Assessing the Performance of Thin-Film Nanofiltration Membranes with Embedded Montmorillonites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Philippine Sodium-montmorillonite and Organo-montmorillonite
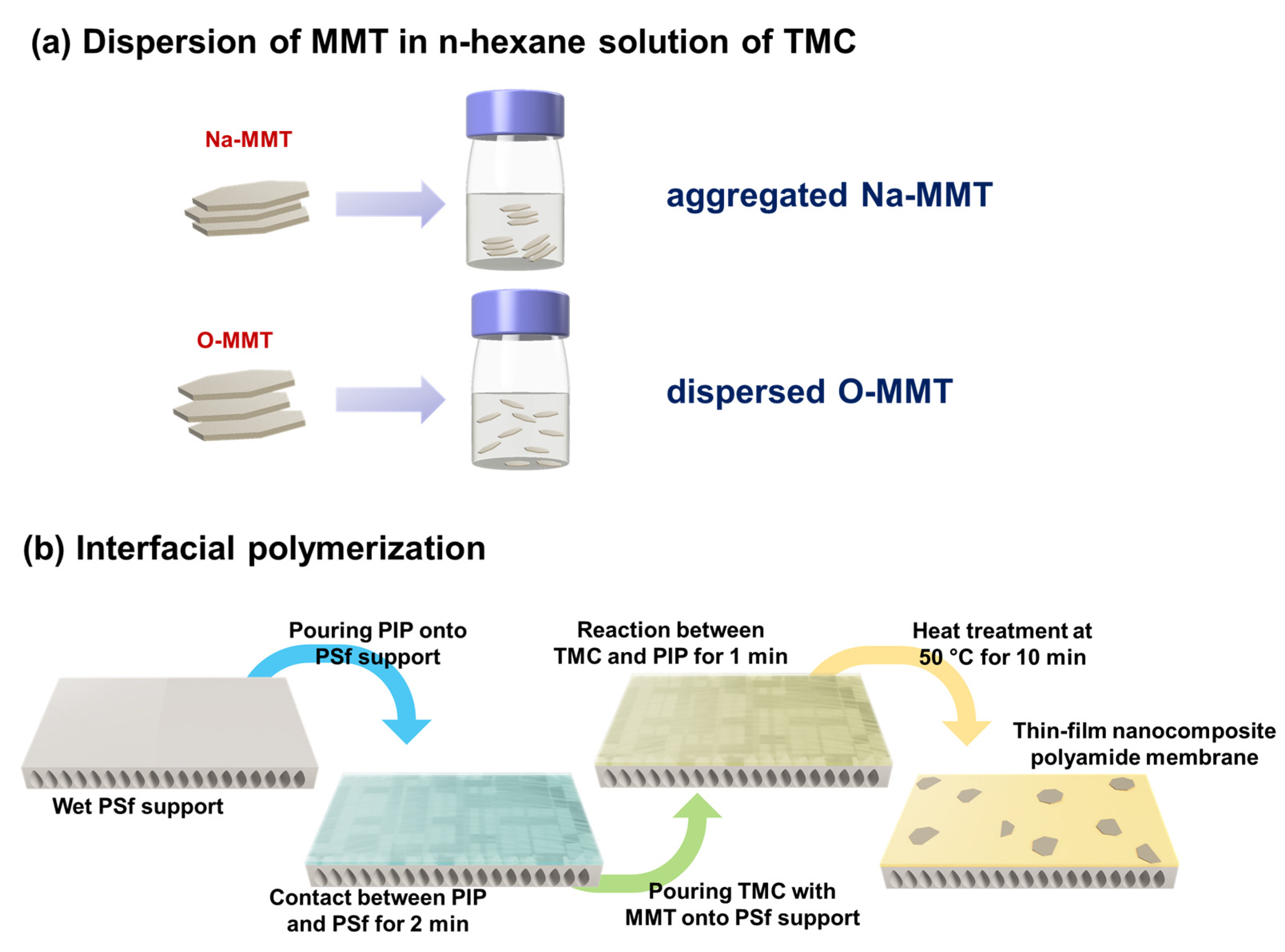
2.3. Preparation of Thin-film Nanocomposite Membranes
2.4. Characterization of Montmorillonites and Composite Membranes
2.5. Evaluation of Nanofiltration Performance
2.6. Evaluation of Antifouling Property and Stability of the Membrane
3. Results and Discussion
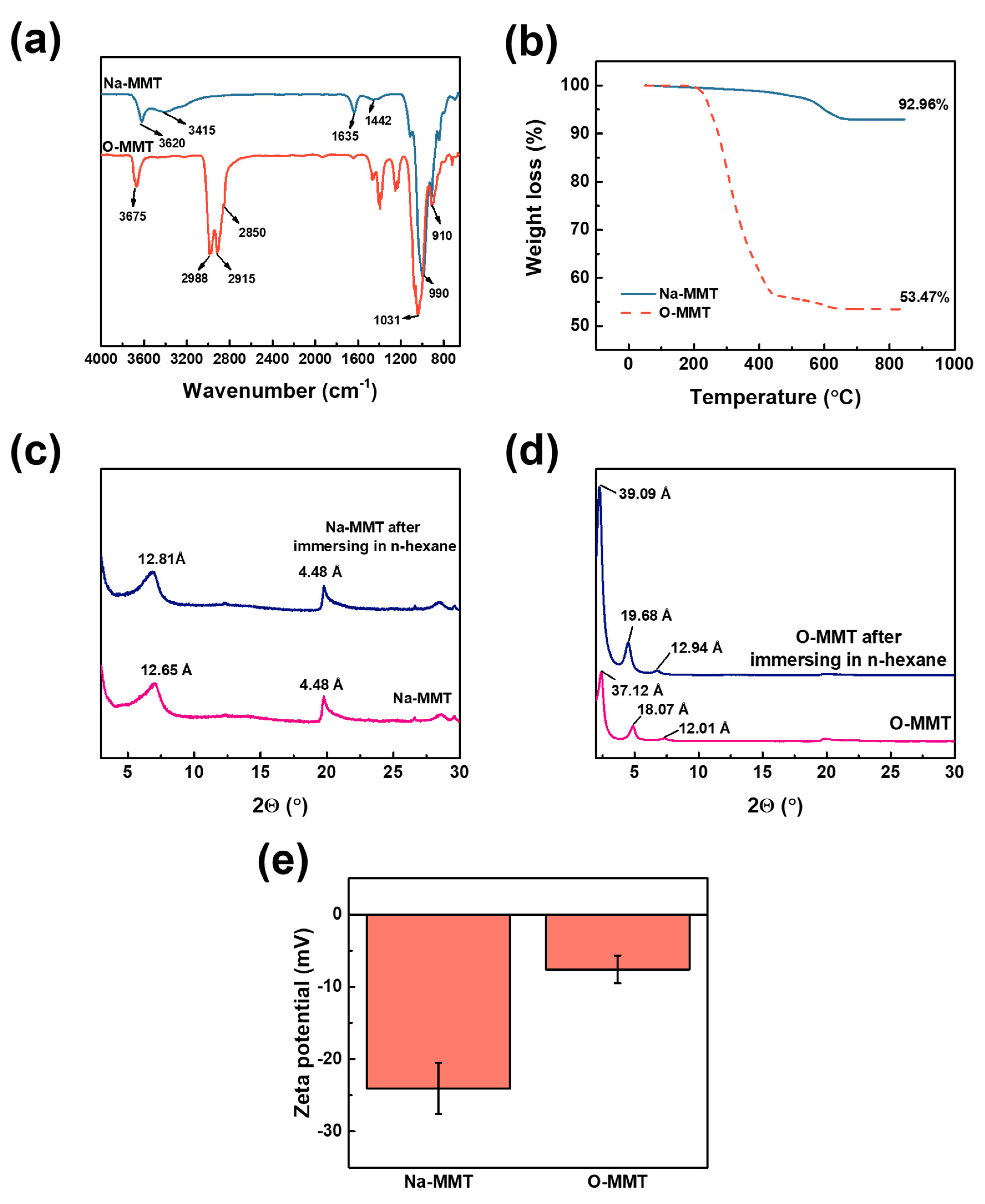
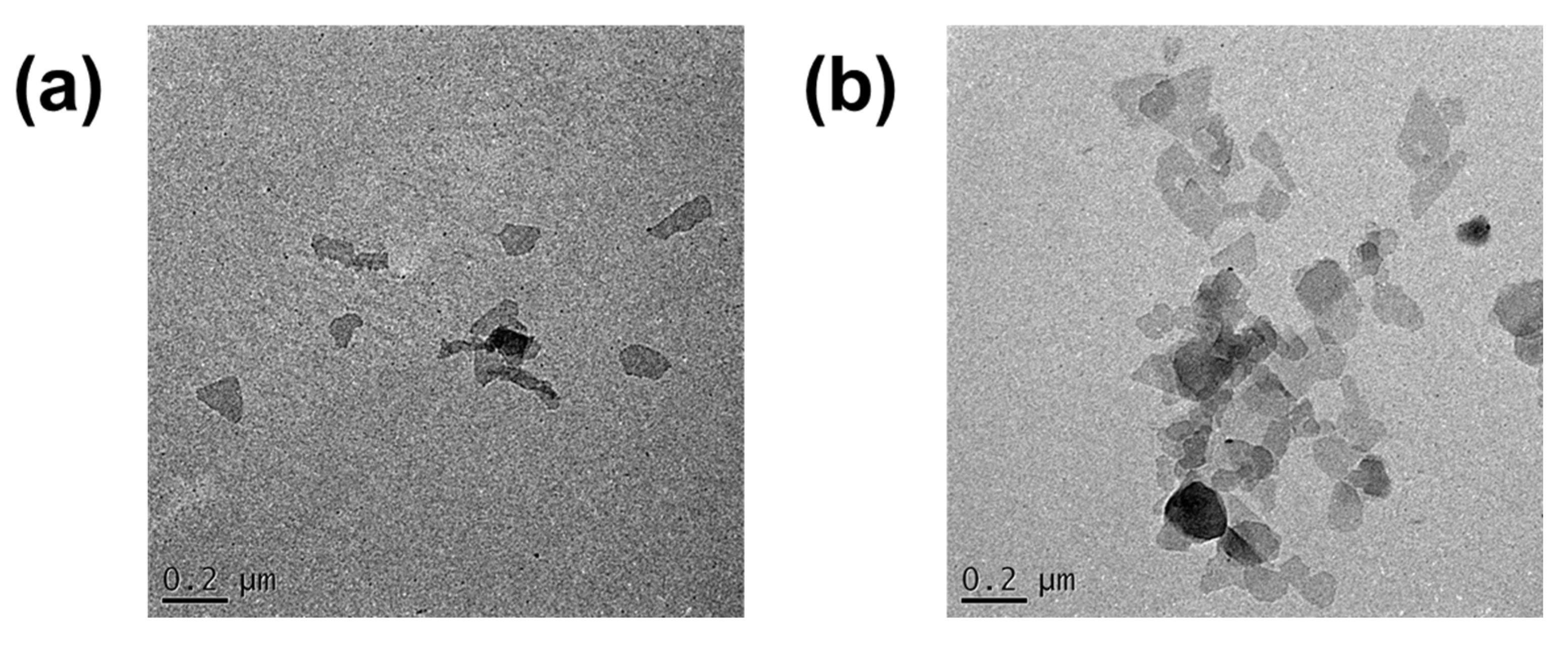
3.1. Characterization of Montmorillonite
3.2. Characterization of the Membranes
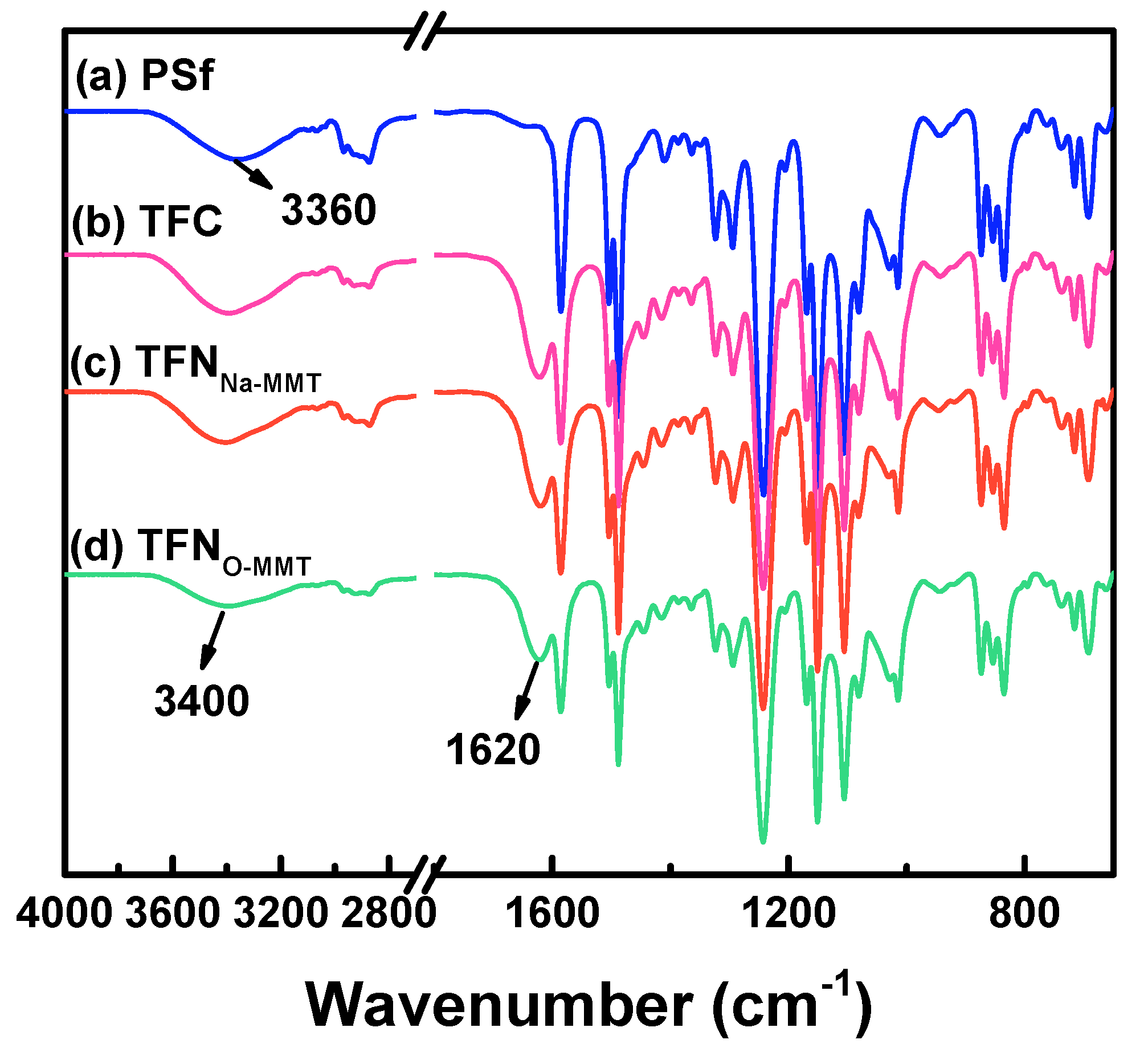
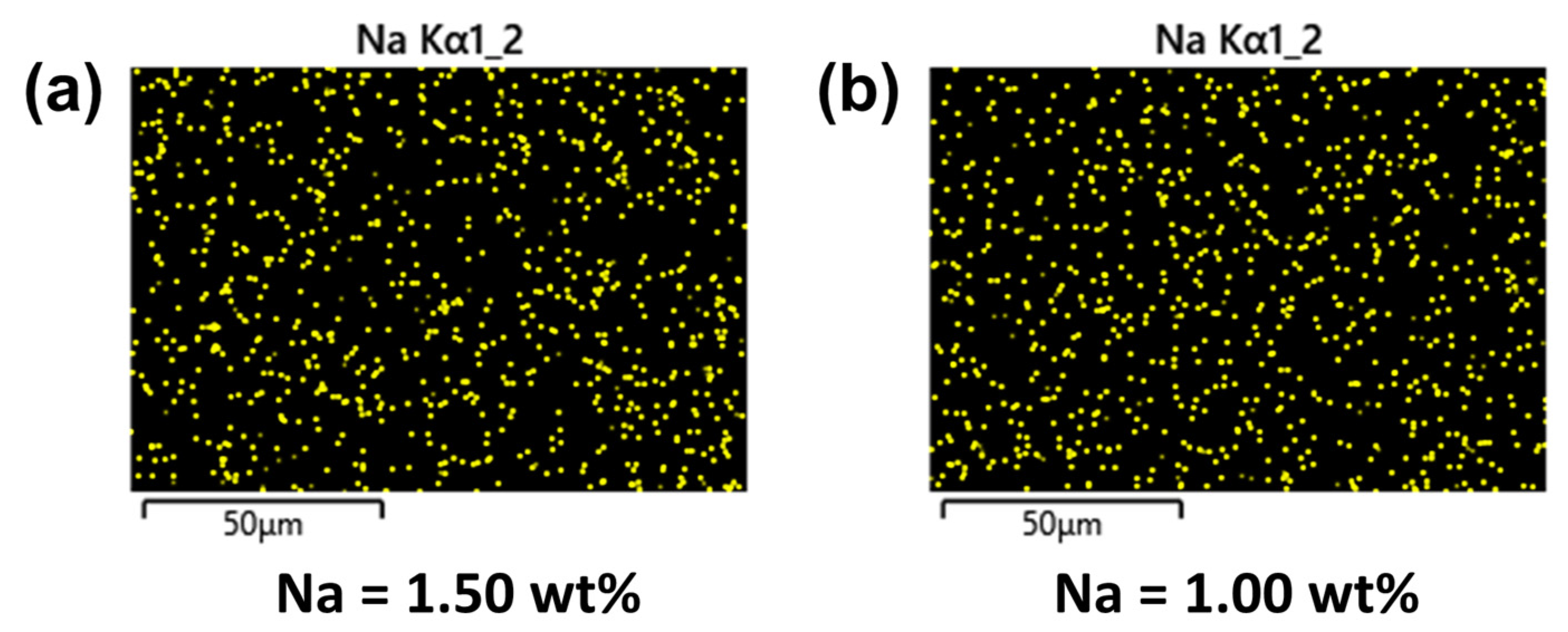
3.2.1. Surface Chemical Composition
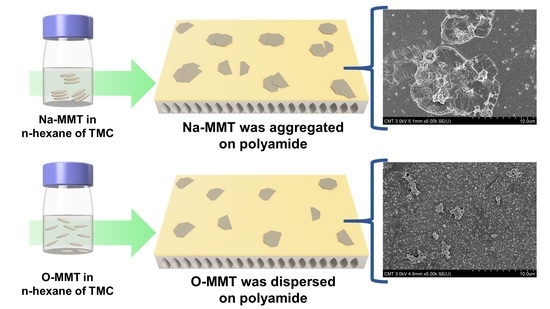
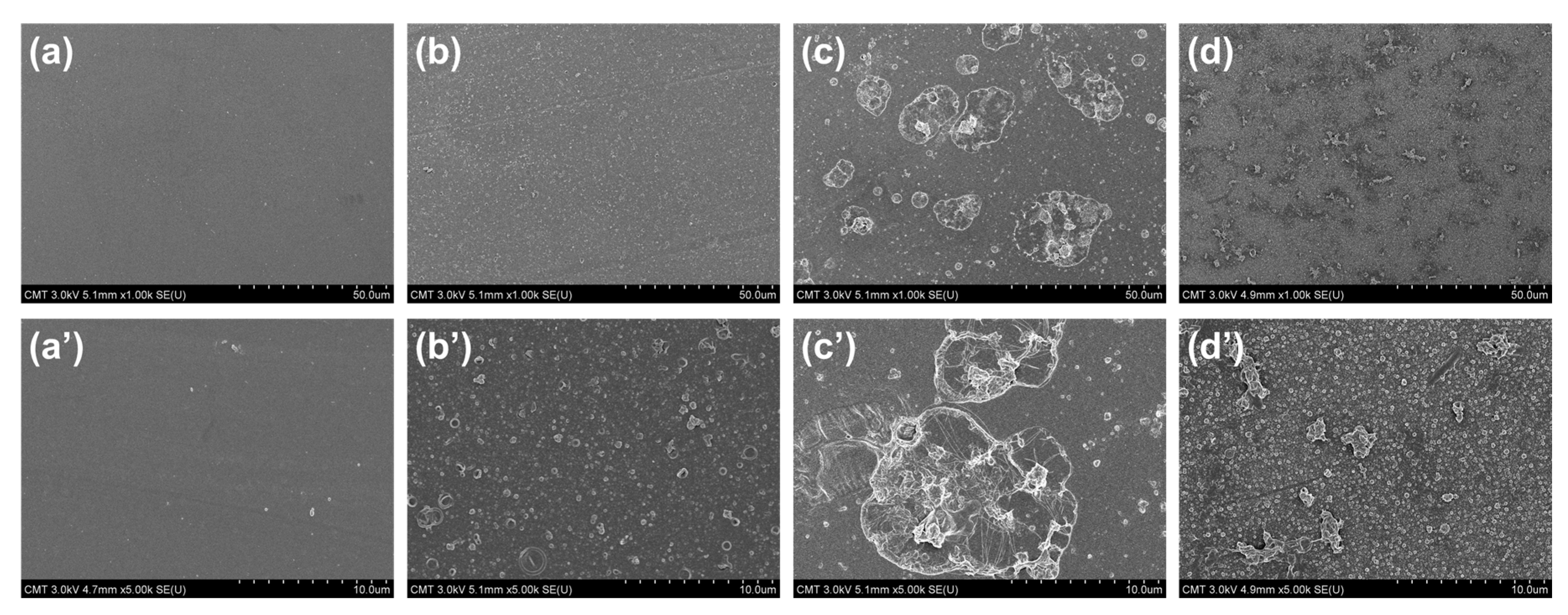
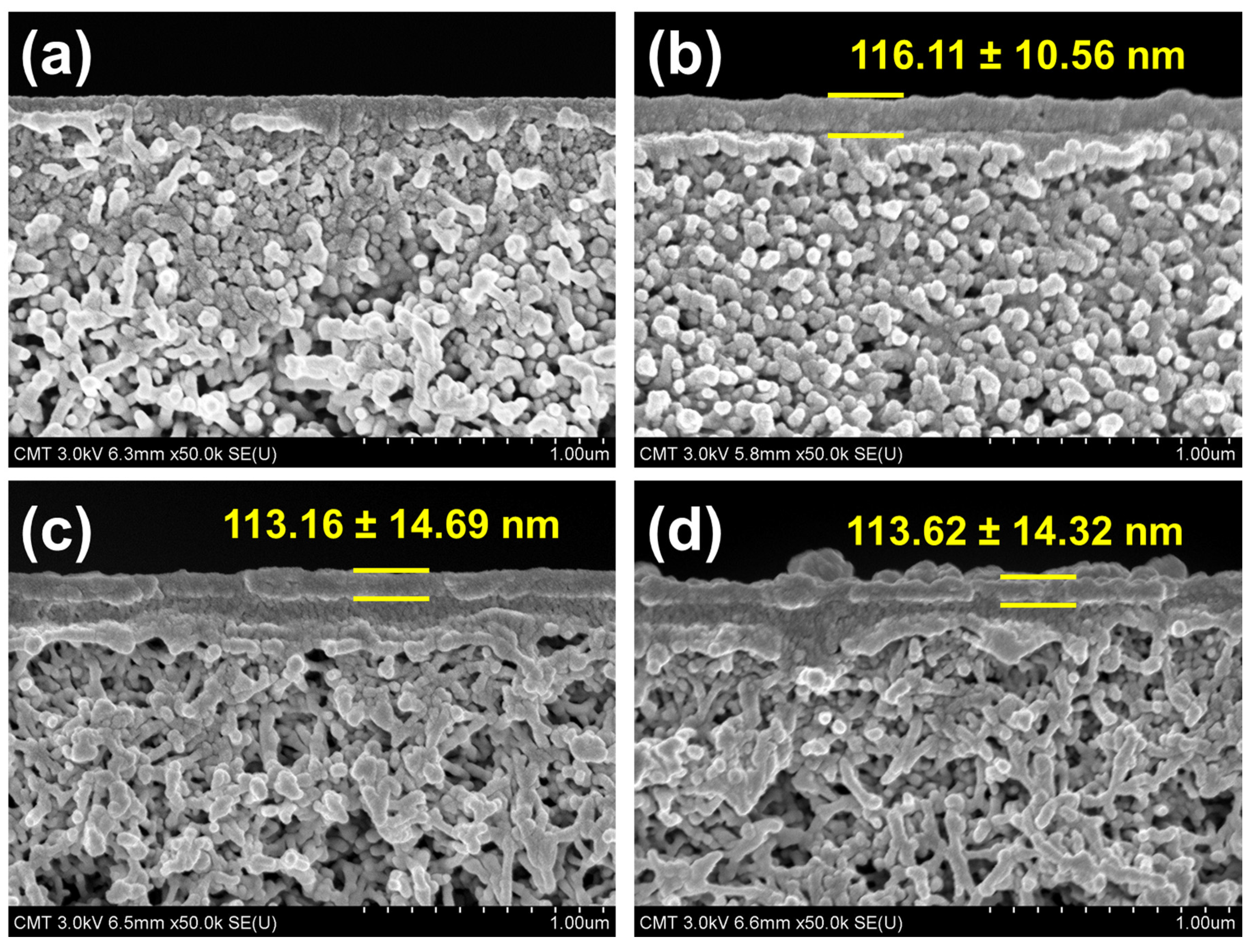
3.2.2. Membrane Morphology and Structure
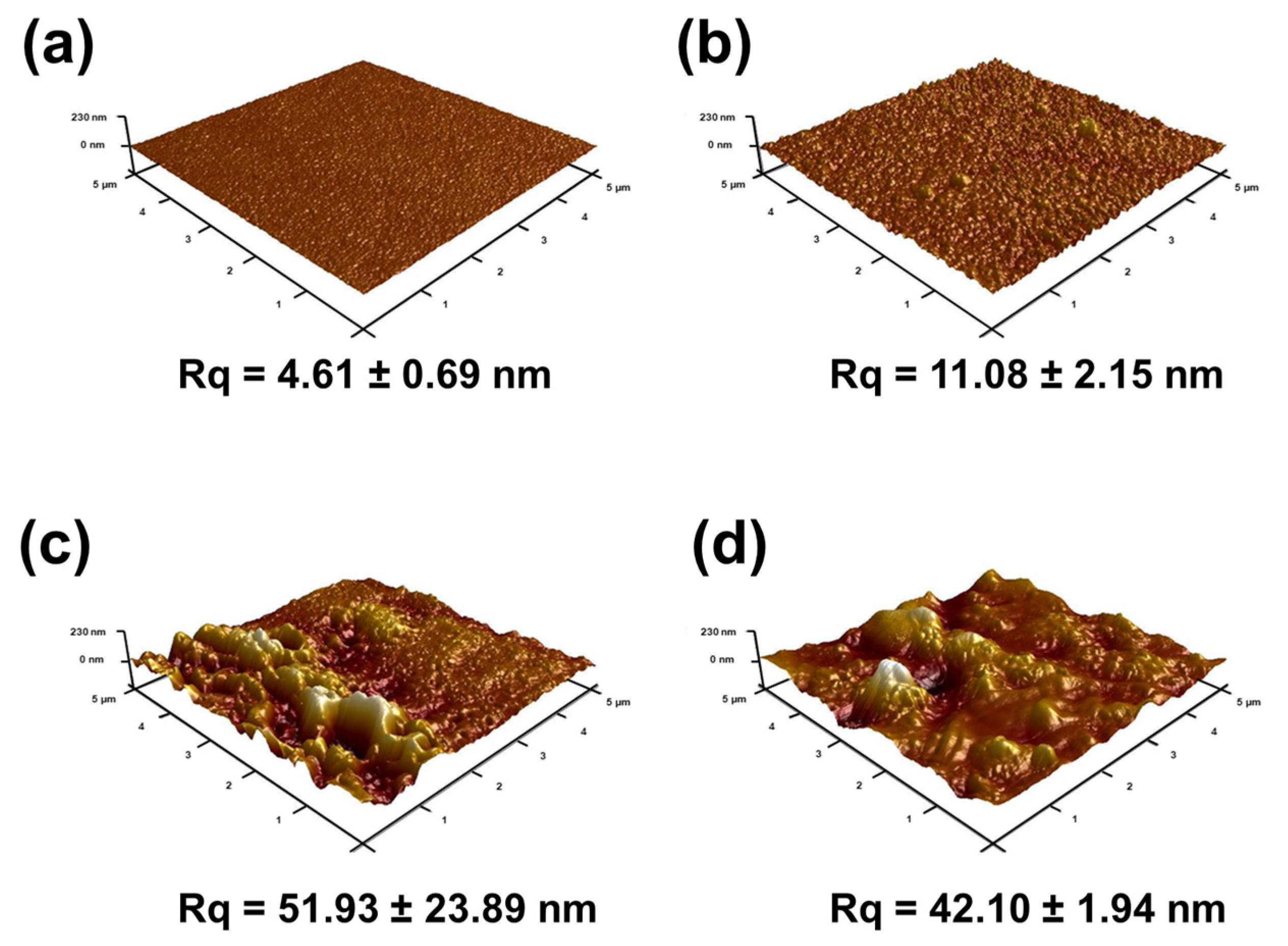
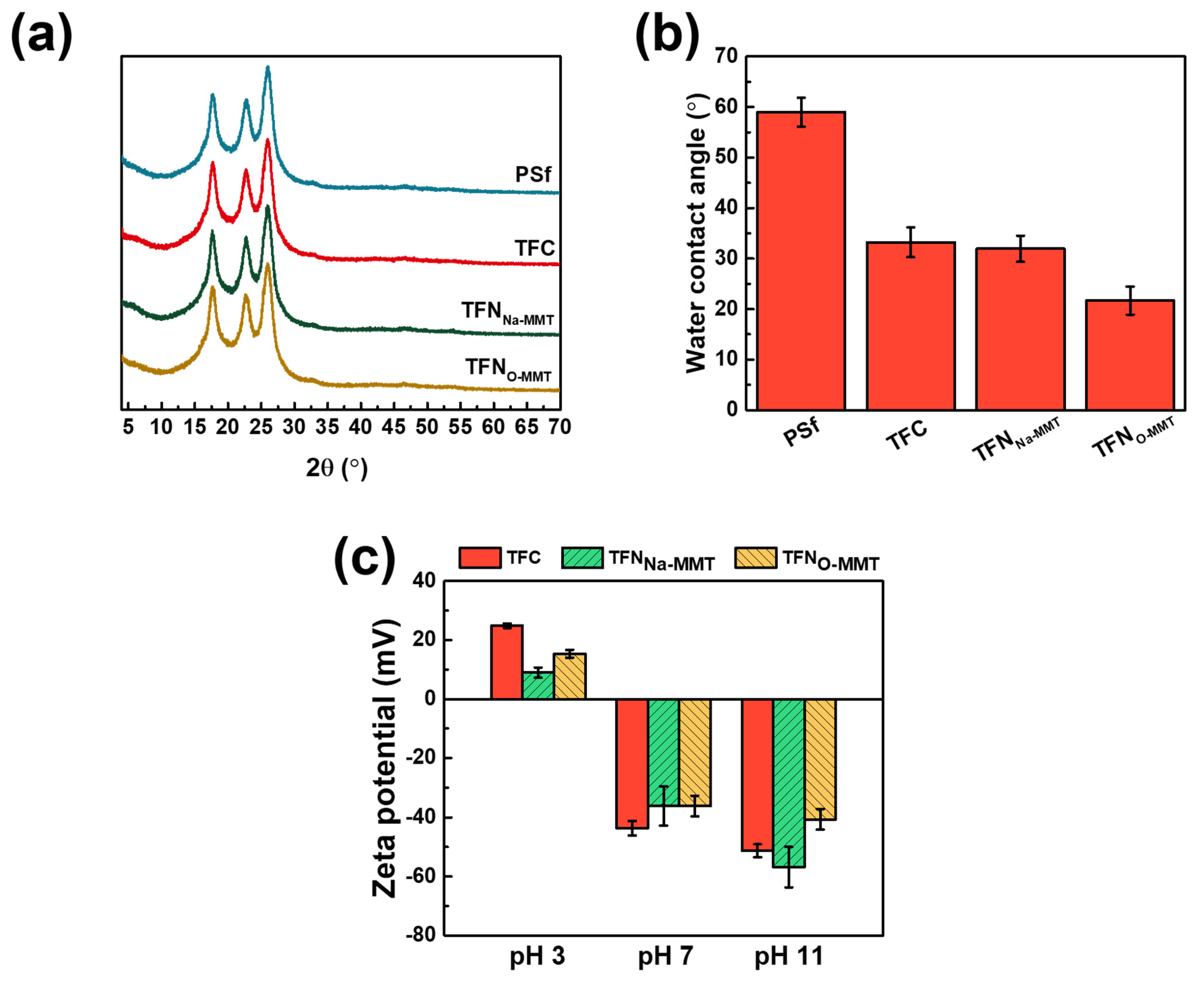
3.2.3. Crystallinity, Wettability and Surface Charge
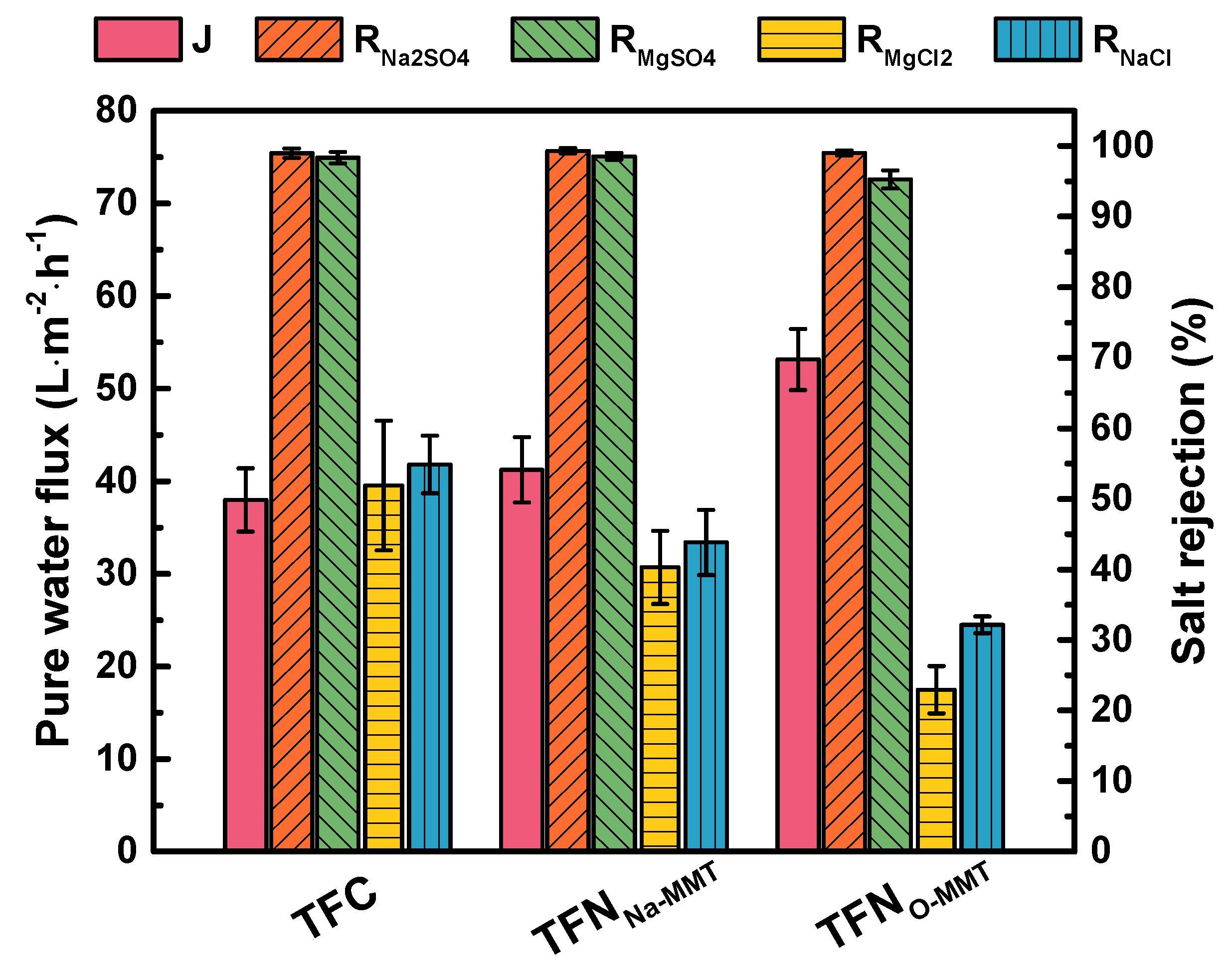
3.3. Nanofiltration Performance
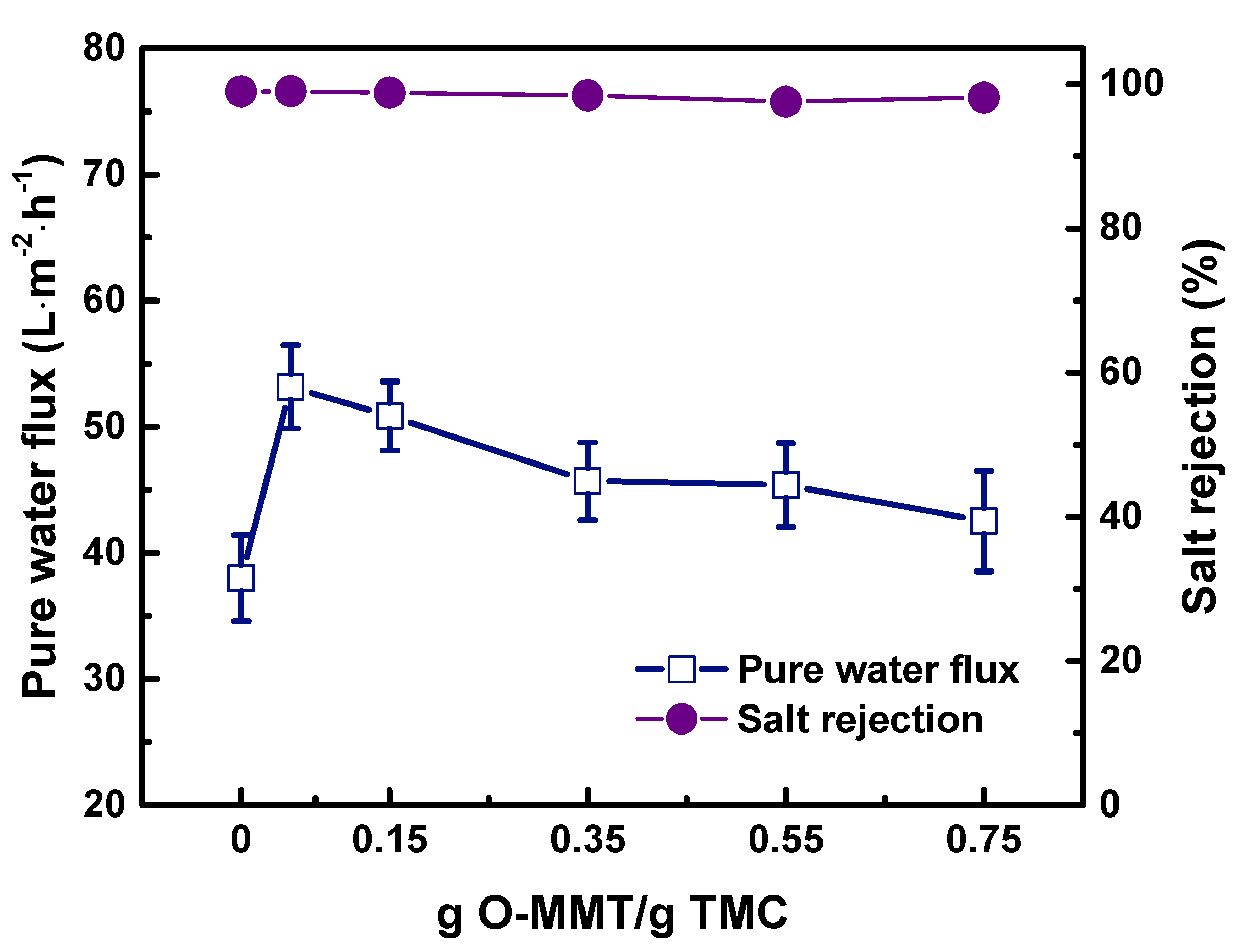
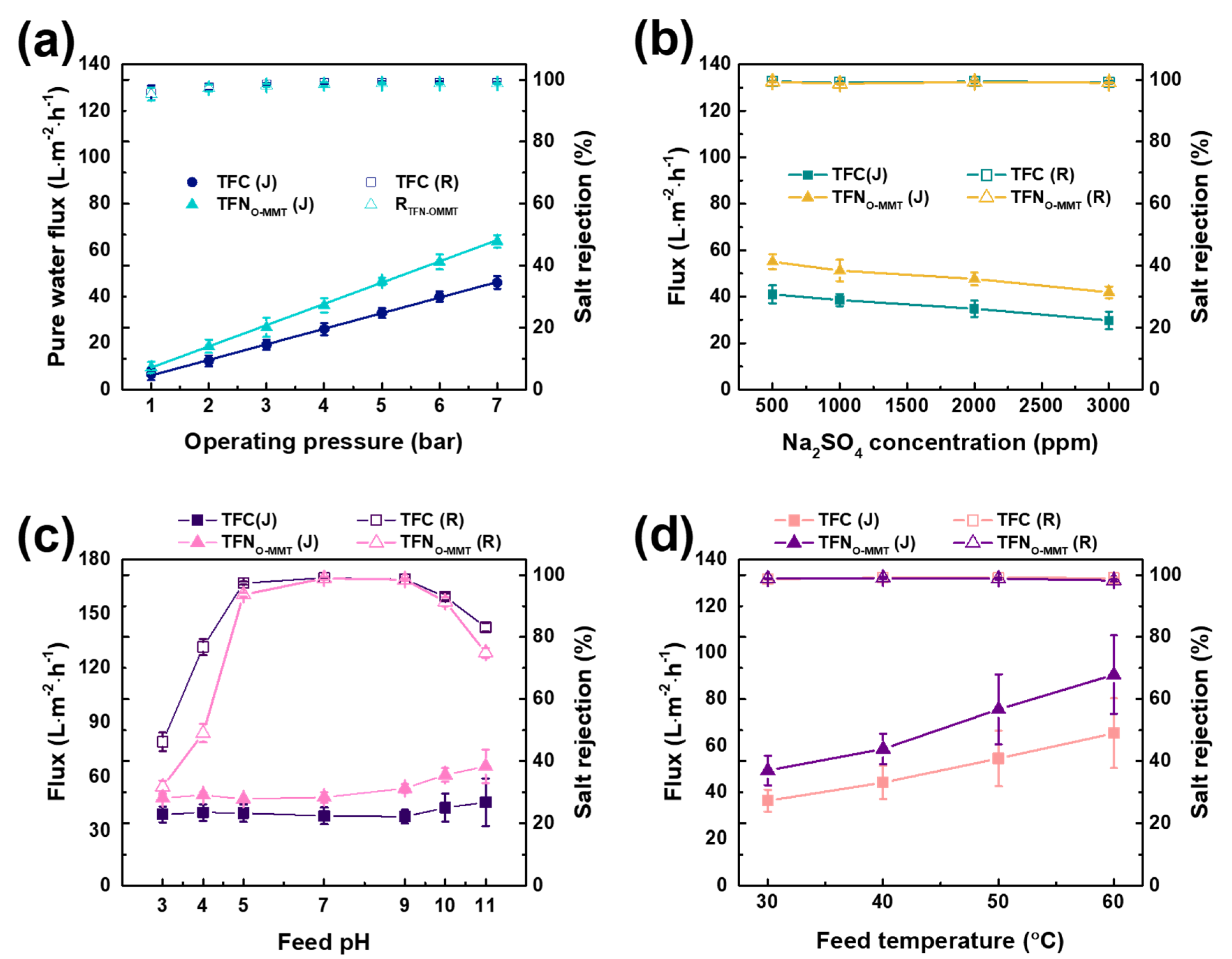
3.4. Operating Conditions
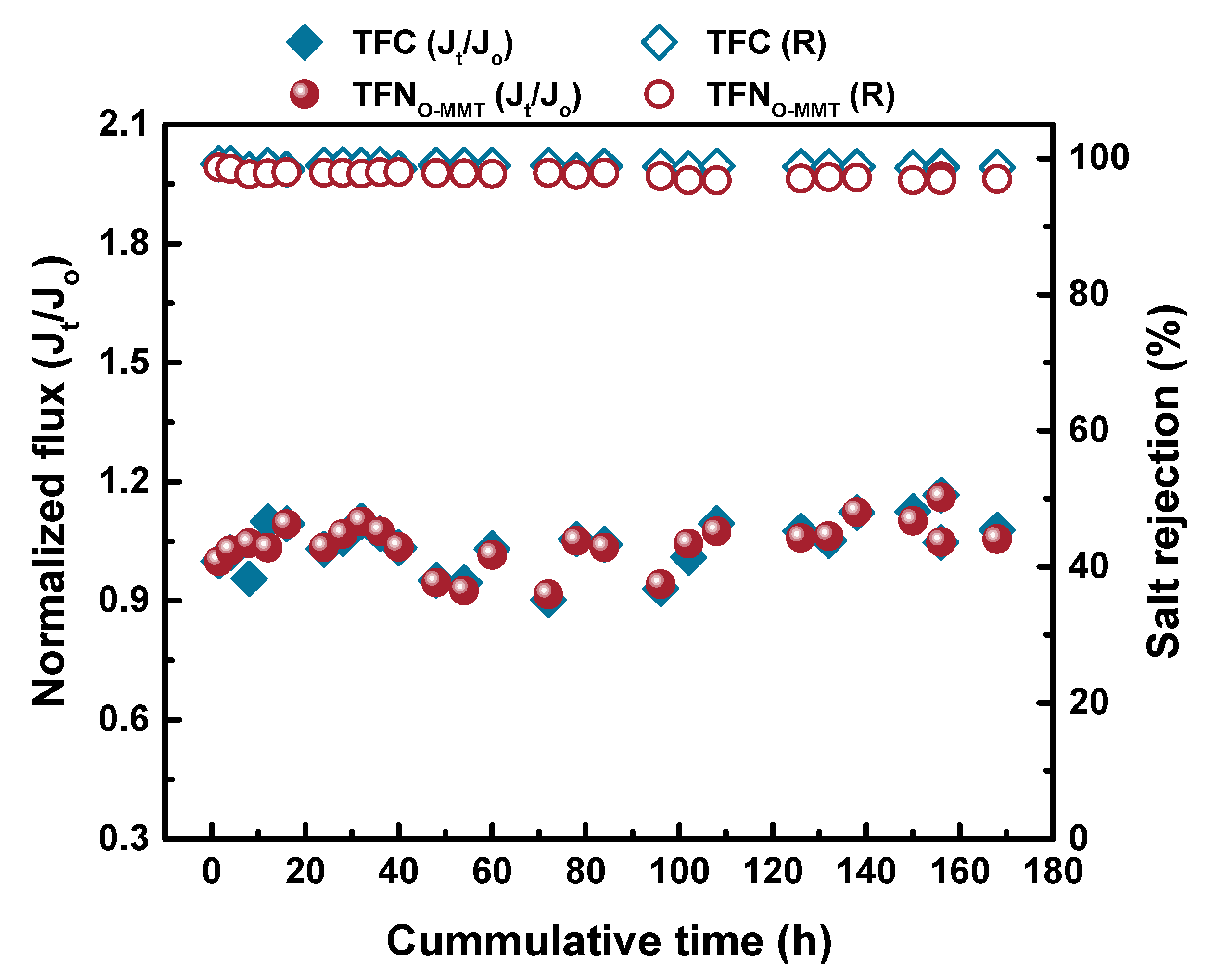
3.5. Antifouling Property and Stability of the Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheremisinoff, P.N. Handbook of Water and Wastewater Treatment Technology; Routledge: Abingdon, UK, 2018. [Google Scholar]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment; IWA Publishing: London, UK, 2016. [Google Scholar]
- Baker, R.; Cussler, E.; Eykamp, W.; Koros, W.; Riley, R.; Strathmann, H. Membrane Separation Systems-Recent Developments and Future Directions; William Andrew Inc.: Westwood, NJ, USA, 1991. [Google Scholar]
