–Flocculation)
3.4. Ultrafiltration Process
3.4.1. Water Flux
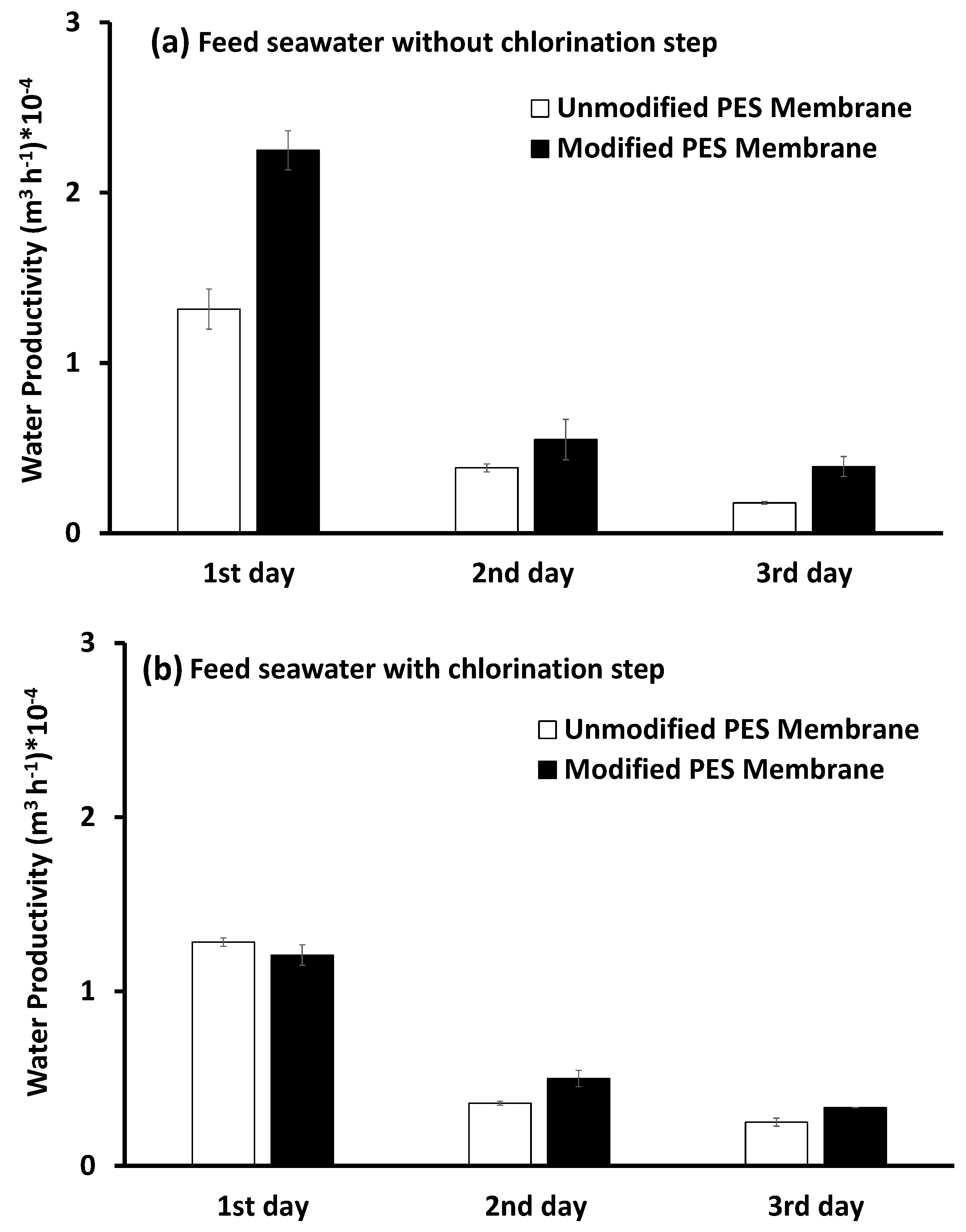
Pretreated Feed Seawater without Bacterial Loading
Pretreated Feed Seawater with Bacterial Loading
3.4.2. Water Productivity
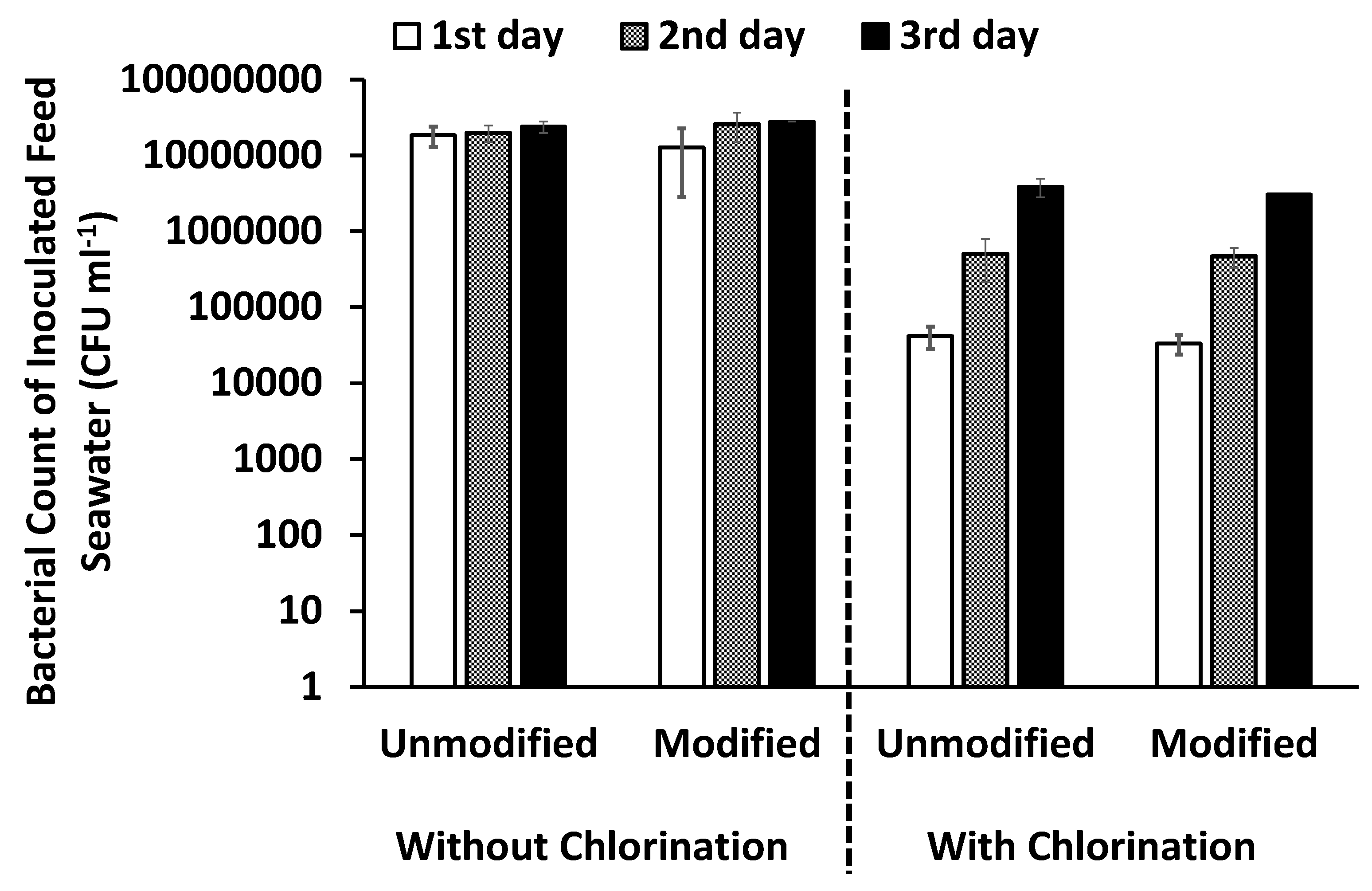
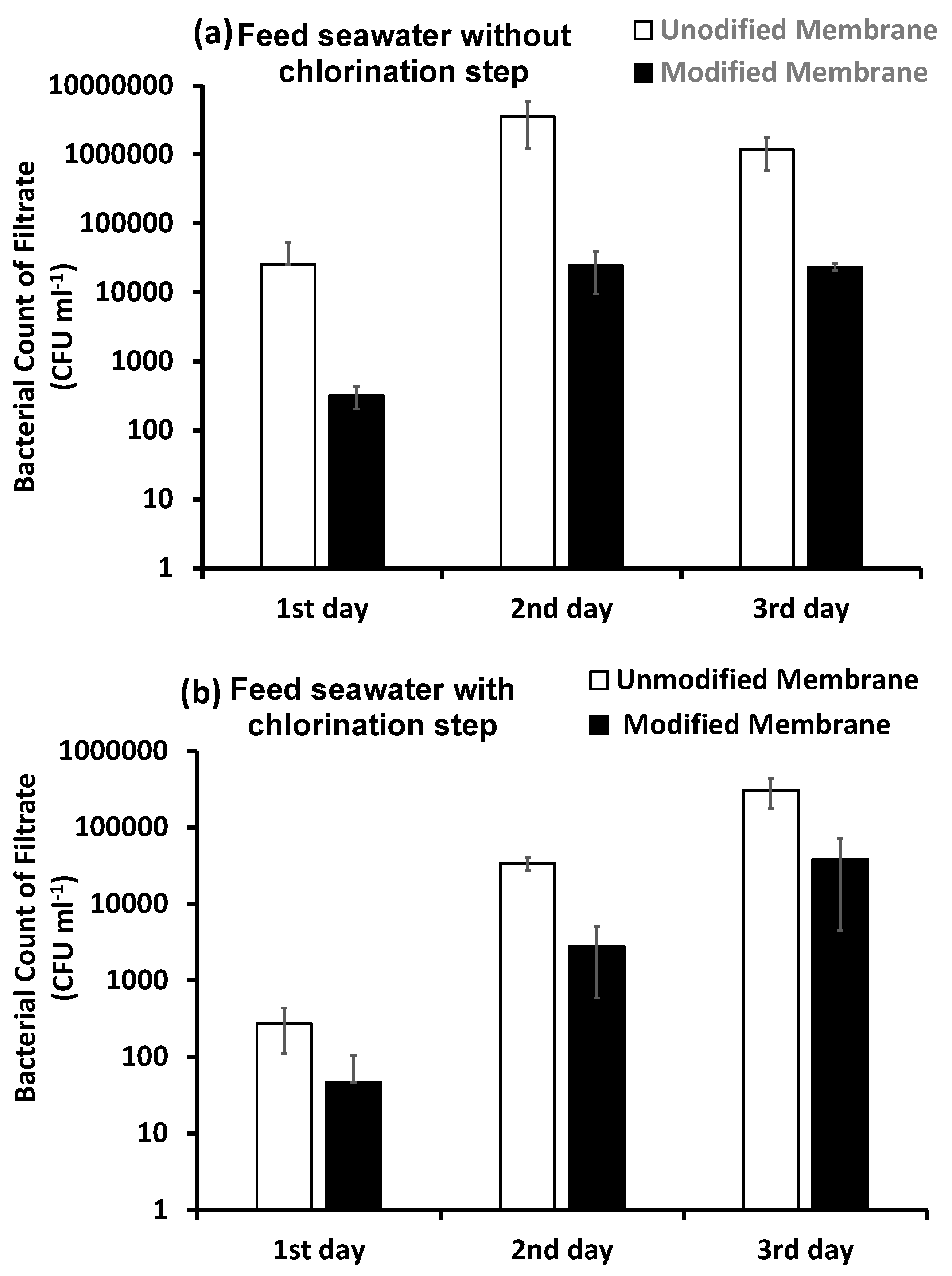
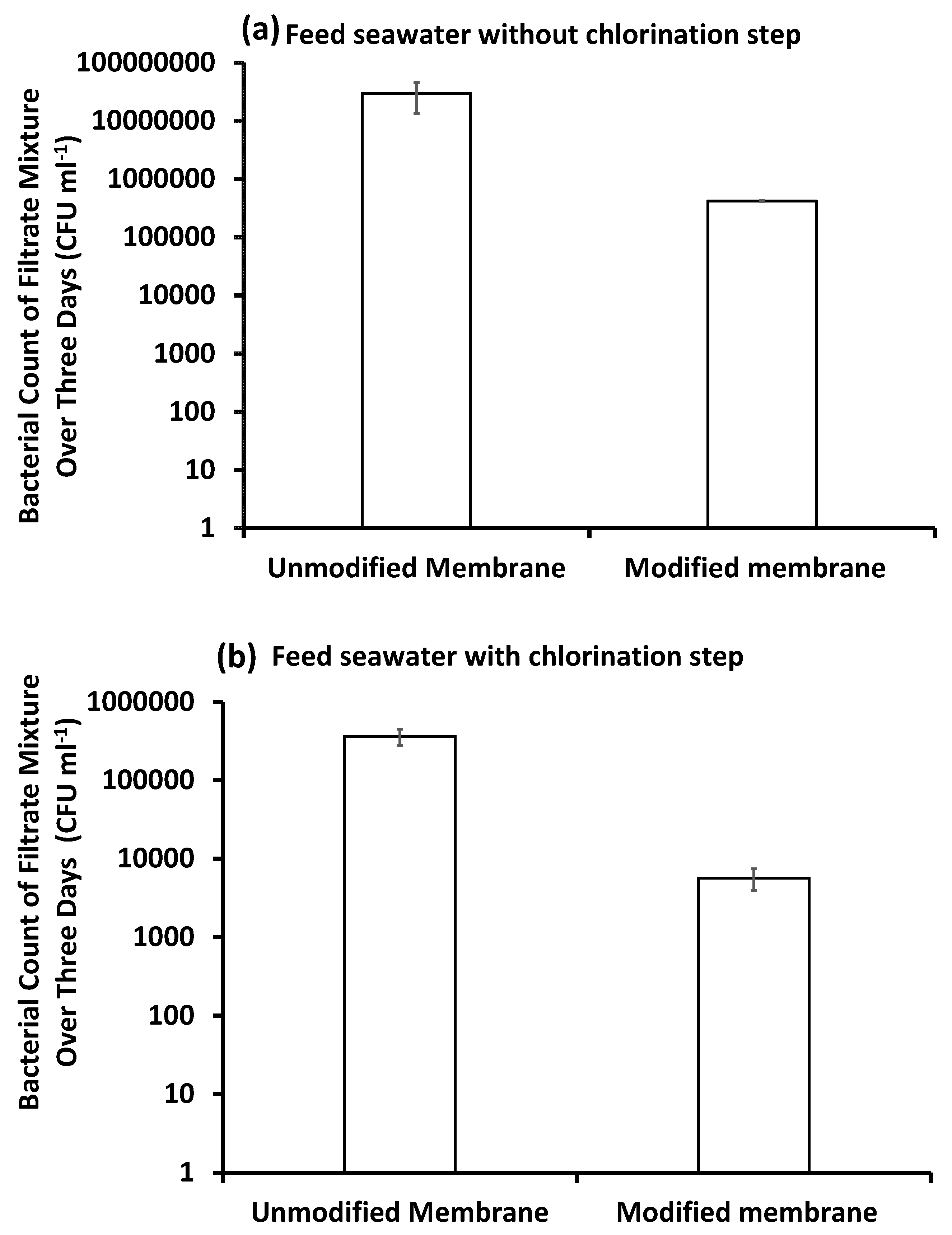
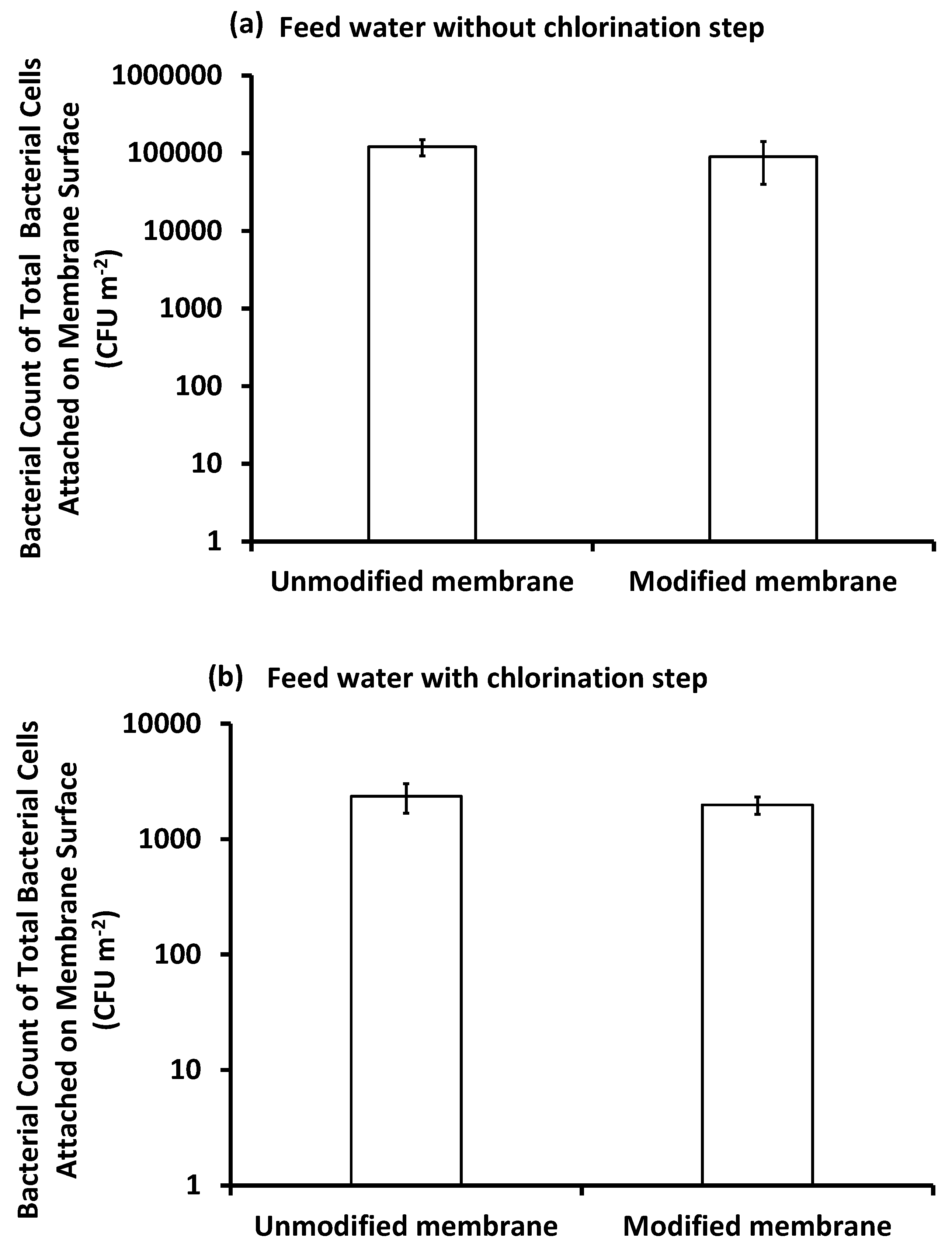
3.4.3. Bacterial Counts
Bacterial Counts in the Pretreated Inoculated Feed Seawater over Three Days
Bacterial Counts in the Filtrate Water over Three Days
Bacterial Count on the Membrane Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Malaeb, L.; Ayoub, G.M. Reverse osmosis technology for water treatment: State of the art review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Khanzada, N.; Khan, S.J.; Davies, P. Performance evaluation of reverse osmosis (RO) pre-treatment technologies for in-land brackish water treatment. Desalination 2017, 406, 44–50. [Google Scholar] [CrossRef]
- Darre, N.C.; Toor, G.S. Desalination of water: A review. Curr. Pollut. Rep. 2018, 4, 104–111. [Google Scholar] [CrossRef]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.J.; Nigmatullin, R. Methods employed for control of fouling in MF and UF membranes: A comprehensive review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State of the art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration membranes for wastewater and water process engineering: A comprehensive statistical review over the past decade. J. Water Process Eng. 2020, 35, 1–14. [Google Scholar] [CrossRef]
- Wolf, P.H.; Siverns, S.; Monti, S. UF membranes for RO desalination pretreatment. Desalination 2005, 182, 293–300. [Google Scholar] [CrossRef]
