Quaternary Ammonium-Bearing Perfluorinated Polymers for Anion Exchange Membrane Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quaternization and Membrane Fabrication
2.3. Characterization
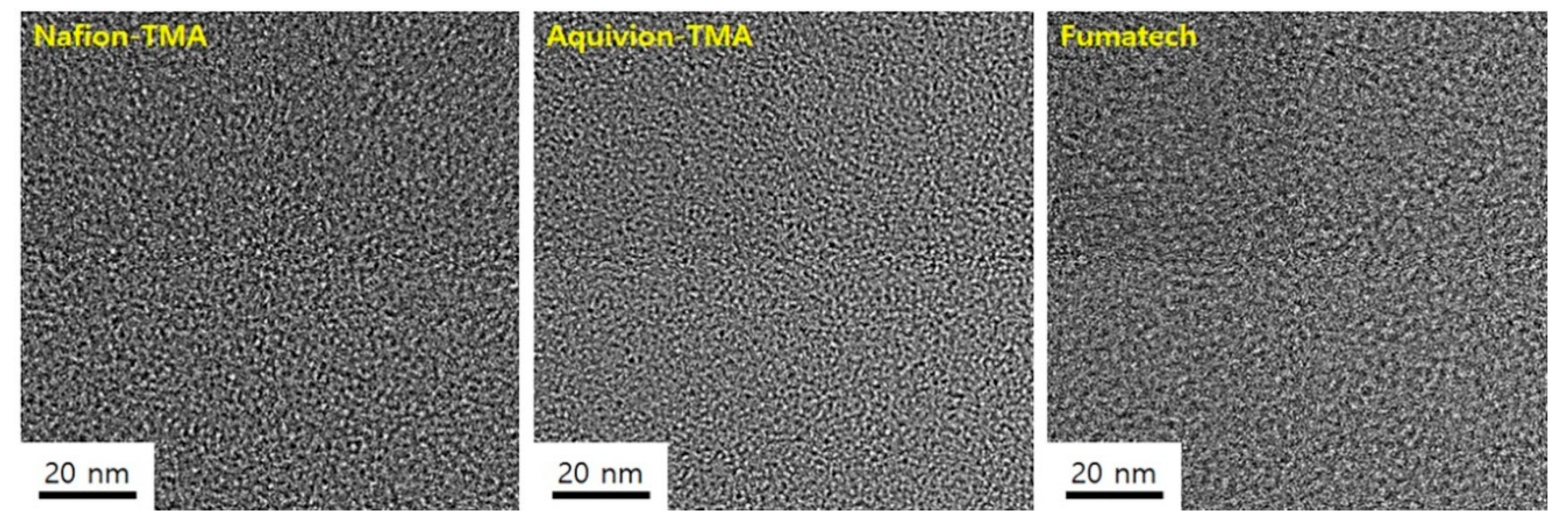
2.4. Transmission Electron Microscopy (TEM)
2.5. Alkaline Stability Tests
2.6. Flow-Electrode Capacitive Deionization (FCDI) Desalination
3. Results
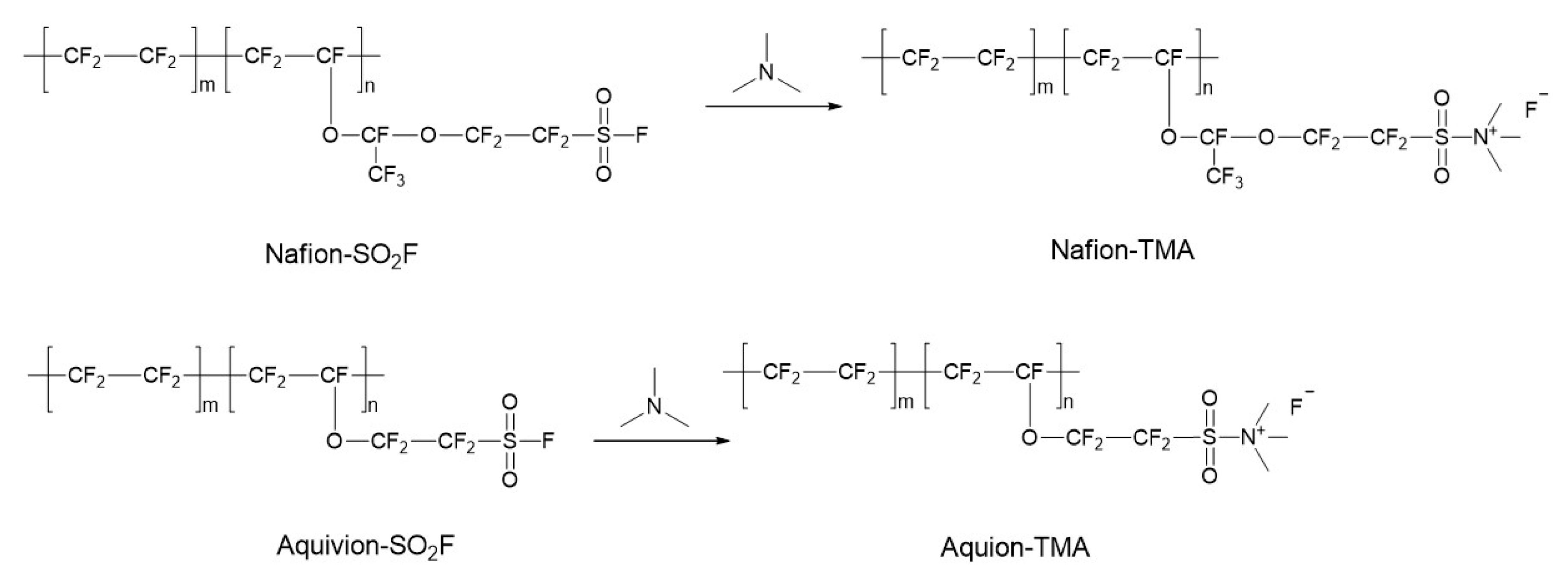
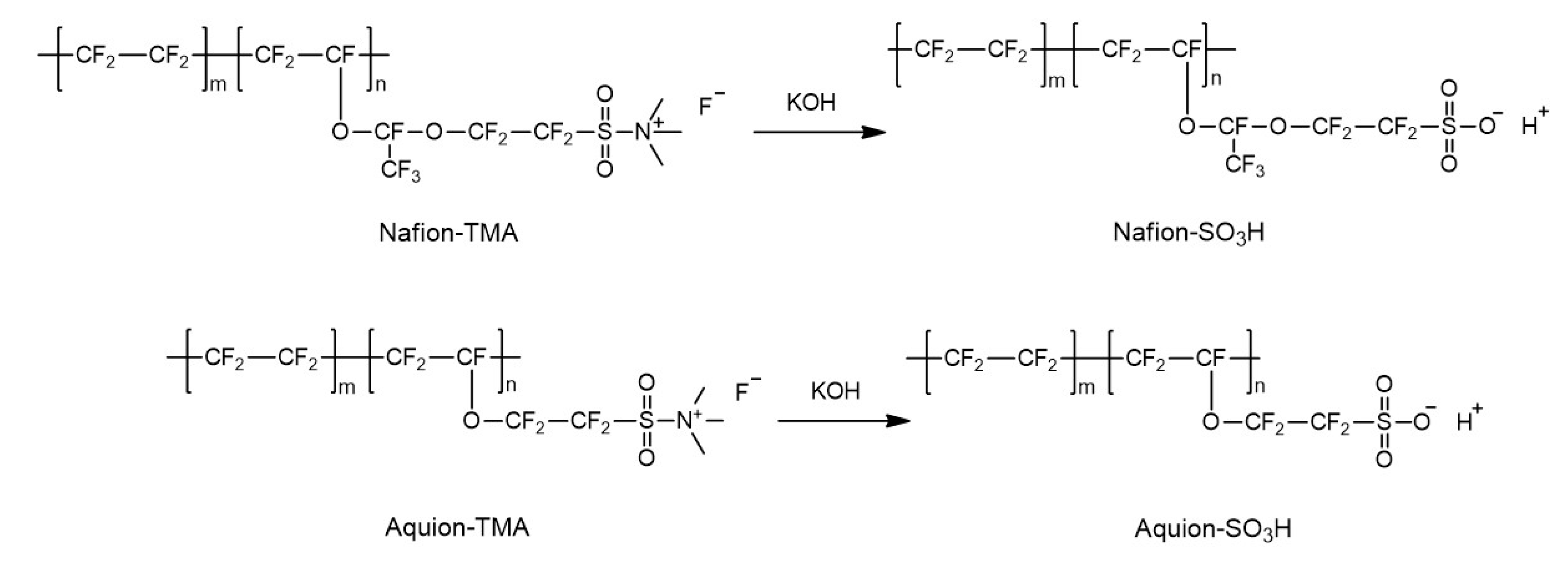
3.1. Syntheses of the Perfluorinated Ionomers
3.2. Solubilities of the Perfluorinated Ionomers and Membrane Preparation
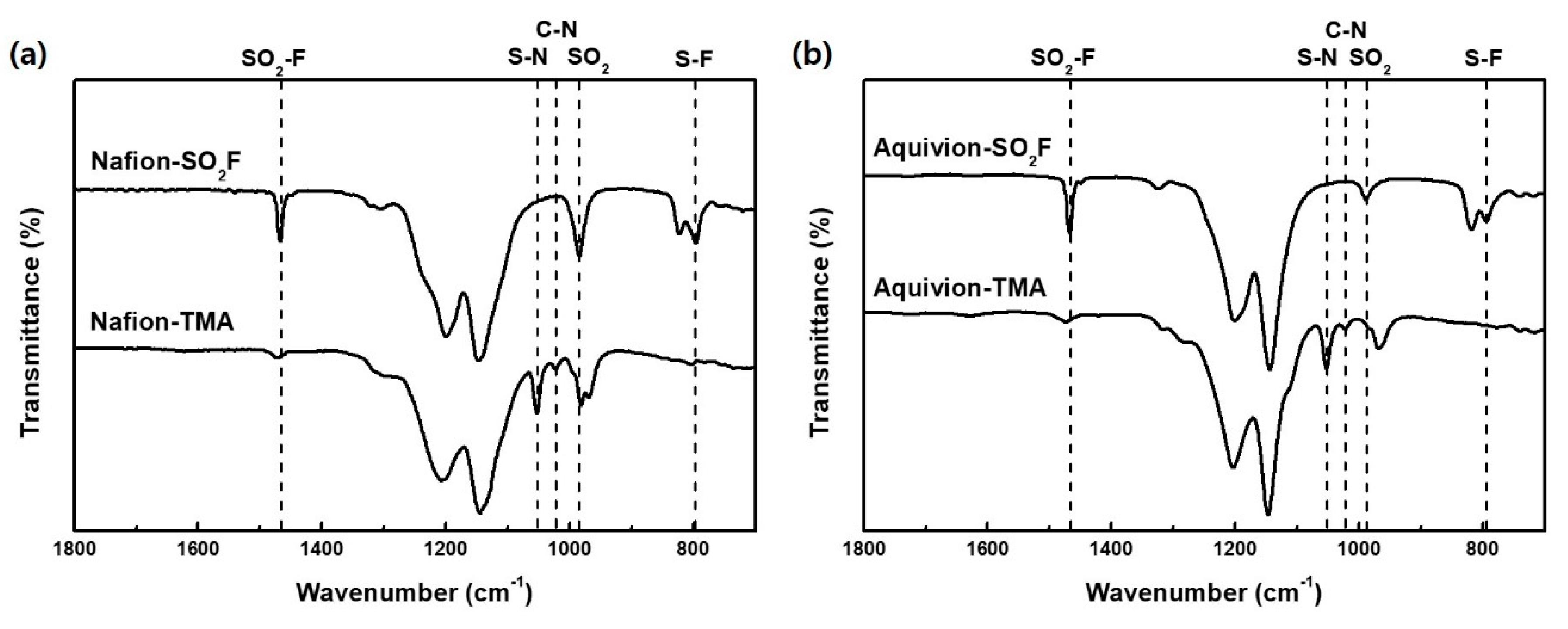
3.3. Physical Properties of the Quaternized Polymers
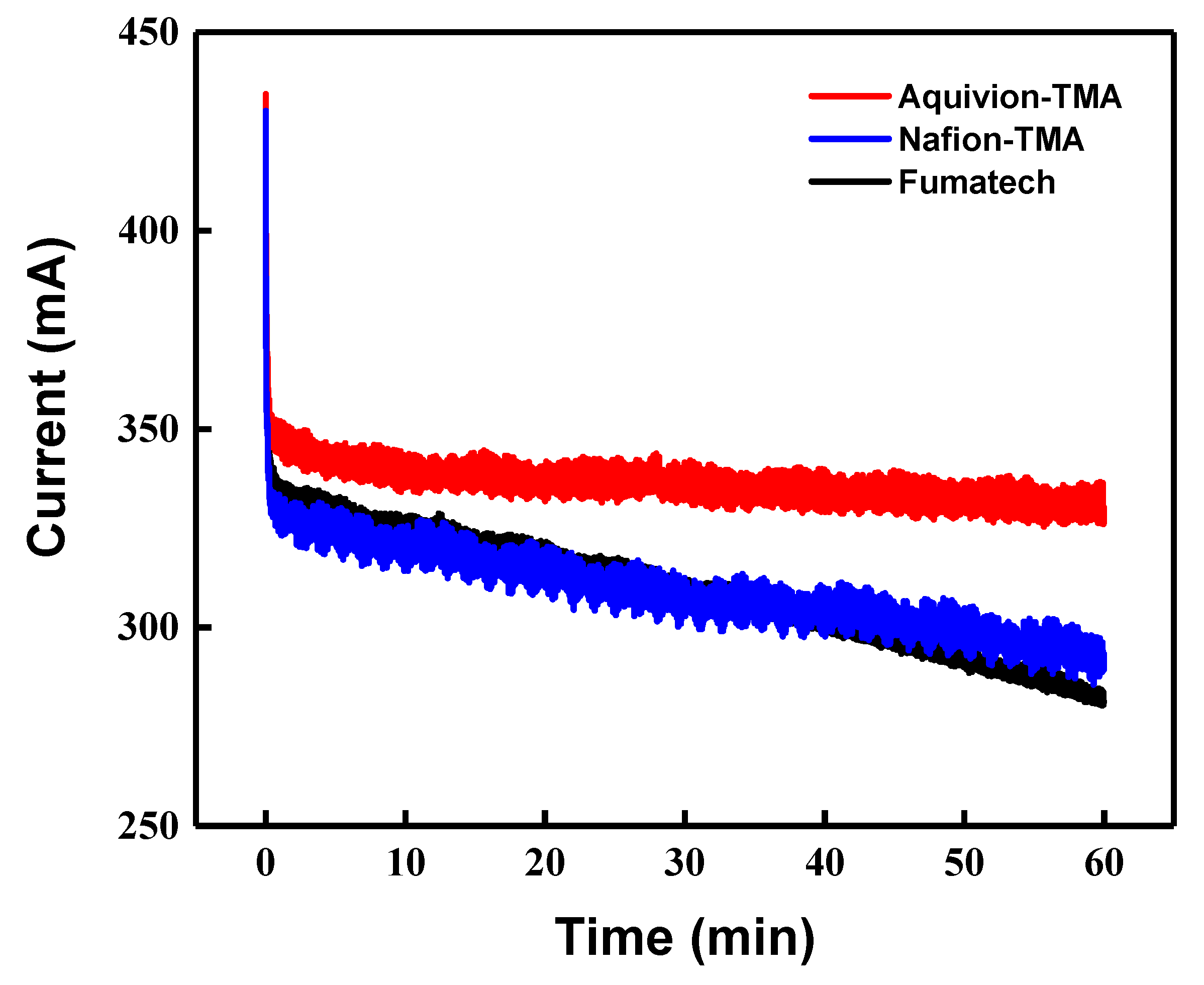
3.4. Flow Electrode Capacitive Deionization Performance and Stability
3.5. Alkaline Stabilities of the Quaternized Polymers Under a NaOH Atmosphere
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eisaman, M.D.; Alvarado, L.; Larner, D.; Wang, P.; Garg, B.; Littau, K.A. CO2 separation using bipolar membrane electrodialysis. Energy Environ. Sci. 2011, 4, 1319–1328. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Hong, J.G.; Chen, Y. Nanocomposite reverse electrodialysis (RED) ion-exchange membranes for salinity gradient power generation. J. Membr. Sci. 2014, 460, 139–147. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, M.; Pan, J.; Sotto, A.; Shen, J.; Gao, C.; der Bruggen, B.V. Separation of divalent ions from seawater concentrate to enhance the purity of coarse salt by electrodialysis with monovalent-selective membranes. Desalination 2017, 411, 28–37. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-Based Polymer Electrolyte Membranes: Importance of Morphology on Ion Transport and Membrane Stability. Chem. Rev. 2017, 117, 4759–4805. [Google Scholar] [CrossRef]
