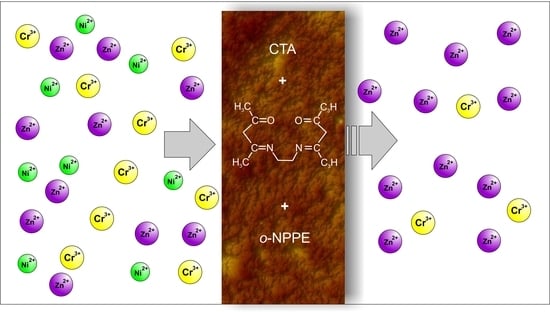
Separation of Zn(II), Cr(III), and Ni(II) Ions Using the Polymer Inclusion Membranes Containing Acetylacetone Derivative as the Carrier
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Apparatus
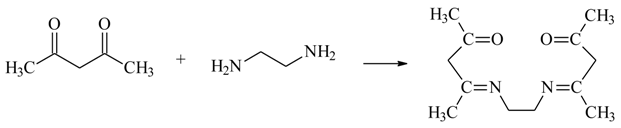
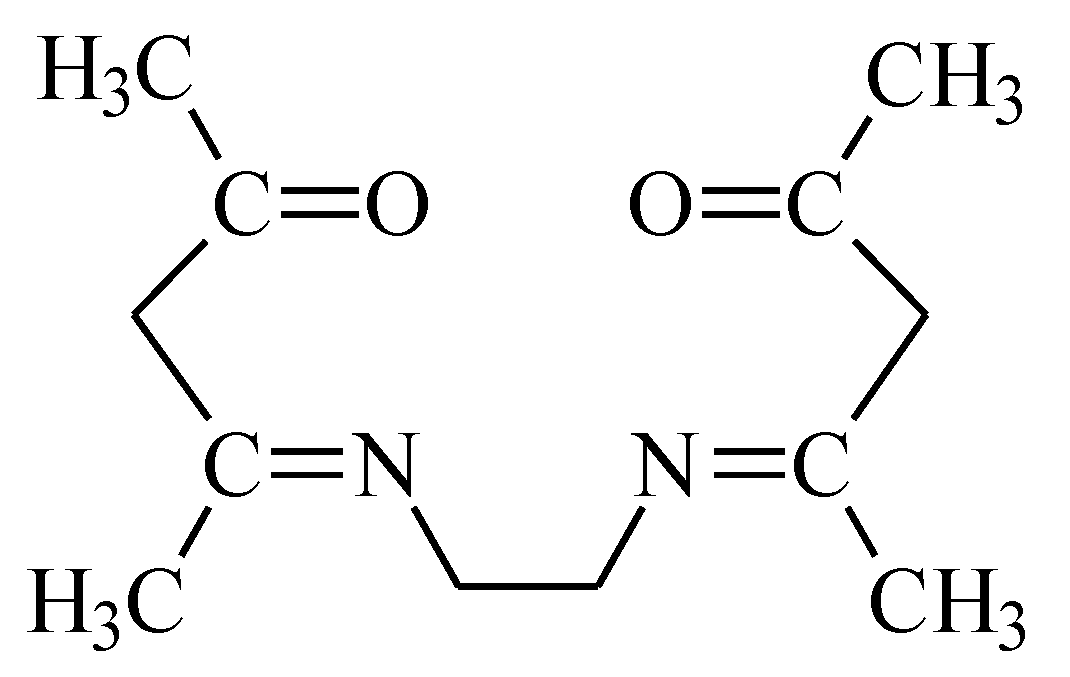
2.2. Polymer Inclusion Membrane Preparation
2.3. Transport Studies
2.4. Applied Calculations Related to the Parameters Characterizing Transport
3. Results and Discussion
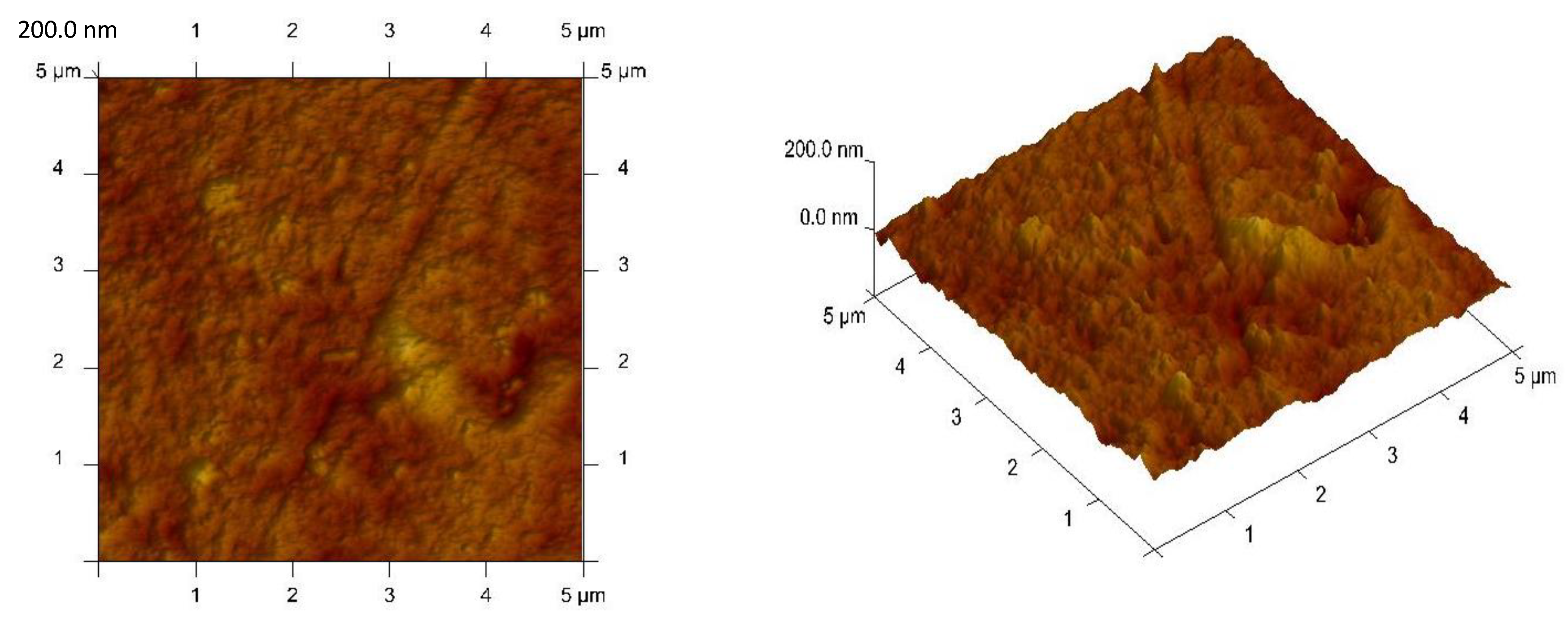
3.1. Membranes Characterization
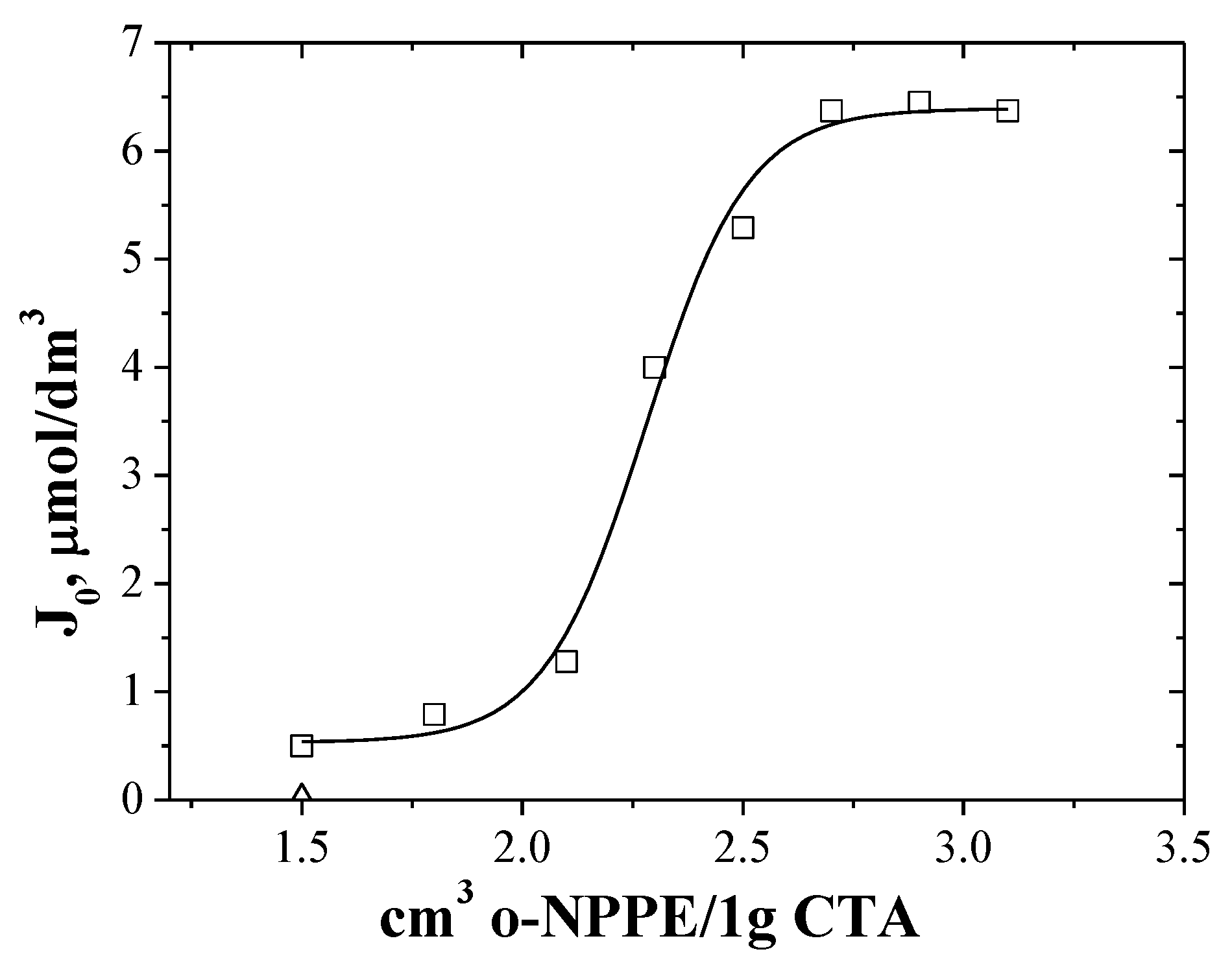
3.2. The Effect of Plasticizer Content on Transport of Zn(II) Ions across PIMs with EDAB-acac
3.3. The Influence of the Carrier Concentration in CTA-o-NPPPE-EDAB-acac Membrane on Transport of Zn(II), Cr(III), and Ni(II) Ions
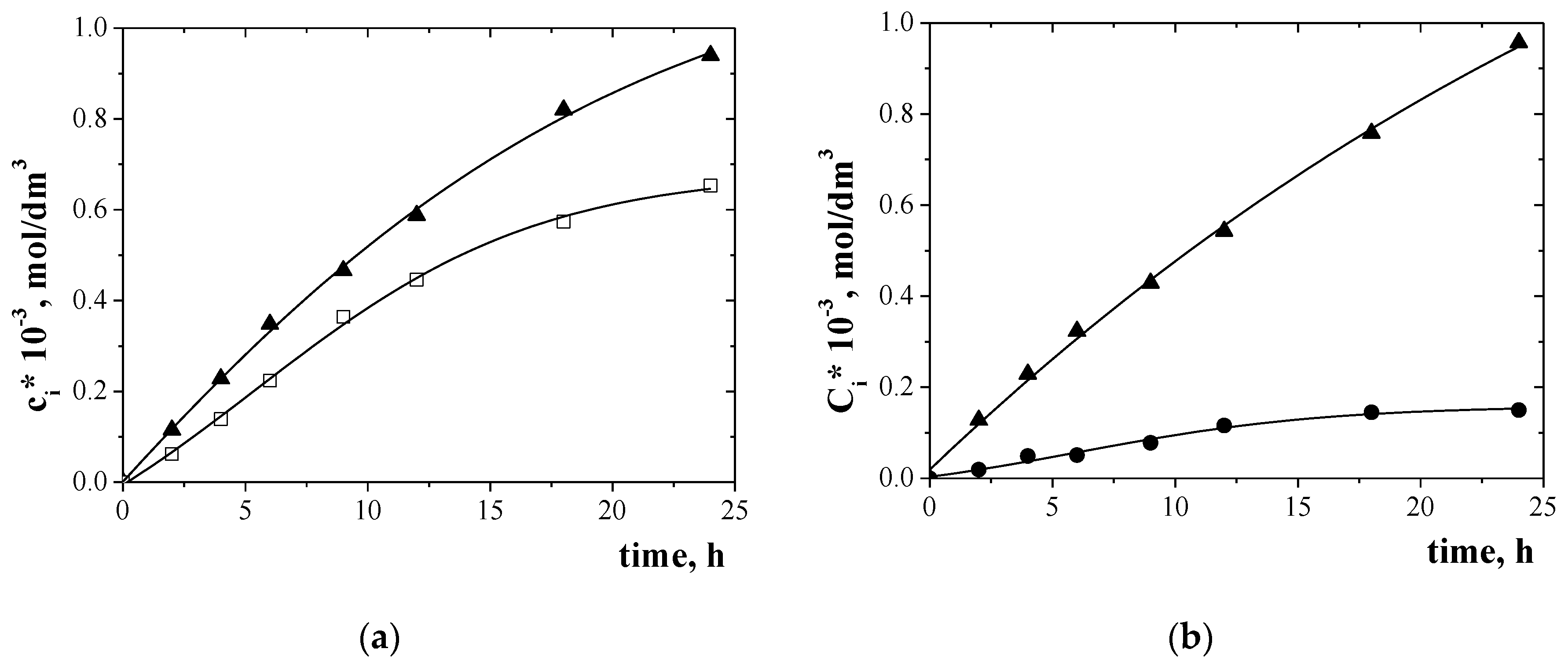
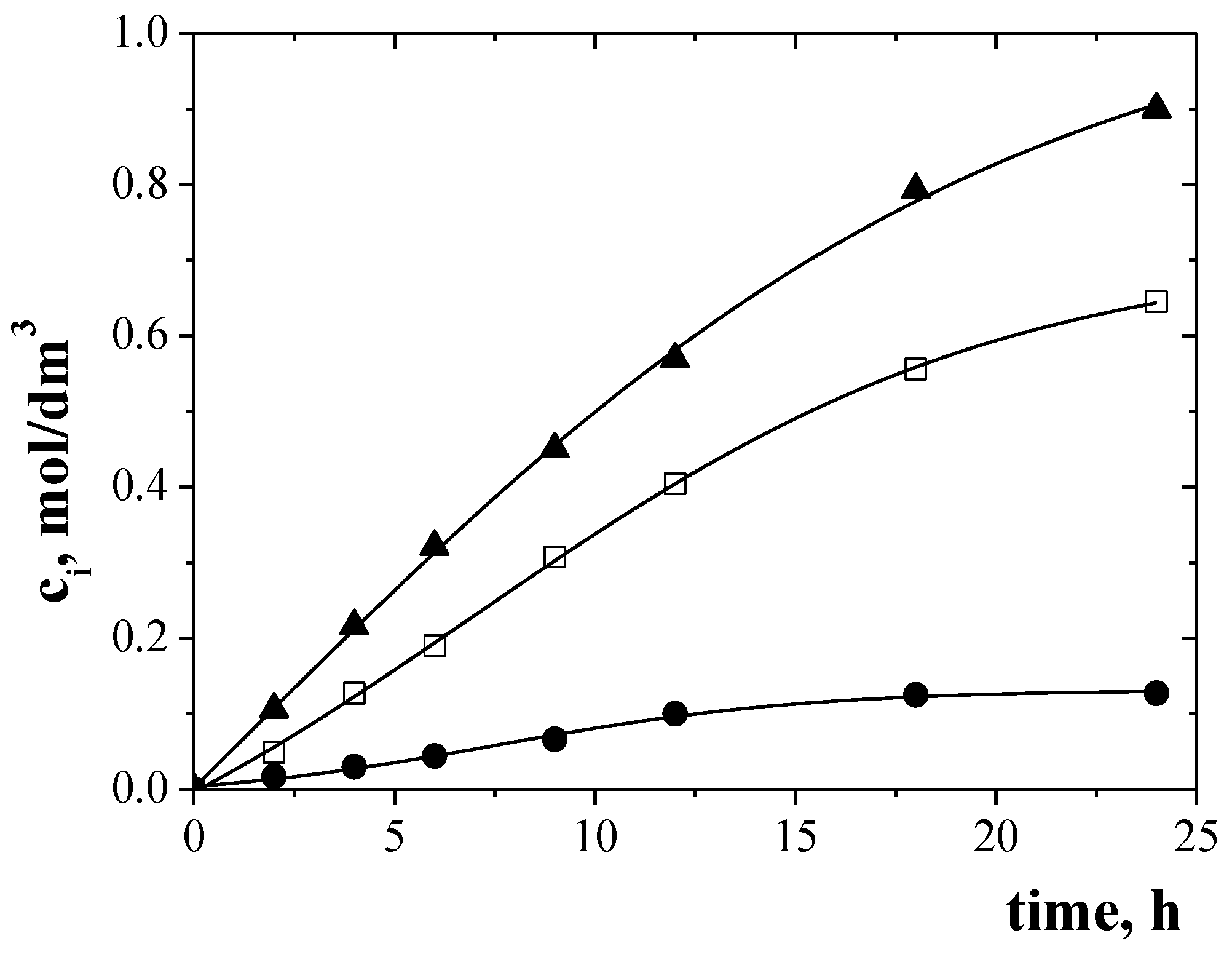
3.4. Separation of Metal Ions from Their Equimolar Solution in Transport across PIMs Doped EDAB-acac
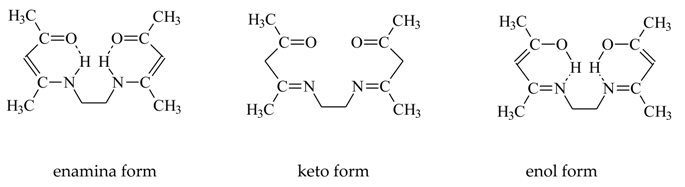
3.5. Recovery of Metal
3.6. Membrane Diffusion Coefficients of Metal Ions Complexes with EDAB-acac
3.7. Studies of Membrane Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de Souza e Silva, P.T.; de Mello, N.T.; Duarte, M.M.M.; Conceicao, M.; Montenegro, B.S.M.; Araujo, A.M.; De Barros Neto, B.; da Silva, V.L. Extraction and recovery of chromium from electroplating sludge. J. Hazard. Mater. 2006, 128, 39–43. [Google Scholar] [CrossRef]
- Giachetti, G.; Sebastiani, L. Metal accumulation in poplar plant grown with industrial wastes. Chemosphere 2006, 64, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Regel-Rosocka, M.; Alguacil, F.J. Recent trends in metals extraction. Rev. Metal. 2013, 49, 292–315. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Xiong, X.; Almeida, M.I.G.S.; Simeonov, S.; Spassov, T.G.; Cattrall, R.W.; Kolev, S.D. The potential of polystyrene-block-polybutadiene-block-polystyrene triblock co-polymer as a base-polymer of polymer inclusion membranes (PIMs). Sep. Purif. Technol. 2019, 229, 115800. [Google Scholar] [CrossRef]
