1. Introduction
Fluorinated polymers have been used extensively due to their high thermal and oxidative stability, excellent chemical resistance, superior electrical insulating ability, and unique optical properties [
1,
2,
3]. Polytetrafluoroethylene (PTFE) and its copolymers with hexafluoropropylene and perfluoropropylvinyl compounds are crystalline and have poor optical transparency and solubility. Since 1989, several classes of amorphous perfluorinated polymers with extraordinary optical and gas transport properties were developed and commercialized by Dupont (as Teflon
TM AF), Solvay (as Hyflon
® AD), and Asahi Glass (as Cytop
TM) [
4,
5,
6].
Over the past 30 years, gas transport properties of these amorphous perfluoropolymers have also been investigated. These perfluoropolymers usually have high gas permeability, significant gas selectivities for some gas pairs (such as He/CO
2 and He/CH
4), and resistance to hydrocarbon-induced plasticization [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17]. They also exhibit better resistance to thin-film physical aging than hydrocarbon polymers [
18,
19]. Due to these attributes, they have been used in membrane separation applications for some gases.
Various new perfluorodioxolane polymers have been synthesized over the past two decades. These perfluoropolymers are not only amorphous and soluble in fluorinated solvents, but also form thin and continuous films. Recently, we have discovered that these perfluorodioxolane films also show extraordinary gas transport properties and low swelling behavior in the presence of vapors and liquids, which make them appealing as gas separation membrane materials [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29].
2. Synthesis and Physical-Chemical Properties of Perfluoropolymers
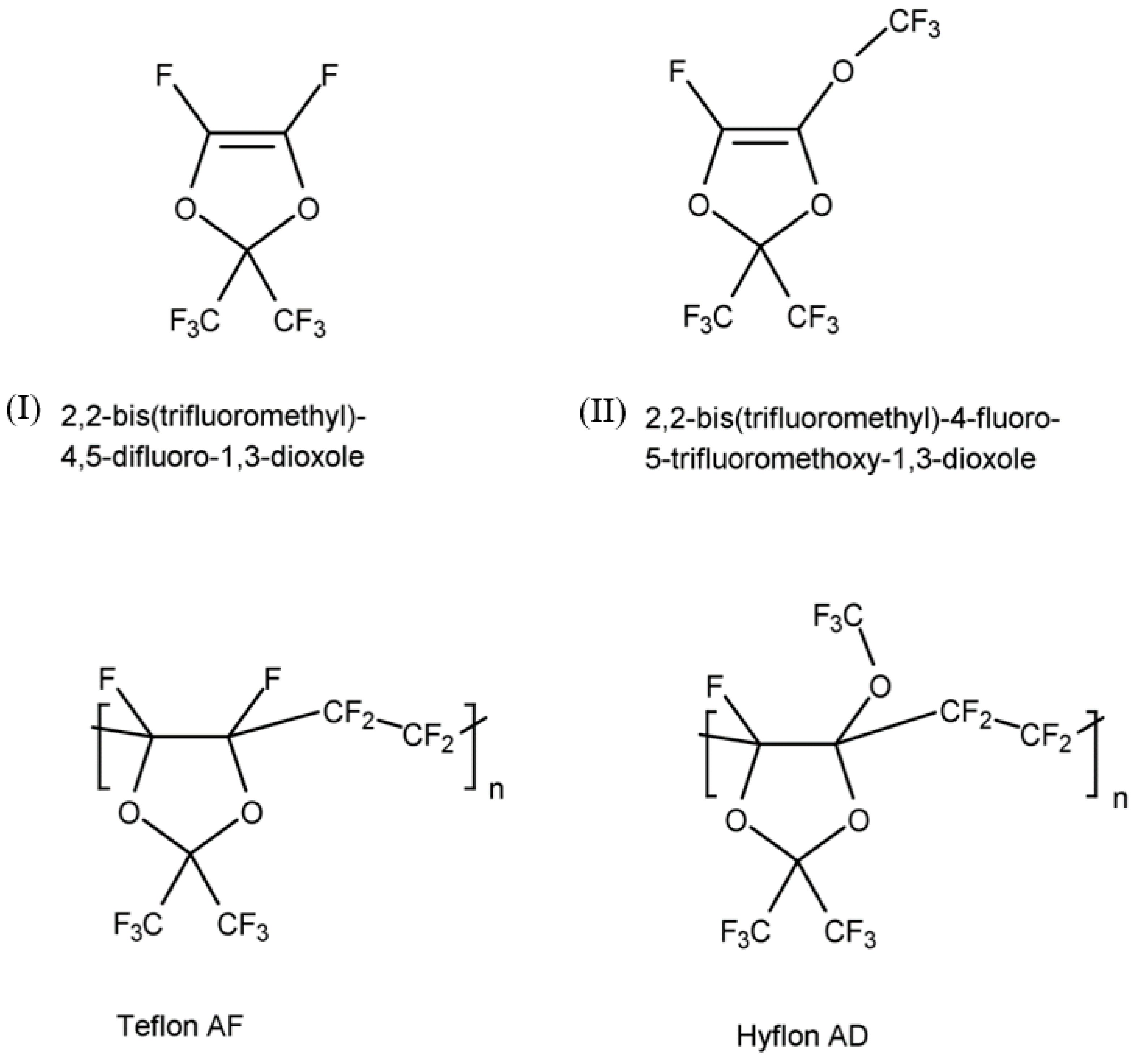
Teflon AF and Hyflon AD are a family of copolymers of tetrafluoroethylene (TFE) with (I) 2,2-bis(trifluoromethyl)-4,5-difluoro-1,3-dioxole and (II) 2,2-bis(trifluoromethyl)-4-fluoro-5-trifluoromethoxy-1,3-dioxole, respectively (
Figure 1). The homopolymers of (I) and (II) are difficult to process as evidenced by their high glass transition temperature (T
g). As a result, copolymerization of these monomers with other olefins is used to improve the processability. In the case of copolymers of TFE with (I) and (II), T
g decreases drastically as the molar percentage of TFE increases. For example, T
gs of Teflon AF 1600 containing 65 mol% dioxole (I) and Teflon AF 2400 containing 87 mol% dioxole become 160 °C and 240 °C, respectively. The T
gs of Hyflon AD 60 containing 60 mol% dioxole (II) and Hyflon AD 80 containing 85 mol% dioxole are 100 °C and 135 °C, respectively, while the T
g of homopolymer (II) reaches 170 °C [
8].