- Atkins, P.; Paula, J.D. Physical Chemistry, 7th ed.; OUP Oxford: Oxford, UK, 2002. [Google Scholar]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Einsla, B.R.; Chempath, S.; Pratt, L.; Boncella, J.; Rau, J.; Macomber, C.; Pivovar, B. Stability of Cations for Anion Exchange Membrane Fuel Cells. ECS Trans. 2019, 11, 1173–1180. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Tignor, S.E.; Krause, J.A.; Choe, Y.-K.; Bae, C. Systematic Alkaline Stability Study of Polymer Backbones for Anion Exchange Membrane Applications. Macromolecules 2016, 49, 3361–3372. [Google Scholar] [CrossRef]
- He, B.; Li, Z.; Zhao, D.; Liu, H.; Zhong, Y.; Ning, J.; Zhang, Z.; Wang, Y.; Hu, Y. Fabrication of porous Cu-doped BiVO4 nanotubes as efficient oxygen-evolving photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2589–2599. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Defected graphene nano-platelets for enhanced hydrophilic nature and visible light-induced photoelectrochemical performances. J. Phys. Chem. Solids 2017, 104, 233–242. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Environmentally sustainable biogenic fabrication of AuNP decorated-graphitic gC 3 N 4 nanostructures towards improved photoelectrochemical performances. RSC Adv. 2018, 8, 13898–13909. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Hickner, M.A.; Herring, A.M.; Coughlin, E.B. Anion exchange membranes: Current status and moving forward. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1727–1735. