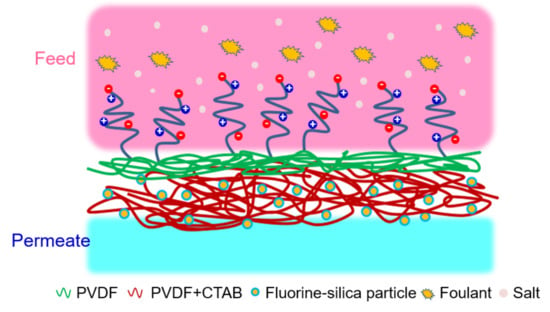
A “Graft to” Electrospun Zwitterionic Bilayer Membrane for the Separation of Hydraulic Fracturing-Produced Water via Membrane Distillation
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Synthesis of the Zwitterion Copolymers
2.3. Fabrication and Modification of MD Membrane
2.3.1. Electrospun Membrane Fabrication
2.3.2. Membrane Modification
2.4. Membrane Characterization
2.5. Membrane Performance
2.6. Antifouling and Antiwetting Investigation with Model Solutions
2.7. Membrane Performance with PW
3. Results and Discussion
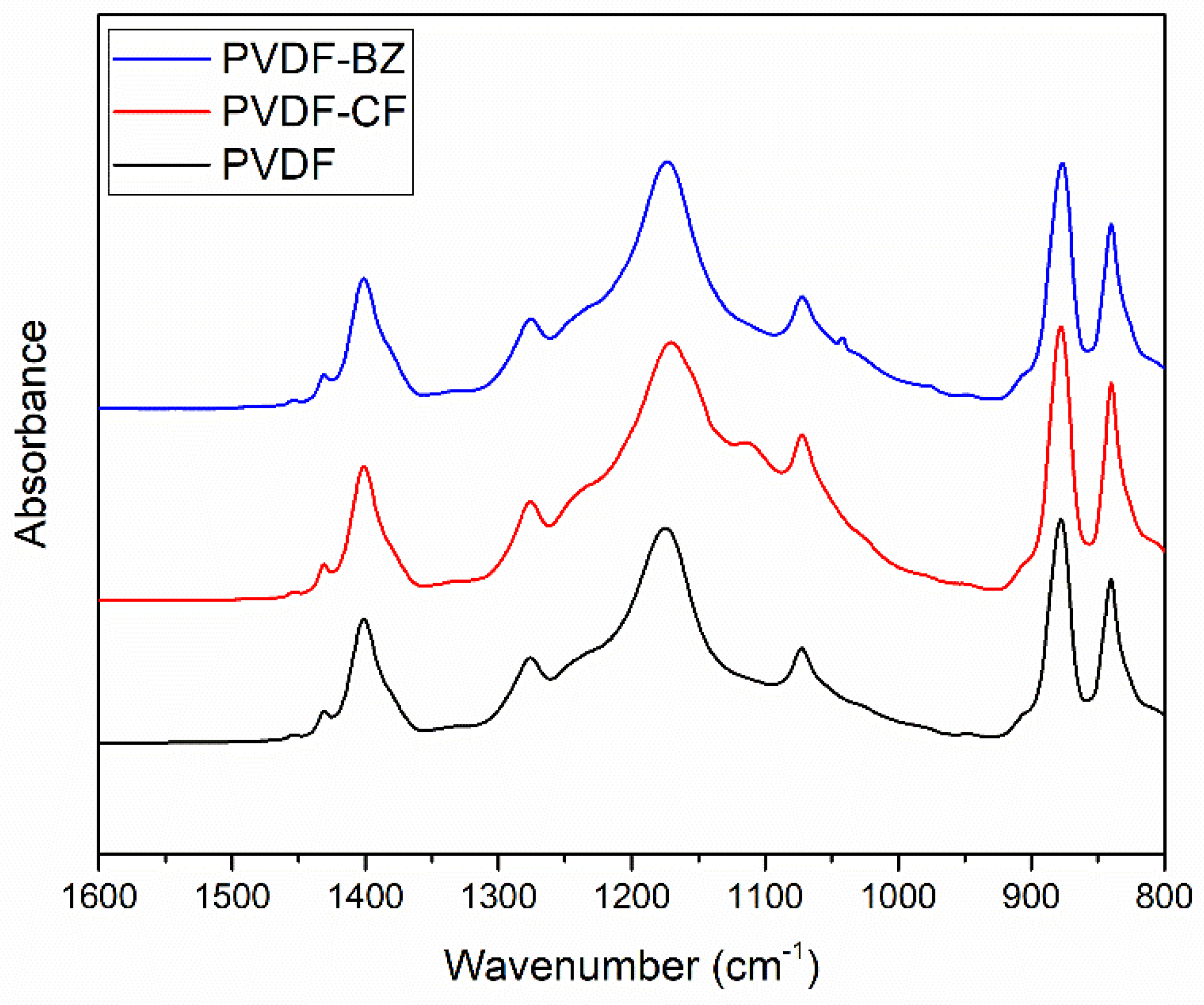
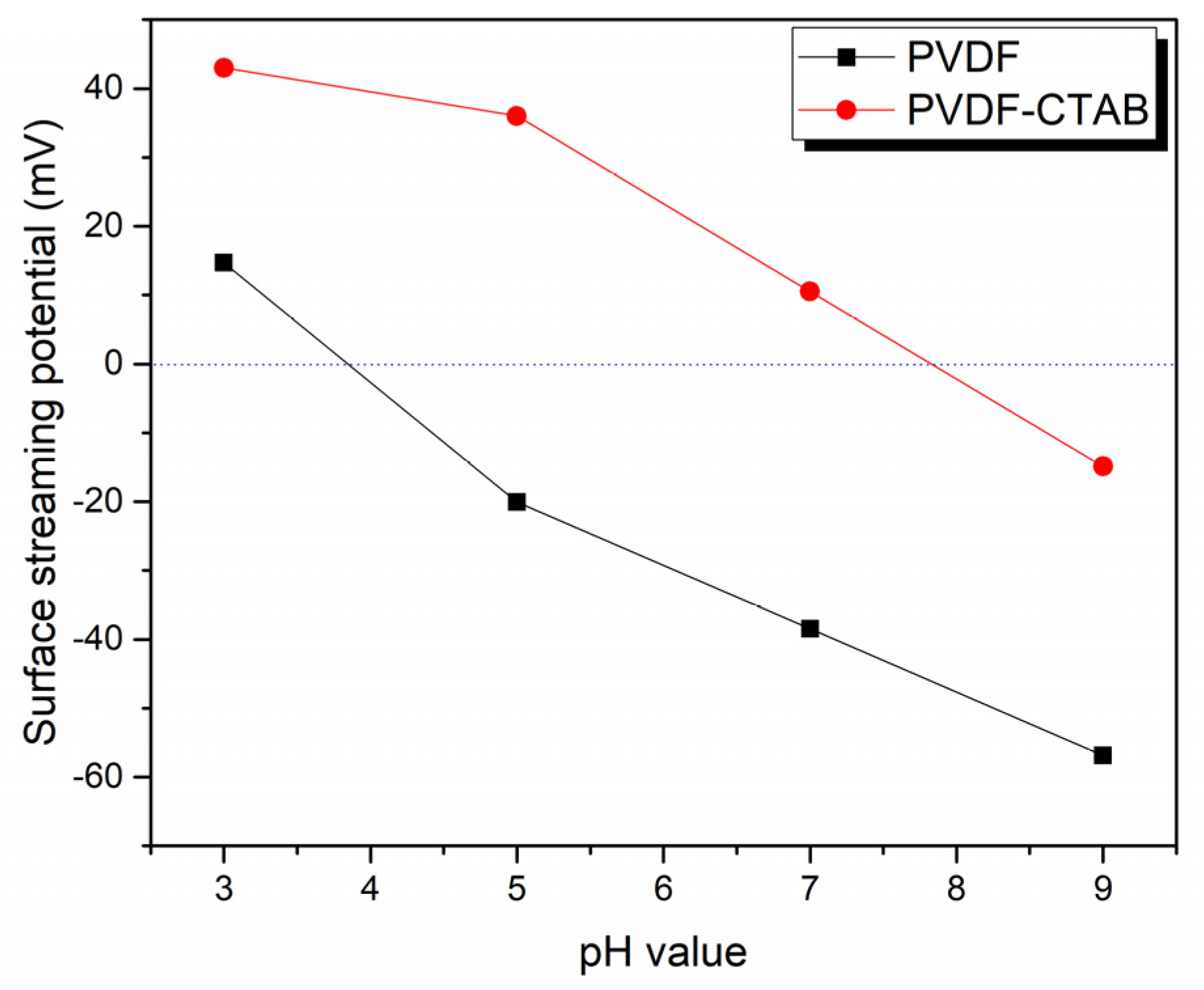
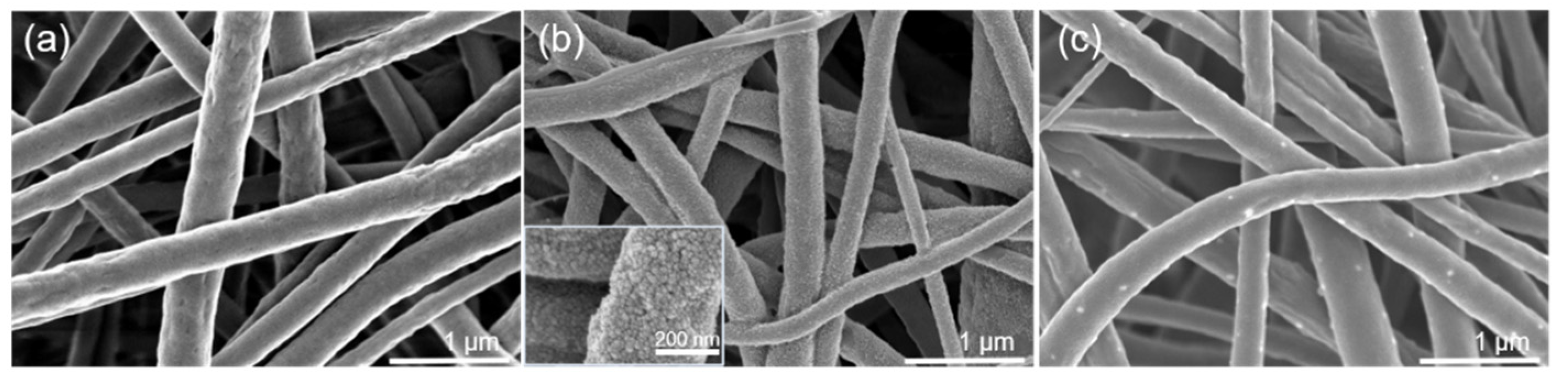
3.1. Physicochemical Properties of the Nanofibrous Membranes
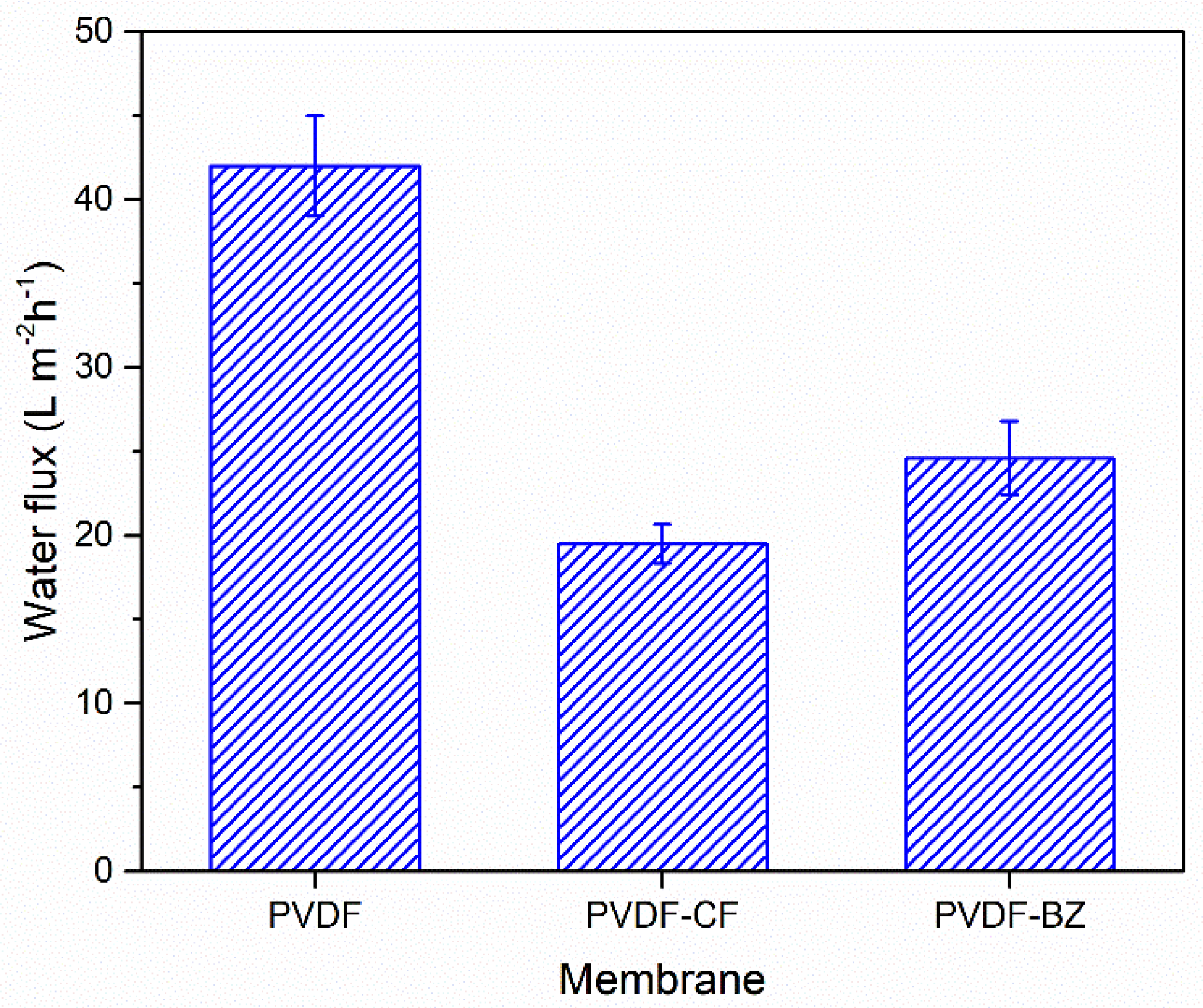
3.2. Intrinsic Membrane Performance
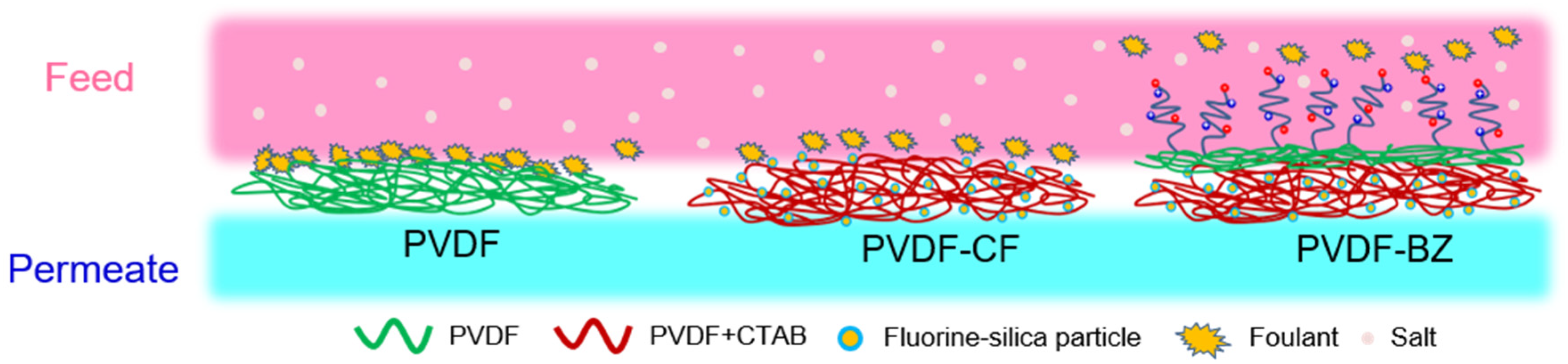
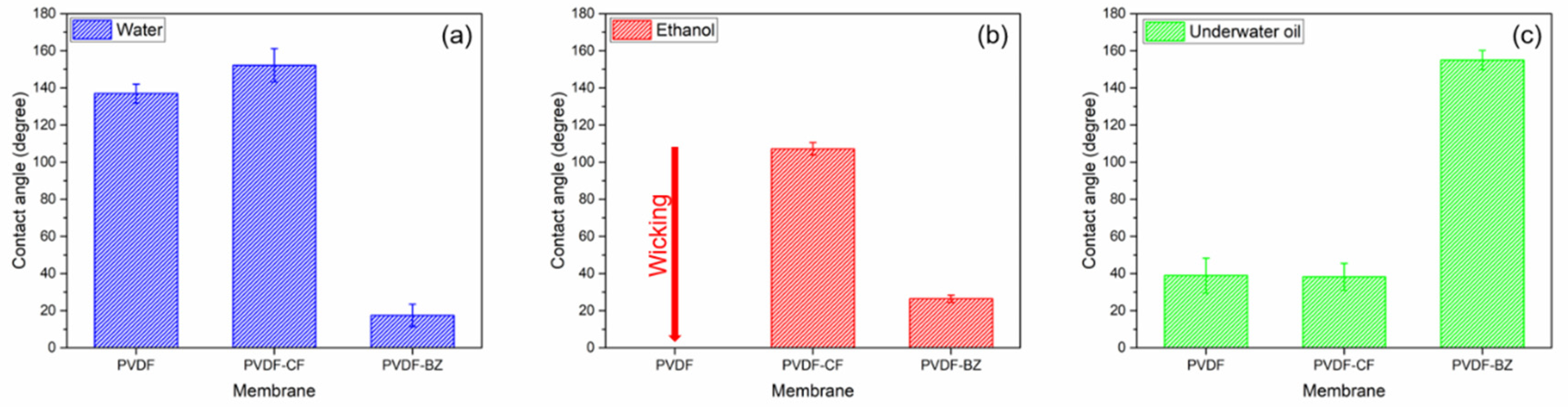
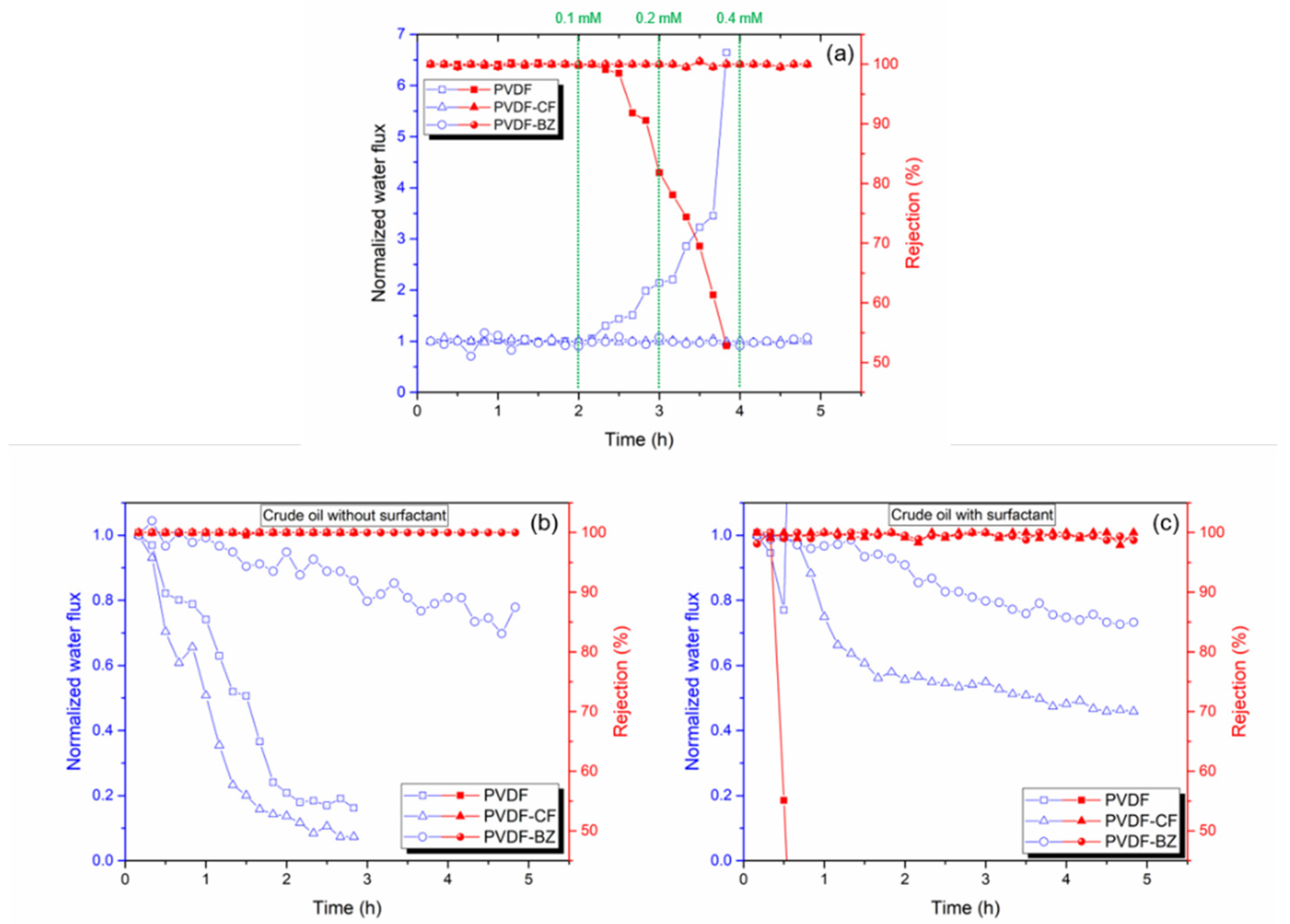
3.3. Antiwetting and Antifouling Behavior
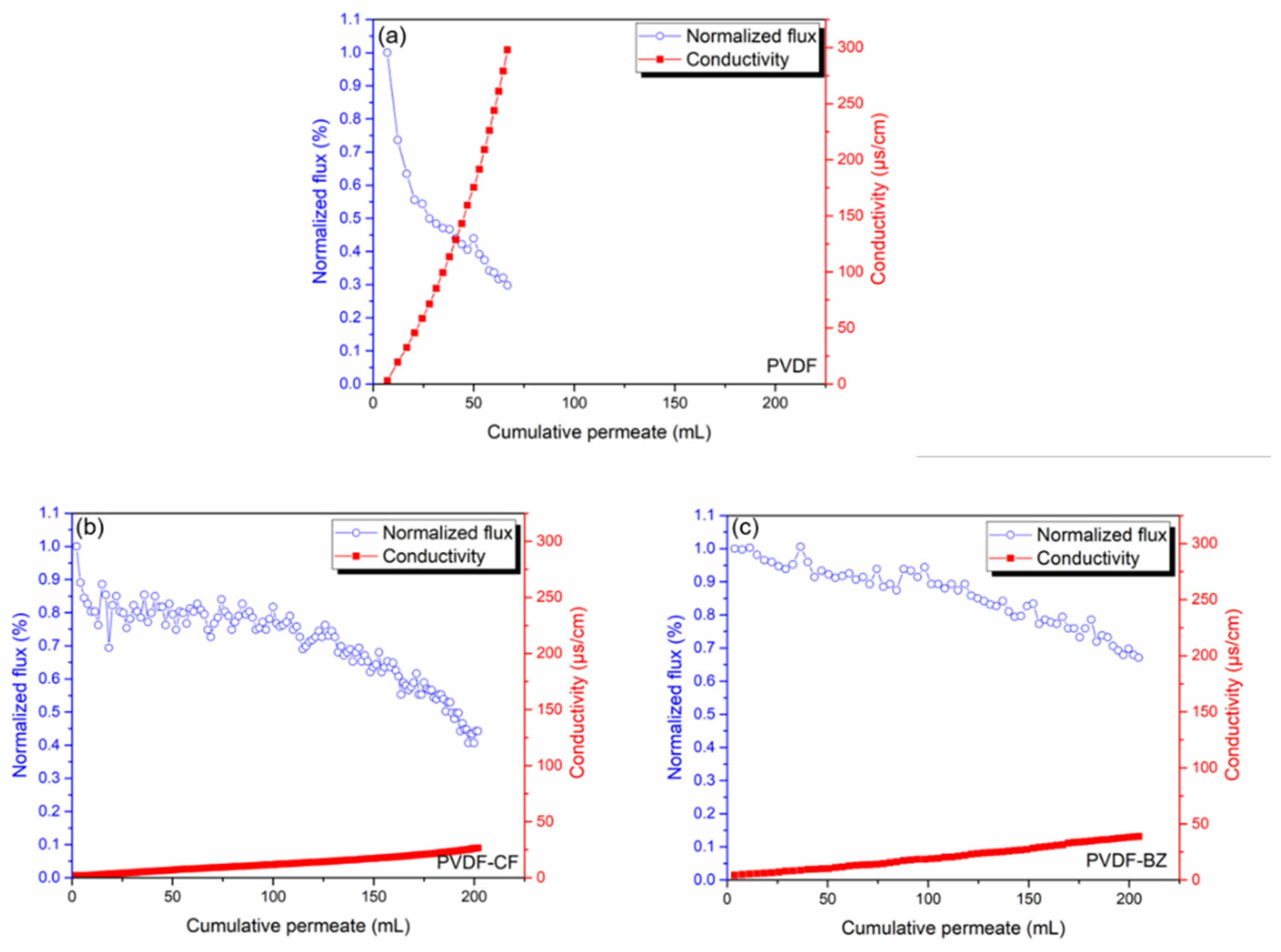
3.4. Treating PW
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dobush, G.U.S. Forecast to Rival Saudi Arabia as World’s Top Oil Exporter by 2024. Fortune, 11 March 2019. [Google Scholar]
- Estrada, J.M.; Bhamidimarri, R. A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 2016, 182, 292–303. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Chen, S.-T.; Patra, T.; Hsu, C.-H.; Sengupta, A.; Hung, W.-S.; Huang, S.