Minimum Net Driving Temperature Concept for Membrane Distillation
Abstract
1. Introduction
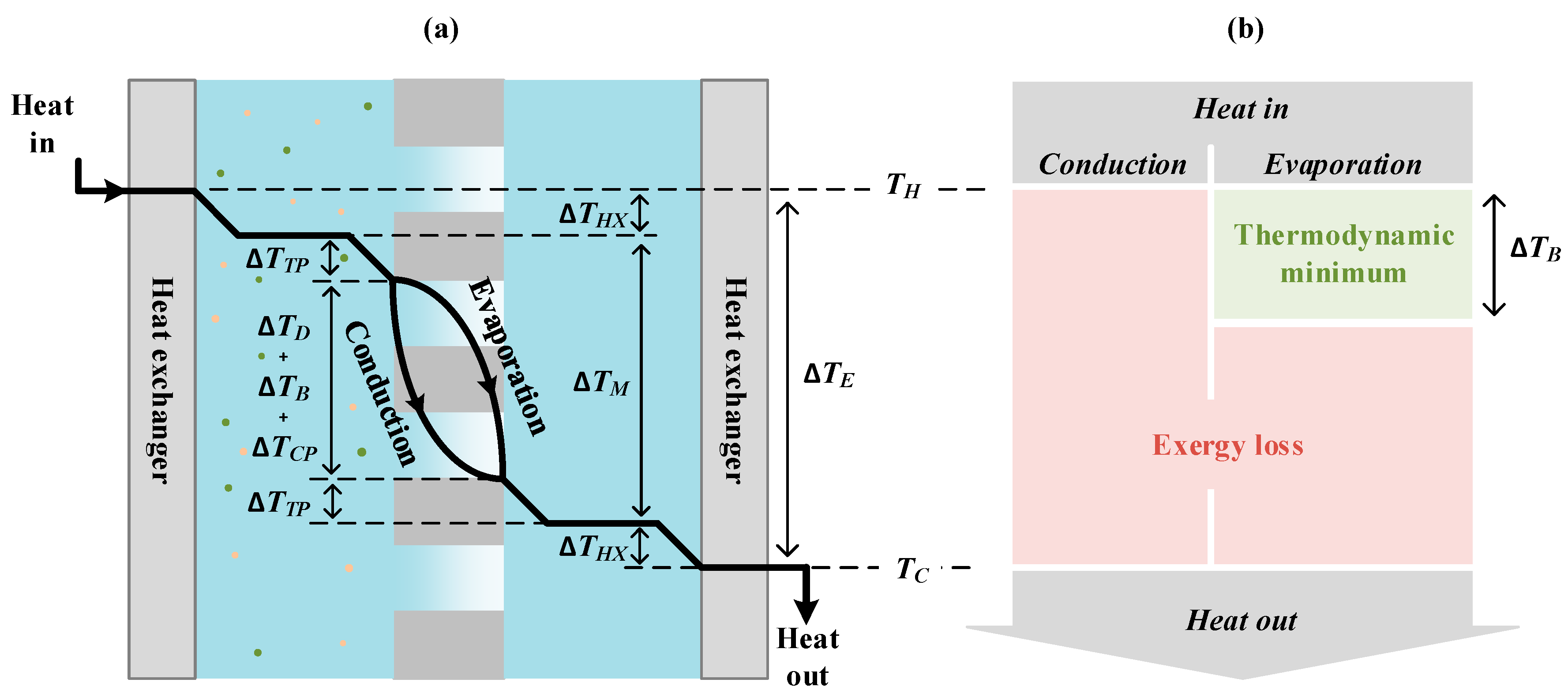
2. Theoretical Background
2.1. Driving Force Efficiency
2.2. Evaporation Efficiency
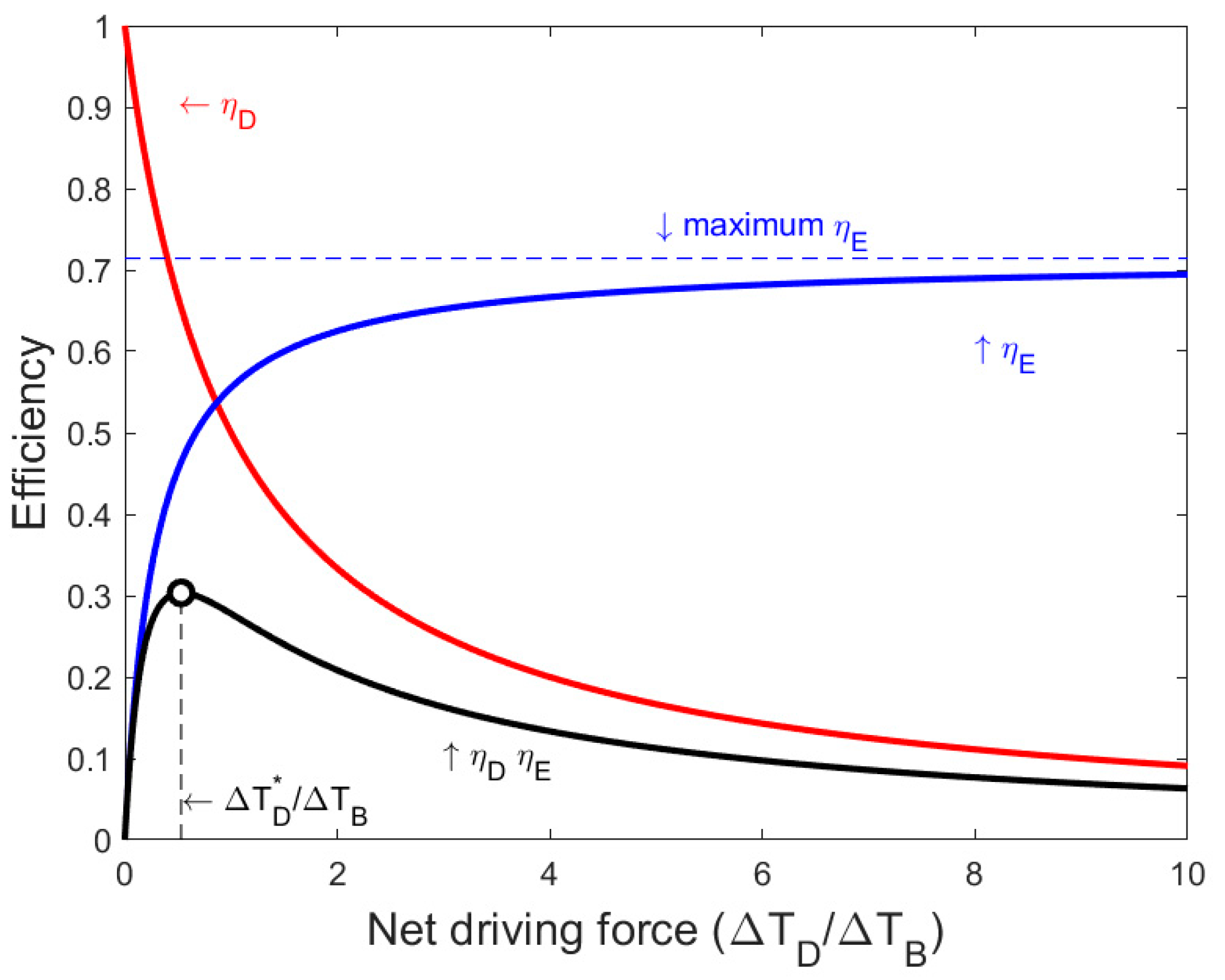
2.3. Net Driving Temperature Difference Maximizing Thermal Efficiency
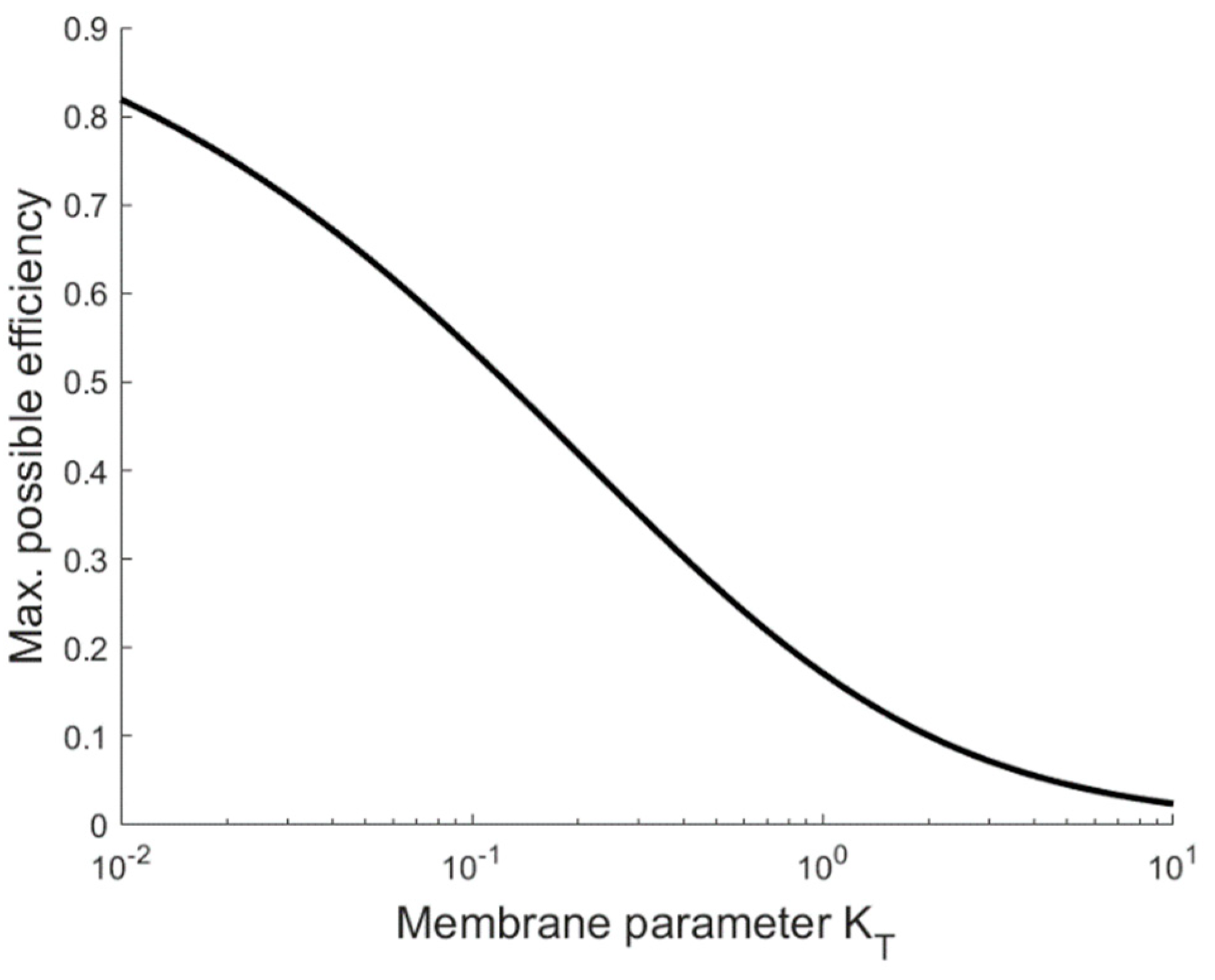
3. Results
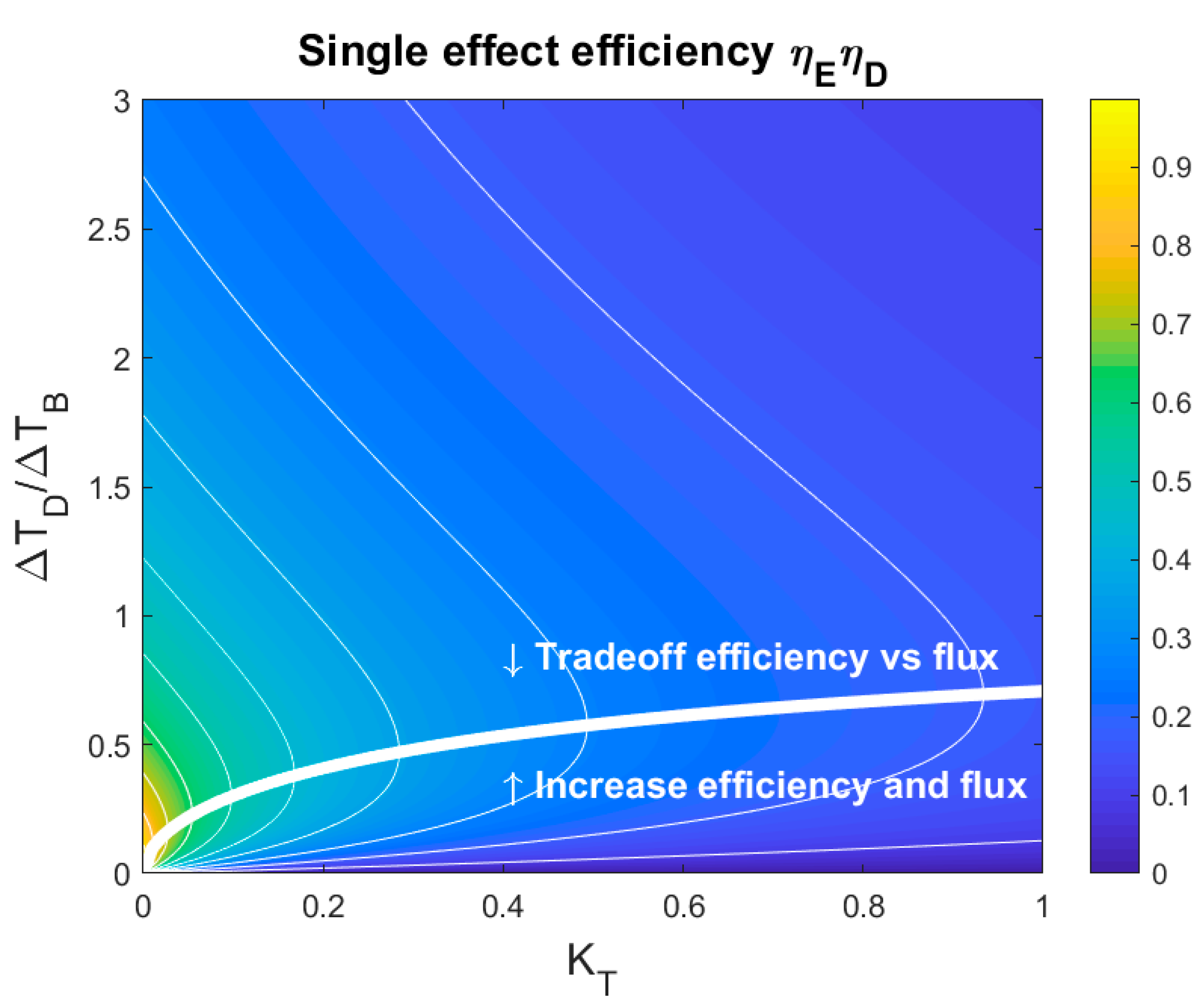
3.1. Minimum Net Driving Temperature Difference for a Single Effect
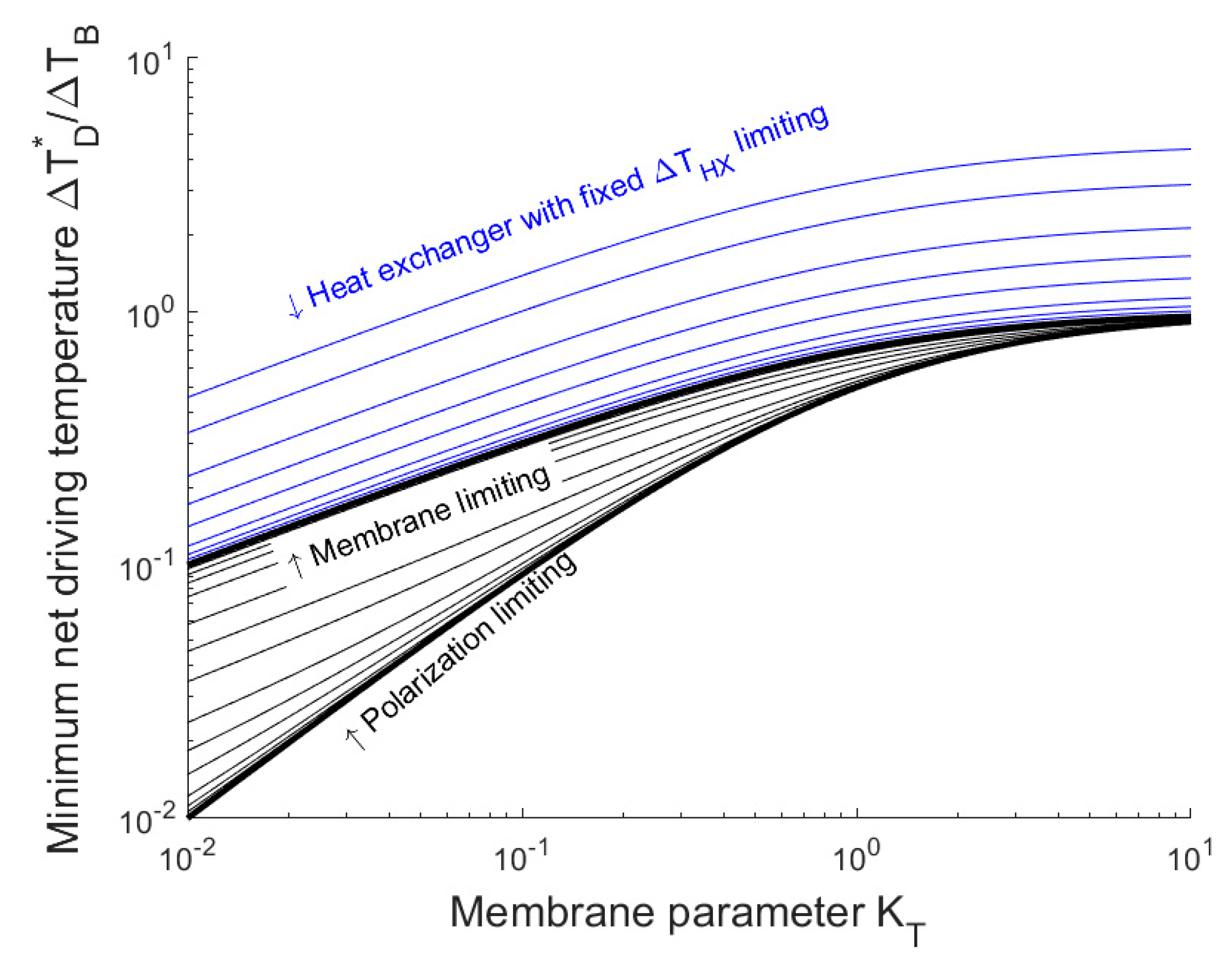
3.2. Influence of Heat Exchanger and Temperature Polarization
3.3. Exergy Efficiency Limitation
4. Discussion
4.1. Implications for Membrane Properties
4.2. Implicit Driving Temperature Variation
4.3. Extension to More Complex Systems
5. Conclusions
- There is a non-zero net driving temperature difference that maximizes the thermodynamic efficiency of a single effect. Below this temperature difference, both the productivity and exergy consumption can improve by increasing the net driving temperature difference. Thus, this is the minimum net driving temperature difference that should be chosen for a rational operational strategy;