- Gin, D.L.; Noble, R.D. Chemistry. Designing the next generation of chemical separation membranes. Science 2011, 332, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Al-Najar, B.; Peters, C.D.; Albuflasa, H.; Hankins, N.P. Pressure and osmotically driven membrane processes: A review of the benefits and production of nano-enhanced membranes for desalination. Desalination 2020, 479, 114323. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Vourch, M.; Balannec, B.; Chaufer, B.; Dorange, G. Nanofiltration and reverse osmosis of model process waters fromthe dairy industry to produce water for reuse. Desalination 2005, 172, 245–256. [Google Scholar] [CrossRef]
- Zhou, D.; Zhu, L.; Fu, Y.; Zhu, M.; Xue, L. Development of lower cost seawater desalination processes using nanofiltration technologies—A review. Desalination 2015, 376, 109–116. [Google Scholar] [CrossRef]
- Diawara, C.K. Nanofiltration process efficiency in water desalination. Sep. Purif. Rev. 2008, 37, 302–324. [Google Scholar] [CrossRef]
- Salehi, F. Current and future applications for nanofiltration technology in the food processing. Food Bioprod. Process. 2014, 92, 161–177. [Google Scholar] [CrossRef]
- Warczok, J.; Ferrando, M.; Lopez, F.; Güell, C. Concentration of apple and pear juices by nanofiltration at low pressures. J. Food Eng. 2004, 63, 63–70. [Google Scholar] [CrossRef]
- Wu, M.; Sun, D.D. Characterization and reduction of membrane fouling during nanofiltration of semiconductor indium phosphide (inp) wastewater. J. Membr. Sci. 2005, 259, 135–144. [Google Scholar] [CrossRef]
- Wu, M. Effect of operating variables on rejection of indium using nanofiltration membranes. J. Membr. Sci. 2004, 240, 105–111. [Google Scholar] [CrossRef]
