Separation of Alcohol-Water Mixtures by a Combination of Distillation, Hydrophilic and Organophilic Pervaporation Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Modelling Schemes
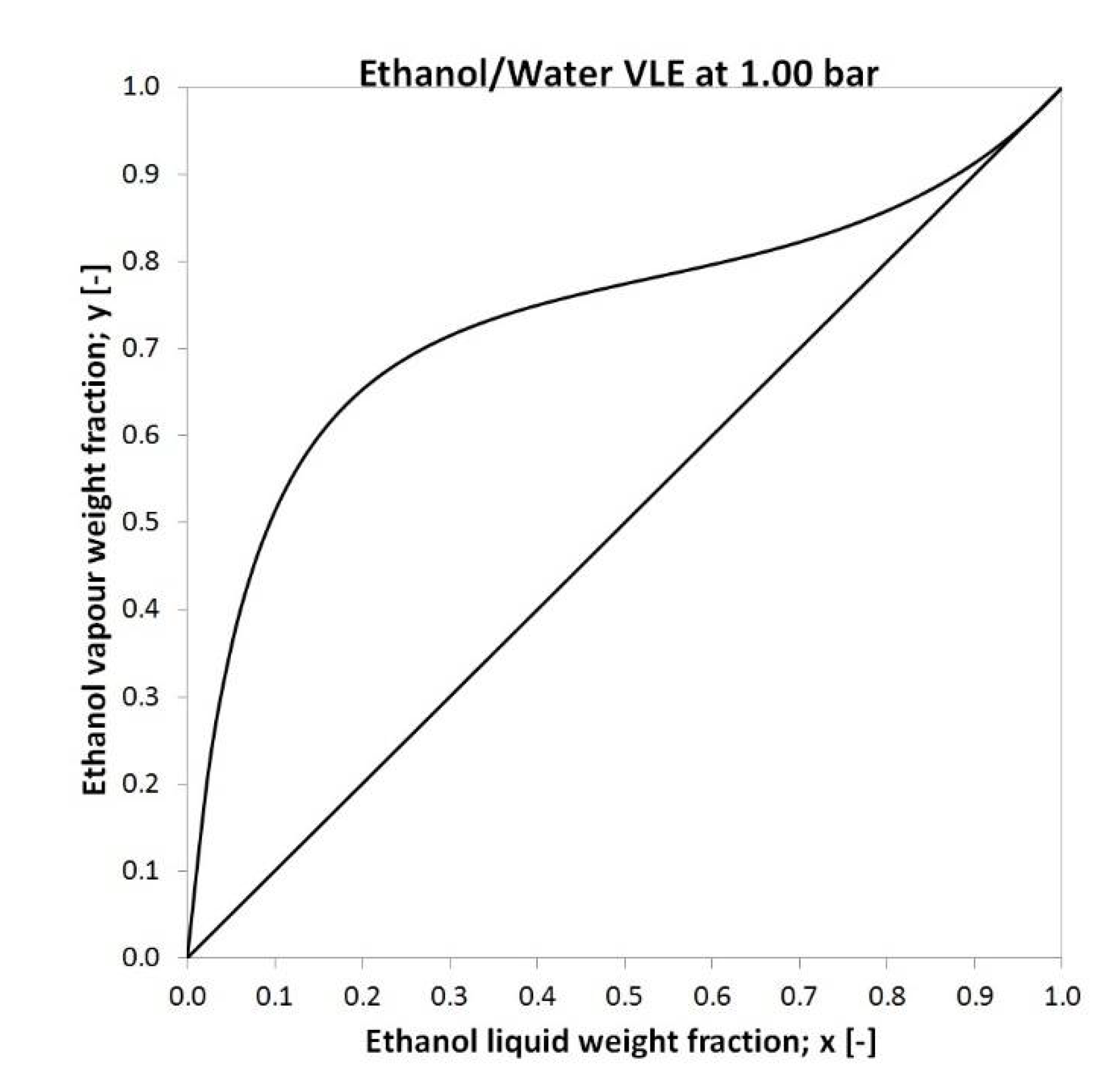
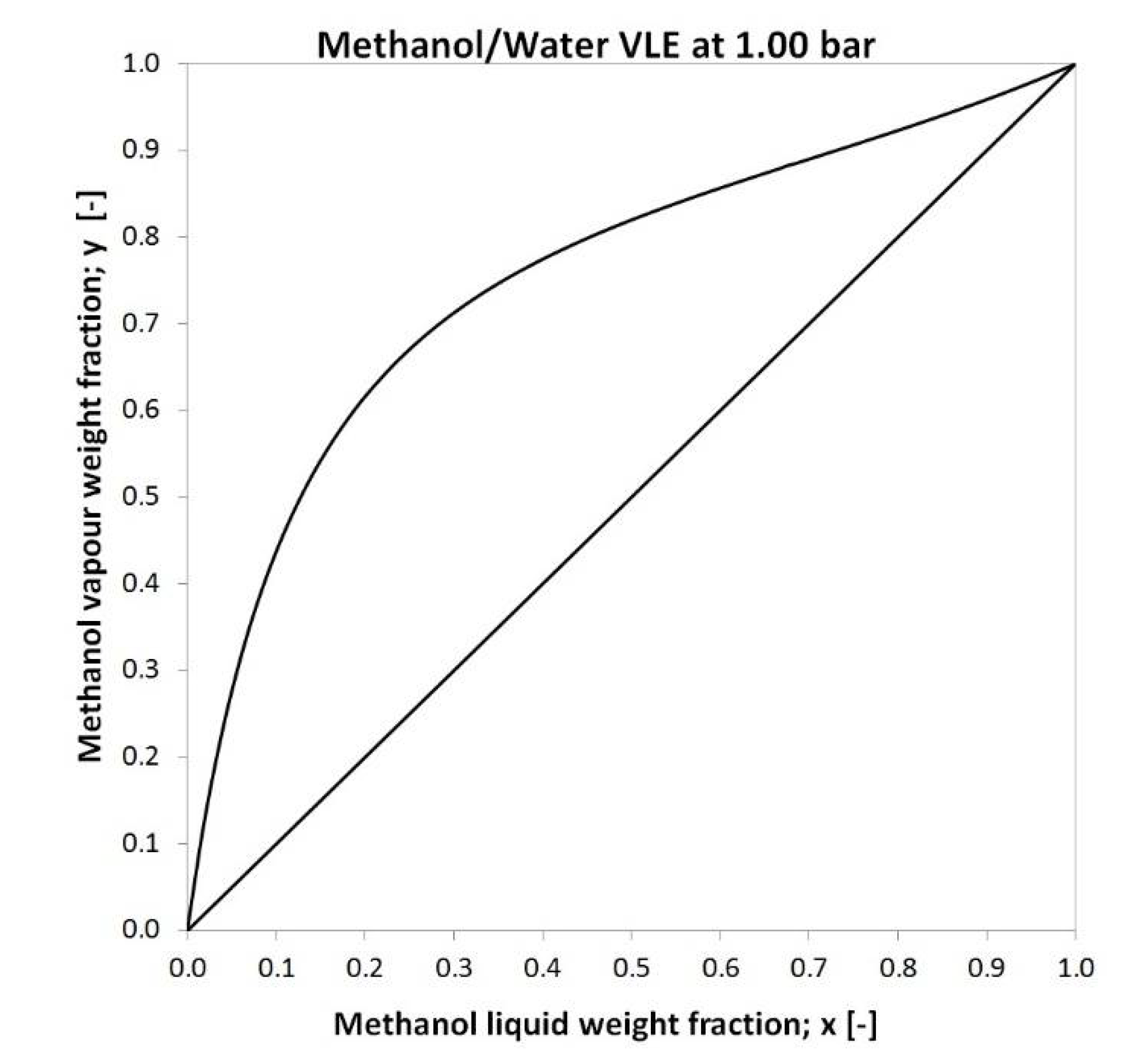
2.2. Membrane Characteristics, Feed Data
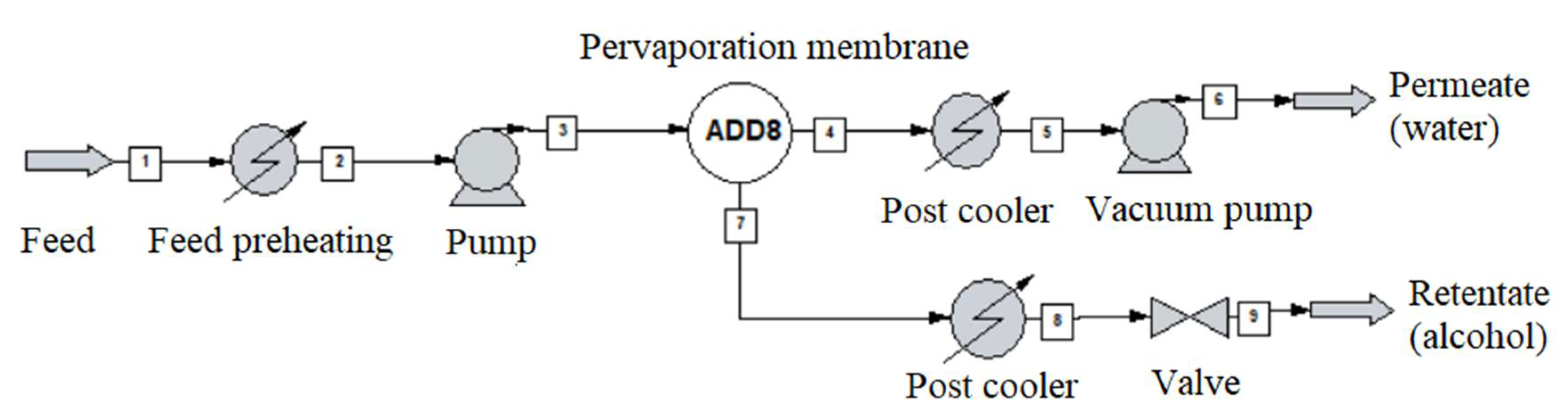
2.3. Pervaporation System
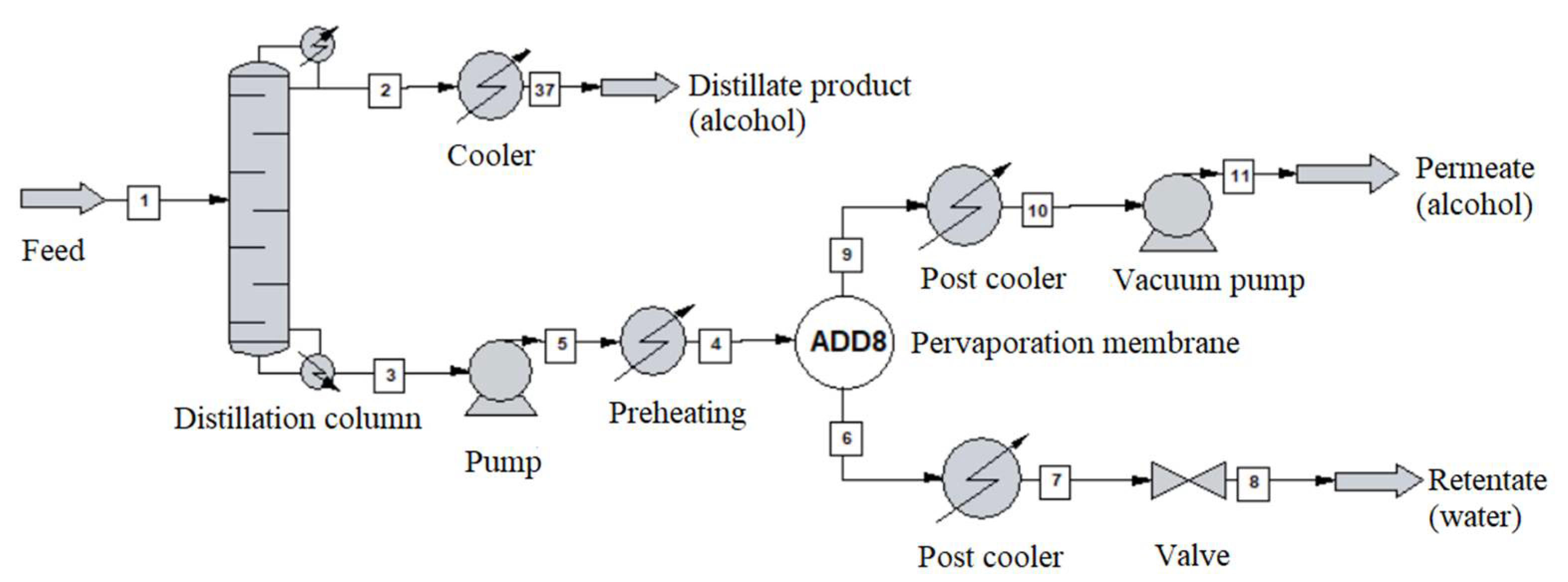
2.4. Hybrid Distillation-Pervaporation System
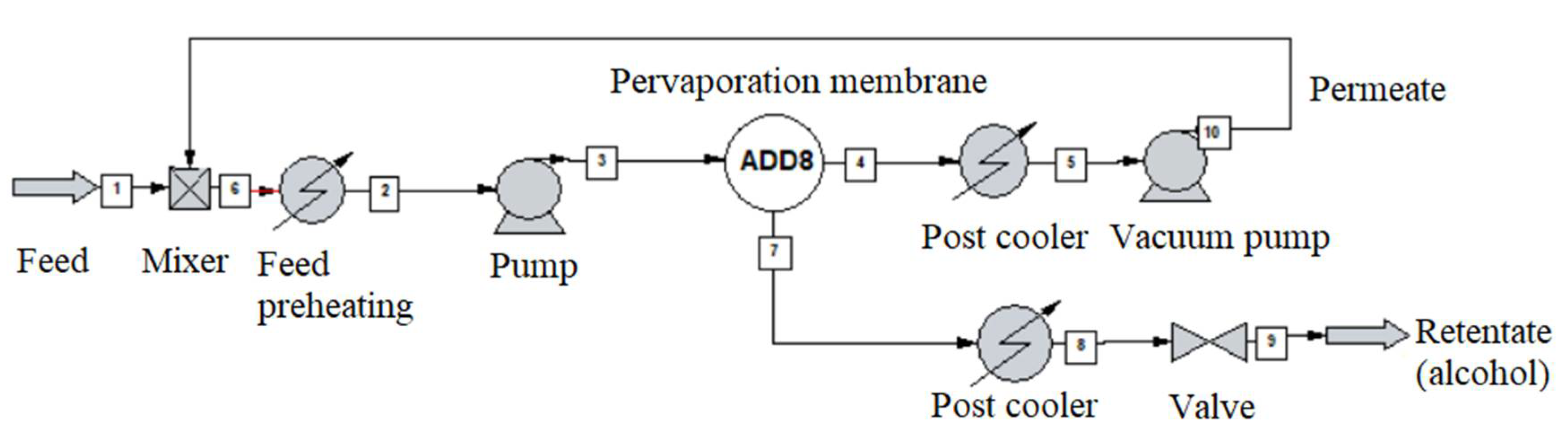
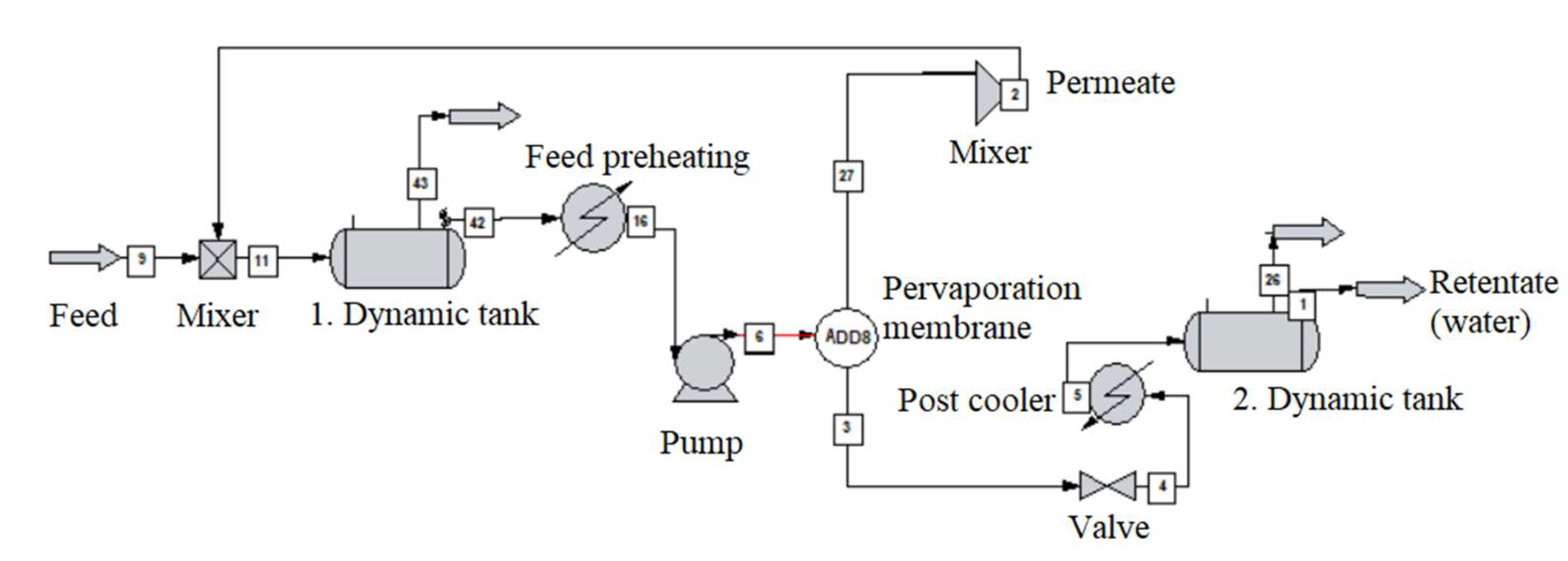
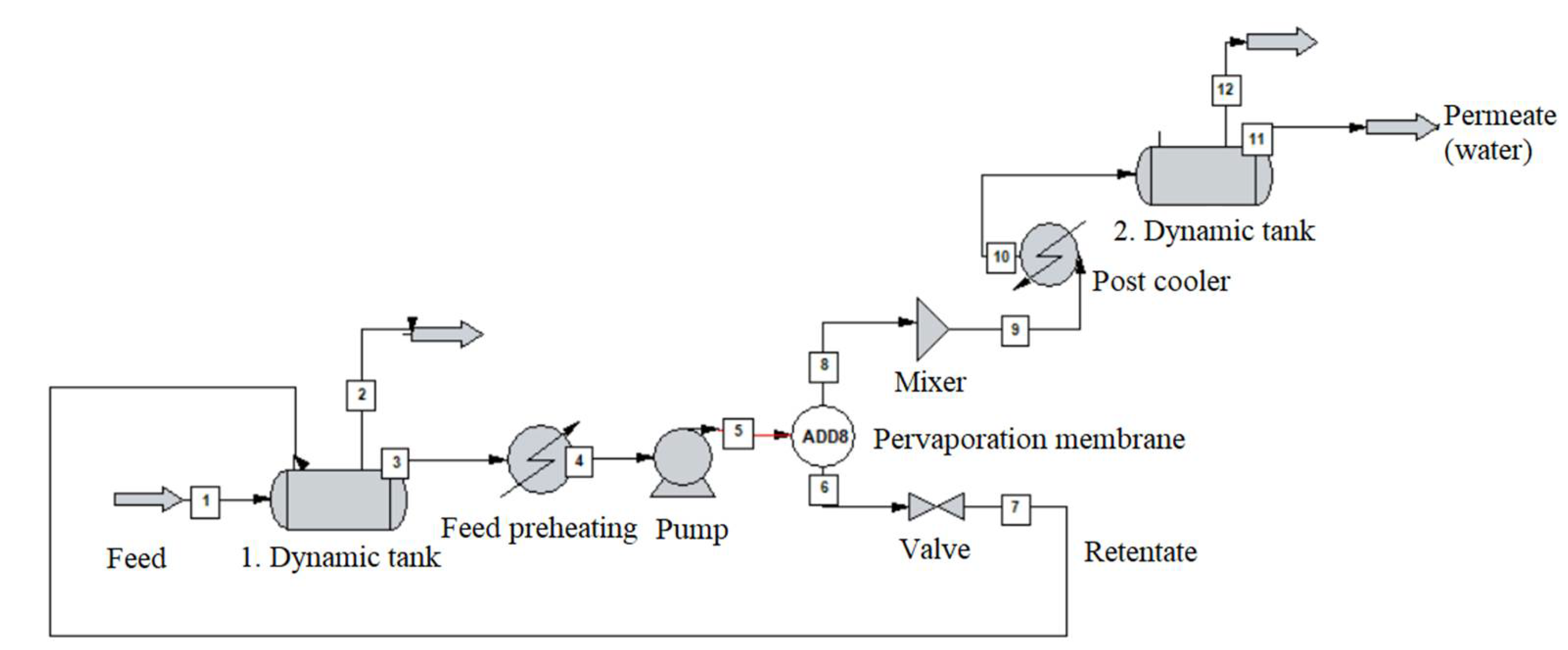
2.5. Dynamic Pervaporation System
3. Results and Discussion
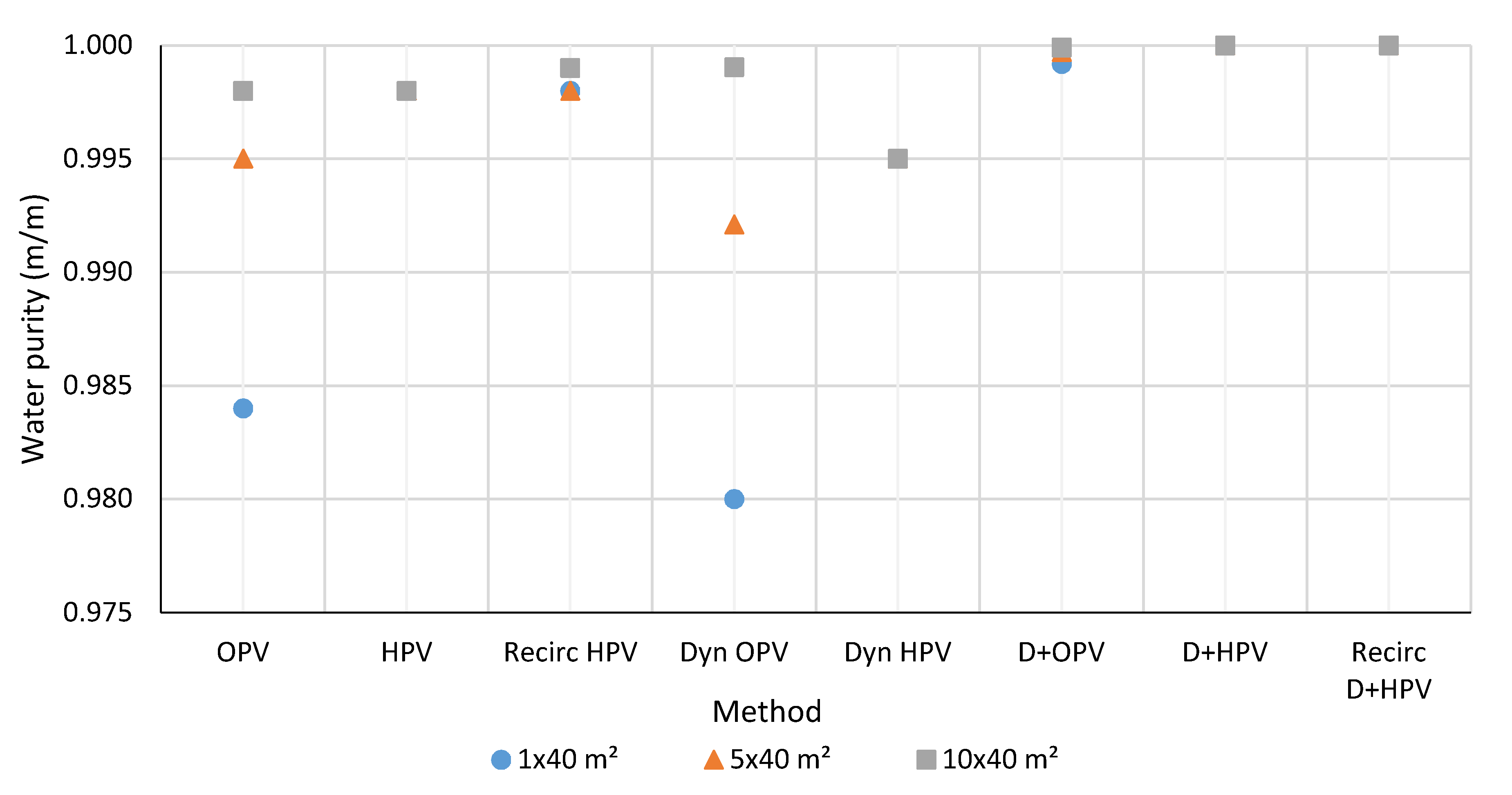
3.1. Water Purity
3.2. Ethanol and Methanol Purity
3.3. Heat Consumptions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Partial flux | |
| Transport coefficient of component i | |
| Q0 | Permeability coefficient of the porous support layer of the membrane |
| Pure i component vapour pressure [bar] | |
| Partial pressure of component i on the vapor phase membrane side [bar] | |
| Partial pressure of component . on the vapour phase membrane side [bar] | |
| Average activity coefficient of component i | |
| Concentration of component i in the feed [m⁄(m%)] | |
| Activation energy of component . in Equation (1) for temperature dependence of the transport coefficient | |
| B | Constant in pervaporation model [-] |
References
- Szanyi, A.; Mizsey, P.; Fonyo, Z. Novel hybrid separation processes for solvent recovery based on positioning the extractive heterogeneous-azeotropic distillation. Chem. Eng. Process. Process Intensif. 2004, 43, 327–338. [Google Scholar] [CrossRef]
- Szanyi, A.; Mizsey, P.; Fonyo, Z. Optimization of Nonideal Separation Structures Based on Extractive Heterogeneous Azeotropic Distillation. Ind. Eng. Chem. Res. 2004, 43, 8269–8274. [Google Scholar] [CrossRef]
- Tóth, A.J.; Szanyi, Á.; Koczka, K.; Mizsey, P. Enhanced separation of highly non-ideal mixtures with extractive heterogeneous-azeotropic distillation. Sep. Sci. Technol. 2016, 51, 1238–1247. [Google Scholar] [CrossRef]
- Toth, A.J.; Haaz, E.; Nagy, T.; Tari, R.; Tarjani, A.J.; Fozer, D.; Szanyi, A.; Koczka, K.-A.; Racz, L.; Ugro, G.; et al. Evaluation of the accuracy of modelling the separation of highly non-ideal mixtures: Extractive heterogeneous-azeotropic distillation. In Computer Aided Chemical Engineering; Espuña, A., Graells, M., Puigjaner, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 40, pp. 241–246. [Google Scholar]