- In experiments where a particular parameter is varied while others remain fixed, the net driving temperature can be consequently varied. The minimum net driving temperature will then correspond to an “optimal” value of another parameter. Thus, we should be aware that, in these cases, the optimal value of the investigated parameter depends on the choice of the other fixed parameters;
- The minimum net driving temperature is proportional to the boiling point elevation (salinity) and is fully determined from three dimensionless constants, which are the ratio of heat transfer coefficients vs. the membrane permeability. Therefore, the treatment of very saline water is relatively more attractive, as this allows a reasonable driving force without incurring a substantial efficiency penalty.
Author Contributions
Funding
Conflicts of Interest
References
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Kiss, A.A.; Readi, O.M.K. An industrial perspective on membrane distillation processes. J. Chem. Technol. Biotechnol. 2018, 93, 2047–2055. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsingerab, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Alsaadi, A.S.; Francis, L.; Maab, H.; Amy, G.L.; Ghaffour, N. Evaluation of air gap membrane distillation process running under sub-atmospheric conditions: Experimental and simulation studies. J. Membr. Sci. 2015, 489, 73–80. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Bundschuh, J.; Ghaffour, N.; Mahmoudi, H.; Goosen, M.; Mushtaq, S.; Hoinkis, J. Low-cost low-enthalpy geothermal heat for freshwater production: Innovative applications using thermal desalination processes. Renew. Sustain. Energy Rev. 2015, 43, 196–206. [Google Scholar] [CrossRef]
- Lee, J.; Alsaadi, A.S.; Ghaffour, N. Multi-stage air gap membrane distillation reversal for hot impaired quality water treatment: Concept and simulation study. Desalination 2019, 450, 1–11. [Google Scholar] [CrossRef]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-based seawater desalination: Present and future prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Ghaffour, N.; Lattemann, S.; Missimer, T.; Ng, K.C.; Sinha, S.; Amy, G. Renewable energy-driven innovative energy-efficient desalination technologies. Appl. Energy 2014, 136, 1155–1165. [Google Scholar] [CrossRef]
- Ghaffour, N.; Soukane, S.; Lee, J.G.; Kim, Y.; Alpatova, A. Membrane distillation hybrids for water production and energy efficiency enhancement: A critical review. Appl. Energy 2019, 254, 113698. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.