-H.; Qian, X.; Wickramasinghe, R.; Chang, Y. Zwitterionic forward osmosis membrane modified by fast second interfacial polymerization with enhanced antifouling and antimicrobial properties for produced water pretreatment. Desalination 2019, 469, 114090. [Google Scholar] [CrossRef]
- Boo, C.; Lee, J.; Elimelech, M. Omniphobic polyvinylidene fluoride (PVDF) membrane for desalination of shale gas produced water by membrane distillation. Environ. Sci. Technol. 2016, 50, 12275–12282. [Google Scholar] [CrossRef] [PubMed]
- Kamaz, M.; Sengupta, A.; Gutierrez, A.; Chiao, Y.-H.; Wickramasinghe, R. Surface modification of PVDF membranes for treating produced waters by direct contact membrane distillation. Int. J. Environ. Res. Public Health 2019, 16, 685. [Google Scholar] [CrossRef]
- Lee, J.; Boo, C.; Ryu, W.H.; Taylor, A.D.; Elimelech, M. Development of Omniphobic Desalination Membranes Using a Charged Electrospun Nanofiber Scaffold. ACS Appl. Mater. Interfaces 2016, 8, 11154–11161. [Google Scholar] [CrossRef]
- Miller, D.J.; Huang, X.; Li, H.; Kasemset, S.; Lee, A.; Agnihotri, D.; Hayes, T.; Paul, D.R.; Freeman, B.D. Fouling-resistant membranes for the treatment of flowback water from hydraulic shale fracturing: A pilot study. J. Membr. Sci. 2013, 437, 265–275. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Carlson, K.H.; Lee, J.; Tong, T. Membrane fouling and reusability in membrane distillation of shale oil and gas produced water: Effects of membrane surface wettability. J. Membr. Sci. 2018, 567, 199–208. [Google Scholar] [CrossRef]
