Characterization and Performance of LbL-Coated Multibore Membranes: Zeta Potential, MWCO, Permeability and Sulfate Rejection
Abstract
1. Introduction
LbL-Modification of Membranes
2. Materials and Methods
2.1. Materials
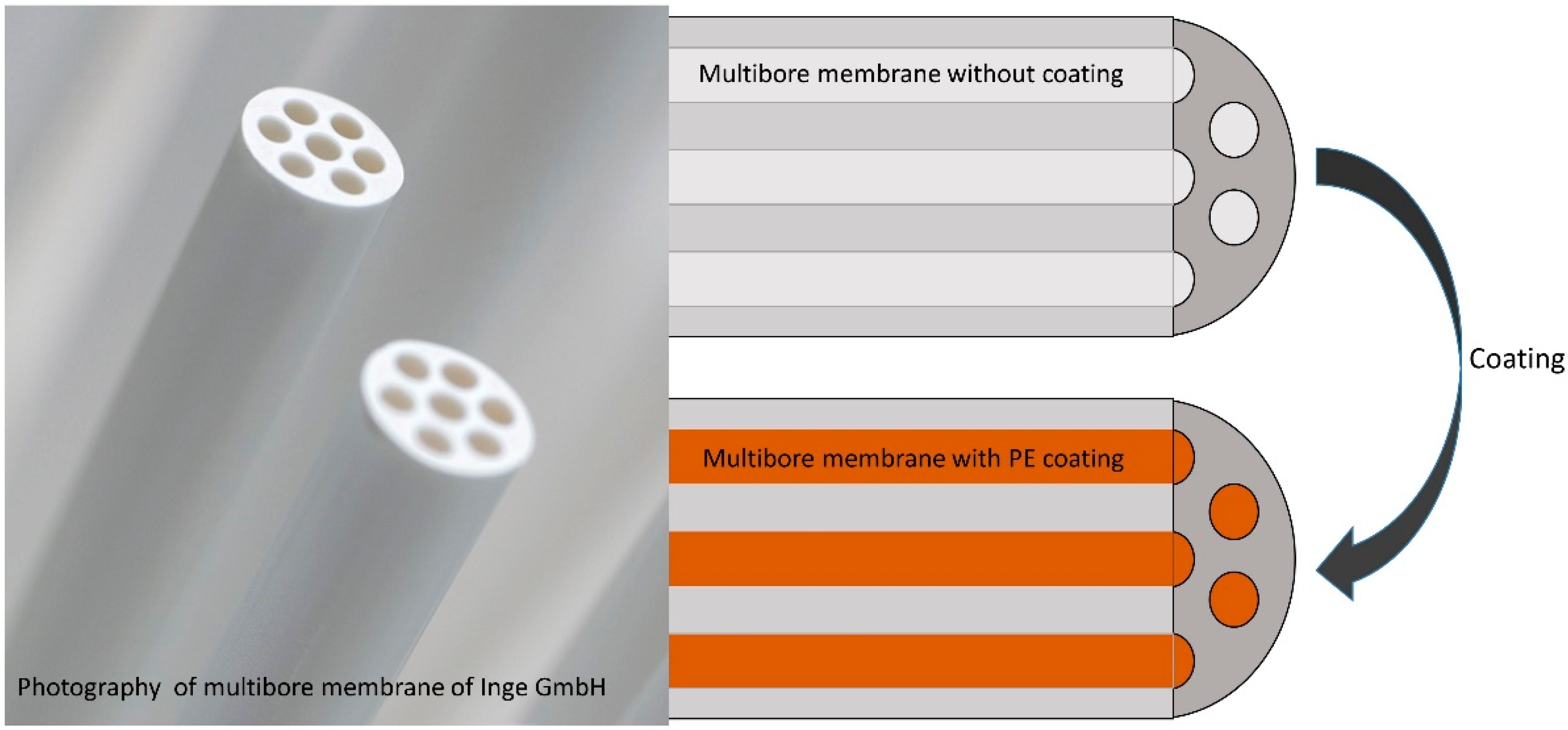
2.2. Membrane
2.3. Coating
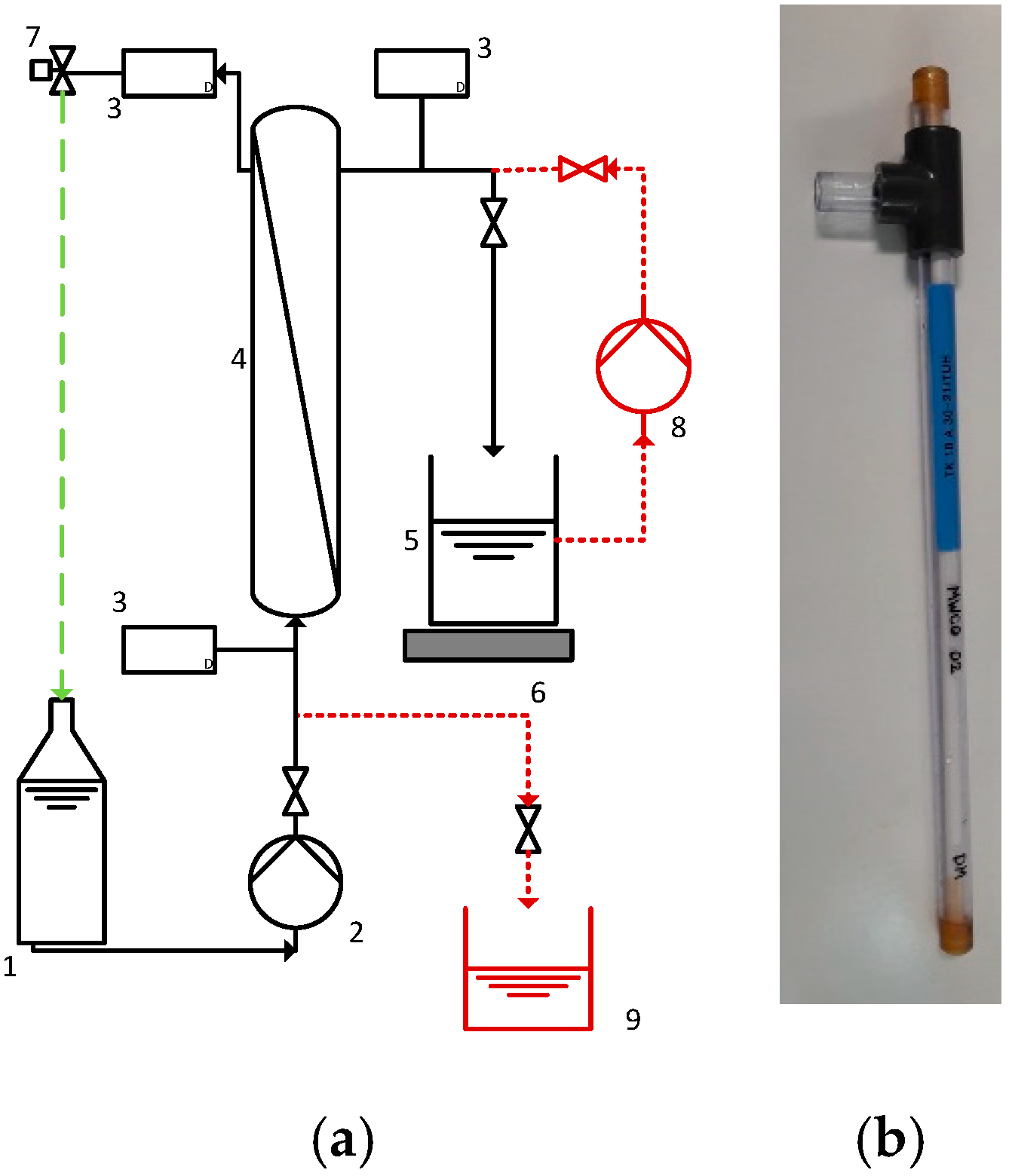
2.4. Filtration Setup and Experiments
2.5. Characterization Methods
2.6. MWCO
2.7. SEM Imaging
2.8. Zeta Potential—Measurement of Flat Sheet Membranes
3. Results and Discussion
3.1. Zeta Potential—Innovative Measurement Method for Multibore Membranes
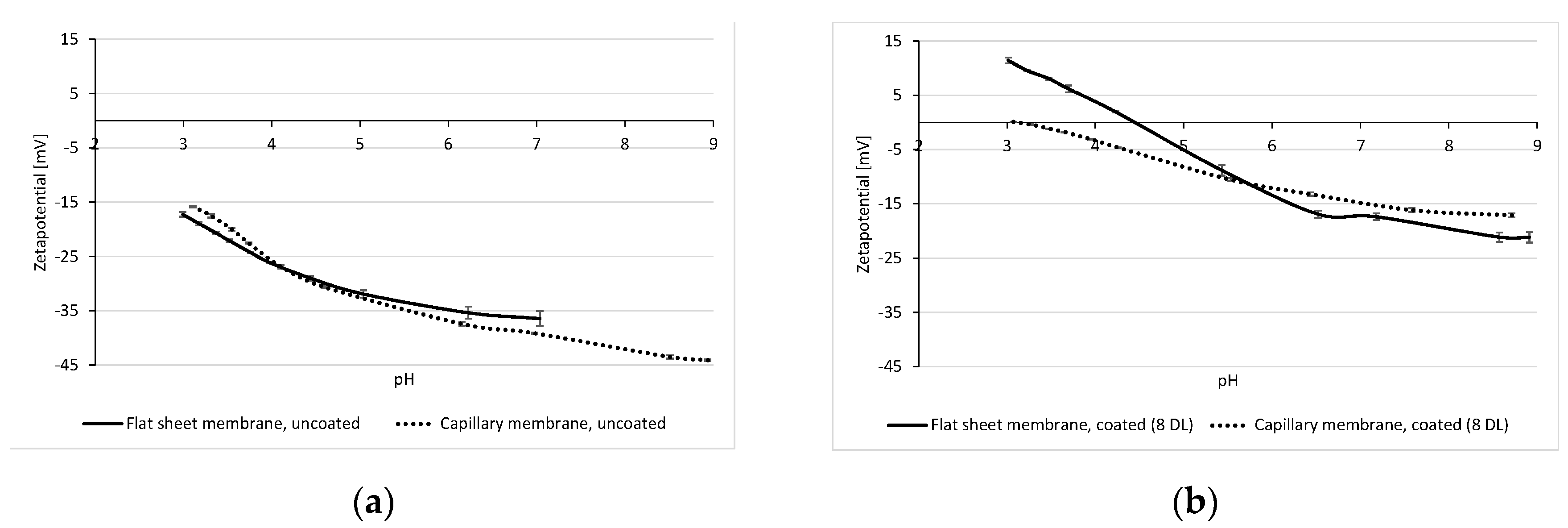
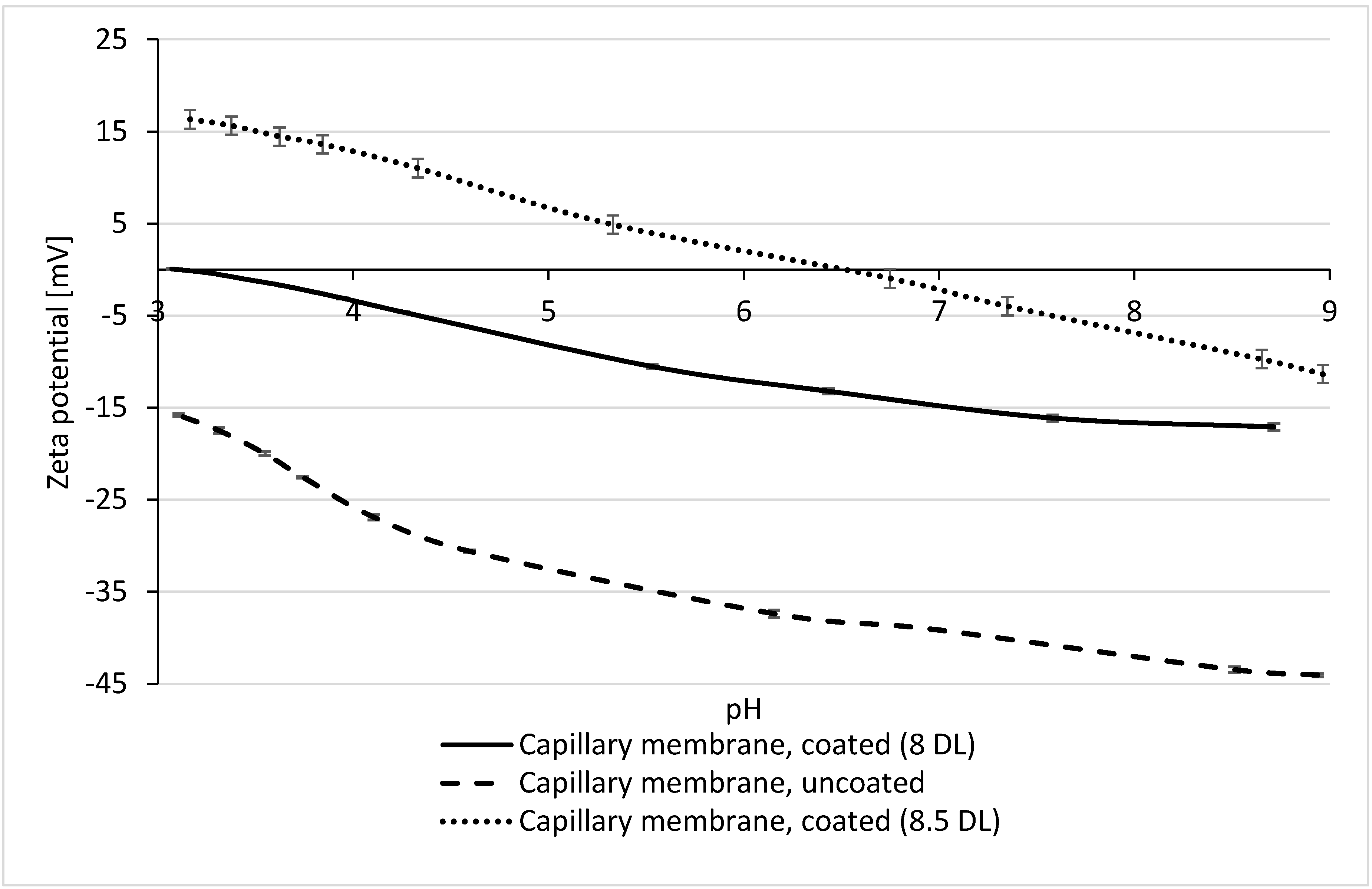
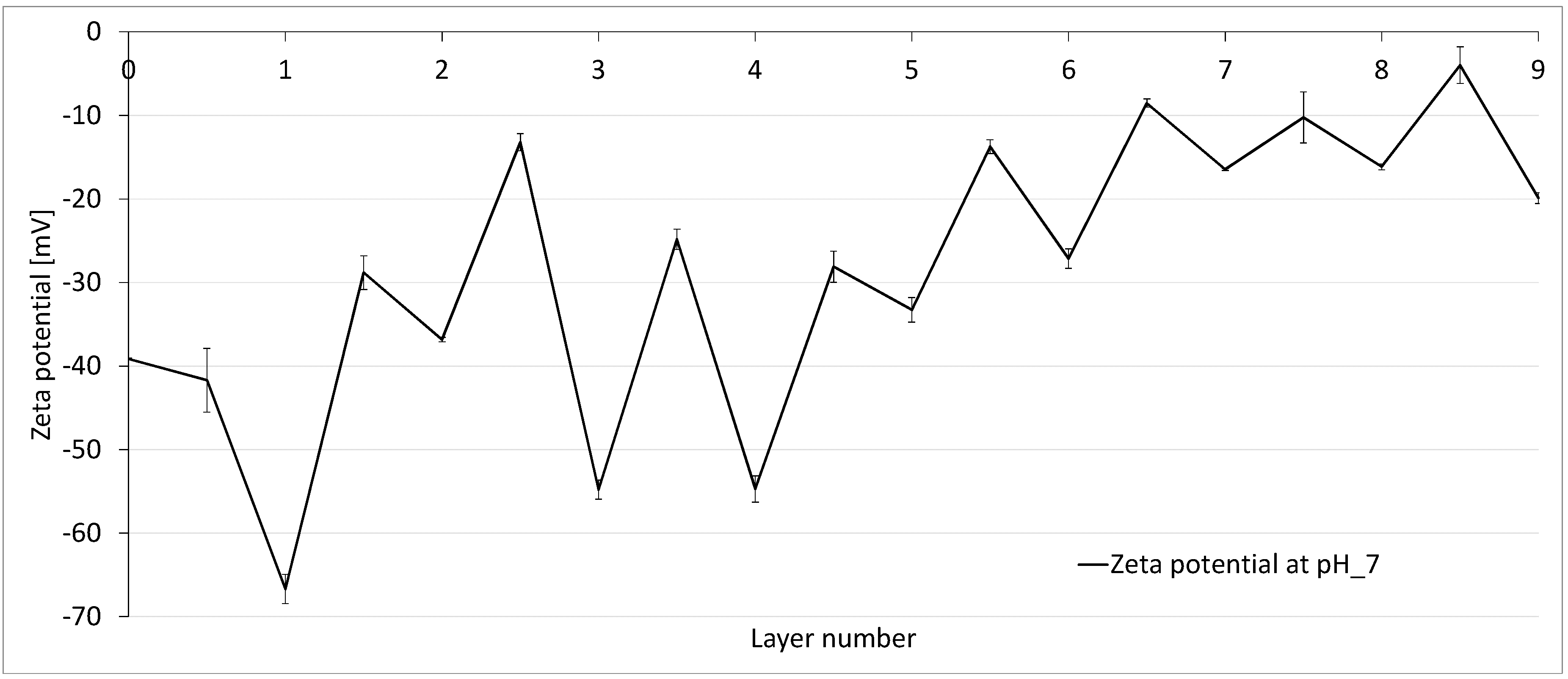