-S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Hitsov, I.; De Sitter, K.; Dotremont, C.; Nopens, I. Economic modelling and model-based process optimization of membrane distillation. Desalination 2018, 436, 125–143. [Google Scholar] [CrossRef]
- Atkins, P.W. Physical Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Dudchenko, A.V.; Chen, C.; Cardenas, A.; Rolf, J.; Jassby, D. Frequency-dependent stability of CNT Joule heaters in ionizable media and desalination processes. Nat. Nanotechnol. 2017, 12, 557–563. [Google Scholar] [CrossRef]
- Anvari, A.; Kekre, K.M.; Yancheshme, A.A.; Yao, Y.; Ronen, A. Membrane distillation of high salinity water by induction heated thermally conducting membranes. J. Membr. Sci. 2019, 589, 117253. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Heat transport and membrane distillation coefficients in direct contact membrane distillation. J. Membr. Sci. 2003, 212, 177–193. [Google Scholar] [CrossRef]
- Qtaishat, M.; Matsuura, T.; Kruczek, B.; Khayet, M. Heat and mass transfer analysis in direct contact membrane distillation. Desalination 2008, 219, 272–292. [Google Scholar] [CrossRef]
- Fane, A.G.; Schofield, R.W.; Fell, C.J.D. The efficient use of membrane distillation. Desalination 1987, 64, 231–243. [Google Scholar] [CrossRef]