- Sardari, K.; Fyfe, P.; Lincicome, D.; Wickramasinghe, S.R. Aluminum electrocoagulation followed by forward osmosis for treating hydraulic fracturing produced waters. Desalination 2018, 428, 172–181. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Robust omniphobic surfaces. Proc. Natl. Acad. Sci. USA 2008, 105, 18200–18205. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, V.A.; Dinachali, S.S.; Nair, A.S.; Ramakrishna, S. Robust Superamphiphobic Film from Electrospun TiO2 Nanostructures. ACS Appl. Mater. Interfaces 2013, 5, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tian, F.; Ding, B.; Yu, J.; Wang, J.; Raza, A. Facile synthesis of robust amphiphobic nanofibrous membranes. Appl. Mater. Interfaces 2013, 276, 750–755. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Wang, Z.; Jin, J.; Lin, S. Novel Janus membrane for membrane distillation with simultaneous fouling and wetting resistance. Environ. Sci. Technol. 2017, 51, 13304–13310. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Xu, Z.; Li, J.; Pan, Z.; Du, Z.; Cheng, F. Preparation of omniphobic PVDF membranes with silica nanoparticles for treating coking wastewater using direct contact membrane distillation: Electrostatic adsorption vs. chemical bonding. J. Memb. Sci. 2019, 574, 349–357. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, J.; Hou, D.; Lin, S. Tailoring surface charge and wetting property for robust oil-fouling mitigation in membrane distillation. J. Memb. Sci. 2016, 516, 113–122. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R. Novel membrane surface modification to enhance anti-oil fouling property for membrane distillation application. J. Memb. Sci. 2013, 447, 26–35. [Google Scholar] [CrossRef]
- An, Q.-F.; Sun, W.-D.; Zhao, Q.; Ji, Y.-L.; Gao, C.-J. Study on a novel nanofiltration membrane prepared by interfacial polymerization with zwitterionic amine monomers. J. Memb. Sci. 2013, 431, 171–179. [Google Scholar] [CrossRef]
- Venault, A.; Chang, Y. Designs of Zwitterionic Interfaces and Membranes. Langmuir 2019, 35, 1714–1726. [Google Scholar] [CrossRef]
- Chou, Y.N.; Venault, A.; Cho, C.H.; Sin, M.C.; Yeh, L.C.; Jhong, J.F.; Chinnathambi, A.; Chang, Y.; Chang, Y. Epoxylated Zwitterionic Triblock Copolymers Grafted onto Metallic Surfaces for General Biofouling Mitigation. Langmuir 2017, 33, 9822–9835. [Google Scholar] [CrossRef] [PubMed]
- Minko, S. Grafting on Solid Surfaces: “Grafting to” and “Grafting from” Methods. In Polymer Surfaces and Interfaces; Springer: Berlin/Heidelberg, Germany, 2008; pp. 215–234. [Google Scholar]
- Ang, M.B.M.Y.; Gallardo, M.R.; Dizon, G.V.C.; De Guzman, M.R.; Tayo, L.L.; Huang, S.-H.; Lai, C.-L.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; et al. Graphene oxide functionalized with zwitterionic copolymers as selective layers in hybrid membranes with high pervaporation performance. J. Memb. Sci. 2019, 587, 117188. [Google Scholar] [CrossRef]