- Toth, A.J.; Szilagyi, B.; Haaz, E.; Solti, S.; Nagy, T.; Tarjani Ariella, J.; Valentinyi, N.; Mizsey, P. Separation of Mixture Containing Maximum Boiling Azeotrope with Extractive Heterogeneous-Azeotropic Distillation. Chem. Eng. Trans. 2018, 69, 571–576. [Google Scholar] [CrossRef]
- Toth, A.J.; Fozer, D.; Nagy, T.; Haaz, E.; Nagy, J.; Mizsey, P. Modelling of extractive heterogeneous-azeotropic distillation in dividing wall column. In Computer Aided Chemical Engineering; Kiss, A.A., Zondervan, E., Lakerveld, R., Özkan, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 46, pp. 235–240. [Google Scholar]
- Toth, A.J.; Szilagyi, B.; Haaz, E.; Solti, S.; Nagy, T.; Szanyi, A.; Nagy, J.; Mizsey, P. Enhanced separation of maximum boiling azeotropic mixtures with extractive heterogeneous-azeotropic distillation. Chem. Eng. Res. Des. 2019, 147, 55–62. [Google Scholar] [CrossRef]
- Laroche, L.; Andersen, H.W.; Morari, M.; Bekiaris, N. Homogeneous azeotropic distillation: Comparing entrainers. Can. J. Chem. Eng. 1991, 69, 1302–1319. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, W.; Shan, J.; Qiu, T. Comparison of Heterogeneous Azeotropic Distillation and Extractive Distillation Methods for Ternary Azeotrope Ethanol/Toluene/Water Separation. Comput. Chem. Eng. 2017, 100. [Google Scholar] [CrossRef]
- Toth, A.J.; Andre, A.; Haaz, E.; Mizsey, P. Modelling of organophilic pervaporation to compete with distillation. In Computer Aided Chemical Engineering; Kravanja, Z., Bogataj, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 343–348. [Google Scholar]
- Tóth, A.J.; Haáz, E.; Nagy, T.; Tarjáni, A.J.; Fózer, D.; André, A.; Valentínyi, N.; Mizsey, P. Treatment of pharmaceutical process wastewater with hybrid separation method: Distillation and hydrophilic pervaporation. Waste Treat. Recovery 2018, 3, 8–13. [Google Scholar] [CrossRef]
- Fontalvo, J.; Keurentjes, J.T.F. A hybrid distillation–pervaporation system in a single unit for breaking distillation boundaries in multicomponent mixtures. Chem. Eng. Res. Des. 2015, 99, 158–164. [Google Scholar] [CrossRef]
- León, J.A.; Schuur, B.; Fontalvo, J. Hybrid distillation-pervaporation in a single unit: Experimental proof of concept in a batch operation. Sep. Purif. Technol. 2020, 252, 117464. [Google Scholar] [CrossRef]
- León, J.A.; Fontalvo, J. Analysis of a hybrid distillation-pervaporation column in a single unit: Intermediate membrane section in the rectifying and stripping section. Can. J. Chem. Eng. 2020, 98, 2227–2237. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Lone, S. Hybrid Process (Pervaporation-Distillation): A Review. Int. J. Sci. Eng. Res. 2012, 3, 549–553. [Google Scholar]