- Lozano, L.J.; Godinez, C.; de los Rios, A.P.; Hernandez-Fernandez, F.J.; Sanchez-Segado, S.; Alguacil, F.J. Recent advances in supported ionic liquid membrane technology. J. Membr. Sci. 2011, 376, 1–14. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Mornane, P.; Potter, L.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R.A. Heavy metal separation with polymer inclusion membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Baczynska, M.; Regel-Rosocka, M.; Coll, M.T.; Fortuny, A.; Sastre, A.M.; Wiśniewski, M. Transport of Zn(II), Fe(II), Fe(III) across polymer inclusion membranes (PIM) and flat sheet supported liquid membranes (SLM) containing phosphonium ionic liquids as metal ion carriers. Sep. Sci. Technol. 2016, 51, 2639–2648. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Applicability of liquid membranes in chromium(VI) transport with amines as ion carriers. J. Membr. Sci. 2005, 266, 143–150. [Google Scholar] [CrossRef]
- Casadellà, A.; Schaetzle, O.; Nijmeijer, K.; Loos, K. Polymer Inclusion Membranes (PIM) for the Recovery of Potassium in the Presence of Competitive Cations. Polymers 2016, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P.; Drioli, E. Fixed sites plasticized cellulose triacetate membranes containing crown ethers for silver(I), copper(II) and gold(III) ions transport. J. Membr. Sci. 2004, 228, 149–157. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole and their Application in the Separation of Non-Ferrous Metal Ions. Polymers 2019, 11, 1780. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Madaeni, S.S.; Khodabakhshi, A.R. Preparation and Characterization of Heterogeneous Cation Exchange Membranes Based on S-Poly Vinyl Chloride and polycarbonate. Sep. Sci. Technol. 2011, 46, 794–808. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Shen, W.; Paimin, R.; Wang, X. The Influence of the Interior Structure of Aliquat 336/PVC Membranes to their Extraction Behavior. Sep. Sci. Technol. 2005, 39, 3527–3539. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E.; Kosciuszko, A.; Gierszewska, M.; Ziuziakowski, K. The Influence of the Morphology and Mechanical Properties of Polymer Inclusion Membranes (PIMs) on Zinc Ion Separation from Aqueous Solutions. Polymers 2018, 10, 134. [Google Scholar] [CrossRef]
- Choi, Y.W.; Moo, S.H. A Study on Supported Liquid Membrane for Selective Separation of Cr(VI). Sep. Sci. Technol. 2005, 39, 1663–1680. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. Extraction of Gold(III) from Hydrochloric Acid Solutions with a PVC-based Polymer Inclusion Membrane (PIM) Containing Cyphos® IL 104. Membranes 2015, 5, 903–914. [Google Scholar] [CrossRef]
- O’Rourke, M.; Duffy, N.; De Marco, R.; Potter, I. Electrochemical Impedance Spectroscopy-A Simple Method for the Characterization of Polymer Inclusion Membranes Containing Aliquat 336. Membranes 2011, 1, 132–148. [Google Scholar] [CrossRef]
- Zulkefeli, N.S.W.; Weng, S.K.; Halim, N.S.A. Removal of heavy metals by polymer inclusion membranes. Curr. Pollut. Rep. 2018, 4, 84–92. [Google Scholar] [CrossRef]
- Bey, S.; Criscuoli, A.; Figoli, A.; Leopold, A.; Simone, S.; Benamor, M.; Drioli, E. Removal of As(V) by PVDF hollow fibers membrane contactors using Aliquat-336 as extractant. Desalination 2010, 264, 193–200. [Google Scholar] [CrossRef]
- Ballinas, M.D.; Rodríguez de San Miguel, E.; Rodríguez, M.T.; Silva, O.; Muñoz, M.; de Gyves, J. Arsenic (V) removal with polymer inclusion membranes from sulfuric acid media using DBBP as carrier. Environ. Sci. Technol. 2004, 38, 886–891. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. The application of membrane extraction in the separation of zinc and cadmium ions. Desal. Water Treat. 2018, 128, 140–147. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Transport of Cr(VI), Zn(II), and Cd(II) ions across polymer inclusion membranes with tridecyl(pyridine) oxide and tri-n-octylamine. Sep. Sci. Technol. 2004, 39, 3127–3141. [Google Scholar] [CrossRef]