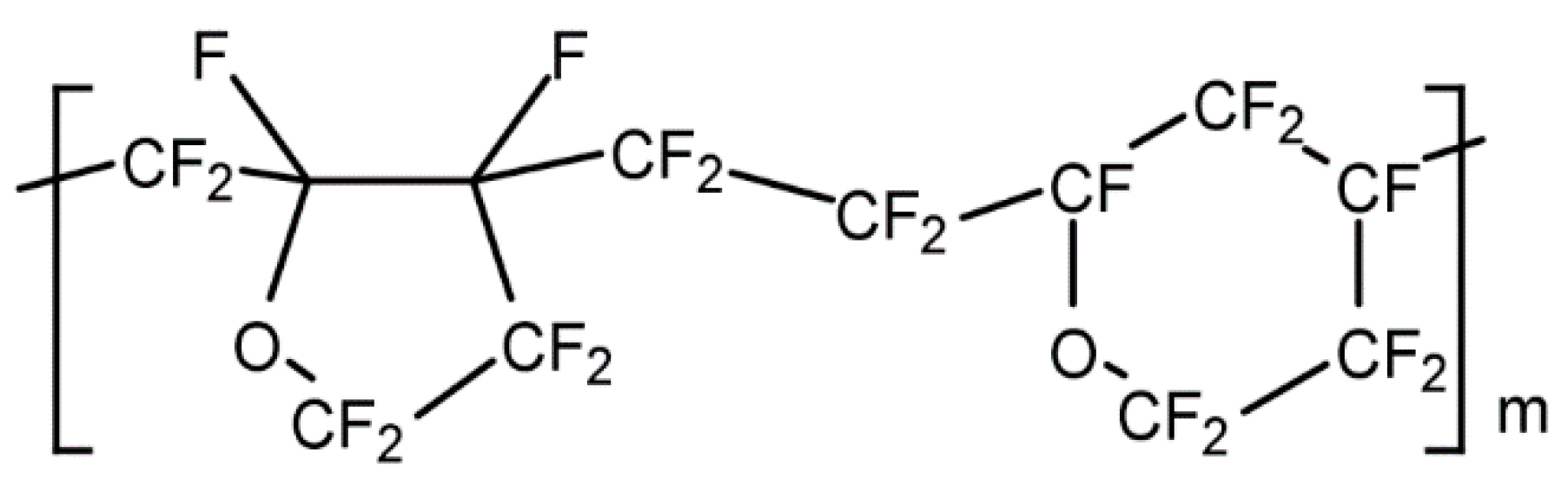
In addition, Cytop is a homopolymer obtained by cyclopolymerization of a perfluorodiene, perfluoro-(4-vinyloxy-1-butene) specifically. Cytop is formed by penta- and hexa-cyclic structures, and the five-membered ring structure is predominant [
8] (
Figure 2).
Table 1 summarizes the physical and optical properties of these commercially available perfluoropolymers. These perfluoropolymers are completely amorphous, contain no hydrogen atoms, show excellent chemical and thermal stability, and are soluble in fluorinated solvents, such as hexafluorobenzene and perfluorohexane. The dielectric constants of these fluoropolymers are low, and they are almost unaffected by humidity.
The commercial amorphous perfluoropolymers exhibit excellent properties in many aspects. However, the preparation methods of these fluorinated polymers are usually complex and expensive, and the T
g of Cytop is relatively low. In order to overcome these limitations, we have synthesized various perfluoro-2-methylene-1,3-dioxolanes, the structures and corresponding T
g of their polymers are shown in
Figure 3 [
22].
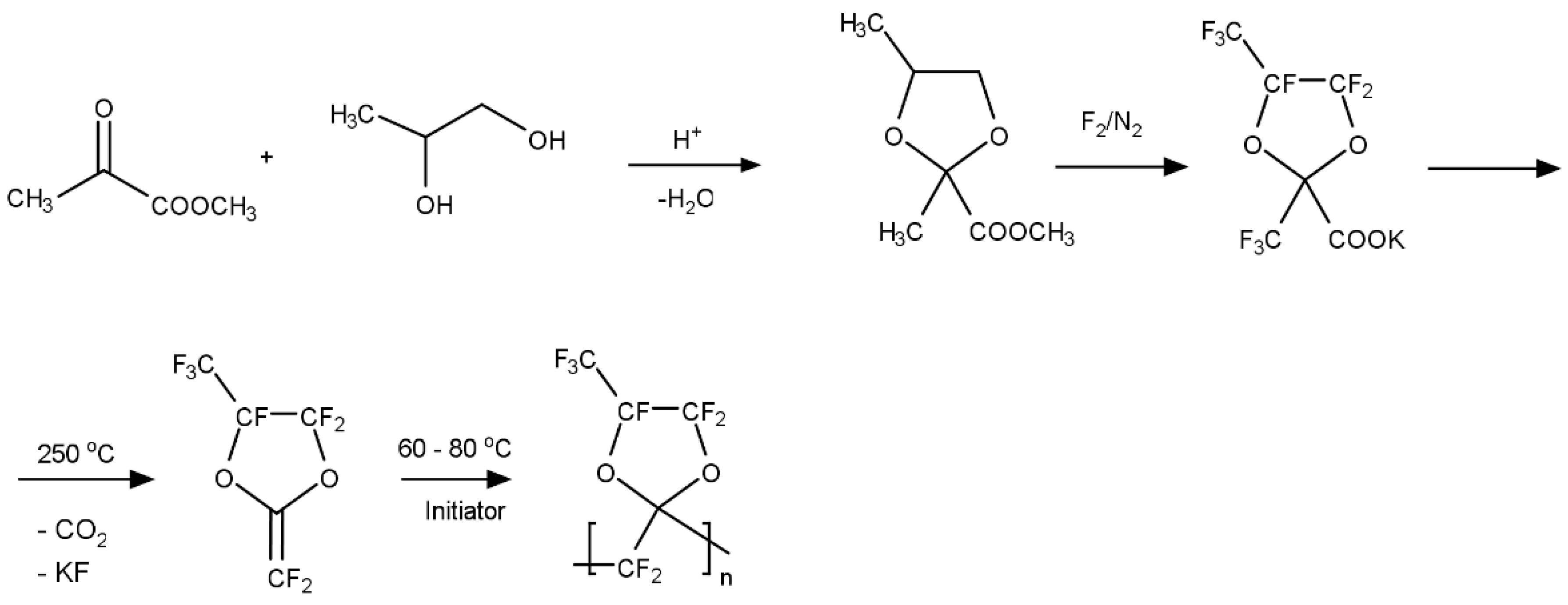
These perfluorodioxolane monomers were prepared by direct fluorination of the corresponding hydrocarbon monomers.
Figure 4 illustrates a typical synthetic scheme for a perfluorodixolane monomer and its polymer. The monomer is polymerized using a free radical initiator, such as a perfluorobenzyl peroxide. The peroxide was decomposed at 60–80 °C by a homolysis mechanism resulting in the formation of pentafluorophenyl radicals, which initiate polymerization and end up as structural units at the polymer chain end. This polyperfluoro(2-methylene-4-methyl-1,3-dioxolane), poly(PFMMD), was also synthesized using a perfluorinated precursor (perfluoro-propyleneoxide) as shown in
Figure 5 [
23].