- Jamaly, S.; Darwish, N.; Ahmed, I.; Hasan, S.A. short review on reverse osmosis pretreatment technologies. Desalination 2014, 354, 30–38. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination. Energy Environ. Sci. 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Biofouling: Lessons from nature. Philos. Trans. R. Soc. A. 2012, 370, 2381–2417. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Sim, L.N.; Chong, T.H.; Taheri, A.H.; Sim, S.; Lai, L.; Krantz, W.B.; Fane, A.G. A review of fouling indices and monitoring techniques for reverse osmosis. Desalination 2018, 434, 169–188. [Google Scholar] [CrossRef]
- Badruzzaman, M.; Voutchkov, N.; Weinrich, L.; Jacangelo, J.G. Selection of pretreatment technologies for seawater reverse osmosis plants: A review. Desalination 2019, 449, 78–91. [Google Scholar] [CrossRef]
- Flemming, H.C. Reverse osmosis membrane biofouling. Exp. Therm. Fluid Sci. 1997, 14, 382–391. [Google Scholar] [CrossRef]
- Al-Ahmad, M.; Aleem, F.A.; Mutiri, A.; Ubaisy, A. Biofouling in RO membrane systems part 1: Fundamentals and control. Desalination 2000, 132, 173–179. [Google Scholar] [CrossRef]
- Prihasto, N.; Liu, Q.F.; Kim, S.H. Pre-treatment strategies for seawater desalination by reverse osmosis system. Desalination 2009, 249, 308–316. [Google Scholar] [CrossRef]
- Matin, A.; Khan, Z.; Zaidi, S.; Boyce, M. Biofouling in reverse osmosis membranes for seawater desalination: Phenomena and prevention. Desalination 2011, 281, 1–16. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef]
- Melo, L.; Bott, T. Biofouling in water systems. Exp. Therm. Fluid Sci. 1997, 14, 375–381. [Google Scholar] [CrossRef]