3.2. Zeta Potential of Multibore Membranes
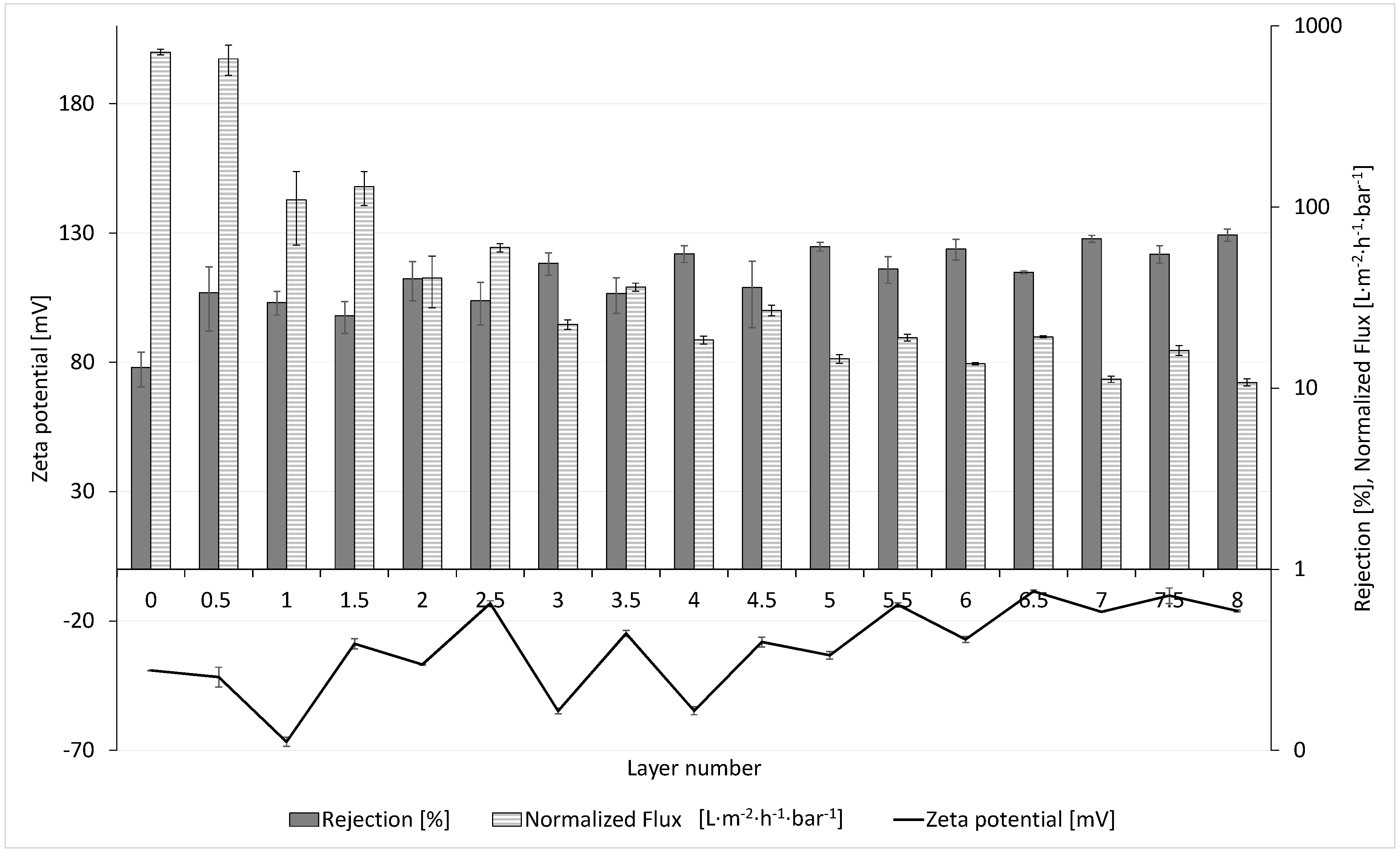
3.3. Influence of Zeta Potential on the Permeability and Rejection
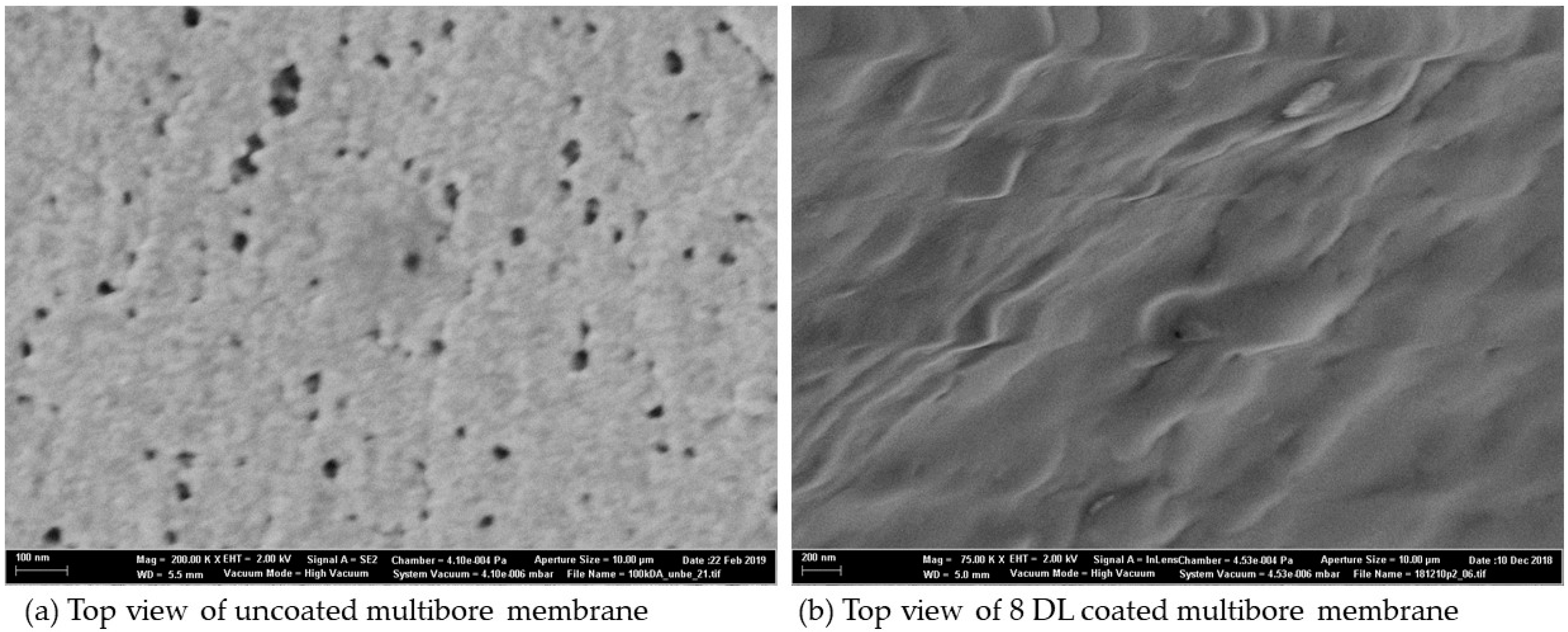
3.4. SEM Analysis of Coated and Uncoated Membranes
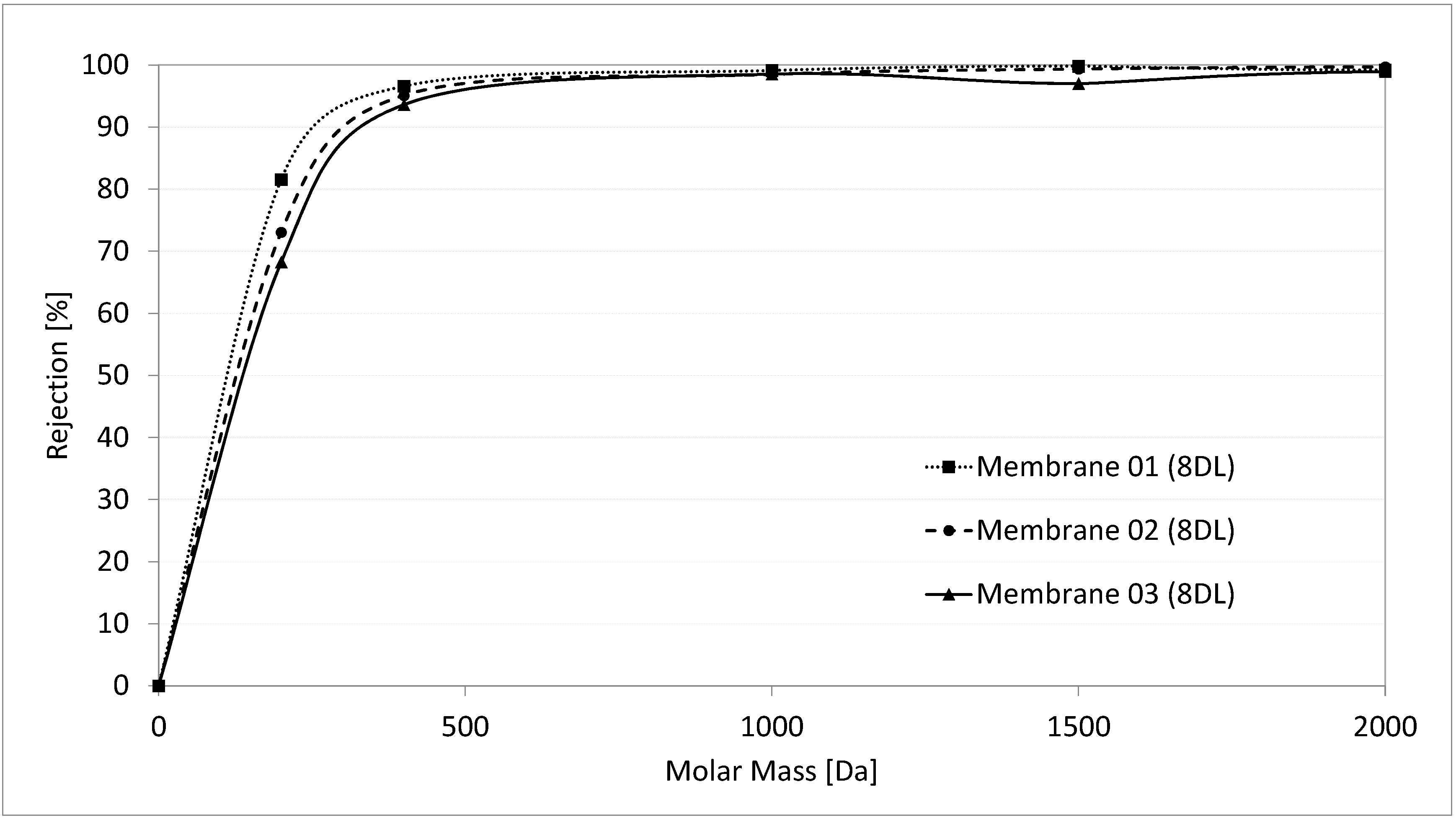
3.5. Determination of the Molecular Weight Cut-Off
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joseph, N.; Ahmadiannamini, P.; Hoogenboom, R.; Vankelecom, I.F.J. Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation. Polym. Chem. 2014, 5, 1817–1831. [Google Scholar] [CrossRef]
- Menne, D. Layer-by-Layer Design of Nanofiltration Membranes: Entwicklung von “Layer-by-Layer” Nanofiltrationsmembranen. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 25 January 2017. [Google Scholar]