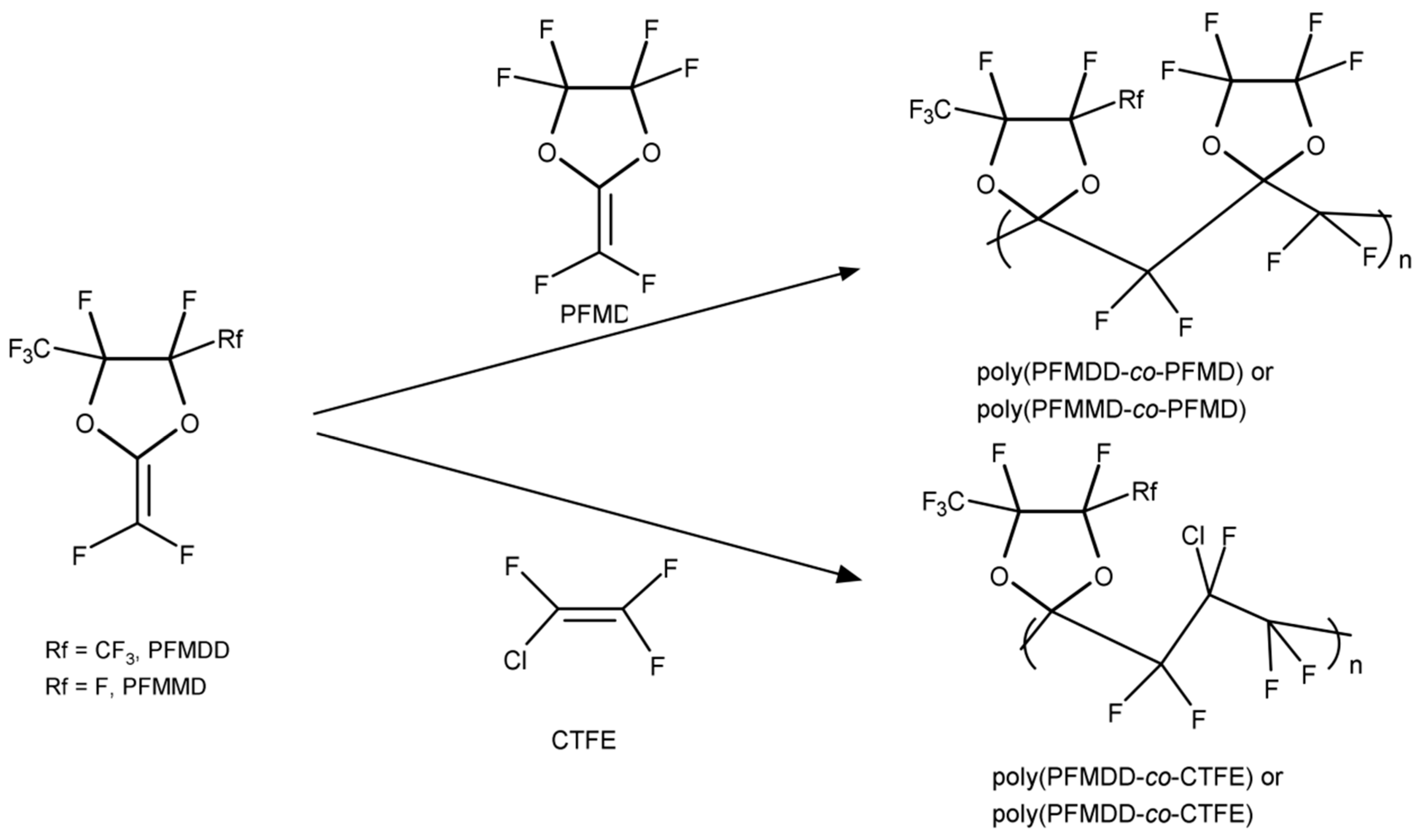
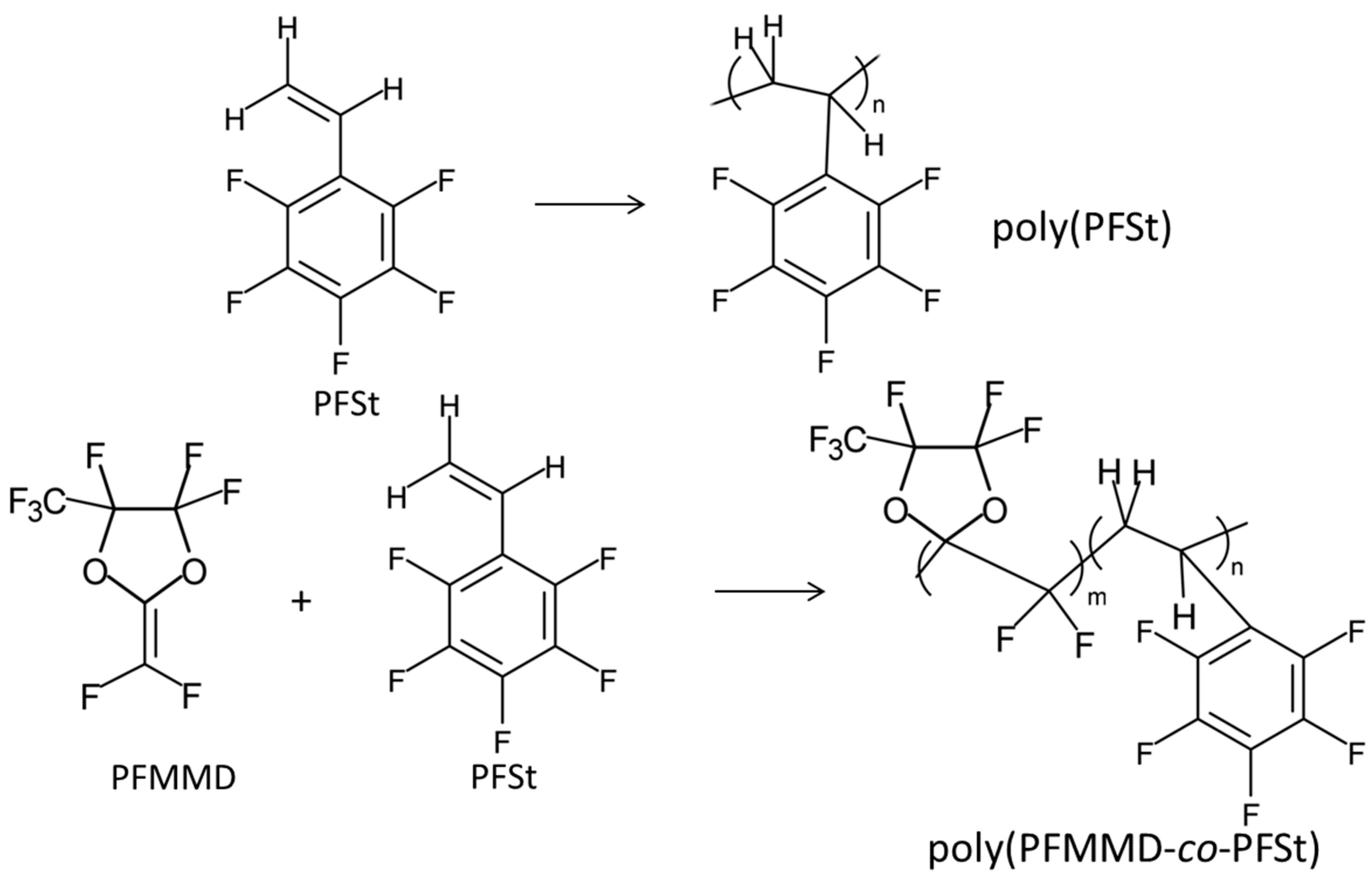
These perfluorodioxolane monomers can be copolymerized with other existed fluorovinyl monomers, as well as with each other (A-H) to form amorphous copolymers that are soluble in fluorinated solvents. The composition determines the T
g of these copolymers. For example, when monomers D and H in
Figure 3 are copolymerized, as the relative amount of monomer D increases from 20 to 75 mol%, the T
g varies from 105 to 155 °C accordingly. The perfluorodioxolane monomers can also be copolymerized with other fluorovinyl monomers such as chlorotrifluoroethylene (CTFE).