- Baker, J.; Dudley, L. Biofouling in membrane systems: A review. Desalination 1998, 118, 81–89. [Google Scholar] [CrossRef]
- Bott, T. Potential physical methods for the control of biofouling in water systems. Chem. Eng. Res. Des. 2001, 79, 484–490. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhang, D.R.; He, Y.; Zhao, X.S.; Bai, R. Modification of membrane surface for anti-biofouling performance: Effect of anti-adhesion and anti-bacteria approaches. J. Membr. Sci. 2010, 346, 121–130. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone) membranes: A mini review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Chang, Y.R.; Lee, Y.J.; Lee, D.J. Membrane fouling during water or wastewater treatments: Current research updated. J. Taiwan Inst. Chem. E. 2019, 94, 88–96. [Google Scholar] [CrossRef]
- Galal-Gorchev, H. Chlorine in water disinfection. Pure Appl. Chem. 1996, 68, 1731–1735. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Kuzmenko, D.; Gitis, N.V.; Vinogradov, M.; Kuiry, S.; Gitis, V. Hypochlorite cleaning causes degradation of polymer membranes. Tribol. Lett. 2007, 28, 109–116. [Google Scholar] [CrossRef]
- Bhojani, G.; Binod Kumar, S.; Kumar Saha, N.; Haldar, S. Membrane biofouling by chlorine resistant Bacillus spp.: Effect of feedwater chlorination on bacteria and membrane biofouling. Biofouling 2018, 34, 426–439. [Google Scholar] [CrossRef]
- Yu, W.; Xu, L.; Graham, N.; Qu, J. Pre-treatment for ultrafiltration: Effect of pre-chlorination on membrane fouling. Sci. Rep. 2014, 4, 5–13. [Google Scholar] [CrossRef]
- Yadav, K.; Morison, K.R. Effects of hypochlorite exposure on flux through polyethersulphone ultrafiltration membranes. Food Bioprod. Process 2010, 88, 419–424. [Google Scholar] [CrossRef]