- Zhang, T.; Li, A.; Xu, X.; Ma, Y.; Xu, D.; Zhang, L.; Gao, J.; Wang, Y. Separation of azeotropic mixture (acetone + n-heptane) by extractive distillation with intermediate and heavy boiling entrainers: Vapour-liquid equilibrium measurements and correlation. J. Chem. Thermodyn. 2021, 152, 106284. [Google Scholar] [CrossRef]
- Zhao, T.; Geng, X.; Qi, P.; Zhu, Z.; Gao, J.; Wang, Y. Optimization of liquid–liquid extraction combined with either heterogeneous azeotropic distillation or extractive distillation processes to reduce energy consumption and carbon dioxide emissions. Chem. Eng. Res. Des. 2018, 132, 399–408. [Google Scholar] [CrossRef]
- Arifin, S.; Chien, I.L. Design and Control of an Isopropyl Alcohol Dehydration Process via Extractive Distillation Using Dimethyl Sulfoxide as an Entrainer. Ind. Eng. Chem. Res. 2008, 47, 790–803. [Google Scholar] [CrossRef]
- Haáz, E.; Szilágyi, B.; Fózer, D.; Tóth, A.J. Combining extractive heterogeneous-azeotropic distillation and hydrophilic pervaporation for enhanced energetic separation of non-ideal ternary mixtures. Front. Chem. Sci. Eng. 2020, 14, 913–927. [Google Scholar] [CrossRef]
- Eliceche, A.M.; Carolina Daviou, M.; Hoch, P.M.; Ortiz Uribe, I. Optimisation of azeotropic distillation columns combined with pervaporation membranes. Comput. Chem. Eng. 2002, 26, 563–573. [Google Scholar] [CrossRef]
- Meng, J.; Li, P.; Cao, B. High-Flux Direct-Contact Pervaporation Membranes for Desalination. Acs Appl. Mater. Interfaces 2019, 11, 28461–28468. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Pervaporation and vacuum membrane distillation processes: Modeling and experiments. Aiche J. 2004, 50, 1697–1712. [Google Scholar] [CrossRef]
- Verhoef, A.; Degrève, J.; Huybrechs, B.; van Veen, H.; Pex, P.; Van der Bruggen, B. Simulation of a hybrid pervaporation–distillation process. Comput. Chem. Eng. 2008, 32, 1135–1146. [Google Scholar] [CrossRef]
- Babaie, O.; Nasr Esfahany, M. Optimization of a new combined approach to reduce energy consumption in the hybrid reactive distillation–pervaporation process. Chem. Eng. Process. Process Intensif. 2020, 151, 107910. [Google Scholar] [CrossRef]
- Tóth, A.J.; Szilágyi, B.; Do Thi, H.T.; Fózer, D.; Selim, A.; Haáz, E. Modelling of Hybrid Method for VOC Removal from Process Wastewater: Distillation and Hydrophilic Pervaporation. Period. Polytech. Chem. Eng. 2020, 64, 364–370. [Google Scholar] [CrossRef]