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Shin, D.; Nugraha, A.F.; Wijaya, F.; Lee, S.; Kim, E.; Choi, J.; Kim, H.-J.; Bae, B. Synthetic approaches for advanced multi-block anion exchange membranes. RSC Adv. 2019, 9, 21106–21115. [Google Scholar] [CrossRef]
- Nugraha, A.F.; Arbi, M.R.; Wijaya, F.; Lee, H.; Shin, D.; Bae, B. Synthesis and characterization of anion-exchange multi-block-copolymer membranes containing highly densified cationic functional groups. Polymer 2020, 210, 122996. [Google Scholar] [CrossRef]
- Nugraha, A.F.; Kim, S.; Wijaya, F.; Bae, B.; Shin, D. Synthetic Approaches for Poly(Phenylene) Block Copolymers via Nickel Coupling Reaction for Fuel Cell Applications. Polymers 2020, 12, 1614. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Li, D.; Liu, B.; Wang, J.; Song, Y. Novel amphoteric ion exchange membranes by blending sulfonated poly(ether ether ketone)/quaternized poly(ether imide) for vanadium redox flow battery applications. J. Mater. Chem. A 2015, 3, 17590–17597. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Li, W.; Xie, X.; Lv, Y. Synthesis and characterization of a fluorinated cross-linked anion exchange membrane. Int. J. Hydrogen Energy 2013, 38, 11045–11052. [Google Scholar] [CrossRef]
- Yan, J.; Moore, H.D.; Hibbs, M.R.; Hickner, M.A. Synthesis and structure–property relationships of poly(sulfone)s for anion exchange membranes. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1790–1798. [Google Scholar] [CrossRef]
- Lee, W.-H.; Park, E.J.; Han, J.; Shin, D.W.; Kim, Y.S.; Bae, C. Poly(terphenylene) Anion Exchange Membranes: The Effect of Backbone Structure on Morphology and Membrane Property. ACS Macro Lett. 2017, 6, 566–570. [Google Scholar] [CrossRef]