- Blázquez, M.; Tellaetxe, A.; de Grooth, J.; Potreck, J.; van Foeken, C.; Borre, F.H.J.; Gascón, A.J. Life cycle assessment of a layer by layer nanofiltration membrane—A case study of the LbLbrane project. In Proceedings of the Avnir conference, Lille, France, 5–6 November 2014. [Google Scholar]
- Menne, D.; Üzüm, C.; Koppelmann, A.; Wong, J.E.; van Foeken, C.; Borre, F.; Dähne, L.; Laakso, T.; Pihlajamäki, A.; Wessling, M. Regenerable polymer/ceramic hybrid nanofiltration membrane based on polyelectrolyte assembly by layer-by-layer technique. J. Membr. Sci. 2016, 520, 924–932. [Google Scholar] [CrossRef]
- Dillmann, S. Untersuchungen zum Einfluss von Beschichtungsparametern auf die Wirksamkeit der LbL-Oberflächenmodifizierung von UF-Kapillarmembranen; Konferenzbeitrag, GDCh Tagung Wasser: Erfurt, Germany, 2019. [Google Scholar]
- Kwon, S.-B.; Lee, J.S.; Kwon, S.J.; Yun, S.-T.; Lee, S.; Lee, J.-H. Molecular layer-by-layer assembled forward osmosis membranes. J. Membr. Sci. 2015, 488, 111–120. [Google Scholar] [CrossRef]
- Quinn, J.F.; Johnston, A.P.R.; Such, G.K.; Zelikin, A.N.; Caruso, F. Next generation, sequentially assembled ultrathin films: Beyond electrostatics. Chem. Soc. Rev. 2007, 36, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Schlenoff, J.B. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kelly, K.D. Kinetics and Application of Polyelectrolyte Membranes and Multilayers. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 10 May 2016. [Google Scholar]
- Ghostine, R.A.; Markarian, M.Z.; Schlenoff, J.B. Asymmetric growth in polyelectrolyte multilayers. J. Am. Chem. Soc. 2013, 135, 7636–7646. [Google Scholar] [CrossRef] [PubMed]
- Adusumilli, M.; Bruening, M.L. Variation of ion-exchange capacity, zeta potential, and ion-transport selectivities with the number of layers in a multilayer polyelectrolyte film. Langmuir ACS J. Surf. Colloids 2009, 25, 7478–7485. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Zhang, G.; Liu, Z.; Peng, Y.; Wang, Z. Evaluations of polyelectrolyte multilayer membranes assembled by a dynamic layer-by-layer technique. Desalination 2008, 234, 300–306. [Google Scholar] [CrossRef]
- Su, B.; Wang, T.; Wang, Z.; Gao, X.; Gao, C. Preparation and performance of dynamic layer-by-layer PDADMAC/PSS nanofiltration membrane. J. Membr. Sci. 2012, 423–424, 324–331. [Google Scholar] [CrossRef]
- Zhang, G.; Gu, W.; Ji, S.; Liu, Z.; Peng, Y.; Wang, Z. Preparation of polyelectrolyte multilayer membranes by dynamic layer-by-layer process for pervaporation separation of alcohol/water mixtures. J. Membr. Sci. 2006, 280, 727–733. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Malaisamy, R.; Talla-Nwafo, A.; Jones, K.L. Polyelectrolyte modification of nanofiltration membrane for selective removal of monovalent anions. Sep. Purif. Technol. 2011, 77, 367–374. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Zembala, M.; Warszyński, P.; Jachimska, B. Characterization of polyelectrolyte multilayers by the streaming potential method. Langmuir ACS J. Surf. Colloids 2004, 20, 10517–10525. [Google Scholar] [CrossRef] [PubMed]
- Reurink, D.M.; Haven, J.P.; Achterhuis, I.; Lindhoud, S.; Roesink, H.D.; de Vos, W.M. Annealing of Polyelectrolyte Multilayers for Control over Ion Permeation. Adv. Mater. Interfaces 2018, 5, 1800651. [Google Scholar] [CrossRef]
- BASF Inge GmbH. Available online: https://www.basf.com/at/de/media/science-around-us/ultrafiltration-creates-clear-conditions.html (accessed on 23 November 2020).