- An, X.; Liu, Z.; Hu, Y. Amphiphobic surface modification of electrospun nanofibrous membranes for anti-wetting performance in membrane distillation. Desalination 2018, 432, 23–31. [Google Scholar] [CrossRef]
- Han, L.; Tan, Y.Z.; Xu, C.; Xiao, T.; Trinh, T.A.; Chew, J.W. Zwitterionic grafting of sulfobetaine methacrylate (SBMA) on hydrophobic PVDF membranes for enhanced anti-fouling and anti-wetting in the membrane distillation of oil emulsions. J. Memb. Sci. 2019, 588, 117196. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Sengupta, A.; Chen, S.-T.; Huang, S.-H.; Hu, C.-C.; Hung, W.-S.; Chang, Y.; Qian, X.; Wickramasinghe, S.R.; Lee, K.-R.; et al. Zwitterion augmented polyamide membrane for improved forward osmosis performance with significant antifouling characteristics. Sep. Purif. Technol. 2019, 212, 316–325. [Google Scholar] [CrossRef]
- Venault, A.; Yang, H.-S.; Chiang, Y.-C.; Lee, B.-S.; Ruaan, R.-C.; Chang, Y. Bacterial resistance control on mineral surfaces of hydroxyapatite and human teeth via surface charge-driven antifouling coatings. ACS Appl. Mater. Interfaces 2014, 6, 3201–3210. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Chen, S.-T.; Sivakumar, M.; Ang, M.B.M.Y.; Patra, T.; Almodovar, J.; Wickramasinghe, S.R.; Hung, W.-S.; Lai, J.-Y. Zwitterionic Polymer Brush Grafted on Polyvinylidene Difluoride Membrane Promoting Enhanced Ultrafiltration Performance with Augmented Antifouling Property. Polymers 2020, 12, 1303. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Gao, S.; Zhang, Z.; Cui, F.; Tang, C.Y. In situ surface modification of thin film composite forward osmosis membranes with sulfonated poly (arylene ether sulfone) for anti-fouling in emulsified oil/water separation. J. Memb. Sci. 2017, 527, 26–34. [Google Scholar] [CrossRef]
- Feng, Y.; Li, W.L.; Hou, Y.F.; Yu, Y.; Cao, W.P.; Zhang, T.D.; Fei, W.D. Enhanced dielectric properties of PVDF-HFP/BaTiO3-nanowire composites induced by interfacial polarization and wire-shape. J. Mater. Chem. C 2015, 3, 1250–1260. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazzella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1622. [Google Scholar] [CrossRef]
- Chou, Y.-N.; Wen, T.-C.; Chang, Y. Zwitterionic surface grafting of epoxylated sulfobetaine copolymers for the development of stealth biomaterial interfaces. Acta Biomater. 2016, 40, 78–91. [Google Scholar] [CrossRef]
- Mirabedini, A. Developing Novel Spinning Methods to Fabricate Continuous Multifunctional Fibres for Bioapplications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Liao, Y.; Wang, R.; Tian, M.; Qiu, C.; Fane, A.G. Fabrication of polyvinylidene fluoride (PVDF) nanofiber membranes by electro-spinning for direct contact membrane distillation. J. Memb. Sci. 2013, 425–426, 30–39. [Google Scholar] [CrossRef]