- Kuzmenko, D.; Arkhangelsky, E.; Belfer, S.; Freger, V.; Gitis, V. Chemical cleaning of UF membranes fouled by BSA. Desalination 2005, 179, 323–333. [Google Scholar] [CrossRef]
- Wolff, S.H.; Zydney, A.L. Effect of bleach on the transport characteristics of polysulfone hemodialyzers. J. Membr. Sci. 2004, 243, 389–399. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Kuzmenko, D.; Gitis, V. Impact of chemical cleaning on properties and functioning of polyethersulfone membranes. J. Membr. Sci. 2007, 305, 176–184. [Google Scholar] [CrossRef]
- Levitsky, I.; Duek, A.; Arkhangelsky, E.; Pinchev, D.; Kadoshian, T.; Shetrit, H.; Naim, R.; Gitis, V. Understanding the oxidative cleaning of UF membranes. J. Membr. Sci. 2011, 377, 206–213. [Google Scholar] [CrossRef]
- Belila, A.; El-Chakhtoura, J.; Otaibi, N.; Muyzer, G.; Gonzalez-Gil, G.; Saikaly, P.E.; Van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Bacterial community structure and variation in a full-scale seawater desalination plant for drinking water production. Water Res. 2016, 94, 62–72. [Google Scholar] [CrossRef]
- Kekec, O.; Gokalsin, B.; Karalti, I.; Kayhan, F.E.; Sesal, N.C. Effects of chlorine stress on Pseudomonas aeruginosa biofilm and analysis of related gene expressions. Curr. Microbiol. 2016, 73, 228–235. [Google Scholar] [CrossRef]
- Flemming, H.C. Biofouling in water systems-cases, causes and countermeasures. Appl. Microbiol. Biotechnol. 2002, 59, 629–640. [Google Scholar] [CrossRef]