- You, W.; Noonan, K.J.T.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Habenicht, B.F.; Paddison, S.J.; Tuckerman, M.E. The effects of the hydrophobic environment on proton mobility in perfluorosulfonic acid systems: An ab initio molecular dynamics study. J. Mater. Chem. 2010, 20, 6342–6351. [Google Scholar] [CrossRef]
- Sung, D.W.; Kim, Y.G.; Bae, Y.C. Ionic conductivities of perfluorosulfonic acid membrane by group contribution method. Polymer 2009, 50, 3686–3692. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Elliott, J.A.; Wu, D.; Paddison, S.J.; Moore, R.B. A unified morphological description of Nafion membranes from SAXS and mesoscale simulations. Soft Matter 2011, 7, 6820–6827. [Google Scholar] [CrossRef]
- Shin, S.-H.; Kodir, A.; Shin, D.; Park, S.-H.; Bae, B. Perfluorinated composite membranes with organic antioxidants for chemically durable fuel cells. Electrochim. Acta 2019, 298, 901–909. [Google Scholar] [CrossRef]
- Shin, S.-H.; Nur, P.J.; Kodir, A.; Kwak, D.-H.; Lee, H.; Shin, D.; Bae, B. Improving the Mechanical Durability of Short-Side-Chain Perfluorinated Polymer Electrolyte Membranes by Annealing and Physical Reinforcement. ACS Omega 2019, 4, 19153–19163. [Google Scholar] [CrossRef]
- Haubold, H.-G.; Vad, T.; Jungbluth, H.; Hiller, P. Nano structure of NAFION: A SAXS study. Electrochim. Acta 2001, 46, 1559–1563. [Google Scholar] [CrossRef]
- Krafft, M.P. Highly fluorinated compounds induce phase separation in, and nanostructuration of liquid media. Possible impact on, and use in chemical reactivity control. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 4251–4258. [Google Scholar] [CrossRef]