- Hou, D.; Ding, C.; Fu, C.; Wang, D.; Zhao, C.; Wang, J. Electrospun nanofibrous omniphobic membrane for anti-surfactant-wetting membrane distillation desalination. Desalination 2019, 468, 114068. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S. Membrane fouling and wetting in membrane distillation and their mitigation by novel membranes with special wettability. Water Res. 2017, 112, 38–47. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Patra, T.; Ang, M.B.M.Y.; Chen, S.-T.; Almodovar, J.; Qian, X.; Wickramasinghe, R.; Hung, W.-S.; Huang, S.-H.; Chang, Y. Zwitterion Co-Polymer PEI-SBMA Nanofiltration Membrane Modified by Fast Second Interfacial Polymerization. Polymers 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Ritt, C.L.; Deshmukh, A.; Wang, Z.; Qin, M.; Epsztein, R.; Elimelech, M. The relative insignificance of advanced materials in enhancing the energy efficiency of desalination technologies. Energy Environ. Sci. 2020, 13, 1694–1710. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, H.; Lee, S.; Lee, S.; Hong, S. Membrane distillation (MD) integrated with crystallization (MDC) for shale gas produced water (SGPW) treatment. Desalination 2017, 403, 172–178. [Google Scholar] [CrossRef]

| Parameter | Unit | Value |
|---|---|---|
| Total dissolved solids (TDS) | mg L−1 | 245,000 |
| Total organic carbon (TOC) | mg L−1 | 120 |
| Total suspended solids (TSS) | mg L−1 | 131 |
| Turbidity | NTU’s | 6.0 |
| pH | - | 6.7 |
| Chloride | mg L−1 | 147,000 |
| Sulfate | mg L−1 | 478 |
| Boron | mg L−1 | 97.4 |
| Calcium | mg L−1 | 30,500 |
| Magnesium | mg L−1 | 5450 |