- Lee, J.; Kim, I.S. Microbial community in seawater reverse osmosis and rapid diagnosis of membrane biofouling. Desalination 2011, 273, 118–126. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Kim, I.S.; Cho, J.; Ngo, H.H. The effect of pretreatment to ultrafiltration of biologically treated sewage effluent: A detailed effluent organic matter (EfOM) characterization. Water Res 2004, 38, 1933–1939. [Google Scholar] [CrossRef]
- Agus, E.; Sedlak, D.L. Formation and fate of chlorination by-products in reverse osmosis desalination systems. Water Res. 2010, 44, 1616–1626. [Google Scholar] [CrossRef]
- Agus, E.; Voutchkov, N.; Sedlak, D.L. Disinfection by-products and their potential impact on the quality of water produced by desalination systems: A literature review. Desalination 2009, 237, 214–237. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic membranes for water purification: Status and future. Angew. Chem. 2015, 54, 368–386. [Google Scholar] [CrossRef]
- Li, D.; Wang, H. Recent developments in reverse osmosis desalination membranes. J. Mater. Chem. 2010, 20, 4551–4566. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Al-Mojjly, A.; Al-Othman, A.; Hilal, N. Membrane separation as a pre-treatment process for oily saline water. Desalination 2018, 447, 182–202. [Google Scholar] [CrossRef]
- Bruggen, B.V.D. Chemical modification of polyethersulfone nanofiltration membranes: A review. J. Appl. Polym. 2009, 114, 630–642. [Google Scholar] [CrossRef]
- Nady, N.; Schroën, K.; Franssen, M.C.; Fokkink, R.; Eldin, M.S.M.; Zuilhof, H.; Boom, R.M. Enzyme-catalyzed modification of PES surfaces: Reduction in adsorption of BSA, dextrin and tannin. J. Colloid Interface Sci. 2012, 378, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Nady, N.; Salem, N.; El-Shazly, A.; Soliman, H.; Kandil, S.H. Fouling-resistant brush-like oligomers of poly(3-aminophenol). Desalin. Water Treat. 2017, 73, 237–248. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Boom, R.M.; Schroën, K. Enzymatic modification of polyethersulfone membranes. Water 2012, 4, 932–943. [Google Scholar] [CrossRef]