- Sommer, S.; Melin, T. Design and Optimization of Hybrid Separation Processes for the Dehydration of 2-Propanol and Other Organics. Ind. Eng. Chem. Res. 2004, 43, 5248–5259. [Google Scholar] [CrossRef]
- Toth, A.J. Comprehensive evaluation and comparison of advanced separation methods on the separation of ethyl acetate-ethanol-water highly non-ideal mixture. Sep. Purif. Technol. 2019, 224, 490–508. [Google Scholar] [CrossRef]
- Parvez, A.M.; Luis, P.; Ooms, T.; Vreysen, S.; Vandezande, P.; Degrève, J.; Van der Bruggen, B. Separation of ethyl acetate–isooctane mixtures by pervaporation and pervaporation-based hybrid methods. Chem. Eng. J. 2012, 210, 252–262. [Google Scholar] [CrossRef]
- Tóth, A.J.; Szilágyi, B.; Fózer, D.; Do Thi, H.T.; Selim, A.K.M.; Haáz, E. Separation of acetone-butanol-ethanol (ABE) fermentation products by pervaporation/Aceton-butanol-etanol (ABE) fermentációs termékek elválasztása pervaporáció segítségével. Circ. Econ. Environ. Prot. Körforgásos Gazdaság És Környezetvédelem 2019, 3, 5–19. [Google Scholar]
- Andre, A.; Nagy, T.; Toth, A.J.; Haaz, E.; Fozer, D.; Tarjani, J.A.; Mizsey, P. Distillation contra pervaporation: Comprehensive investigation of isobutanol-water separation. J. Clean. Prod. 2018, 187, 804–818. [Google Scholar] [CrossRef]
- Omidali, M.; Raisi, A.; Aroujalian, A. Separation and purification of isobutanol from dilute aqueous solutions by a hybrid hydrophobic/hydrophilic pervaporation process. Chem. Eng. Process. Process Intensif. 2014, 77, 22–29. [Google Scholar] [CrossRef]
- Cséfalvay, E.; Szitkai, Z.; Mizsey, P.; Fonyó, Z. Experimental data based modelling and simulation of isopropanol dehydration by pervaporation. Desalination 2008, 229, 94–108. [Google Scholar] [CrossRef]
- Kim, H.-G.; Na, H.-R.; Lee, H.R.; Kim, M.I.; Lim, C.-S.; Seo, B. Distillation-pervaporation membrane hybrid system for epichlorohydrin and isopropyl alcohol recovery in epoxy resin production process. Sep. Purif. Technol. 2021, 254, 117678. [Google Scholar] [CrossRef]
- Van Hoof, V.; Van den Abeele, L.; Buekenhoudt, A.; Dotremont, C.; Leysen, R. Economic comparison between azeotropic distillation and different hybrid systems combining distillation with pervaporation for the dehydration of isopropanol. Sep. Purif. Technol. 2004, 37, 33–49. [Google Scholar] [CrossRef]
- Hassankhan, B.; Raisi, A. Separation of isobutanol/water mixtures by hybrid distillation-pervaporation process: Modeling, simulation and economic comparison. Chem. Eng. Process. Process Intensif. 2020, 155, 108071. [Google Scholar] [CrossRef]