| Potassium | mg L−1 | 4330 |
| Sodium | mg L−1 | 55,900 |
| Conductivity | µS/cm | 323,000 |
| Total nitrogen | mg L−1 | 43.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiao, Y.-H.; Yap Ang, M.B.M.; Huang, Y.-X.; DePaz, S.S.; Chang, Y.; Almodovar, J.; Wickramasinghe, S.R. A “Graft to” Electrospun Zwitterionic Bilayer Membrane for the Separation of Hydraulic Fracturing-Produced Water via Membrane Distillation. Membranes 2020, 10, 402. https://doi.org/10.3390/membranes10120402
Chiao Y-H, Yap Ang MBM, Huang Y-X, DePaz SS, Chang Y, Almodovar J, Wickramasinghe SR. A “Graft to” Electrospun Zwitterionic Bilayer Membrane for the Separation of Hydraulic Fracturing-Produced Water via Membrane Distillation. Membranes. 2020; 10(12):402. https://doi.org/10.3390/membranes10120402
Chicago/Turabian StyleChiao, Yu-Hsuan, Micah Belle Marie Yap Ang, Yu-Xi Huang, Sandrina Svetlana DePaz, Yung Chang, Jorge Almodovar, and S. Ranil Wickramasinghe. 2020. "A “Graft to” Electrospun Zwitterionic Bilayer Membrane for the Separation of Hydraulic Fracturing-Produced Water via Membrane Distillation" Membranes 10, no. 12: 402. https://doi.org/10.3390/membranes10120402
APA StyleChiao, Y.-H., Yap Ang, M. B. M., Huang, Y.-X., DePaz, S. S., Chang, Y., Almodovar, J., & Wickramasinghe, S. R. (2020). A “Graft to” Electrospun Zwitterionic Bilayer Membrane for the Separation of Hydraulic Fracturing-Produced Water via Membrane Distillation. Membranes, 10(12), 402. https://doi.org/10.3390/membranes10120402