- Shams Ashaghi, K.; Ebrahimi, M.; Czermak, P.J.O.E.S. Ceramic ultra-and nanofiltration membranes for oilfield produced water treatment: A mini review. Open Environ. Sci. 2007, 1, 1–8. [Google Scholar] [CrossRef]
- Nair, R.R.; Protasova, E.; Bilstad, T.; Strand, S. Evaluation of nanofiltration membrane process for smartwater production in carbonate reservoirs from deoiled produced water and seawater. SPE Prod. Oper. 2019, 34, 409–420. [Google Scholar] [CrossRef]
- Foureaux, A.F.S.; Reis, E.O.; Lebron, Y.; Moreira, V.; Santos, L.V.; Amaral, M.S.; Lange, L.C. Rejection of pharmaceutical compounds from surface water by nanofiltration and reverse osmosis. Sep. Purif. Technol. 2019, 212, 171–179. [Google Scholar] [CrossRef]
- Licona, K.P.M.; Geaquinto, L.R.d.O.; Nicolini, J.V.; Figueiredo, N.G.; Chiapetta, S.C.; Habert, A.C.; Yokoyama, L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process Eng. 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Lau, W.-J.; Ismail, A.F. Polymeric nanofiltration membranes for textile dye wastewater treatment: Preparation, performance evaluation, transport modelling, and fouling control—A review. Desalination 2009, 245, 321–348. [Google Scholar] [CrossRef]
- Han, G.; Chung, T.S.; Weber, M.; Maletzko, C. Low-pressure nanofiltration hollow fiber membranes for effective fractionation of dyes and inorganic salts in textile wastewater. Environ. Sci. Technol. 2018, 52, 3676–3684. [Google Scholar] [CrossRef]
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Tul Muntha, S.; Kausar, A.; Siddiq, M. Advances in polymeric nanofiltration membrane: A review. Polym. Plast. Technol. Eng. 2016, 56, 841–856. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. A review on semi-aromatic polyamide tfc membranes prepared by interfacial polymerization: Potential for water treatment and desalination. Sep. Purif. Technol. 2017, 181, 159–182. [Google Scholar] [CrossRef]