- Schofield, R.W.; Fane, A.G.; Fell, C.J.D. Heat and mass transfer in membrane distillation. J. Membr. Sci. 1987, 33, 299–313. [Google Scholar] [CrossRef]
- Deshmukh, A.; Elimelech, M. Understanding the impact of membrane properties and transport phenomena on the energetic performance of membrane distillation desalination. J. Membr. Sci. 2017, 539, 458–474. [Google Scholar] [CrossRef]
- Alsaadi, A.S.; Francis, L.; Amy, G.L.; Ghaffour, N. Experimental and theoretical analyses of temperature polarization effect in vacuum membrane distillation. J. Membr. Sci. 2014, 471, 138–148. [Google Scholar] [CrossRef]
- Brogioli, D.; la Mantia, F.; Yip, N.Y. Thermodynamic analysis and energy efficiency of thermal desalination processes. Desalination 2018, 428, 29–39. [Google Scholar] [CrossRef]
- Xu, J.; Singh, Y.B.; Amy, G.L.; Ghaffour, N. Effect of operating parameters and membrane characteristics on air gap membrane distillation performance for the treatment of highly saline water. J. Membr. Sci. 2016, 512, 73–82. [Google Scholar] [CrossRef]
- Lee, J.G.; Lee, E.J.; Jeong, S.; Guo, J.; An, A.K.; Guo, H.; Kim, J.; Leiknes, T.; Ghaffour, N. Theoretical modeling and experimental validation of transport and separation properties of carbon nanotube electrospun membrane distillation. J. Membr. Sci. 2017, 526, 395–408. [Google Scholar] [CrossRef]