Figure 6 shows typical examples of the copolymers [
24,
27].
These copolymers are also amorphous and soluble in only fluorinated solvents, and their physical properties are summarized in
Table 2. When PFMMD was copolymerized with pentafluorostyrene (PFSt), the copolymers obtained were soluble in common organic solvents such as acetone and THF (
Figure 7).
3. Gas Transport Properties
The performance of non-porous polymers is evaluated in terms of permeability and selectivity. Permeability (
P) is defined in Equation (1) with the pressure difference (∆
p), thickness (
), and normalized flux (
n) [
28,
30,
31]:
Permeability has units of Barrer, where 1 Barrer = 10−10 cm3 (STP) cm/(cm2 s cmHg). For asymmetric membranes with an unknown selective layer thickness, the gas permeation property is characterized using the term of permeance (P/l), which is expressed in units of GPU, where 1 GPU = 10−6 cm3(STP)/(cm2 s cmHg).
In the solution-diffusion model, permeability can be expressed as the product of the concentration-averaged diffusion coefficient (
, cm
2/s) and the sorption coefficient (
S, cm
3(STP) cm
−3 atm
−1), which represent the kinetic and thermodynamic components of the transport process, respectively [
30]:
The selectivity,
, is defined as the ratio of the permeabilities of two permeating species,
A and
B:
where
DA/DB is the diffusivity selectivity, and
SA/SB is the solubility selectivity. Modern polymeric membrane materials are designed and engineered by tailoring the sorption and diffusion behavior for selectivity improvement [
31,
32].
4. Gas Separation Properties of Perfluorodioxolane Polymers
Two perfluorodioxolane polymers, poly(PFMD) and poly(PFMMD) (as shown in
Figure 6), were fabricated into 10–50 µm thin films by solvent casting or melt pressing. The films were completely dried by heating at 10 °C below the T
g of the polymers in air before the gas permeation tests.
Figure 8 shows the gas permeability of CH
4, N
2, Ar, CO
2, H
2, and He in these two perfluorodioxolane films as a function of feed pressure at 35 °C. Gas permeability is independent of the feed pressure, suggesting that the films were defect-free [
28].
Table 3 summarizes the gas separation properties of the perfluoropolymers. Poly(PFMD) and Cytop have similar fractional free volume (FFV) and T
g. However, poly(PFMD) is less permeable (except for He) and much more selective than Cytop. Similarly, poly(PFMMD) has lower N
2 and CO
2 permeability than Hyflon AD 80 and much higher selectivity, while their FFV and T
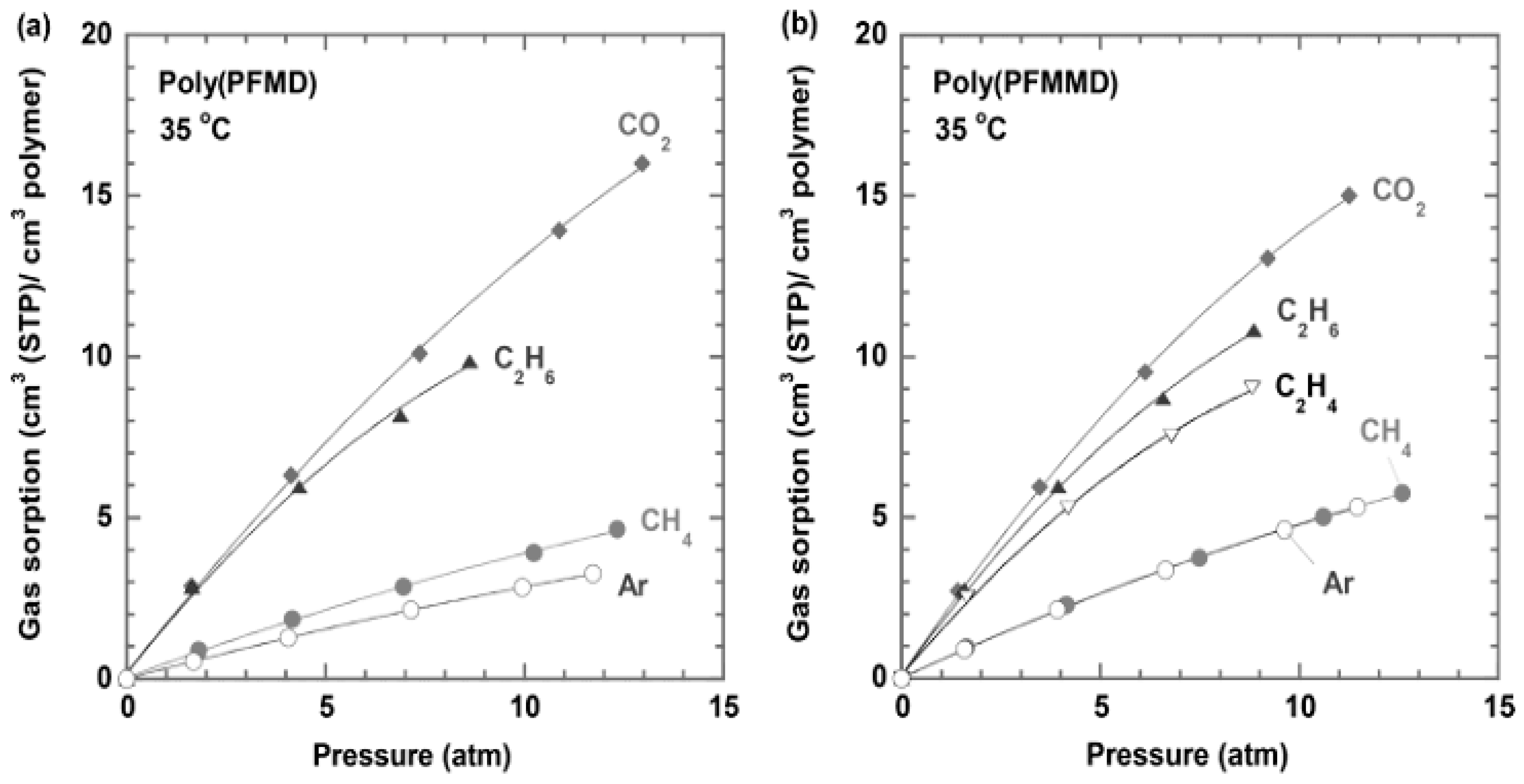
g are similar. Gas solubility of these perfluorodioxolane polymers is generally low and depends on the applied pressure, as shown in
Figure 9 [
28].
Poly(PFMMD) was directly compared with its analog hydrocarbon polymer, poly(4-methyl-2-methylene-1,3-dioxolane) (poly(MMD)) [
16]. Poly(PFMMD) with an FFV of 0.23 exhibits much higher gas permeability than poly(MMD) with an FFV of 0.095. For example, the CO
2 permeabilities of poly(PFMMD) and poly(MMD) at 35 °C are 58 Barrer and 1.3 Barrer, respectively. The bulky –CF
3 and –CF
2 groups hinder perfluoropolymers chain packing and result in much higher FFV, and therefore increased permeability.