- Koczka, K.; Manczinger, J.; Mizsey, P.; Fonyo, Z. Novel hybrid separation processes based on pervaporation for THF recovery. Chem. Eng. Process. Process Intensif. 2007, 46, 239–246. [Google Scholar] [CrossRef]
- Haelssig, J.B.; Thibault, J.; Tremblay, A.Y. Numerical investigation of Membrane Dephlegmation: A hybrid pervaporation–distillation process for ethanol recovery. Chem. Eng. Process. Process Intensif. 2011, 50, 1226–1236. [Google Scholar] [CrossRef]
- Meng, D.; Dai, Y.; Xu, Y.; Wu, Y.; Cui, P.; Zhu, Z.; Ma, Y.; Wang, Y. Energy, economic and environmental evaluations for the separation of ethyl acetate/ethanol/water mixture via distillation and pervaporation unit. Process Saf. Environ. Prot. 2020, 140, 14–25. [Google Scholar] [CrossRef]
- Kunnakorn, D.; Rirksomboon, T.; Siemanond, K.; Aungkavattana, P.; Kuanchertchoo, N.; Chuntanalerg, P.; Hemra, K.; Kulprathipanja, S.; James, R.B.; Wongkasemjit, S. Techno-economic comparison of energy usage between azeotropic distillation and hybrid system for water–ethanol separation. Renew. Energy 2013, 51, 310–316. [Google Scholar] [CrossRef]
- Luis, P.; Amelio, A.; Vreysen, S.; Calabro, V.; Van der Bruggen, B. Simulation and environmental evaluation of process design: Distillation vs. hybrid distillation–pervaporation for methanol/tetrahydrofuran separation. Appl. Energy 2014, 113, 565–575. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 927068. [Google Scholar] [CrossRef]
- Tusel, G.; Ballweg, A. Method and Apparatus for Dehydrating Mixtures of Organic Liquids and Water. US Patent 4405409A, 1983. [Google Scholar]
- Zeng, W.; Li, B.; Li, H.; Jin, H.; Wu, D.; Li, Y. A pervaporation-crystallization (PC) process for simultaneous recovery of ethanol and sodium pyruvate from waste centrifugal mother liquid. J. Membr. Sci. 2021, 619, 118749. [Google Scholar] [CrossRef]
- Johnson, R.A.; Sun, J.C.; Sun, J. A pervaporation–microfiltration–osmotic distillation hybrid process for the concentration of ethanol–water extracts of the Echinacea plant. J. Membr. Sci. 2002, 209, 221–232. [Google Scholar] [CrossRef]
- Rautenbach, R.; Herion, C.; Franke, M. Dehydration of multicomponent organic systems by a reverse osmosis pervaporation-hybrid process-module-, process design and economics. Desalination 1988, 70, 445–453. [Google Scholar] [CrossRef]
- Roza, M.; Maus, E. Industrial experience with hybrid distillation-pervaporation or vapor permeation applications. Distillation & Absorption 2006.