- Ehsan Yakavalangi, M.; Rimaz, S.; Vatanpour, V. Effect of surface properties of polysulfone support on the performance of thin film composite polyamide reverse osmosis membranes. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E.M.V. Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Li, W.; Bian, C.; Fu, C.; Zhou, A.; Shi, C.; Zhang, J. A poly(amide-co-ester) nanofiltration membrane using monomers of glucose and trimesoyl chloride. J. Membr. Sci. 2016, 504, 185–195. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.-S. Design and synthesis of a fluoro-silane amine monomer for novel thin film composite membranes to dehydrate ethanol via pervaporation. J. Mater. Chem. A 2013, 1, 9814–9826. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Xu, Z.-L.; Xue, S.-M.; Wei, Y.-M.; Yang, H. A chlorine-tolerant nanofiltration membrane prepared by the mixed diamine monomers of pip and bhttm. J. Membr. Sci. 2016, 498, 374–384. [Google Scholar] [CrossRef]
- An, Q.-F.; Sun, W.-D.; Zhao, Q.; Ji, Y.-L.; Gao, C.-J. Study on a novel nanofiltration membrane prepared by interfacial polymerization with zwitterionic amine monomers. J. Membr. Sci. 2013, 431, 171–179. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Ang, M.B.M.Y.; Lai, C.-L.; Trilles, C.A.; Pereira, J.M.; Aquino, R.R.; Huang, S.-H.; Lee, K.-R. Choice of apposite dispersing medium for silica nanoparticles leading to their effective embedment in nanocomposite nanofiltration membranes. Ind. Eng. Chem. Res. 2019, 58, 17937–17944. [Google Scholar] [CrossRef]