- Roche, E.; Pineri, M.; Duplessix, R. Phase separation in perfluorosulfonate ionomer membranes. J. Polym. Sci. Polym. Phys. Ed. 1982, 20, 107–116. [Google Scholar] [CrossRef]
- Mittelsteadt, C.K.; Staser, J. Simultaneous water uptake, diffusivity and permeability measurement of perfluorinated sulfonic acid polymer electrolyte membranes. ECS Trans. 2011, 41, 101. [Google Scholar] [CrossRef]
- Takamatsu, T.; Hashiyama, M.; Eisenberg, A. Sorption phenomena in Nafion membranes. J. Appl. Polym. Sci. 1979, 24, 2199–2220. [Google Scholar] [CrossRef]
- Yeo, S.C.; Eisenberg, A. Physical properties and supermolecular structure of perfluorinated ion-containing (Nafion) polymers. J. Appl. Polym. Sci. 1977, 21, 875–898. [Google Scholar] [CrossRef]
- Park, A.M.; Owczarczyk, Z.R.; Garner, L.E.; Yang-Neyerlin, A.C.; Long, H.; Antunes, C.M.; Sturgeon, M.R.; Lindell, M.J.; Hamrock, S.J.; Yandrasits, M.; et al. Synthesis and Characterization of Perfluorinated Anion Exchange Membranes. ECS Trans. 2017, 80, 957–966. [Google Scholar] [CrossRef]
- Vandiver, M.A.; Horan, J.L.; Yang, Y.; Tansey, E.T.; Seifert, S.; Liberatore, M.W.; Herring, A.M. Synthesis and characterization of perfluoro quaternary ammonium anion exchange membranes. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1761–1769. [Google Scholar] [CrossRef]
- Vandiver, M.A.; Seifert, S.; Liberatore, M.W.; Herring, A.M. Synthesis and Characterization of Perfluoro Quaternary Ammonium Ion Exchange Membranes for Fuel Cell Applications. ECS Trans. 2013, 50, 2119–2127. [Google Scholar] [CrossRef]