- Wang, L.; Paimin, R.; Cattrall, R.W.; Shen, W.; Kolev, S.D. The extraction of cadmium (II) and copper (II) from hydrochloric acid solutions using an Aliquat 336/PVC membrane. J. Membr. Sci. 2000, 176, 105–111. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Selective transport of Cu(II) across a polymer inclusion membrane with 1-alkylimidazole from nitrate solutions. Sep. Sci. Technol. 2012, 47, 1113–1118. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The application of polymer inclusion membranes based on CTA with 1-alkylimidazole for the separation of zinc(II) and manganese(II) ions from aqueous solutions. Polymers 2019, 11, 242. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H. Specific membrane transport of silver and copper as Ag(CN)32− and Cu(CN)43− ions through a supported liquid, brane using K+-crown ether as a carrier. Desalination 2003, 151, 87–94. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Bautista-Flores, A.N.; San Miguel, E.R.; Muhammed, M.; Gyves, J. Transport characterization of a PIM system used for the extraction of Pb(II) using D2EHPA as carrier. J. Membr. Sci. 2005, 250, 247–257. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Bourceanu, G.; Olariu, R.I.; Arsene, C. Removal of lead(II) from aqueous solutions by a polyvinyl-chloride inclusion membrane without added plasticizer. J. Membr. Sci. 2011, 377, 167–174. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Bourceanu, G.; Timpu, D. Experimental and modelling studies of lead(II) sorption onto a polyvinyl-chloride inclusion membrane. Chem. Eng. J. 2011, 172, 817–827. [Google Scholar] [CrossRef]
- Tor, A.; Arslan, G.; Muslu, H.; Celikas, A.; Cengeloglu, Y.; Ersoz, M. Facilitated transport of Cr(III) thought polymer inclusion membrane with di(2-ethylhexyl)phosphoric acid (DEHPA). J. Membr. Sci. 2009, 329, 169–174. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W. Removal of chromium (VI) from aqueous solutions by polymer inclusion membranes. Water Res. 2002, 36, 4870–4876. [Google Scholar] [CrossRef]