- Gregurec, D.; Olszyna, M.; Politakos, N.; Yate, L.; Dahne, L.; Moya, S.E. Stability of polyelectrolyte multilayers in oxidizing media: A critical issue for the development of multilayer based membranes for nanofiltration. Colloid Polym. Sci. 2015, 293, 381–388. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Chichester, West Sussex, UK; Hoboken, NJ, USA, 2012. [Google Scholar]
- Luxbacher, T. The Zeta Potential for Solid Surface Analysis: A Practical Guide to Streaming Potential Measurement; Anton Paar GmbH: Graz, Austria, 2014. [Google Scholar]
- Hach Lange. Sulfate, SulfaVer 4 Method 8051, Powder Pillows; Hach Lange: Loveland, CO, USA, 2009. [Google Scholar]
- Crittenden, J.C. MWH’s Water Treatment: Principles and Design, 3rd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Luxbacher, T. Assessment of Surface Charge for Polymer Hollow Fibre Membranes. Procedia Eng. 2012, 44, 1440–1442. [Google Scholar] [CrossRef]
- de Grooth, J.; Oborný, R.; Potreck, J.; Nijmeijer, K.; de Vos, W.M. The role of ionic strength and odd-even effects on the properties of polyelectrolyte multilayer nanofiltration membranes. J. Membr. Sci. 2015, 475, 311–319. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Ng, C.Y.; Leo, C.P.; Rohani, R. Development of nanofiltration membrane with high salt selectivity and performance stability using polyelectrolyte multilayers. Desalination 2014, 351, 19–26. [Google Scholar] [CrossRef]
- Miller, M.D.; Bruening, M.L. Correlation of the Swelling and Permeability of Polyelectrolyte Multilayer Films. Chem. Mater. 2005, 17, 5375–5381. [Google Scholar] [CrossRef]
- Dubas, S.T.; Schlenoff, J.B. Swelling and Smoothing of Polyelectrolyte Multilayers by Salt. Langmuir 2001, 17, 7725–7727. [Google Scholar] [CrossRef]
- McCormick, M.; Smith, R.N.; Graf, R.; Barrett, C.J.; Reven, L.; Spiess, H.W. NMR Studies of the Effect of Adsorbed Water on Polyelectrolyte Multilayer Films in the Solid State. Macromolecules 2003, 36, 3616–3625. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dillmann, S.; Kaushik, S.A.; Stumme, J.; Ernst, M. Characterization and Performance of LbL-Coated Multibore Membranes: Zeta Potential, MWCO, Permeability and Sulfate Rejection. Membranes 2020, 10, 412. https://doi.org/10.3390/membranes10120412
Dillmann S, Kaushik SA, Stumme J, Ernst M. Characterization and Performance of LbL-Coated Multibore Membranes: Zeta Potential, MWCO, Permeability and Sulfate Rejection. Membranes. 2020; 10(12):412. https://doi.org/10.3390/membranes10120412
Chicago/Turabian StyleDillmann, Saskia, Shambhavi Arvind Kaushik, Jakob Stumme, and Mathias Ernst. 2020. "Characterization and Performance of LbL-Coated Multibore Membranes: Zeta Potential, MWCO, Permeability and Sulfate Rejection" Membranes 10, no. 12: 412. https://doi.org/10.3390/membranes10120412
APA StyleDillmann, S., Kaushik, S. A., Stumme, J., & Ernst, M. (2020). Characterization and Performance of LbL-Coated Multibore Membranes: Zeta Potential, MWCO, Permeability and Sulfate Rejection. Membranes, 10(12), 412. https://doi.org/10.3390/membranes10120412