- Arges, C.G.; Jung, M.-S.; Johnson, G.; Parrondo, J.; Ramani, V. Anion Exchange Membranes (AEMs) with Perfluorinated and Polysulfone Backbones with Different Cation Chemistries. ECS Trans. 2011, 41, 1795–1816. [Google Scholar] [CrossRef]
- Divekar, A.G.; Kuo, M.-C.; Park, A.M.; Motz, A.R.; Page-Belknap, Z.S.; Owczarczyk, Z.; Long, H.; Seifert, S.; Maupin, C.M.; Yandrasits, M.A.; et al. The impact of alkyl tri-methyl ammonium side chains on perfluorinated ionic membranes for electrochemical applications. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 700–712. [Google Scholar] [CrossRef]
- Jung, M.-s.J.; Arges, C.G.; Ramani, V. A perfluorinated anion exchange membrane with a 1,4-dimethylpiperazinium cation. J. Mater. Chem. 2011, 21, 6158–6160. [Google Scholar] [CrossRef]
- Kim, D.S.; Fujimoto, C.H.; Hibbs, M.R.; Labouriau, A.; Choe, Y.-K.; Kim, Y.S. Resonance Stabilized Perfluorinated Ionomers for Alkaline Membrane Fuel Cells. Macromolecules 2013, 46, 7826–7833. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Bae, C. Systematic Analysis of Cation Stability in Alkaline Exchange Membrane Fuel Cells. ECS Trans. 2014, 64, 1221–1228. [Google Scholar] [CrossRef]
- Kim, E.; Lee, S.; Woo, S.; Park, S.-H.; Yim, S.-D.; Shin, D.; Bae, B. Synthesis and characterization of anion exchange multi-block copolymer membranes with a fluorine moiety as alkaline membrane fuel cells. J. Power Sources 2017, 359, 568–576. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, K.S.; Yang, S.; Choi, J.; Park, H.-r.; Kim, D.K. A novel three-dimensional desalination system utilizing honeycomb-shaped lattice structures for flow-electrode capacitive deionization. Energy Environ. Sci. 2017, 10, 1746–1750. [Google Scholar] [CrossRef]