Table 4 compares the solubility and diffusivity of CO
2 and CH
4 in the perfluorodioxolane polymers, Hyflon AD 80, and Cytop [
28]. Gas diffusivity can be calculated using Equation (2), and it is usually influenced by the polymer FFV and chain rigidity. Poly(PFMD) exhibits CO
2 diffusivity one order of magnitude lower and CO
2/CH
4 diffusivity ratio 80% higher than poly(PFMMD) because poly(PFMMD) has a bulky CF
3 substituent on the dioxolane ring and thus higher T
g and FFV.
Interestingly, poly(PFMD) shows lower gas diffusivity than its counterpart commercial perfluoropolymer (Cytop) despite their similar T
g and FFV, and poly(PFMMD) exhibits much lower diffusivity than Hyflon AD 80. On the other hand, the perfluorodioxolane polymers show higher diffusivity selectivity (stronger size-sieving ability) than the commercial perfluoropolymers with similar free volume. These results can be ascribed to the narrower pore connections and larger microcavities in perfluorodioxolane polymers compared to their commercial counterparts, as evidenced by the d-spacing values from the WAXD measurements [
28].
Poly(PFMD) and poly(PFMMD) also exhibit a solubility selectivity up to 65% larger relative to commercial perfluoropolymers, especially for separations involving CO
2. The molecular origin of this behavior, which has been investigated using the lattice fluid theory [
29], lies in the more favorable CO
2 interaction with poly(PFMD) and poly(PFMMD) than commercial perfluoropolymers. This favorable interaction stems, in turn, from the higher oxygen/carbon ratio exhibited by poly(PFMD) and poly(PFMMD) relative to Teflon AF. Specifically, the oxygen/carbon ratio, which is 2:4 for poly(PFMD), 2:5 for poly(PFMMD), and 2:5.3 for Teflon AF 2400. Polar C–O–C bonds are known to interact favorably with CO
2 molecules, which is consistent with the solubility selectivity increasing in the order: poly(PFMD) > poly(PFMMD) > Teflon AF 2400. Based on lattice fluid modeling, the CO
2-polymer binary interaction becomes more favorable in the order: poly(PFMD) > poly(PFMMD) > Teflon AF 2400. These results mirror the orders of the oxygen/carbon ratio values, as well as of the Hildebrand solubility parameters. Specifically, the Hildebrand solubility parameter exhibited by poly(PFMD) is larger relative to poly(PFMMD), that is, it is closer to the CO
2 value (i.e., 21.8 MPa
0.5).
Equally important, the polymer cohesive energy density, which is represented by the characteristic pressure, p*, in the lattice fluid framework, is the key parameter to tailor solubility selectivity, all other factors (i.e., interactional pattern, free volume) being equal. Typically, solubility selectivity increases with increasing polymer cohesive energy density [
29]. This conclusion is consistent with the lattice fluid characteristic pressure, p*, increasing in the order: poly(PFMD) > poly(PFMMD) > Teflon AF.
5. Gas Separation Properties of Copolymers of Perfluorodioxolane
The gas permeability of these perfluorodioxolane polymers were investigated and summarized in
Table 5. PFMDD (
Figure 6) has two trifluoromethyl groups on the 4′ and 5′ position with
trans and
cis isomers. In this case, the ratio of
trans to
cis isomer is 73 and 27 mol%, and the polymer obtained was completely amorphous and soluble in fluorinated solvent with a T
g of 165 °C. PFMD was also polymerized by a free radical initiator and the obtained polymer is semicrystalline. The T
g and melting point are 106 and 228 °C, respectively. The copolymers of PFDMM and PFMMD with PFMD were obtained (
Figure 6). The monomer reactivity ratios are almost equal and thus the resulting copolymers form an ideal random structure.
Table 5 shows that increasing the PFMD content in the copolymers decreases the gas permeances and increases the gas/CH
4 selectivity due to the more efficient polymer chain packing for the less hindered PFMD, which results in stronger sieving ability [
24].
Table 6 summarizes the gas permeation properties of perfluorodioxolane copolymers with CTFE [
25,
27]. The T
gs of the copolymers decreased as the amount of CTFE was increased. The molecular weights of the copolymers are over 200,000, and they are still amorphous and soluble in fluorinated solvents (up to 50 mol% CTFE). The flexibility and fracture due to elongation of the films increases with increasing CTFE content in the copolymers. Increasing the CTFE content in the copolymers also increases the gas selectivity, especially for some gas pairs, such as He/CH
4 and H
2/CH
4. The introduction of small polar monomers, such as CTFE, increases the efficiency of chain packing, leading to enhanced size sieving ability. Additionally, polychlorotrifluoroethylene (PCTFE) exhibits higher solubility parameter and, thus, higher CO
2/gas solubility selectivity than PTFE [
33], as well as better separation performance for He/H
2 and He/CH
4.
Gas transport properties of various polystyrene polymers have also been investigated. The permeability of gases such as CO
2, CH
4, and N
2 is much higher in pentafluorostyrene (PFSt) than in other substituted polystyrenes [
34]. Pentafluorostyrene is soluble in common organic solvents such as acetone and THF. The T
g is 110 °C and it is thermally stable. The copolymers of PFMMD and PFSt were prepared (
Figure 7). When PFSt content is more than 20 mol% in the copolymer, the polymer was soluble in acetone, and the polymer films obtained were clear and transparent. The gas permeability and selectivity of the polymer are measured. The typical physical data for the copolymer PFMMD and PFSt are shown in
Table 7 [
35].