- Zarzo, D. 11-Beneficial uses and valorization of reverse osmosis brines. In Emerging Technologies for Sustainable Desalination Handbook; Gude, V.G., Ed.; Butterworth-Heinemann: MS, USA, 2018; pp. 365–397. [Google Scholar] [CrossRef]
- Cséfalvay, E.; Deák, A.; Farkas, T.; Hanák, L.; Mika, L.T.; Mizsey, P.; Sawinsky, J.; Simándi, B.; Szánya, T.; Székely, E.; et al. Vegyipari Műveletek II.: Anyagátadó műveletek és kémiai reaktorok; Budapest University of Technology and Economics, 2012; pp. 501–518. [Google Scholar]
- Figoli, A.; Santoro, S.; Galiano, F.; Basile, A. Pervaporation membranes: Preparation, characterization, and application. In Pervaporation, Vapour Permeation and Membrane Distillation; Basile, A., Figoli, A., Khayet, M., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 19–63. [Google Scholar] [CrossRef]
- Crespo, J.G.; Brazinha, C. Fundamentals of pervaporation. In Pervaporation, Vapour Permeation and Membrane Distillation; Basile, A., Figoli, A., Khayet, M., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 3–17. [Google Scholar] [CrossRef]
- Soriïn, M.; Ayotte-sauvé, E.; Sadeghiï, F.; Rheault, F. Thermodynamic Equipartition and Energy Efficient Membrane Networks. Int. J. Thermodyn. 2010, 13, 9–13. [Google Scholar]
- Rhim, J.-W.; Park, H.B.; Lee, C.-S.; Jun, J.-H.; Kim, D.S.; Lee, Y.M. Crosslinked poly(vinyl alcohol) membranes containing sulfonic acid group: Proton and methanol transport through membranes. J. Membr. Sci. 2004, 238, 143–151. [Google Scholar] [CrossRef]
- Hsueh, C.L.; Kuo, J.F.; Huang, Y.H.; Wang, C.C.; Chen, C.Y. Separation of ethanol–water solution by poly(acrylonitrile-co-acrylic acid) membranes. Sep. Purif. Technol. 2005, 41, 39–47. [Google Scholar] [CrossRef]
- Nik, O.G.; Moheb, A.; Mohammadi, T. Separation of Ethylene Glycol/Water Mixtures using NaA Zeolite Membranes. Chem. Eng. Technol. 2006, 29, 1340–1346. [Google Scholar] [CrossRef]
- Araki, S.; Gondo, D.; Imasaka, S.; Yamamoto, H. Permeation properties of organic compounds from aqueous solutions through hydrophobic silica membranes with different functional groups by pervaporation. J. Membr. Sci. 2016, 514, 458–466. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Removal of hazardous volatile organic compounds from water by vacuum pervaporation with hydrophobic ceramic membranes. J. Membr. Sci. 2015, 474, 11–19. [Google Scholar] [CrossRef]
- Ki Hong, Y.; Hi Hong, W. Influence of ceramic support on pervaporation characteristics of IPA/water mixtures using PDMS/ceramic composite membrane. J. Membr. Sci. 1999, 159, 29–39. [Google Scholar] [CrossRef]
- González-Velasco, J.R.; González-Marcos, J.A.; López-Dehesa, C. Pervaporation of ethanol—Water mixtures through poly(1-trimethylsilyl-1-propyne) (PTMSP) membranes. Desalination 2002, 149, 61–65. [Google Scholar] [CrossRef]
- Liang, L.; Dickson, J.M.; Jiang, J.; Brook, M.A. Effect of low flow rate on pervaporation of 1,2-dichloroethane with novel polydimethylsiloxane composite membranes. J. Membr. Sci. 2004, 231, 71–79. [Google Scholar] [CrossRef]
- Mandal, S.; Pangarkar, V.G. Separation of methanol–benzene and methanol–toluene mixtures by pervaporation: Effects of thermodynamics and structural phenomenon. J. Membr. Sci. 2002, 201, 175–190. [Google Scholar] [CrossRef]
- Cunha, V.S.; Paredes, M.L.L.; Borges, C.P.; Habert, A.C.; Nobrega, R. Removal of aromatics from multicomponent organic mixtures by pervaporation using polyurethane membranes: Experimental and modeling. J. Membr. Sci. 2002, 206, 277–290. [Google Scholar] [CrossRef]
- Smitha, B.; Suhanya, D.; Sridhar, S.; Ramakrishna, M. Separation of organic–organic mixtures by pervaporation—A review. J. Membr. Sci. 2004, 241, 1–21. [Google Scholar] [CrossRef]