- Cho, Y.; Yoo, C.-Y.; Lee, S.W.; Yoon, H.; Lee, K.S.; Yang, S.; Kim, D.K. Flow-electrode capacitive deionization with highly enhanced salt removal performance utilizing high-aspect ratio functionalized carbon nanotubes. Water Res. 2019, 151, 252–259. [Google Scholar] [CrossRef]
- Salerno, H.L.S.; Elabd, Y.A. Anion exchange membranes derived from nafion precursor for the alkaline fuel cell: Effect of cation type on properties. J. Appl. Polym. Sci. 2013, 127, 298–307. [Google Scholar] [CrossRef]
- Liu, X.; Gao, H.; Chen, X.; Hu, Y.; Pei, S.; Li, H.; Zhang, Y. Synthesis of perfluorinated ionomers and their anion exchange membranes. J. Membr. Sci. 2016, 515, 268–276. [Google Scholar] [CrossRef]
- Moukheiber, E.; De Moor, G.; Flandin, L.; Bas, C. Investigation of ionomer structure through its dependence on ion exchange capacity (IEC). J. Membr. Sci. 2012, 389, 294–304. [Google Scholar] [CrossRef]
- Ghielmi, A.; Vaccarono, P.; Troglia, C.; Arcella, V. Proton exchange membranes based on the short-side-chain perfluorinated ionomer. J. Power Sources 2005, 145, 108–115. [Google Scholar] [CrossRef]
- Ugo, P.; Bertoncello, P.; Vezzà, F. Langmuir–Blodgett films of different ionomeric polymers deposited on electrode surfaces. Electrochim. Acta 2004, 49, 3785–3793. [Google Scholar] [CrossRef][Green Version]
- Chempath, S.; Einsla, B.R.; Pratt, L.R.; Macomber, C.S.; Boncella, J.M.; Rau, J.A.; Pivovar, B.S. Mechanism of tetraalkylammonium headgroup degradation in alkaline fuel cell membranes. J. Phys. Chem. C 2008, 112, 3179–3182. [Google Scholar] [CrossRef]