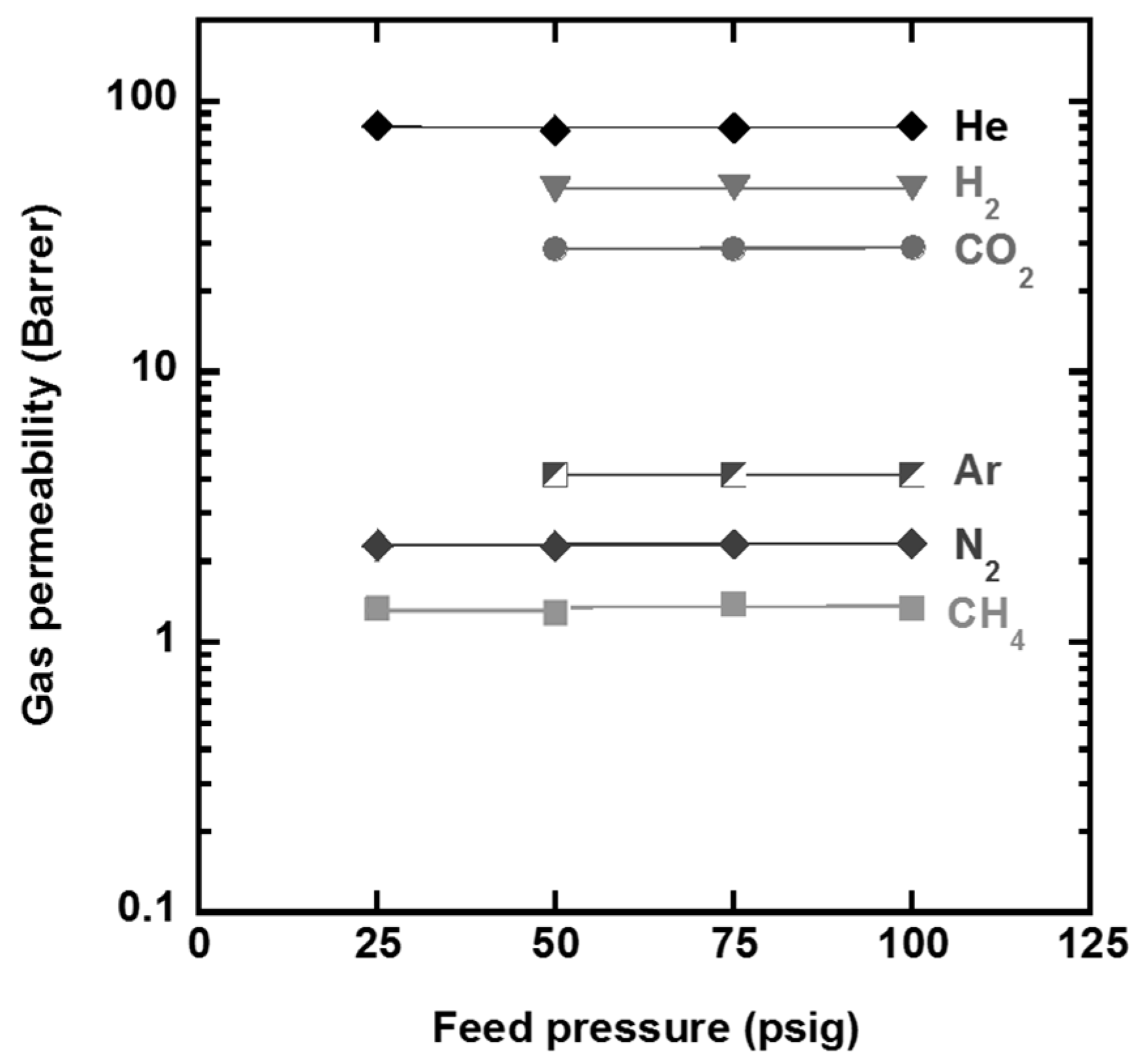
Figure 10 shows the gas permeability of the PFMMD-
co-PFSt film as function of feed pressure and
Table 8 summarizes the typical gas permeability of the film. PFMMD-
co-PFSt exhibits much higher gas selectivity as compared with the neat PFSt polymer, but it shows lower gas selectivity than other PFMMD copolymers.
6. Mechanical Properties of Polyperfluorodioxolane Polymers
Polyperfluorodioxolane polymers show superior gas separation properties. However, the polymer films show some brittleness and can crack upon bending. Polymer mechanical properties can be improved by the addition of plasticizers. Perfluoropolyether (PFPE) is chemically and thermally stable and used as a lubricant in the aerospace and automotive industries. Poly(PFMMD) is relatively compatible with PFPE, and the blended films of poly(PFMMD) and PFPE are clear and transparent.
Table 9 records the mechanical and gas transport properties of the blends of poly(PFMMD) and PFPE, including tensile strength (
σ, MPa) and Young’s modulus (
E, MPa) [
37].
As the PFPE content increases from 0 to 5 wt% and 10 wt%, the breaking elongation of the blends increases from 1.3% to 4.1% and 7.36%, respectively. Young’s modulus decreases with increasing PFPE content, indicating reduced brittleness and increased flexibility. The gas permeance, especially N
2 and O
2, increases with increasing PFPE content, while gas selectivity decreases (
Table 9). This finding is consistent with general transport behavior in plasticized glassy polymers. The plasticizer increases polymer chain motions and thus gas diffusion coefficients, particularly for large molecules. As a result, the size-sieving ability of the plasticized polymeric membrane is reduced, resulting in a decrease in selectivity.
Another way to improve the film flexibility is to copolymerize with a smaller monomer to reduce the steric crowding along the polymer chain while ensuring the amount of the smaller monomer is suitable to retain the amorphous nature. The best co-monomer choice would be TFE. However, TFE is not easy to handle in a common chemical laboratory. On the other hand, chlorotrifluoroethylene (CTFE) can be an attractive alternative because it is commercially available and safer to handle in academic laboratories than TFE. CTFE is the most widely used fluoroalkene after TFE and vinylidene fluoride (VDF) and is readily copolymerized with various vinyl monomers to yield novel copolymers [
33]. We have prepared copolymers of PFMMD and CTFE (cf.
Figure 6).
Table 10 summarizes the physical properties of PFMMD and its copolymers with CTFE. The copolymers are thermally stable near 300 °C under an air atmosphere. Increasing the CTFE content in the copolymers decreases the T
g and Young’s modulus, and increases both tensile strength and elongation at break of the copolymer.
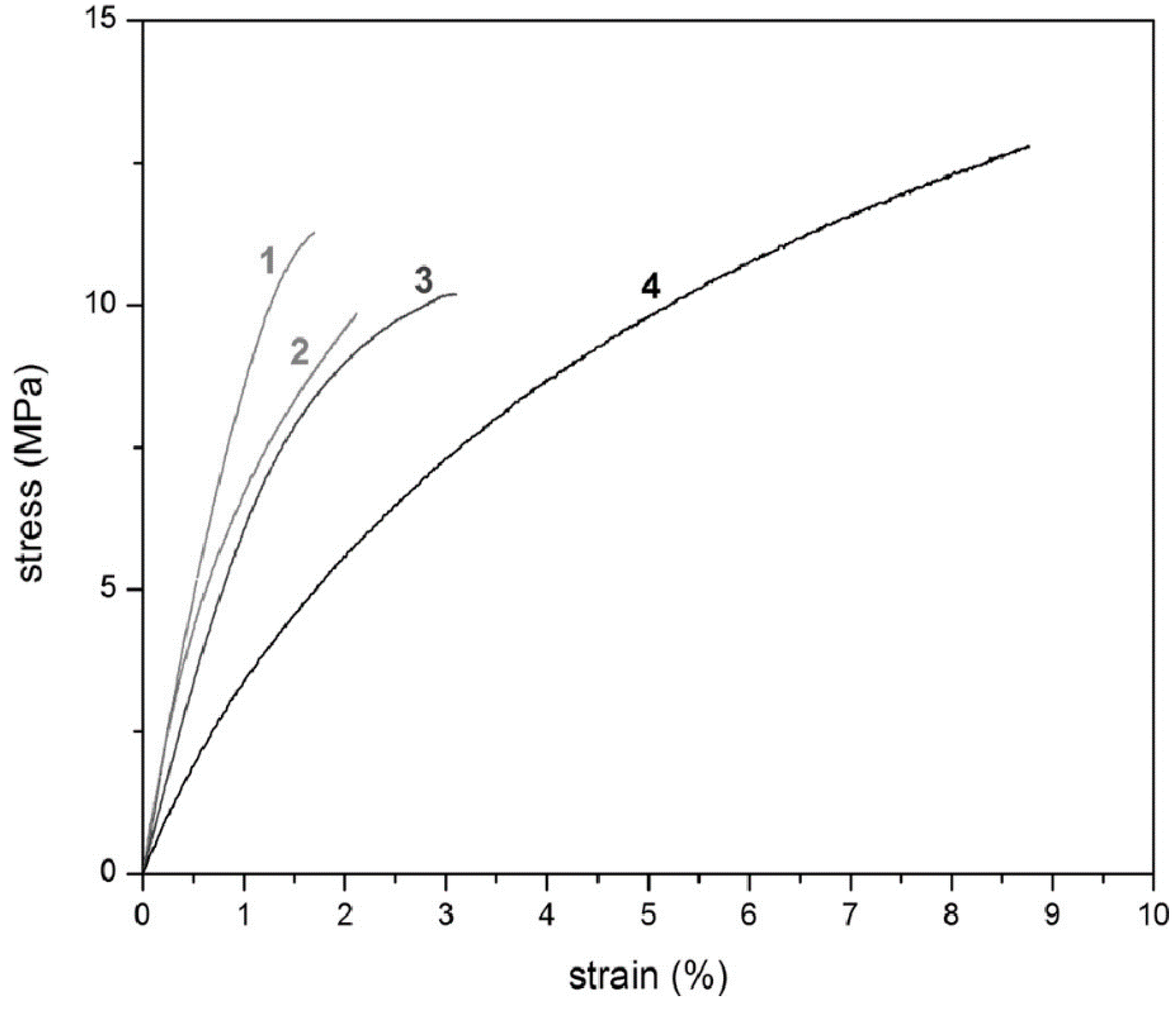
Figure 11 also depicts the stress–strain curves for these copolymers. These results validate that the brittleness of poly(PFMMD) films can be reduced, and film flexibility can be improved when CTFE is introduced in the polymer chains [
25].
