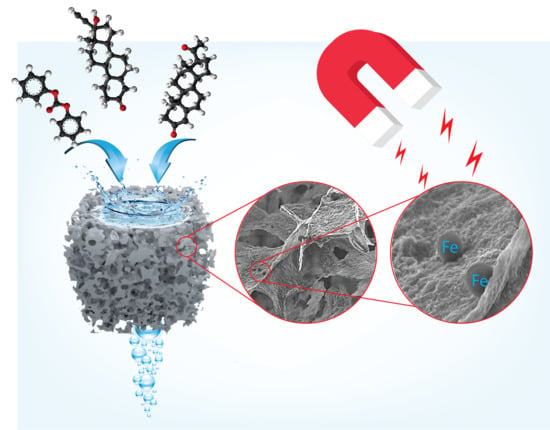
Reduced Graphene Oxide-Based Foam as an Endocrine Disruptor Adsorbent in Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Graphene Oxide Synthesis
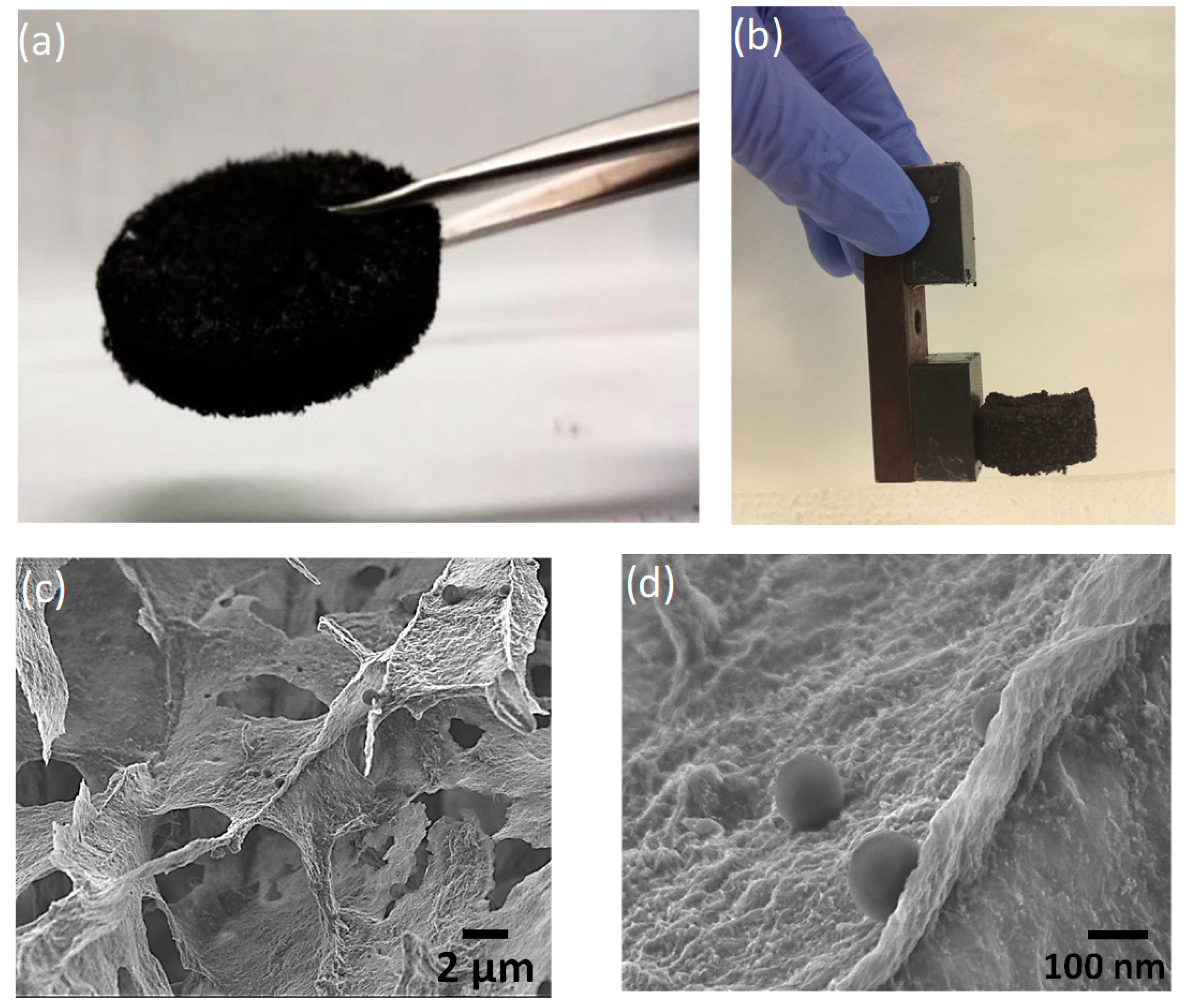
2.3. 3D Porous Materials Preparation (GO–PEI–FeNPs)
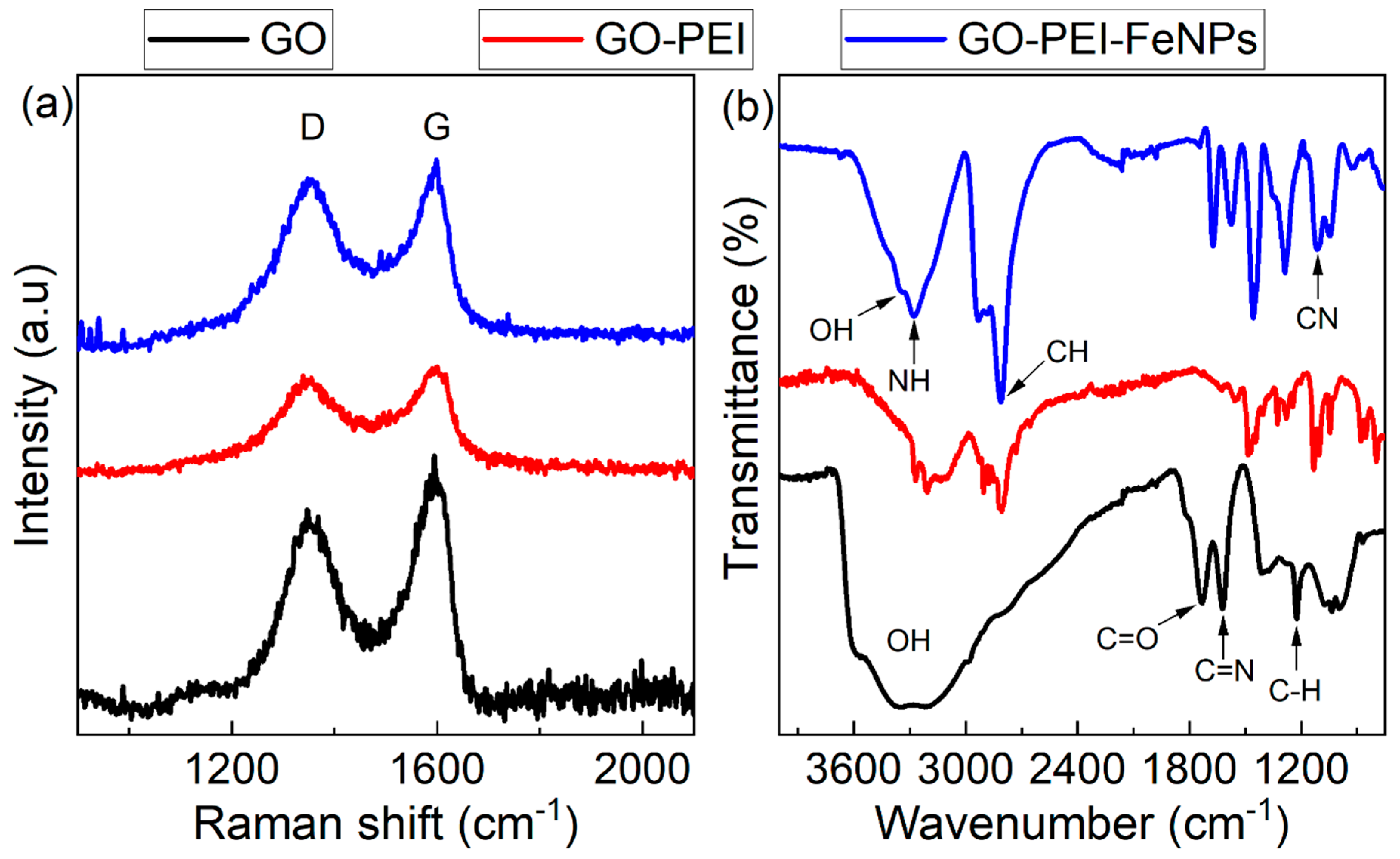
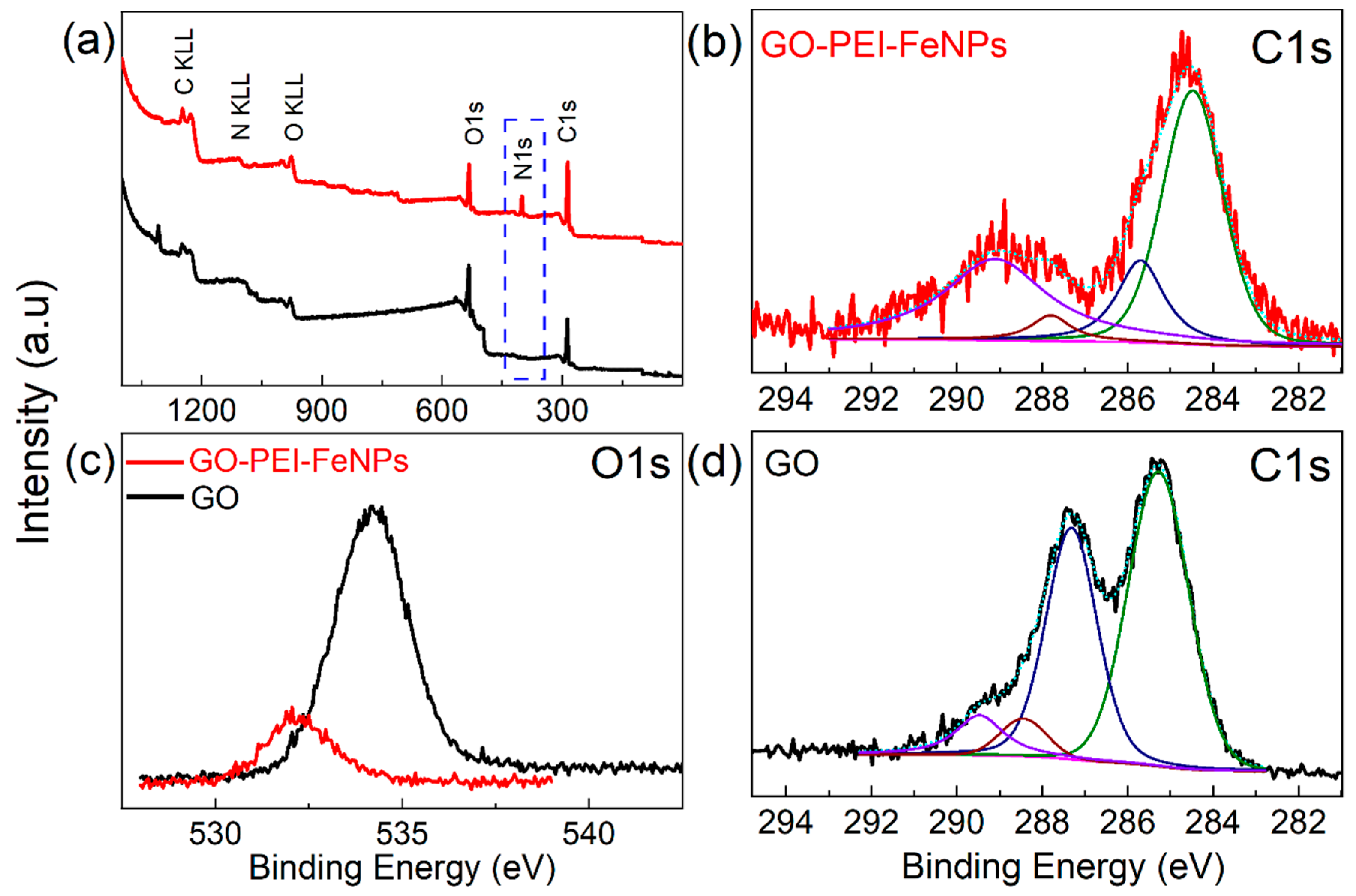
2.4. Instrumental Characterization
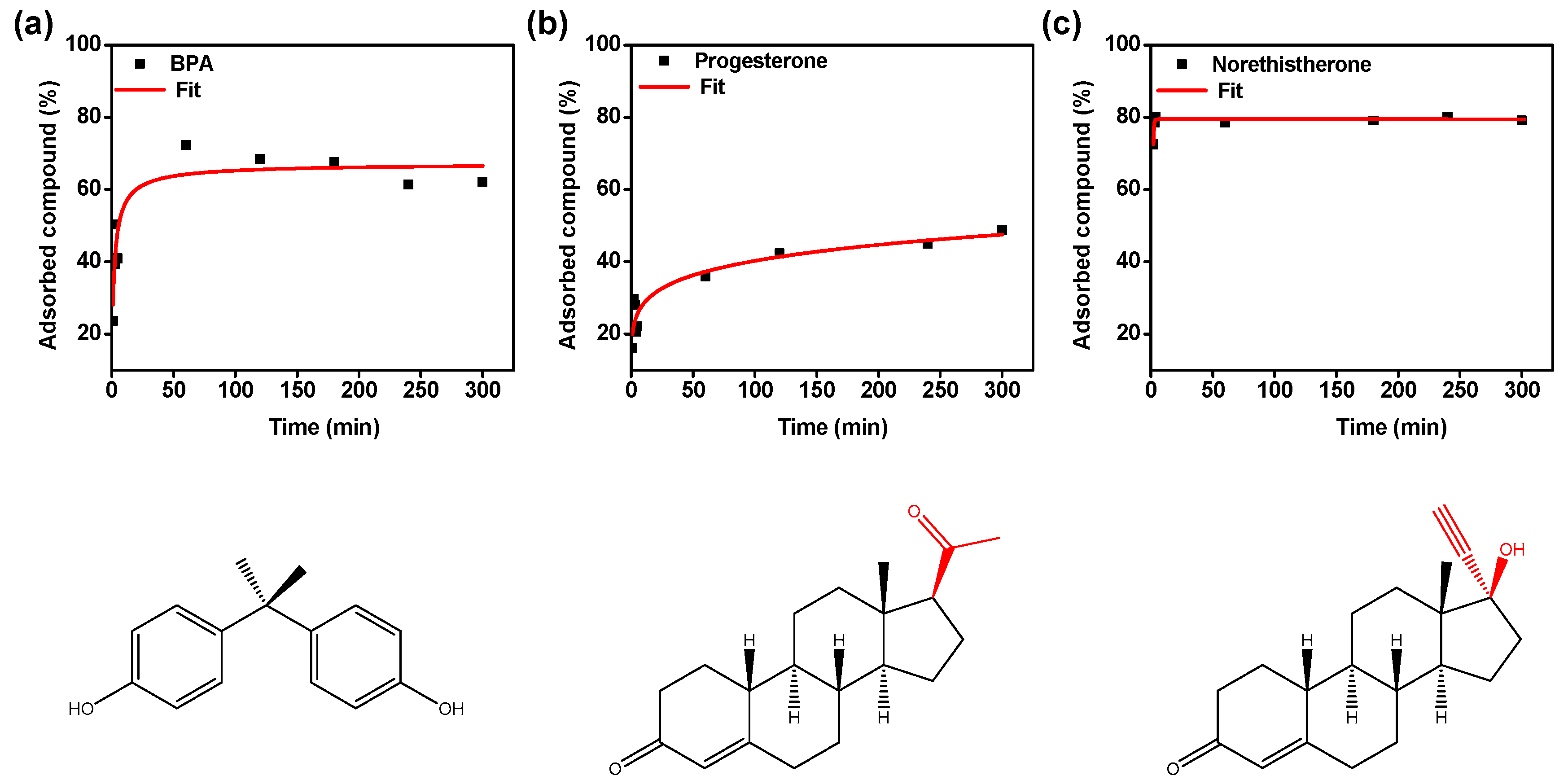
2.5. Adsorption of BPA, Progesterone and Norethisterone
3. Results and Discussion
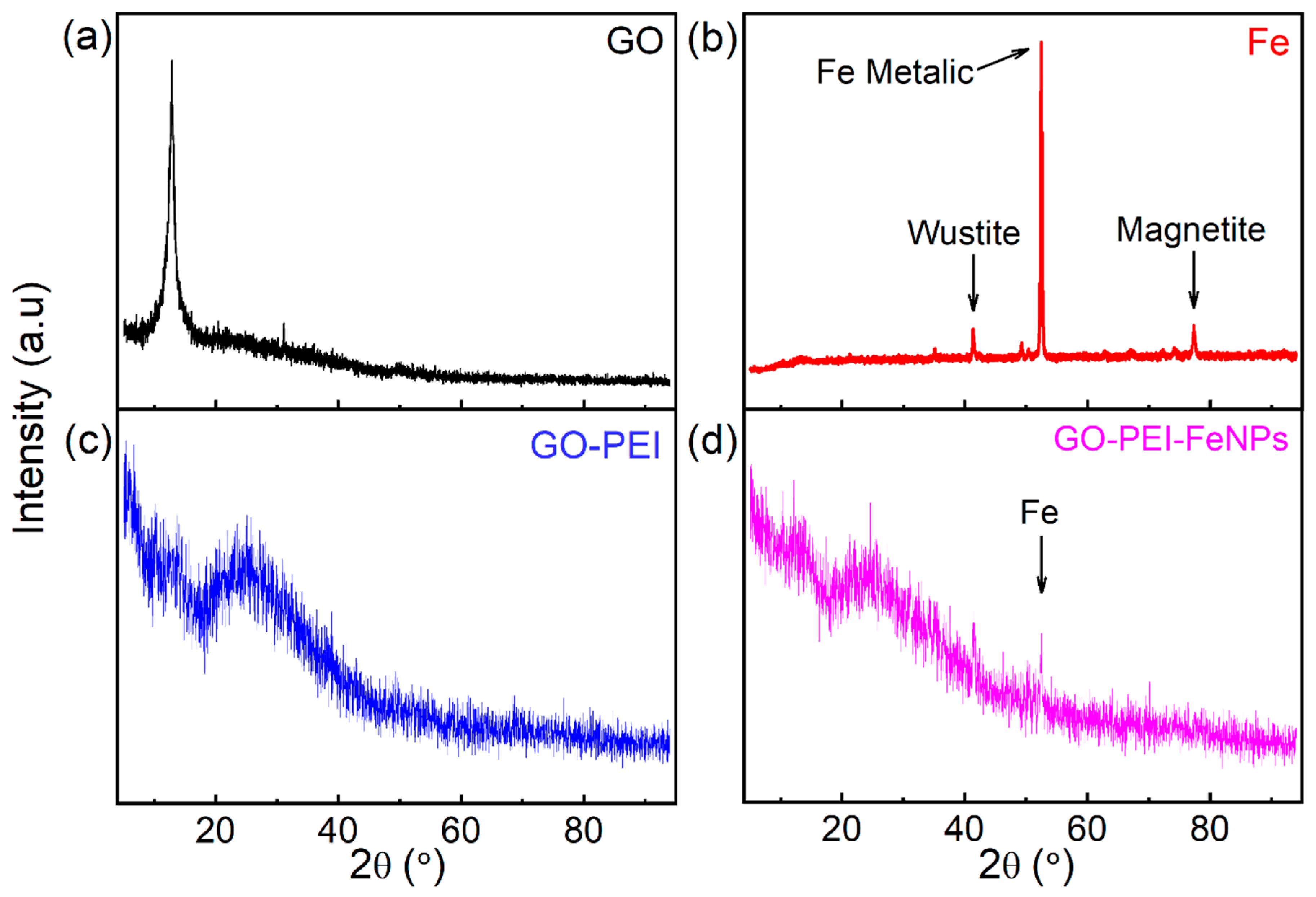
3.1. Membrane Characterization
3.2. Study of Adsorption of BPA, Progesterone and Norethisterone
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newbold, R.R.; Hanson, R.B.; Jefferson, W.N.; Bullock, B.C.; Haseman, J.; McLachlan, J.A. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis 2000, 21, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Dixon, D.; Yates, M.; Moore, A.B.; Ma, L.; Maas, R.; Korach, K.S. Estrogen receptor-α knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev. Biol. 2001, 238, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 2006, 147, s11–s17. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.F.; Andrade, L.S.; Rocha-Filho, R.C.; Bocchi, N.; Biaggio, S.R. Electrochemical determination of bisphenol A using a boron-doped diamond electrode. Electrochim. Acta 2012, 82, 3–8. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, C.Y.; Hung, C.H. Electrochemical remediation of BPA in a soil matrix by Pd/Ti and RuO2/Ti electrodes. J. Appl. Electrochem. 2013, 43, 1163–1174. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef]
- Bautista-Toledo, I.; Ferro-García, M.A.; Rivera-Utrilla, J.; Moreno-Castilla, C.; Vegas Fernández, F.J. Bisphenol A removal from water by activated carbon. Effects of carbon characteristics and solution chemistry. Environ. Sci. Technol. 2005, 39, 6246–6250. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, C.; Zhu, L.; Liang, N.; Liu, X.; Jia, J.; Zhang, C.; Zhai, S.; Zhang, B. Adsorption of bisphenol A to a carbon nanotube reduced its endocrine disrupting effect in mice male offspring. Int. J. Mol. Sci. 2014, 15, 15981–15993. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. On the Atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.Y.; Cui, Y.; Zhu, J.H.; Han, B.H. Preparation of three-dimensional graphene oxide–polyethylenimine porous materials as dye and gas adsorbents. ACS Appl. Mater. Interfaces 2013, 5, 9172–9179. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nano 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, W.; Piner, R.D.; Velamakanni, A.; Ruoff, R.S. Transparent self-assembled films of reduced graphene oxide platelets. Appl. Phys. Lett. 2009, 95, 103104. [Google Scholar] [CrossRef]
- N’Diaye, J.; Poorahong, S.; Hmam, O.; Izquierdo, R.; Siaj, M. Facile synthesis rhodium nanoparticles decorated single layer graphene as an enhancement hydrogen peroxide sensor. J. Electroanal. Chem. 2017, 789, 85–91. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.; Müllen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Watcharotone, S.; Dikin, D.A.; Stankovich, S.; Piner, R.; Jung, I.; Dommett, G.H.B.; Evmenenko, G.; Wu, S.E.; Chen, S.F.; Liu, C.P.; et