- Yilmaz, A.; Arslan, G.; Tor, A.; Akin, I. Selectively facilitated transport of Zn(II) through a novel polymer inclusion membrane containing Cyanex 272 as a carrier reagent. Desalination 2011, 277, 301–307. [Google Scholar] [CrossRef]
- Rodrigues, M.A.S.; Amado, F.D.R.; Bischoff, M.R.; Ferreira, C.A.; Bernardes, A.M.; Ferreira, J.Z. Transport of zinc complexes through an anion exchange membrane. Desalination 2008, 227, 241–252. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Bringas, E.; Tan, N.R.; Ortiz, I.; Ghahramani, M.; Shahmirzadi, M.A.A. Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review. J. Water Proc. Eng. 2016, 9, 78–110. [Google Scholar] [CrossRef]
- Gasser, M.S.; El-Hefny, N.E.; Rizk, S.E.; Daoud, J.A. Transfer and Separation of Zn(II)/Co(II) by Supported Liquid Membrane Containing CYANEX 925 in Kerosene as Carrier. J. Phys. Sci. 2013, 24, 63–81. [Google Scholar]
- Ulewicz, M.; Kozłowski, C.; Walkowiak, W. Removal of Zn(II), Cd(II) and Cu(II) ions by polymer inclusion membrane with side-armed diphosphaza-16-crown-6-ethers. Physicochem. Probl. Miner. Process. 2004, 38, 131–138. [Google Scholar]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The use of 1-alkylimidzoles for selective separation of zinc ions in the transport process across a polymeric inclusion membrane. Physicochem. Probl. Miner. Process. 2014, 50, 131–142. [Google Scholar] [CrossRef]
- Kozlowska, J.; Kozłowski, C.A.; Koziol, J.J. Transport of Zn(II), Cd(II), and Pb(II) across CTA plasticized membranes containing organophosphorous acids as an ion carriers. Sep. Purif. Technol. 2007, 57, 430–434. [Google Scholar] [CrossRef]
- Baczynska, M.; Regel-Rosocka, M.; Nowicki, M.; Wisniewski, M. Effect of the structure of polymer inclusion membranes on Zn(II) transport from chloride aqueous solutions. J. Appl. Polym. Sci. 2015, 132, 42319–42329. [Google Scholar] [CrossRef]
- Ulewicz, M.; Sadowska, K.; Biernat, J.F. Selective transport of Pb(II) across polymer inclusion membrane using imidazole azocrown ethers as carriers. Physicochem. Probl. Miner. Process. 2007, 41, 133–143. [Google Scholar]