- van der Veen, V.; Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Boom, R.M.; Abee, T. Listeria monocytogenes repellence by enzymatically modified PES surfaces. J. Appl. Polym. Sci. 2015, 132, 41576. [Google Scholar] [CrossRef]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef]
- Nady, N.; El-Shazly, A. Laccase-catalysed modification of PES membranes using amine-bearing modifiers. Desalination Water Treat. 2015, 55, 2996–3002. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington DC, USA, 2012; pp. 183–190. [Google Scholar]
- Shon, H.; Kim, S.; Vigneswaran, S.; Aim, R.B.; Lee, S.; Cho, J. Physicochemical pretreatment of seawater: Fouling reduction and membrane characterization. Desalination 2009, 238, 10–21. [Google Scholar] [CrossRef]
- Wef, A.A. Standard Methods for the Examination of water and Wastewater, 21st ed.; American Public Health Association, American Water Works Association, Water Environmental Federation: Washington, DC, USA, 2005; p. 1360. [Google Scholar]
- Jett, B.D.; Hatter, K.L.; Huycke, M.M.; Gilmore, M.S. Simplified agar plate method for quantifying viable bacteria. Biotechniques 1997, 23, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Bloem, J.; Bär-Gilissen, M.J.B.; Cappenberg, T.E. Fixation, counting, and manipulation of heterotrophic nanoflagellates. Appl. Environ. Microbiol. 1986, 52, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Nessim, R.B.; Tadros, H.R.; Taleb, A.E.A.; Moawad, M.N. Chemistry of the Egyptian Mediterranean coastal waters. Egypt. J. Aquat. Res. 2015, 41, 1–10. [Google Scholar] [CrossRef]
