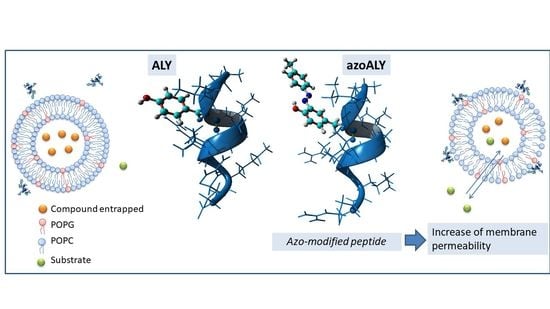
Study of the Interaction of a Novel Semi-Synthetic Peptide with Model Lipid Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of the Reference Peptide ALY
2.2. Molecular Dynamics (MD) Simulations
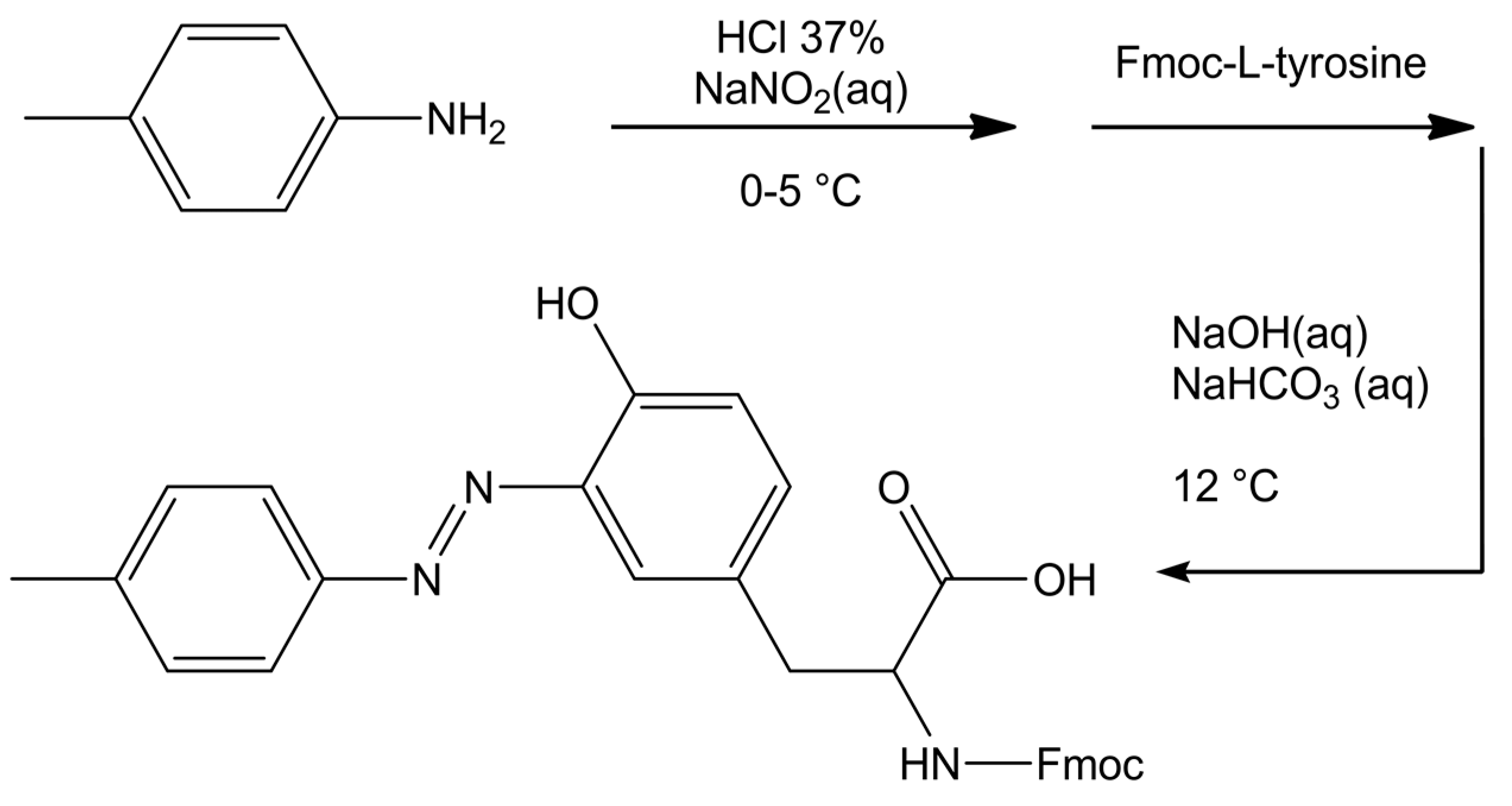
2.3. Synthesis of Azobenzene-Modified Tyr (azoTyr) and the Peptides ALY and azoALY
2.4. Permeability Tests with POPC/POPG (9:1) Vesicles
2.4.1. Materials
2.4.2. Calcein Release Test with Giant Unilamellar Vesicles (GUVs)