- Ulewicz, M.; Szczygelska-Tao, J.; Biernat, J.F. Selectivity of Pb(II) transport across polymer inclusion membranes doped with imidazole azothiacrown ethers. J. Membrane Sci. 2009, 344, 32–38. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of polymer and supported membranes with 1-decyl-4-methylimidazole for pertraction of transition metal ions. Sep. Sci. Technol. 2014, 49, 1713–1721. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, R.; Ulewicz, M. Zinc recovery from model and waste solutions using polymer inclusion membrane (PIMs) with 1-octyl-4-methylimidazole. Desalin. Water Treat. 2018, 102, 211–219. [Google Scholar] [CrossRef]
- Takeuchi, T.; Böttcher, A.; Quezada, C.M.; Meade, T.J.; Gray, H.B. Inhibition of thermolysin and human α-thrombin by cobalt(III) Schiff base complexes. Bioorganic Med. Chem. 1999, 7, 815–819. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Transport of metal ions across polymer inclusion membrane with 1-alkylimidazole. Physicochem. Probl. Miner. Process. 2011, 46, 119–130. [Google Scholar]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1984, 19, 857–894. [Google Scholar] [CrossRef]
- Visser, H.C.; Reinhoudt, D.N.; de Jong, F. Carrier-mediated transport through liquid membranes. Chem. Soc. Rev. 1994, 23, 75–81. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K.; Urbaniak, W. Selective transport of zinc ions through a novel polymer inclusion membranes (PIMs) containing β-diketone derivatives as a carrier reagents. Sep. Sci. Technol. 2016, 51, 2620–2627. [Google Scholar] [CrossRef]
- Gherrou, A.; Kerdjoudj, H.; Molinari, R.; Seta, P. Preparation and characterization of polymer plasticized membranes (PPM) embedding a crown ether carrier application to copper ions transport. Mat. Sci. Eng. C 2005, 25, 436–443. [Google Scholar] [CrossRef]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver(I) and copper(II) ions transport mechanisms in a supported and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Pawlicki, G.; Staniszewski, B.; Witt, K.; Urbaniak, W.; Lis, S. Complexation studies of 3-substituted β-diketones with selected d- and f-metal ions. Chem. Pap. 2011, 65, 221–225. [Google Scholar] [CrossRef]