- Kotlhao, K.; Lawal, I.A.; Moutloali, R.M.; Klink, M.J. Antifouling properties of silver-zinc oxide polyamide thin film composite membrane and rejection of 2-chlorophenol and 2,4-dichlorophenol. Membranes 2019, 9, 96. [Google Scholar] [CrossRef]
- Yin, J.; Kim, E.-S.; Yang, J.; Deng, B. Fabrication of a novel thin-film nanocomposite (tfn) membrane containing mcm-41 silica nanoparticles (nps) for water purification. J. Membr. Sci. 2012, 423–424, 238–246. [Google Scholar] [CrossRef]
- Liu, S.; Fang, F.; Wu, J.; Zhang, K. The anti-biofouling properties of thin-film composite nanofiltration membranes grafted with biogenic silver nanoparticles. Desalination 2015, 375, 121–128. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Kim, S.-J.; Lee, K.-H. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. A 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Graphene oxide (go) enhanced polyamide (pa) thin-film nanocomposite (tfn) membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Lind, M.L.; Ghosh, A.K.; Jawor, A.; Huang, X.; Hou, W.; Yang, Y.; Hoek, E.M. Influence of zeolite crystal size on zeolite-polyamide thin film nanocomposite membranes. Langmuir 2009, 25, 10139–10145. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, B.; Hu, X.; Nair, S.; Chen, Y. Thin film nanocomposite membrane containing zeolitic imidazolate framework-8 via interfacial polymerization for highly permeable nanofiltration. J. Taiwan Inst. Chem. Eng. 2017, 83, 159–167. [Google Scholar] [CrossRef]
- Li, N.; Yu, L.; Xiao, Z.; Jiang, C.; Gao, B.; Wang, Z. Biofouling mitigation effect of thin film nanocomposite membranes immobilized with laponite mediated metal ions. Desalination 2020, 473, 114162. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, D.L.; Chung, T.S. Nanoclays-incorporated thin-film nanocomposite membranes for reverse osmosis desalination. Adv. Mater. Interfaces 2020, 7, 1902108. [Google Scholar] [CrossRef]