al. Graphene−silica composite thin films as transparent conductors. Nano Lett. 2007, 7, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Chergui, S.; Rhili, K.; Poorahong, S.; Siaj, M. Graphene Oxide Membrane Immobilized Aptamer as a Highly Selective Hormone Removal. Membranes 2020, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Soin, N.; Bajpai, R.; Misra, D.S.; McLaughlin, J.A.; Roy, S.S. Graphene oxide for electrochemical sensing applications. J. Mater. Chem. 2011, 21, 14725–14731. [Google Scholar] [CrossRef]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv. Funct. Mater. 2012, 22, 4421–4425. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Ladrón-de-Guevara, A.; Boscá, A.; Pedrós, J.; Climent-Pascual, E.; de Andrés, A.; Calle, F.; Martínez, J. Reduced graphene oxide/polyaniline electrochemical supercapacitors fabricated by laser. Appl. Surf. Sci. 2019, 467, 691–697. [Google Scholar] [CrossRef]
- Poorahong, S.; Harding, D.J.; Siaj, M. Hollow molybdenum oxide-graphene oxide spheres as a binder-free electrocatalyst membrane with enhanced hydrogen evolution efficiency. Mater. Lett. 2020, 272, 127872. [Google Scholar] [CrossRef]
- El maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Activated carbon for dyes removal: Modeling and understanding the adsorption process. J. Chem. 2020, 2020, 2096834. [Google Scholar] [CrossRef]
- Elmorsi, T.M. Equilibrium isotherms and kinetic studies of removal of methylene blue dye by adsorption onto Miswak leaves as a natural adsorbent. J. Environ. Prot. 2011, 6, 11. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Yang, X.; Chen, C.; Li, J.; Zhao, G.; Ren, X.; Wang, X. Graphene oxide-iron oxide and reduced graphene oxide-iron oxide hybrid materials for the removal of organic and inorganic pollutants. RSC Adv. 2012, 2, 8821–8826. [Google Scholar] [CrossRef]
- Chen, B.; Yue, W.; Zhao, H.; Long, F.; Cao, Y.; Pan, X. Simultaneous capture of methyl orange and chromium(vi) from complex wastewater using polyethylenimine cation decorated magnetic carbon nanotubes as a recyclable adsorbent. RSC Adv. 2019, 9, 4722–4734. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Czikkely, M.; Neubauer, E.; Fekete, I.; Ymeri, P.; Fogarassy, C. Review of heavy metal adsorption processes by several organic matters from wastewaters. Water 2018, 10, 1377. [Google Scholar] [CrossRef]
- Wu, T.; Chen, M.; Zhang, L.; Xu, X.; Liu, Y.; Yan, J.; Wang, W.; Gao, J. Three-dimensional graphene-based aerogels prepared by a self-assembly process and its excellent catalytic and absorbing performance. J. Mater. Chem. A 2013, 1, 7612–7621. [Google Scholar] [CrossRef]
- Eissa, S.; N’diaye, J.; Brisebois, P.; Izquierdo, R.; Tavares, A.C.; Siaj, M. Probing the influence of graphene oxide sheets size on the performance of label-free electrochemical biosensors. Sci. Rep. 2020, 10, 13612. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, X.; Qiu, J.; Lin, J.; Xu, D.; Zhang, C.; Lv, M.; Yang, X. Three-dimensional graphene-based hydrogel/aerogel materials. Rev. Adv. Mater. Sci. 2014, 36, 137–151. [Google Scholar]
- Cui, P.; Lee, J.; Hwang, E.; Lee, H. One-pot reduction of graphene oxide at subzero temperatures. Chem. Commun. 2011, 47, 12370–12372. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H. Ueber die Adsorption in Loesungen; Engelmann: Leipzig, Germany, 1906. [Google Scholar]