- Miyake, Y.; Ohtsuki, K.; Teramoto, M. Effect of alkyl chain length of 3-alkylpentane-2,4-dione on the extraction of copper. Solvent Extr. Ion Exch. 1990, 8, 799–815. [Google Scholar] [CrossRef]
- Kislik, V.S. Liquid Membranes. Principles and Application in Chemical Separation and Wastewater Treatment; Elsevier: Burlington, VT, USA, 2010. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Supported Liquid (SLM) and Polymer Inclusion (PIM) Membranes Pertraction of Copper(II) from Aqueous Nitrate Solutions by 1-Hexyl-2-Methylimidazole. Sep. Sci. Technol. 2012, 47, 1383–1389. [Google Scholar] [CrossRef]

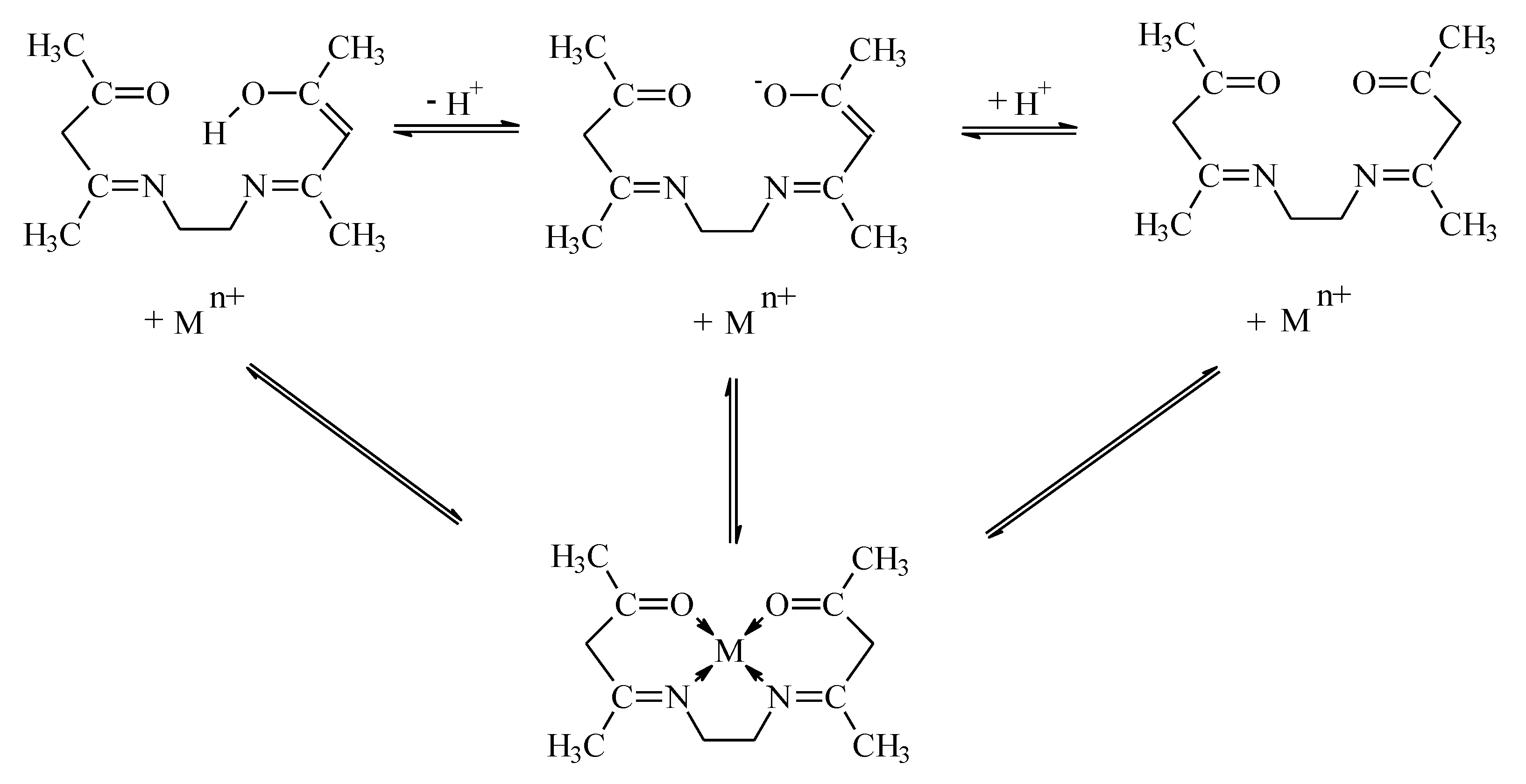
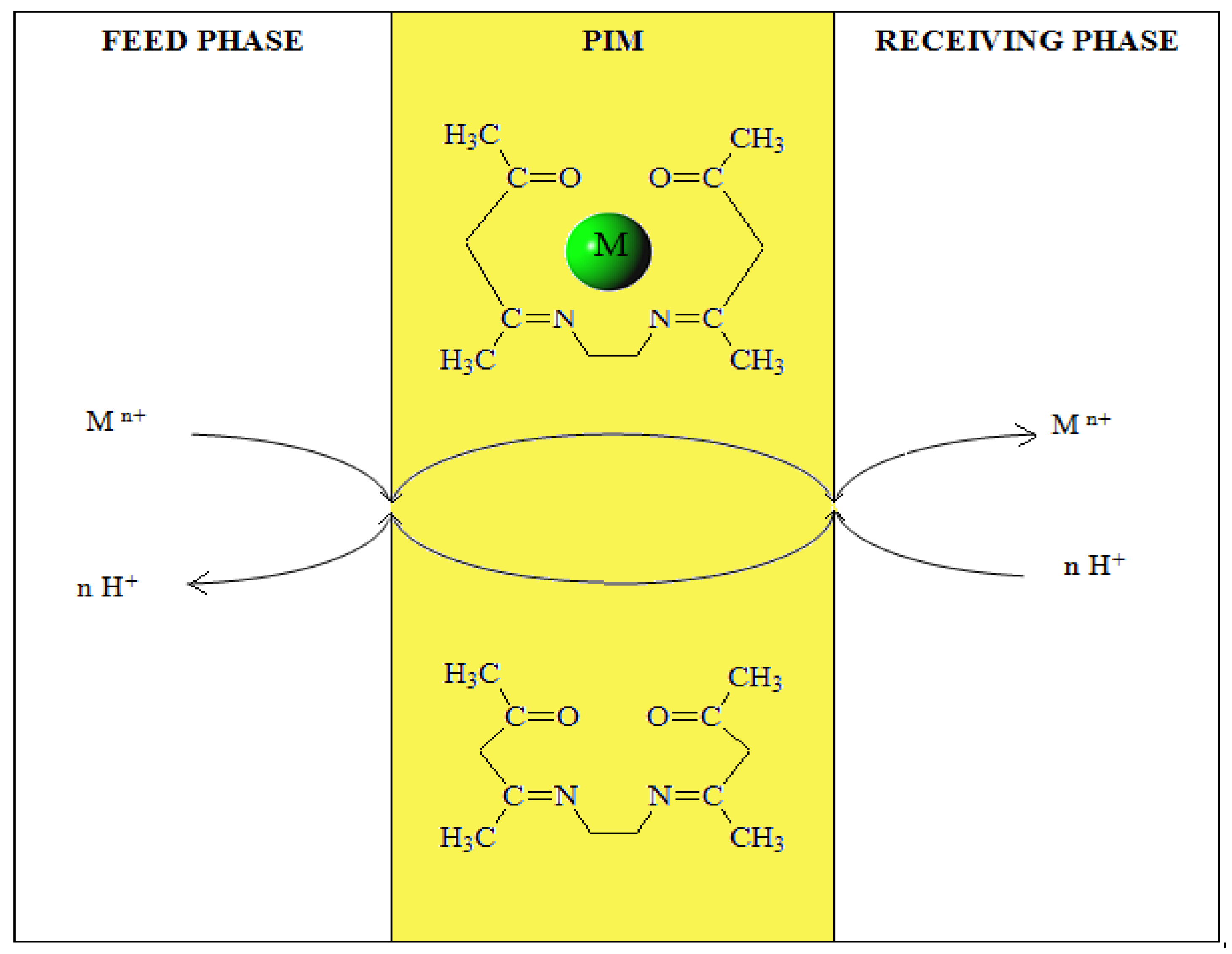
| The initial flux (Ji) | V-feed phase volume, m3 A-effective area of the membrane, m2 | |
| The selectivity coefficient (S) | Ji-initial flux metal M1 or M2, µmol/m2∙s | |
| The recovery coefficient (RF) | c-metal ions concentration in the feed phase at a given time, (mol/dm3) ci-initial metal ions concentration in the feed phase, (mol/dm3) |
| Membrane | Average Thickness, µm | Effective Pore Size, µm | Tortuosity | Roughness (Rq), nm | Ref. |
|---|---|---|---|---|---|
| CTA-o-NPPE-0.8 mol/dm3 EDAB-acac | 30 | 0.058 | 2.60 | 4.40 | - |
| PVC-DAO-60% acac | 27 | 0.063 | - | 3.55 | [16] |
| PVC-DAO-60% 3-propyl-acac | 32 | 0.060 | - | 1.76 | [50] |
| PVC-DAO-60% 3-benzyl-acac | 33 | 0.066 | - | 2.05 | [50] |
| Concentration of Carrier, mol/dm3 | Metal Ions | Initial Flux, Ji µmol/m2∙s | RF after 24 h, % |
|---|---|---|---|
| 0.2 | Zn(II) | 0.08 | 14 |
| Cr(III) | 0.05 | 6 | |
| Ni(II) | 0.01 | - | |
| 0.4 | Zn(II) | 1.32 | 25 |
| Cr(III) | 0.72 | 11 | |
| Ni(II) | 0.02 | 1 | |
| 0.6 | Zn(II) | 5.26 | 77 |
| Cr(III) | 3.40 | 52 | |
| Ni(II) | 0.32 | 4 | |
| 0.8 | Zn(II) | 6.37 | 90 |
| Cr(III) | 5.53 | 65 | |
| Ni(II) | 0.40 | 6 | |
| 1.0 | Zn(II) | 6.32 | 83 |
| Cr(III) | 5.49 | 61 | |