- Kadhom, M.; Deng, B. Thin film nanocomposite membranes filled with bentonite nanoparticles for brackish water desalination: A novel water uptake concept. Microporous Mesoporous Mater. 2019, 279, 82–91. [Google Scholar] [CrossRef]
- Maalige, N.R.; Aruchamy, K.; Mahto, A.; Sharma, V.; Deepika, D.; Mondal, D.; Nataraj, S.K. Low operating pressure nanofiltration membrane with functionalized natural nanoclay as antifouling and flux promoting agent. Chem. Eng. J. 2019, 358, 821–830. [Google Scholar] [CrossRef]
- Tajuddin, M.H.; Yusof, N.; Wan Azelee, I.; Wan Salleh, W.N.; Ismail, A.F.; Jaafar, J.; Aziz, F.; Nagai, K.; Razali, N.F. Development of copper-aluminum layered double hydroxide in thin film nanocomposite nanofiltration membrane for water purification process. Front. Chem. 2019, 7, 3. [Google Scholar] [CrossRef]
- Tajuddin, M.H.; Yusof, N.; Abdullah, N.; Abidin, M.N.Z.; Salleh, W.N.W.; Ismail, A.F.; Matsuura, T.; Hairom, N.H.H.; Misdan, N. Incorporation of layered double hydroxide nanofillers in polyamide nanofiltration membrane for high performance of salts rejections. J. Taiwan Inst. Chem. Eng. 2019, 97, 1–11. [Google Scholar] [CrossRef]
- Dong, H.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Clay nanosheets as charged filler materials for high-performance and fouling-resistant thin film nanocomposite membranes. J. Membr. Sci. 2015, 494, 92–103. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Tien, H.N.; Khivantsev, K.; Song, Z.; Zhou, F.; Yu, M. Polyamide/nitrogen-doped graphene oxide quantum dots (n-goqd) thin film nanocomposite reverse osmosis membranes for high flux desalination. Desalination 2019, 451, 125–132. [Google Scholar] [CrossRef]