- Ahalya, N.; Kanamadi, R.D.; Ramachandra, T.V. Biosorption of chromium (VI) from aqueous solutions by the husk of Bengal gram (Cicer arientinum). Electron. J. Biotechnol. 2005, 8, 258–264. [Google Scholar] [CrossRef]
- Desta, M.B. Batch sorption experiments: Langmuir and freundlich isotherm studies for the adsorption of textile metal ions onto teff straw (Eragrostis tef) agricultural waste. J. Thermodyn. 2013, 2013, 6. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Graphene-based adsorbents for the removal of toxic organic pollutants: A review. J. Environ. Manag. 2019, 244, 370–382. [Google Scholar] [CrossRef] [PubMed]
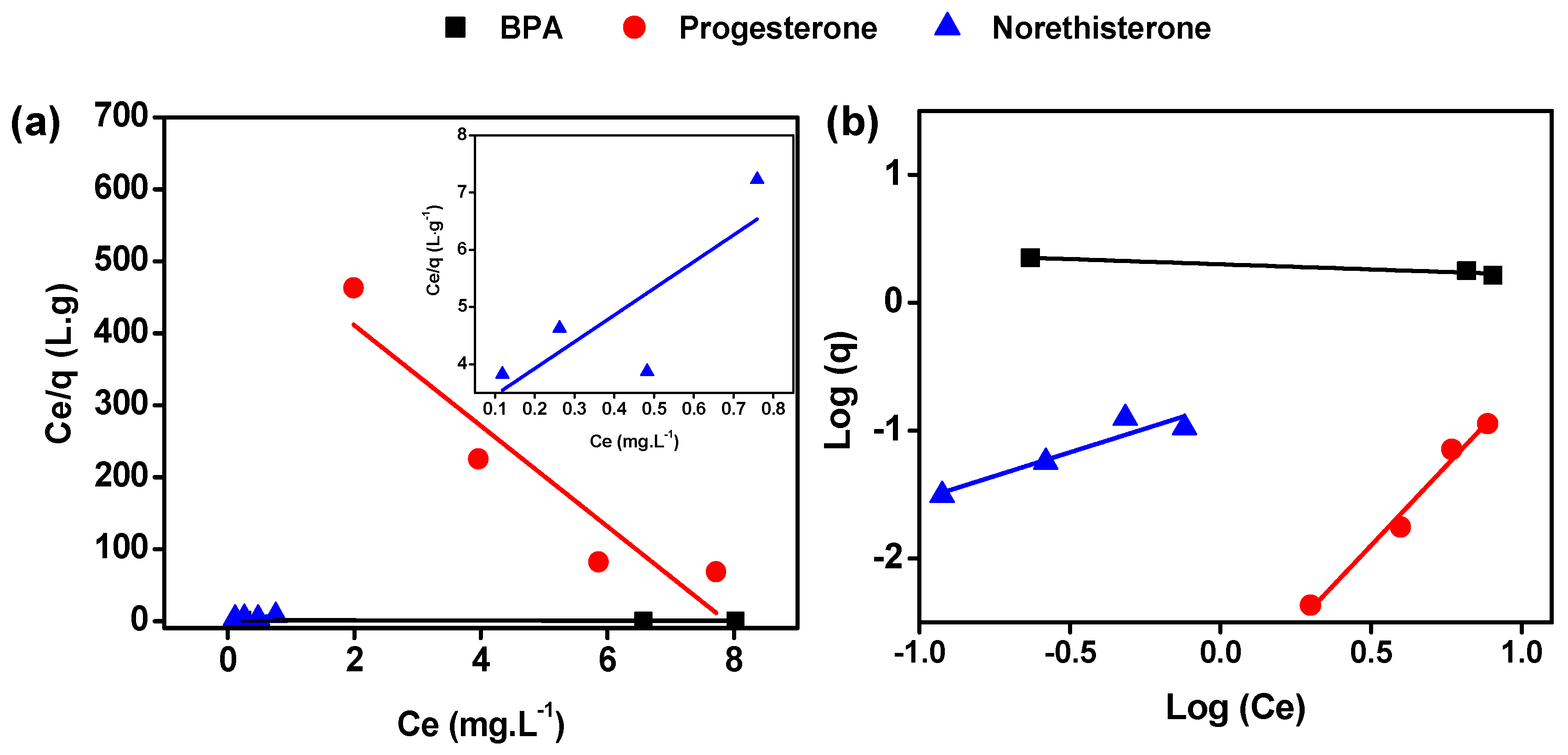
| Adsorption Model/Analyte | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| b | qmax | R2 | n | K | R2 | |
| (L∙mg−1) | (mg∙g−1) | |||||
| BPA | 0.13 | 6.61 | 0.93 | −12.43 | 0.5 | 0.92 |
| Progesterone | −0.13 | −0.01 | 0.82 | 0.40 | 1396.4 | 0.98 |