8. A relevant Application of Perfluoropolymers: Recovery of Helium from Natural Gas
Helium (He) is extensively used in many industrial, medical, and scientific fields. Helium’s main source is from natural gas. The content of He in natural gas can vary from 0.01 to 4.0 mol% [
41]. Presently, the main industrial method of He production is cryogenic distillation, which is very energy consumable. Another more efficient method for recovering He is proposed by using gas separation membranes, which has attracted research in these fields for many years [
36,
42,
43,
44,
45,
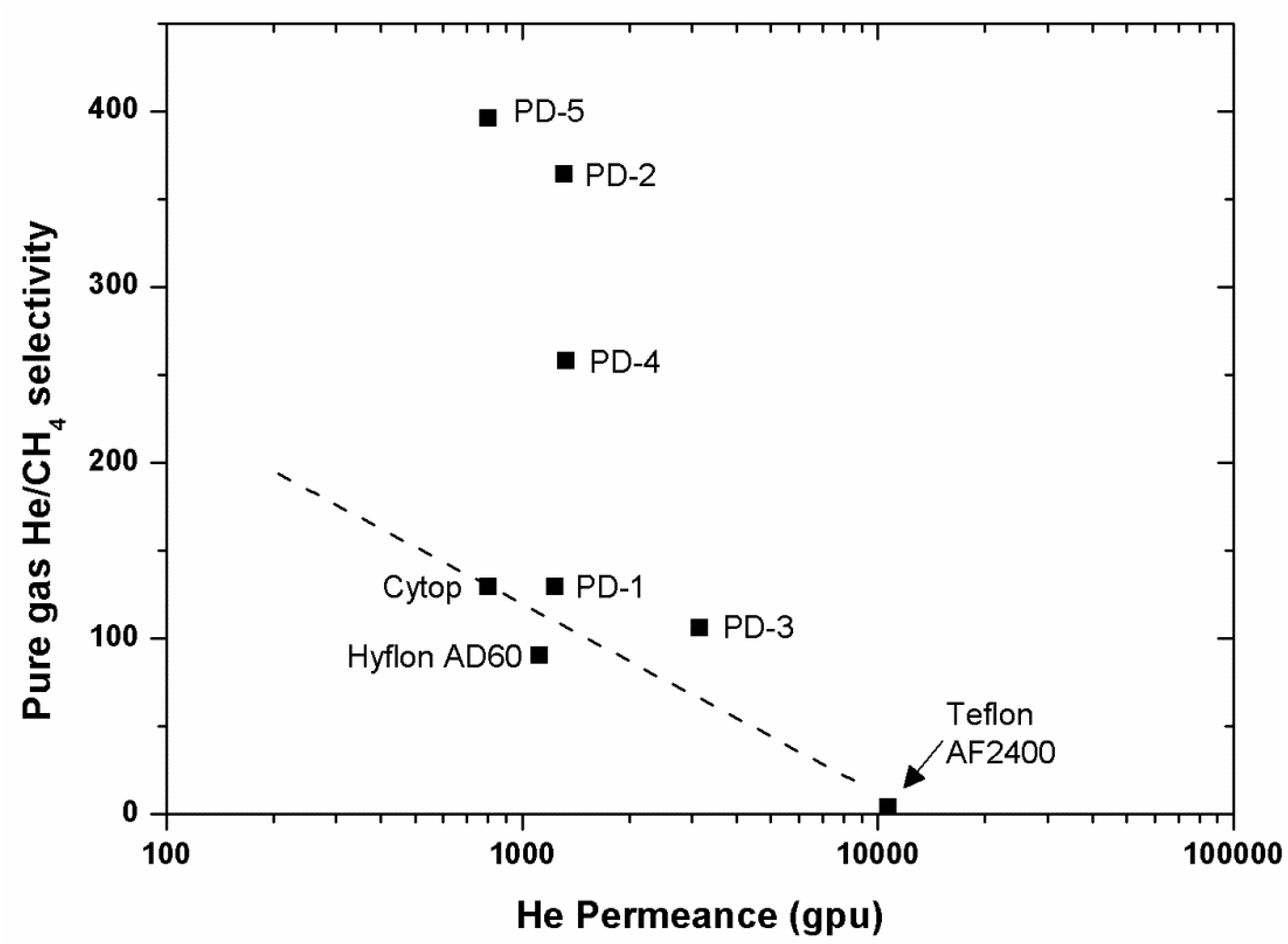
46]. Perfluorodioxolane polymers exhibit much higher He/CH
4 selectivity compared to commercial perfluoropolymers and seem promising. The obtained data points are shown to be above the Robeson upper bounds (
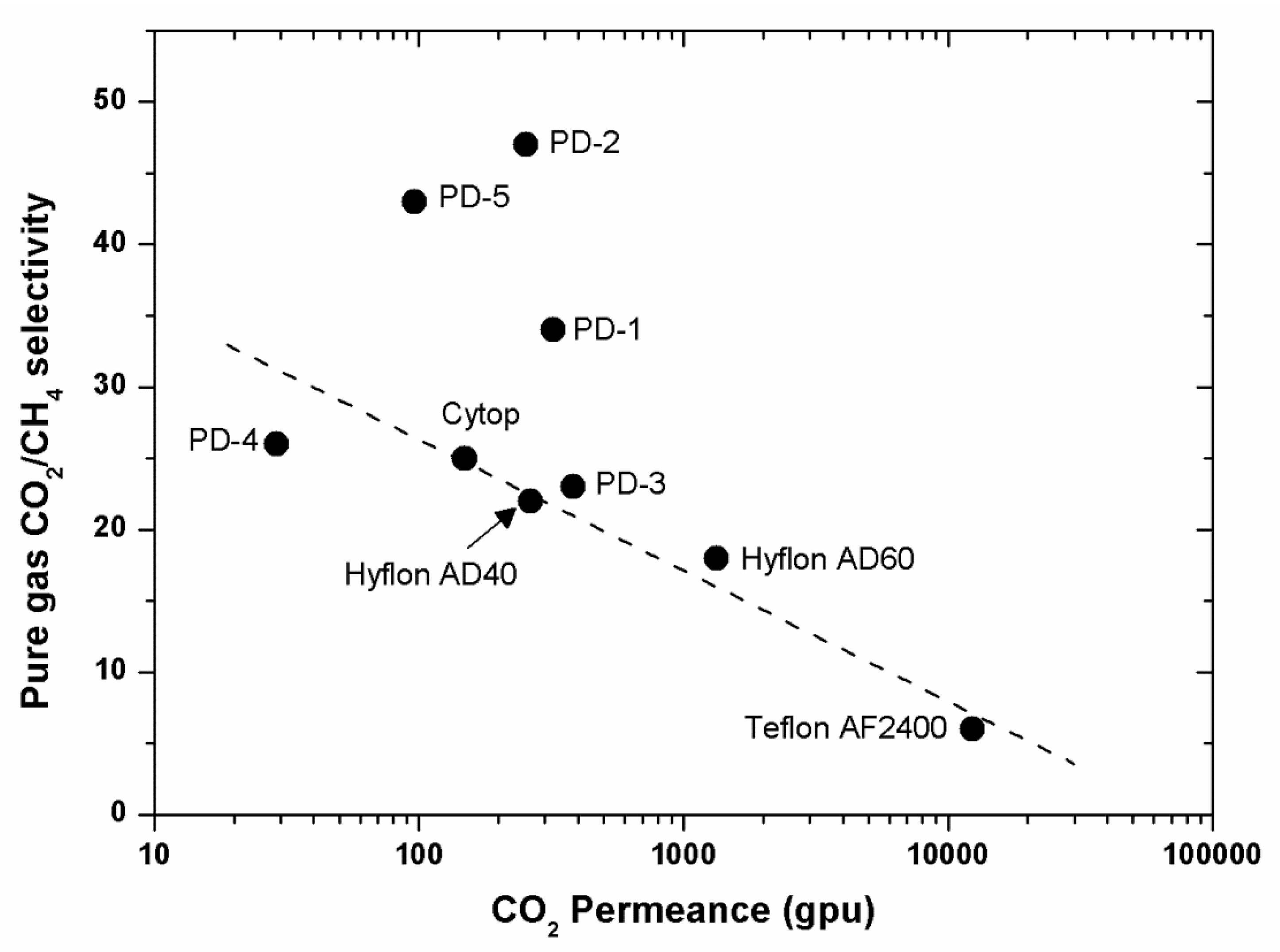
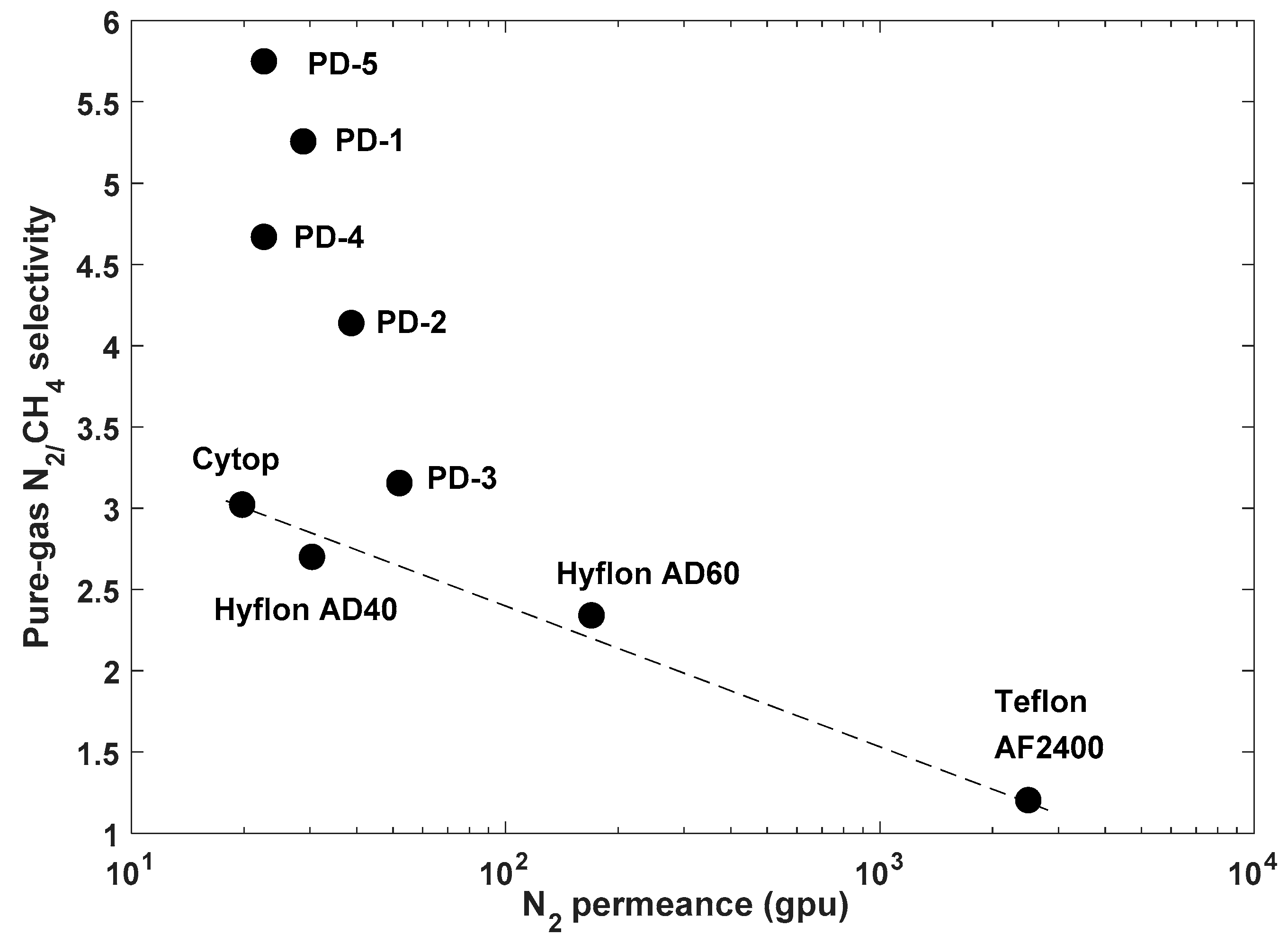
Figure 12,
Figure 13 and
Figure 14).
Fluoropolymers and fluorinated liquids exhibit higher He/gas solubility selectivity than their hydrocarbon analogs [
17,
38], which is consistent with perfluoropolymers having unfavorable interaction with hydrocarbons including CH
4. As a result, perfluoropolymers are very promising materials for He/CH
4 separation.
9. Conclusions
Perfluorodioxolane polymers are highly stable both chemically and thermally. These polymers have the features of being amorphous and soluble in fluorinated solvents. They have also shown relatively high permeability and selectivity for gas pairs such as N2, CO2, H2, He, with CH4 compared with commercial perfluoropolymers, including Teflon AF, Hyflon AD, and Cytop. The gas separation properties of these polymers tend to be near or above the upper bound for a number of important gas pairs. In addition, the preparation of the perfluorodioxolane polymers is rather simple and practical, and it is affordable at large scale. For these reasons, perfluorodioxolane polymer-based membranes are currently being developed for a number of membrane gas separations including helium recovery and CO2 removal from natural gas resource.