- Lee, J.G.; Alsaadi, A.S.; Karam, A.M.; Francis, L.; Soukane, S.; Ghaffour, N. Total water production capacity inversion phenomenon in multi-stage direct contact membrane distillation: A theoretical study. J. Membr. Sci. 2017, 544, 126–134. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blankert, B.; Vrouwenvelder, J.S.; Witkamp, G.-J.; Ghaffour, N. Minimum Net Driving Temperature Concept for Membrane Distillation. Membranes 2020, 10, 100. https://doi.org/10.3390/membranes10050100
Blankert B, Vrouwenvelder JS, Witkamp G-J, Ghaffour N. Minimum Net Driving Temperature Concept for Membrane Distillation. Membranes. 2020; 10(5):100. https://doi.org/10.3390/membranes10050100
Chicago/Turabian StyleBlankert, Bastiaan, Johannes S. Vrouwenvelder, Geert-Jan Witkamp, and Noreddine Ghaffour. 2020. "Minimum Net Driving Temperature Concept for Membrane Distillation" Membranes 10, no. 5: 100. https://doi.org/10.3390/membranes10050100
APA StyleBlankert, B., Vrouwenvelder, J. S., Witkamp, G.-J., & Ghaffour, N. (2020). Minimum Net Driving Temperature Concept for Membrane Distillation. Membranes, 10(5), 100. https://doi.org/10.3390/membranes10050100