- Ghoreyshi, A.A.; Jahanshahi, M.; Peyvandi, K. Modeling of volatile organic compounds removal from water by pervaporation process. Desalination 2008, 222, 410–418. [Google Scholar] [CrossRef]
- Xu, Z.-K.; Dai, Q.-W.; Liu, Z.-M.; Kou, R.-Q.; Xu, Y.-Y. Microporous polypropylene hollow fiber membranes: Part II. Pervaporation separation of water/ethanol mixtures by the poly(acrylic acid) grafted membranes. J. Membr. Sci. 2003, 214, 71–81. [Google Scholar] [CrossRef]
- Mohammadi, T.; Aroujalian, A.; Bakhshi, A. Pervaporation of dilute alcoholic mixtures using PDMS membrane. Chem. Eng. Sci. 2005, 60, 1875–1880. [Google Scholar] [CrossRef]
- Luis, P.; Van der Bruggen, B. Pervaporation modeling: State of the art and future trends. In Pervaporation, Vapour Permeation and Membrane Distillation; Basile, A., Figoli, A., Khayet, M., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 87–106. [Google Scholar] [CrossRef]
- Beebe, A.H.; Coulter, K.E.; Lindsay, R.A.; Baker, E.M. Equilibria in Ethanol-Water System at Pressures Less Than Atmospheric. Ind. Eng. Chem. 1942, 34, 1501–1504. [Google Scholar] [CrossRef]
- Shah, D.; Kissick, K.; Ghorpade, A.; Hannah, R.; Bhattacharyya, D. Pervaporation of alcohol–water and dimethylformamide–water mixtures using hydrophilic zeolite NaA membranes: Mechanisms and experimental results. J. Membr. Sci. 2000, 179, 185–205. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Sun, W.; Yang, J.; Ren, Z.-Q. The Study on Pervaporation Behaviors of Dilute Organic Solution Through PDMS/PTFE Composite Membrane. Appl. Biochem. Biotechnol. 2009, 160, 156. [Google Scholar] [CrossRef]
- Haaz, E.; Toth, A.J. Methanol dehydration with pervaporation: Experiments and modelling. Sep. Purif. Technol. 2018, 205, 121–129. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Deng, X. Studies on the pervaporation membrane of permeation water from methanol/water mixture. J. Membr. Sci. 2008, 325, 192–198. [Google Scholar] [CrossRef]
- Kujawski, W. Pervaporative Removal of Organics from Water Using Hydrophobic Membranes. Binary Mixtures. Sep. Sci. Technol. 2000, 35, 89–108. [Google Scholar] [CrossRef]
- Rautenbach, R.; Herion, C.; Meyer-Blumentoth, U. Pervaporation Membrane Separation Processes; Elsevier: New York, NY, USA, 1991; Volume 1, pp. 181–191. [Google Scholar]
- Valentínyi, N.; Cséfalvay, E.; Mizsey, P. Modelling of pervaporation: Parameter estimation and model development. Chem. Eng. Res. Des. 2013, 91, 174–183. [Google Scholar] [CrossRef]
- Haáz, E.; Valentínyi, N.; Tarjáni, A.J.; Fózer, D.; André, A.; Selim, A.; Khaled, M.; Fuad, R.; Nagy, T.; Deák, C.; et al. Platform Molecule Removal from Aqueous Mixture with Organophilic Pervaporation: Experiments and Modelling. Period. Polytech. Chem. Eng. 2019, 63, 138–146. [Google Scholar] [CrossRef]
- Toth, A.J.; Mizsey, P. Methanol removal from aqueous mixture with organophilic pervaporation: Experiments and modelling. Chem. Eng. Res. Des. 2015, 98, 123–135. [Google Scholar] [CrossRef]



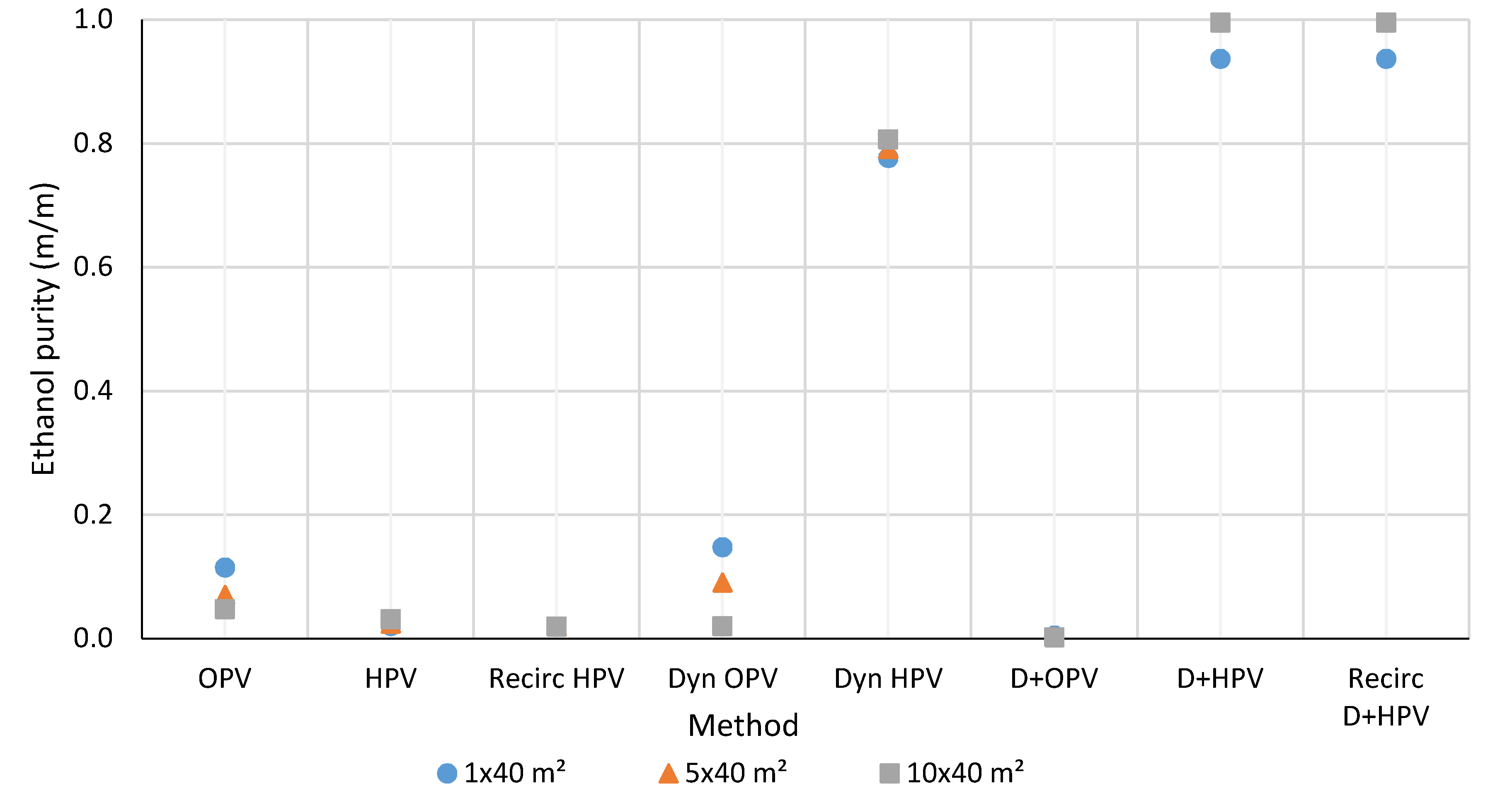
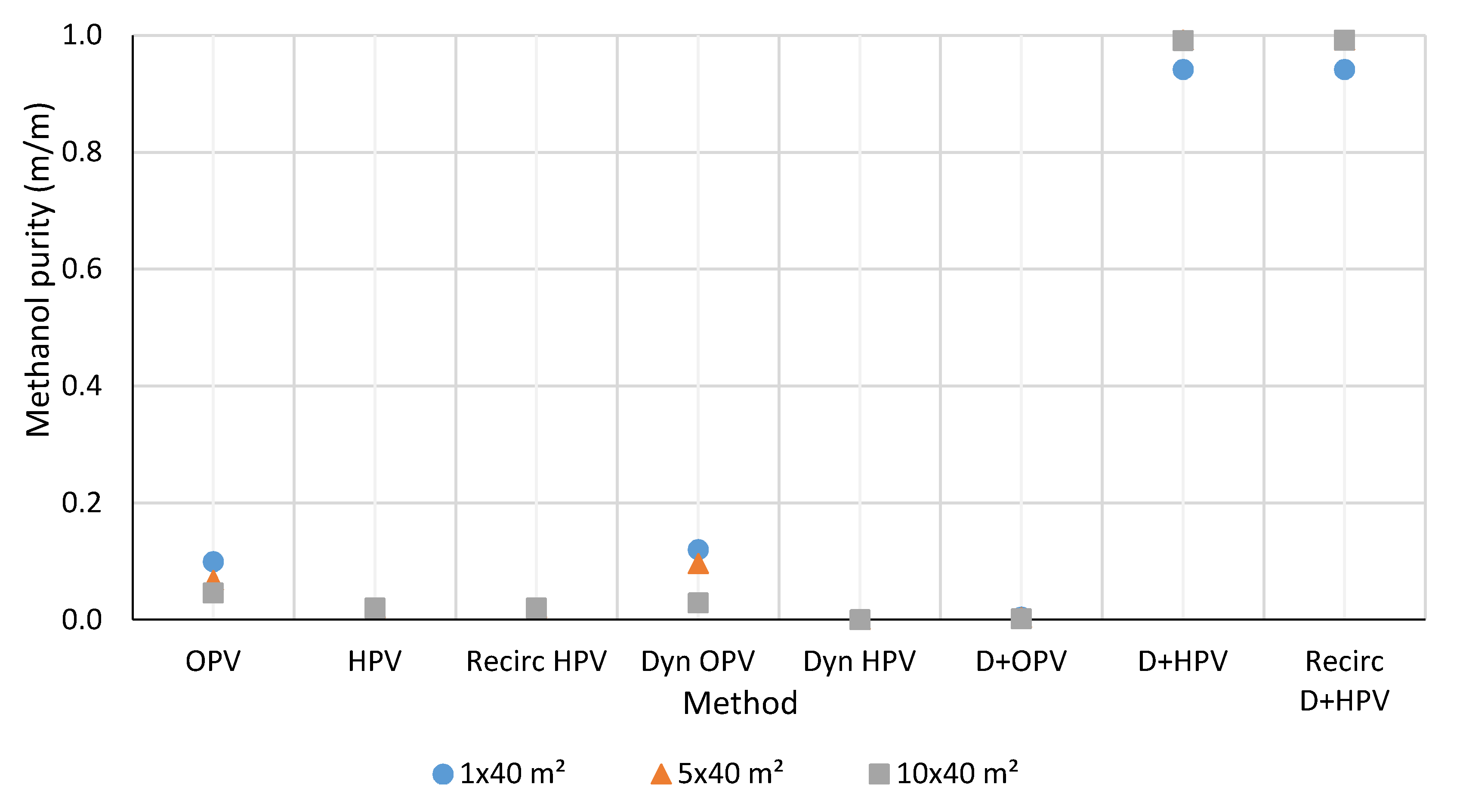
| Method | Abbreviation |
|---|---|
| Organophilic pervaporation | OPV |
| Hydrophilic pervaporation | HPV |
| Hydrophilic pervaporation with recirculation | Recirc HPV |
| Dynamic organophilic pervaporation | Dyn OPV |
| Dynamic hydronophilic pervaporation | Dyn HPV |
| Hybrid distillation-organophilic pervaporation | D + OPV |
| Hybrid distillation-hydrophilic pervaporation | D + HPV |
| Hybrid distillation-hydrophilic pervaporation with recirculation | Recirc D + HPV |
| Pervaporation Units | Value | Unit | |
|---|---|---|---|
| Permeate pressure | 0.008 | bar | |
| Inlet pressure drop | 0.1 | bar | |
| Permeability | 108 | kmol/m2 hbar | |
| Transport coefficient | Water | 0.000202 | kmol/m2 h |
| Ethanol | 0.0000193 | ||
| Activity energy | Water | 77,877 | kJ/kmol |
| Ethanol | 128,572 | ||