- Sun, H.; Wu, P. Tuning the functional groups of carbon quantum dots in thin film nanocomposite membranes for nanofiltration. J. Membr. Sci. 2018, 564, 394–403. [Google Scholar] [CrossRef]
- Kim, E.-S.; Hwang, G.; Gamal El-Din, M.; Liu, Y. Development of nanosilver and multi-walled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394–395, 37–48. [Google Scholar] [CrossRef]
- Zarrabi, H.; Yekavalangi, M.E.; Vatanpour, V.; Shockravi, A.; Safarpour, M. Improvement in desalination performance of thin film nanocomposite nanofiltration membrane using amine-functionalized multiwalled carbon nanotube. Desalination 2016, 394, 83–90. [Google Scholar] [CrossRef]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A.F. A review on polyamide thin film nanocomposite (tfn) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-H.; Hoek, E.M.V.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Buruga, K.; Song, H.; Shang, J.; Bolan, N.; Jagannathan, T.K.; Kim, K.H. A review on functional polymer-clay based nanocomposite membranes for treatment of water. J. Hazard. Mater. 2019, 379, 120584. [Google Scholar] [CrossRef]
- Sanyal, O.; Liu, Z.; Yu, J.; Meharg, B.M.; Hong, J.S.; Liao, W.; Lee, I. Designing fouling-resistant clay-embedded polyelectrolyte multilayer membranes for wastewater effluent treatment. J. Membr. Sci. 2016, 512, 21–28. [Google Scholar] [CrossRef]
- Dlamini, D.S.; Li, J.; Mamba, B.B. Critical review of montmorillonite/polymer mixed-matrix filtration membranes: Possibilities and challenges. Appl. Clay Sci. 2019, 168, 21–30. [Google Scholar] [CrossRef]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- 231, E.H.C. Bentonite, Kaloline, and Selected clay Minerals. Available online: www.inchem.org (accessed on 20 March 2020).