| Norethisterone | 1.56 | 0.21 | 0.49 | 1.35 | 6.3 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
N’Diaye, J.; Poorahong, S.; Hmam, O.; Jiménez, G.C.; Izquierdo, R.; Siaj, M. Reduced Graphene Oxide-Based Foam as an Endocrine Disruptor Adsorbent in Aqueous Solutions. Membranes 2020, 10, 340. https://doi.org/10.3390/membranes10110340
N’Diaye J, Poorahong S, Hmam O, Jiménez GC, Izquierdo R, Siaj M. Reduced Graphene Oxide-Based Foam as an Endocrine Disruptor Adsorbent in Aqueous Solutions. Membranes. 2020; 10(11):340. https://doi.org/10.3390/membranes10110340
Chicago/Turabian StyleN’Diaye, Jeanne, Sujittra Poorahong, Ons Hmam, Gastón Contreras Jiménez, Ricardo Izquierdo, and Mohamed Siaj. 2020. "Reduced Graphene Oxide-Based Foam as an Endocrine Disruptor Adsorbent in Aqueous Solutions" Membranes 10, no. 11: 340. https://doi.org/10.3390/membranes10110340
APA StyleN’Diaye, J., Poorahong, S., Hmam, O., Jiménez, G. C., Izquierdo, R., & Siaj, M. (2020). Reduced Graphene Oxide-Based Foam as an Endocrine Disruptor Adsorbent in Aqueous Solutions. Membranes, 10(11), 340. https://doi.org/10.3390/membranes10110340