| Parameter “B” | Water | 2.63 | - |
| Ethanol | −8.68 | ||
| Pervaporation Units | Value | Unit | |
|---|---|---|---|
| Permeate pressure | 0.008 | bar | |
| Inlet pressure drop | 0.1 | bar | |
| Permeability | 108 | kmol/m2 hbar | |
| Transport coefficient | Water | 0.026 | kmol/m2 h |
| Ethanol | 0.077 | ||
| Activity energy | Water | 31,363 | kJ/kmol |
| Ethanol | 33,090 | ||
| Parameter “B” | Water | −0.73 | - |
| Ethanol | −0.04 | ||
| Pervaporation Units | Value | Unit | |
|---|---|---|---|
| Permeate pressure | 0.008 | bar | |
| Inlet pressure drop | 0.1 | bar | |
| Permeability | 108 | kmol/m2 hbar | |
| Transport coefficient | Water | 0.167 | kmol/m2 h |
| Methanol | 0.00018 | ||
| Activity energy | Water | 23,498 | kJ/kmol |
| Methanol | 30,795 | ||
| Parameter “B” | Water | −6.51 | - |
| Methanol | −2.4 | ||
| Pervaporation Units | Value | Unit | |
|---|---|---|---|
| Permeate pressure | 0.008 | bar | |
| Inlet pressure drop | 0.1 | bar | |
| Permeability | 108 | kmol/m2 hbar | |
| Transport coefficient | Water | 0.00246 | kmol/m2 h |
| Methanol | 0.0458 | ||
| Activity energy | Water | 44,170 | kJ/kmol |
| Methanol | 45,646 | ||
| Parameter “B“ | Water | 1.19 | - |
| Methanol | −5.64 | ||
| Parameters | |
|---|---|
| Thermodynamic model | UNIQUAC |
| Column type | SCDS |
| Column material | Carbon steel |
| Plate type | Valve, SS304 |
| Plate material | Carbon steel |
| Distillation product ethanol (or methanol) | target min. 0.9 m/m |
| Bottom product water | 0.9999 m/m |
| Parameters | Value | Unit | |
|---|---|---|---|
| Dynamic tank | diameter | 5 | m |
| cylinder height | 10 | m | |
| pressure | 1 | bar | |
| initial fluid level 1 | 2 | m | |
| initial fluid level 2 | 10−10 | m | |
| Time | 600 | min | |
| Characteristics | Value | Unit | |
|---|---|---|---|
| Feed pressure | 1 | bar | |
| Feed temperature | 20 | °C | |
| Feed flow | 1000 | kg/h | |
| Feed composition | Water | 0.98 | m/m |
| Ethanol (or methanol) | 0.02 | m/m | |
| Methods | Total Heat Consumptions (MJ/h) | |||||
|---|---|---|---|---|---|---|
| Ethanol-Water | Methanol-Water | |||||
| 1 × 40 m2 | 5 × 40 m2 | 10 × 40 m2 | 1 × 40 m2 | 5 × 40 m2 | 10 × 40 m2 | |
| OPV | −7.00 | −177.73 | −207.12 | −7.47 | −219.14 | −248.12 |
| HPV | −8.47 | −37.54 | −68.54 | −0.34 | −2.64 | −3.29 |
| Recirc HPV | −0.22 | −0.28 | −0.34 | −0.21 | −1.99 | −2.00 |
| Dyn OPV | −75763.30 | −73057.06 | −73389.00 | −75904.13 | −73030.71 | −73247.39 |
| Dyn HPV | −1.85 × 106 | −1.79 × 106 | −1.73 × 106 | −9.96 × 106 | −9.96 × 106 | −9.96 × 106 |
| D + OPV | −6.66 | −173.07 | −202.19 | −7.25 | −215.19 | −243.90 |
| D + HPV | 325.86 | 325.65 | 325.55 | 325.83 | 324.36 | 324.24 |
| Recirc D + HPV | 326.30 | 326.71 | 326.63 | 326.32 | 325.41 | 325.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do Thi, H.T.; Mizsey, P.; Toth, A.J. Separation of Alcohol-Water Mixtures by a Combination of Distillation, Hydrophilic and Organophilic Pervaporation Processes. Membranes 2020, 10, 345. https://doi.org/10.3390/membranes10110345
Do Thi HT, Mizsey P, Toth AJ. Separation of Alcohol-Water Mixtures by a Combination of Distillation, Hydrophilic and Organophilic Pervaporation Processes. Membranes. 2020; 10(11):345. https://doi.org/10.3390/membranes10110345
Chicago/Turabian StyleDo Thi, Huyen Trang, Peter Mizsey, and Andras Jozsef Toth. 2020. "Separation of Alcohol-Water Mixtures by a Combination of Distillation, Hydrophilic and Organophilic Pervaporation Processes" Membranes 10, no. 11: 345. https://doi.org/10.3390/membranes10110345
APA StyleDo Thi, H. T., Mizsey, P., & Toth, A. J. (2020). Separation of Alcohol-Water Mixtures by a Combination of Distillation, Hydrophilic and Organophilic Pervaporation Processes. Membranes, 10(11), 345. https://doi.org/10.3390/membranes10110345