- Salerno, H.L.; Beyer, F.L.; Elabd, Y.A. Anion exchange membranes derived from nafion precursor for the alkaline fuel cell. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 552–562. [Google Scholar] [CrossRef]




| Ionomer | IEC [meq/g] | Water Uptake (wt%) | Swelling Ratio (%) | Conductivity @ 25 °C (mS/cm) | CF (mS/cm) | ||
|---|---|---|---|---|---|---|---|
| Theoretical | Titration | Area | Thickness | ||||
| Nafion-TMA | 0.91 | 0.88 | 21 | 12 | 5 | 27 | 1.31 |
| Aquivion-TMA | 1.15 | 1.06 | 23 | 15 | 5 | 38 | 1.67 |
| Fumatech | 1.6-2.1 | 1.84 | 34 | 17 | 7 | 10 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Lee, H.; Yang, T.-H.; Bae, B.; Tran, N.A.T.; Cho, Y.; Jung, N.; Shin, D. Quaternary Ammonium-Bearing Perfluorinated Polymers for Anion Exchange Membrane Applications. Membranes 2020, 10, 306. https://doi.org/10.3390/membranes10110306
Lee S, Lee H, Yang T-H, Bae B, Tran NAT, Cho Y, Jung N, Shin D. Quaternary Ammonium-Bearing Perfluorinated Polymers for Anion Exchange Membrane Applications. Membranes. 2020; 10(11):306. https://doi.org/10.3390/membranes10110306
Chicago/Turabian StyleLee, Seunghyun, Hyejin Lee, Tae-Hyun Yang, Byungchan Bae, Nguyen Anh Thu Tran, Younghyun Cho, Namgee Jung, and Dongwon Shin. 2020. "Quaternary Ammonium-Bearing Perfluorinated Polymers for Anion Exchange Membrane Applications" Membranes 10, no. 11: 306. https://doi.org/10.3390/membranes10110306
APA StyleLee, S., Lee, H., Yang, T.-H., Bae, B., Tran, N. A. T., Cho, Y., Jung, N., & Shin, D. (2020). Quaternary Ammonium-Bearing Perfluorinated Polymers for Anion Exchange Membrane Applications. Membranes, 10(11), 306. https://doi.org/10.3390/membranes10110306