| Ni(II) | 0.36 | 5 |
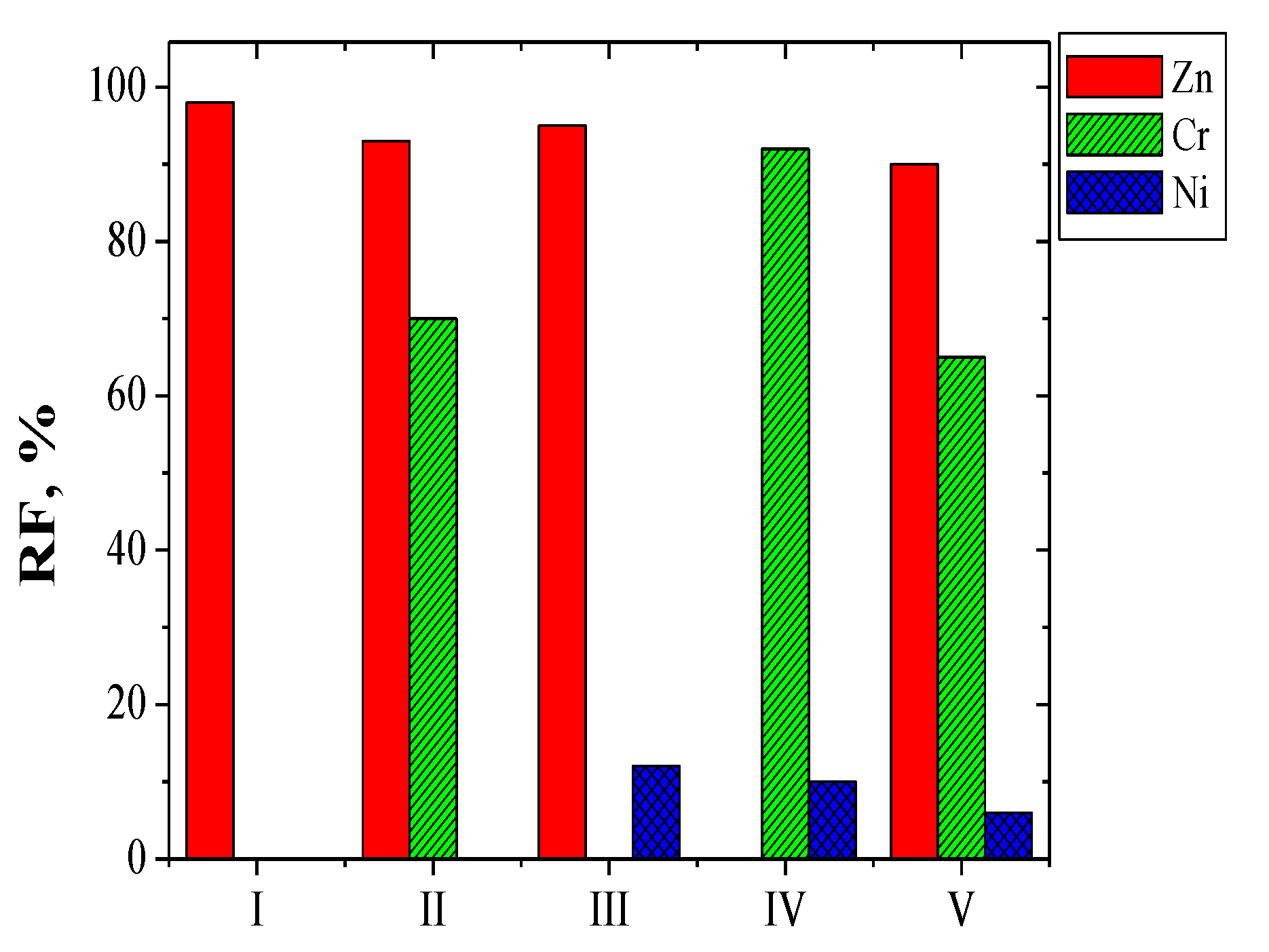
| Solutions | Metal Ions | Initial Flux, Ji µmol/m2∙s | Selectivity Order and Selectivity Coefficients |
|---|---|---|---|
| I | Zn(II) | 11.25 | - |
| II | Zn(II) Cr(III) | 8.40 5.98 | Zn(II) > Cr(III) 1.4 |
| III | Zn(II) Ni(II) | 9.16 0.52 | Zn(II) > Ni(II) 17.6 |
| IV | Cr(III) Ni(II) | 7.27 0.61 | Cr(III) > Ni(II) 11.9 |
| V | Zn(II) Cr(III) Ni(II) | 6.37 5.53 0.40 | Zn(II) > Cr(III) > Ni(II) 1.2 15.9 |
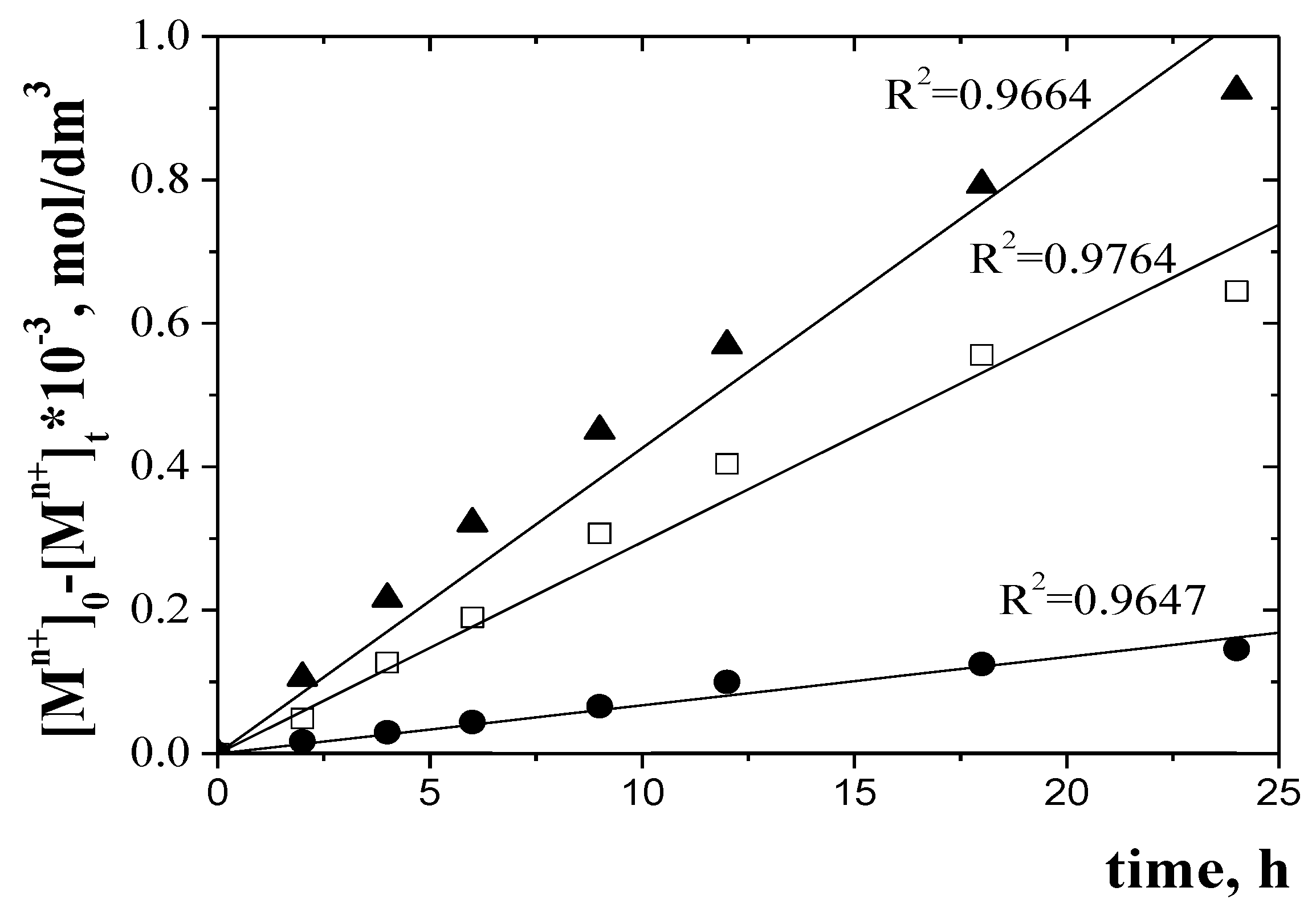
| the diffusion coefficient (Do) | Do = do/Δo | do-the thickness of the membrane (Table 2); Δo could be seen from Figure 10 |
| the normalized membrane diffusion coefficient (Do,n) | Do,n= Do∙(ε/τ) |
| Metal ion | Δo, s/m | Do, cm2/s | Do,n cm2/s |
|---|---|---|---|
| Zn(II) | 107.33 | 4.061 × 10−8 | 1.97 × 10−9 |
| Cr(III) | 106.52 | 6.765 × 10−10 | 4.58 × 10−11 |
| Ni(II) | 1010.41 | 2.362 × 10−12 | 6.25 × 10−13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzyminska-Lenarcik, E.; Pyszka, I.; Ulewicz, M. Separation of Zn(II), Cr(III), and Ni(II) Ions Using the Polymer Inclusion Membranes Containing Acetylacetone Derivative as the Carrier. Membranes 2020, 10, 88. https://doi.org/10.3390/membranes10050088
Radzyminska-Lenarcik E, Pyszka I, Ulewicz M. Separation of Zn(II), Cr(III), and Ni(II) Ions Using the Polymer Inclusion Membranes Containing Acetylacetone Derivative as the Carrier. Membranes. 2020; 10(5):88. https://doi.org/10.3390/membranes10050088
Chicago/Turabian StyleRadzyminska-Lenarcik, Elzbieta, Ilona Pyszka, and Malgorzata Ulewicz. 2020. "Separation of Zn(II), Cr(III), and Ni(II) Ions Using the Polymer Inclusion Membranes Containing Acetylacetone Derivative as the Carrier" Membranes 10, no. 5: 88. https://doi.org/10.3390/membranes10050088
APA StyleRadzyminska-Lenarcik, E., Pyszka, I., & Ulewicz, M. (2020). Separation of Zn(II), Cr(III), and Ni(II) Ions Using the Polymer Inclusion Membranes Containing Acetylacetone Derivative as the Carrier. Membranes, 10(5), 88. https://doi.org/10.3390/membranes10050088