- Borghini, M.; Bryden, H.; Schroeder, K.; Sparnocchia, S.; Vetrano, A. The Mediterranean is becoming saltier. Ocean Sci. 2014, 10, 693–700. [Google Scholar] [CrossRef]
- Almasi, A.; Dargahi, A.; Motlagh, Z.J.; Mahmoodi, M.; Mohammadi, M.; Rezaei, S.; Janjani, H.; Amirian, T. Evaluation of common coagulants and polymeric coagulant aid in the removal of suspended particles and colloidal turbidity of raw water of Gavoshan dam. J. Chem. Pharm. 2016, 9, 3178–3181. [Google Scholar]
- Matilainen, A.; Vepsalainen, M.; Sillanpaa, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef]
- Baghvand, A.; Zand, A.D.; Mehrdadi, N.; Karbassi, A. Optimizing coagulation process for low to high turbidity waters using aluminum and iron salts. Am. J. Environ. Sci. 2010, 6, 442–448. [Google Scholar] [CrossRef]
- Goosen, M.; Sablani, S.; Al-Hinai, H.; Al-Obeidani, S.; Al-Belushi, R.; Jackson, A. Fouling of reverse osmosis and ultrafiltration membranes: A critical review. Sep. Sci. Technol. 2005, 39, 2261–2297. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Mansouri, J.; Harrisson, S.; Chen, V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 2010, 20, 4567–4586. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Dominguez, D.C. Calcium signalling in bacteria. Mol. Microbiol. 2004, 54, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Patrauchan, M.A.; Sarkisova, S.; Sauer, K.; Franklin, M.J. Calcium influences cellular and extracellular product formation during biofilm-associated growth of a marine Pseudoalteromonas. Microbiology 2005, 151, 2885–2897. [Google Scholar] [CrossRef]