- Brigatti, M.F.; Galan, E.; Theng, B. Structures and mineralogy of clay minerals. Dev. Clay Sci. 2006, 1, 19–86. [Google Scholar]
- Basilia, B.A.; Mendoza, H.D.; Cada, L.G. Synthesis and characterization of rpet/organomontmorillonite nanocomposites. Philipp. Eng. J. 2002, 23, 19–34. [Google Scholar]
- Inglethorpe, S.; Morgan, D.; Highley, D.; Bloodworth, J. Industrial minerals laboratory manual. In British Geological Survey Technical Report; WG/93/20, Mineralogy and Petrology Series; Natural Environment Research Council: Nottingham, UK, 1993. [Google Scholar]
- Favre, H.; Lagaly, G. Organo-bentonites with quaternary alkylammonium ions. Clay Miner. 1991, 26, 19–32. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Trilles, C.A.; De Guzman, M.R.; Pereira, J.M.; Aquino, R.R.; Huang, S.-H.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Improved performance of thin-film nanocomposite nanofiltration membranes as induced by embedded polydopamine-coated silica nanoparticles. Sep. Purif. Technol. 2019, 224, 113–120. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, H.; Wu, H.; Zhang, Z.; Liao, L. Influence of the surfactants’ nature on the structure and rheology of organo-montmorillonite in oil-based drilling fluids. Appl. Clay Sci. 2017, 135, 244–252. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, D.; Zhang, H.; Lu, S.; Chen, L.; Yu, X. Impact of environmental conditions on the sorption behavior of pb(ii) in na-bentonite suspensions. J. Hazard. Mater. 2010, 183, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Motawie, A.M.; Madany, M.M.; El-Dakrory, A.Z.; Osman, H.M.; Ismail, E.A.; Badr, M.M.; El-Komy, D.A.; Abulyazied, D.E. Physico-chemical characteristics of nano-organo bentonite prepared using different organo-modifiers. Egypt. J. Pet. 2014, 23, 331–338. [Google Scholar] [CrossRef]
- Tan, X.L.; Hu, J.; Zhou, X.; Yu, S.M.; Wang, X.K. Characterization of lin‘an montmorillonite and its application in the removal of ni2+ from aqueous solutions. Radiochim. Acta 2008, 96, 487–495. [Google Scholar] [CrossRef]
- Atia, A. Adsorption of chromate and molybdate by cetylpyridinium bentonite. Appl. Clay Sci. 2008, 41, 73–84. [Google Scholar] [CrossRef]
- Ahmad, M.B.; Gharayebi, Y.; Salit, M.S.; Hussein, M.Z.; Shameli, K. Comparison of in situ polymerization and solution-dispersion techniques in the preparation of polyimide/montmorillonite (mmt) nanocomposites. Int. J. Mol. Sci. 2011, 12, 6040–6050. [Google Scholar] [CrossRef] [PubMed]
- Lagaly, G. Interaction of alkylamines with different types of layered compounds. Solid State Ionics 1986, 22, 43–51. [Google Scholar] [CrossRef]
- Ltifi, I.; Ayari, F.; Chehimi, D.B.H.; Ayadi, M.T. Physicochemical characteristics of organophilic clays prepared using two organo-modifiers: Alkylammonium cation arrangement models. Appl. Water Sci. 2018, 8, 91. [Google Scholar] [CrossRef]
- Brum, M.C.; Capitaneo, J.L.; Oliveira, J.F. Removal of hexavalent chromium from water by adsorption onto surfactant modified montmorillonite. Miner. Eng. 2010, 23, 270–272. [Google Scholar] [CrossRef]
- Marras, S.I.; Tsimpliaraki, A.; Zuburtikudis, I.; Panayiotou, C. Thermal and colloidal behavior of amine-treated clays: The role of amphiphilic organic cation concentration. J. Colloid Interface Sci. 2007, 315, 520–527. [Google Scholar] [CrossRef]
- Kwon, Y.; Leckie, J. Hypochlorite degradation of crosslinked polyamide membranesii. Changes in hydrogen bonding behavior and performance. J. Membr. Sci. 2006, 282, 456–464. [Google Scholar] [CrossRef]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide ro and nf membranes. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Lau, V.J.; Ji, Y.L.; Huang, S.H.; An, Q.F.; Caparanga, A.R.; Tsai, H.A.; Hung, W.S.; Hu, C.C.; Lee, K.R.; et al. Correlating psf support physicochemical properties with the formation of piperazine-based polyamide and evaluating the resultant nanofiltration membrane performance. Polymers 2017, 9, 505. [Google Scholar] [CrossRef]
- Xiang, J.; Xie, Z.; Hoang, M.; Zhang, K. Effect of amine salt surfactants on the performance of thin film composite poly(piperazine-amide) nanofiltration membranes. Desalination 2013, 315, 156–163. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Zhang, X. Preparation and characterization of poly(piperazineamide) composite nanofiltration membrane by interfacial polymerization of 3,3′,5,5′-biphenyl tetraacyl chloride and piperazine. J. Membr. Sci. 2009, 335, 133–139. [Google Scholar] [CrossRef]
- Meihong, L.; Sanchuan, Y.; Yong, Z.; Congjie, G. Study on the thin-film composite nanofiltration membrane for the removal of sulfate from concentrated salt aqueous: Preparation and performance. J. Membr. Sci. 2008, 310, 289–295. [Google Scholar] [CrossRef]
- BA, F.; AE, Y. Removal of cationic dye (basic red 18) from aqueous solution using natural turkish clay. Glob. Nest J. 2013, 15, 529–541. [Google Scholar]
- Liu, H.; Zhang, M.; Zhao, H.; Jiang, Y.; Liu, G.; Gao, J. Enhanced dispersibility of metal–organic frameworks (mofs) in the organic phase via surface modification for tfn nanofiltration membrane preparation. RSC Adv. 2020, 10, 4045–4057. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K. Few-layers mos2 nanosheets modified thin film composite nanofiltration membranes with improved separation performance. J. Membr. Sci. 2020, 595, 117526. [Google Scholar] [CrossRef]
- Hu, J.; Lv, Z.; Xu, Y.; Zhang, X.; Wang, L. Fabrication of a high-flux sulfonated polyamide nanofiltration membrane: Experimental and dissipative particle dynamics studies. J. Membr. Sci. 2016, 505, 119–129. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Yaroshchuk, A. Non-steric mechanisms of nanofiltration: Superposition of donnan and dielectric exclusion. Sep. Purif. Technol. 2001, 22–23, 143–158. [Google Scholar] [CrossRef]
- Spiegler, K.S.; Kedem, O. Thermodynamics of hyperfiltration (reverse osmosis): Criteria for efficient membranes. Desalination 1966, 1, 311–326. [Google Scholar] [CrossRef]
- García-Martín, N.; Silva, V.; Carmona, F.J.; Palacio, L.; Hernández, A.; Prádanos, P. Pore size analysis from retention of neutral solutes through nanofiltration membranes. The contribution of concentration–polarization. Desalination 2014, 344, 1–11. [Google Scholar] [CrossRef]
- Lu, P.; Liang, S.; Zhou, T.; Xue, T.; Mei, X.; Wang, Q. Layered double hydroxide nanoparticle modified forward osmosis membranes via polydopamine immobilization with significantly enhanced chlorine and fouling resistance. Desalination 2017, 421, 99–109. [Google Scholar] [CrossRef]
- Lu, P.; Li, W.; Yang, S.; Liu, Y.; Wang, Q.; Li, Y. Layered double hydroxide-modified thin–film composite membranes with remarkably enhanced chlorine resistance and anti-fouling capacity. Sep. Purif. Technol. 2019, 220, 231–237. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Ji, Y.L.; Huang, S.H.; Lee, K.R.; Lai, J.Y. A facile and versatile strategy for fabricating thin-film nanocomposite membranes with polydopamine-piperazine nanoparticles generated in situ. J. Membr. Sci. 2019, 579, 79–89. [Google Scholar] [CrossRef]
- Shen, L.; Feng, S.; Li, J.; Chen, J.; Li, F.; Lin, H.; Yu, G. Surface modification of polyvinylidene fluoride (pvdf) membrane via radiation grafting: Novel mechanisms underlying the interesting enhanced membrane performance. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]

| C (%) | O (%) | N (%) | Other Elements (%) a | |
|---|---|---|---|---|
| TFC | 70.3 | 16.9 | 12.9 | |
| TFNNA-MMT | 74.8 | 14.5 | 7.64 | 3.02 |
| TFNO-MMT | 71.3 | 16.6 | 8.69 | 3.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ang, M.B.M.Y.; Deang, A.B.G.; Aquino, R.R.; Basilia, B.A.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Assessing the Performance of Thin-Film Nanofiltration Membranes with Embedded Montmorillonites. Membranes 2020, 10, 79. https://doi.org/10.3390/membranes10050079
Ang MBMY, Deang ABG, Aquino RR, Basilia BA, Huang S-H, Lee K-R, Lai J-Y. Assessing the Performance of Thin-Film Nanofiltration Membranes with Embedded Montmorillonites. Membranes. 2020; 10(5):79. https://doi.org/10.3390/membranes10050079
Chicago/Turabian StyleAng, Micah Belle Marie Yap, Amira Beatriz Gaces Deang, Ruth R. Aquino, Blessie A. Basilia, Shu-Hsien Huang, Kueir-Rarn Lee, and Juin-Yih Lai. 2020. "Assessing the Performance of Thin-Film Nanofiltration Membranes with Embedded Montmorillonites" Membranes 10, no. 5: 79. https://doi.org/10.3390/membranes10050079
APA StyleAng, M. B. M. Y., Deang, A. B. G., Aquino, R. R., Basilia, B. A., Huang, S.-H., Lee, K.-R., & Lai, J.-Y. (2020). Assessing the Performance of Thin-Film Nanofiltration Membranes with Embedded Montmorillonites. Membranes, 10(5), 79. https://doi.org/10.3390/membranes10050079