- Berchtold, M.W.; Villalobo, A. The many faces of calmodulin in cell proliferation, programmed cell death, autophagy, and cancer. Biochim. Biophys. Acta 2014, 1843, 398–435. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nady, N.; Salem, N.; Amer, R.; El-Shazly, A.; Kandil, S.H.; Hassouna, M.S.E.-D. Comparison between a Conventional Anti-Biofouling Compound and a Novel Modified Low-Fouling Polyethersulfone Ultrafiltration Membrane: Bacterial Anti-Attachment, Water Quality and Productivity. Membranes 2020, 10, 227. https://doi.org/10.3390/membranes10090227
Nady N, Salem N, Amer R, El-Shazly A, Kandil SH, Hassouna MSE-D. Comparison between a Conventional Anti-Biofouling Compound and a Novel Modified Low-Fouling Polyethersulfone Ultrafiltration Membrane: Bacterial Anti-Attachment, Water Quality and Productivity. Membranes. 2020; 10(9):227. https://doi.org/10.3390/membranes10090227
Chicago/Turabian StyleNady, Norhan, Noha Salem, Ranya Amer, Ahmed El-Shazly, Sherif H. Kandil, and Mohamed Salah El-Din Hassouna. 2020. "Comparison between a Conventional Anti-Biofouling Compound and a Novel Modified Low-Fouling Polyethersulfone Ultrafiltration Membrane: Bacterial Anti-Attachment, Water Quality and Productivity" Membranes 10, no. 9: 227. https://doi.org/10.3390/membranes10090227
APA StyleNady, N., Salem, N., Amer, R., El-Shazly, A., Kandil, S. H., & Hassouna, M. S. E.-D. (2020). Comparison between a Conventional Anti-Biofouling Compound and a Novel Modified Low-Fouling Polyethersulfone Ultrafiltration Membrane: Bacterial Anti-Attachment, Water Quality and Productivity. Membranes, 10(9), 227. https://doi.org/10.3390/membranes10090227