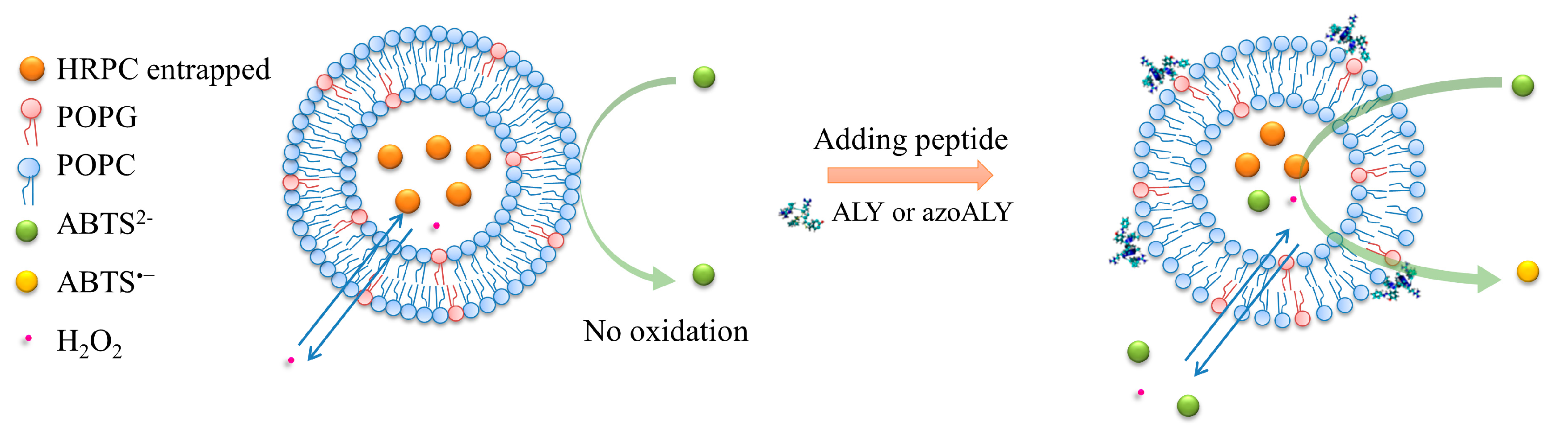
2.4.3. Enzymatic Permeability Assay with Large Unilamellar Vesicles (LUVs)
3. Results and Discussion
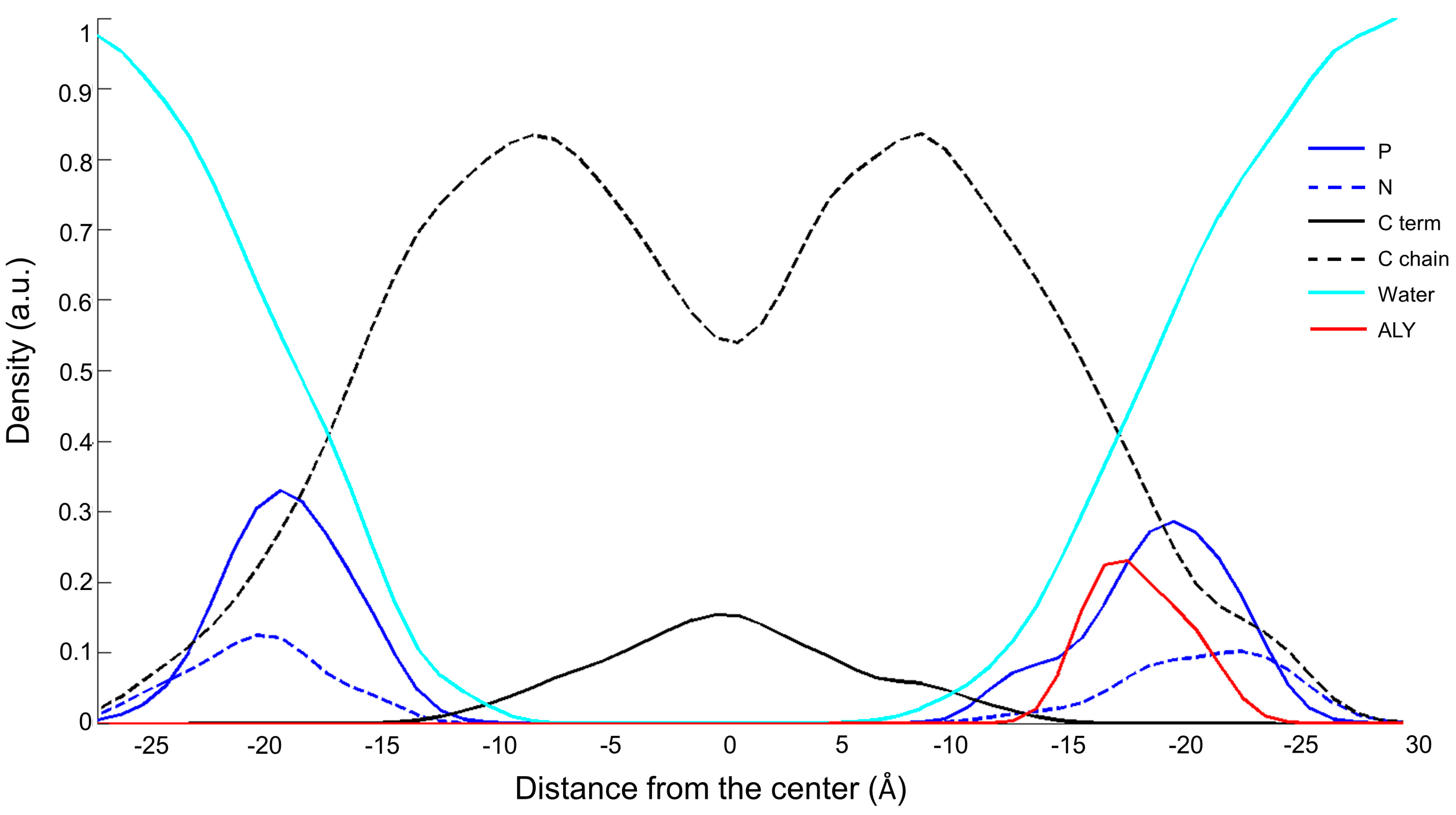
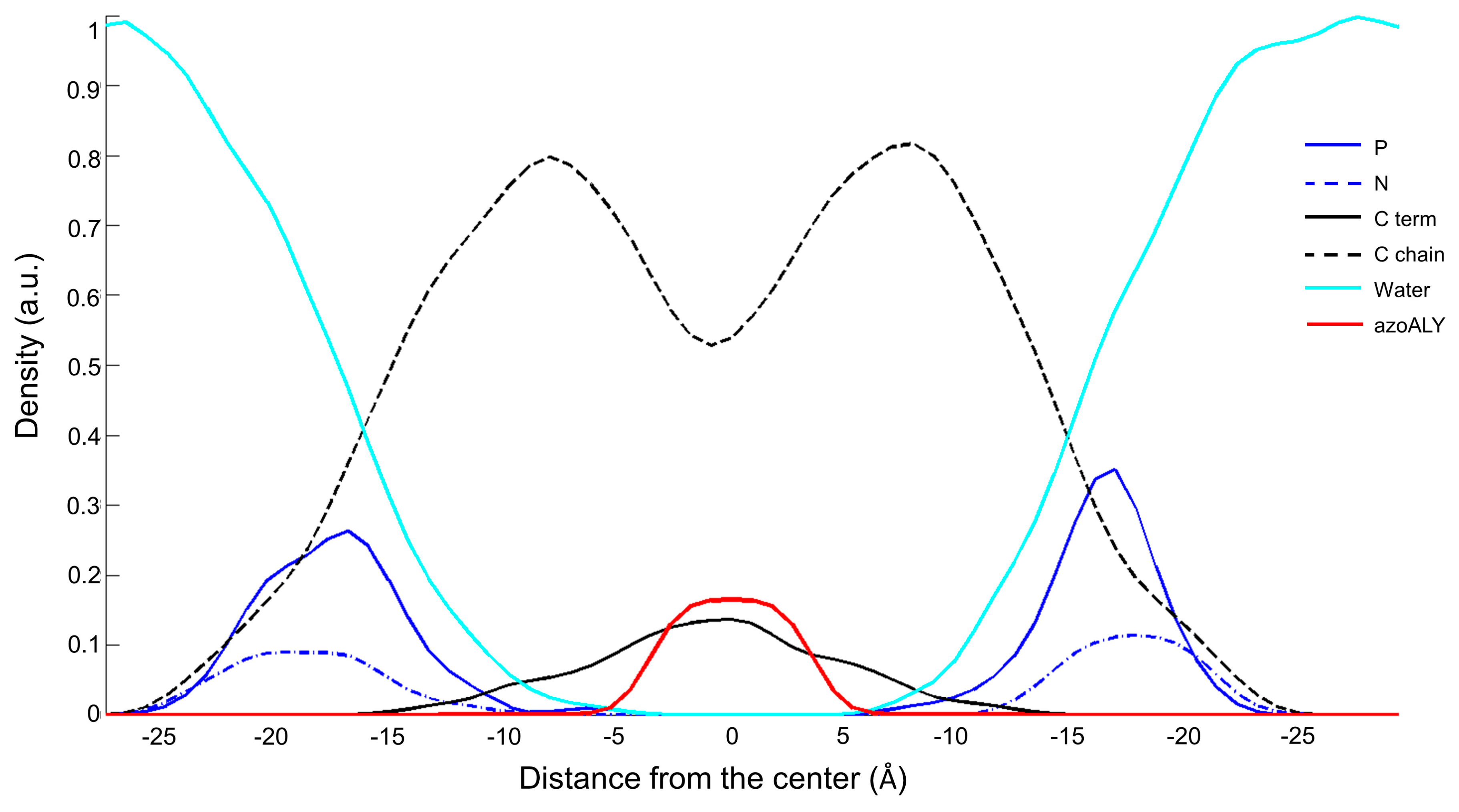
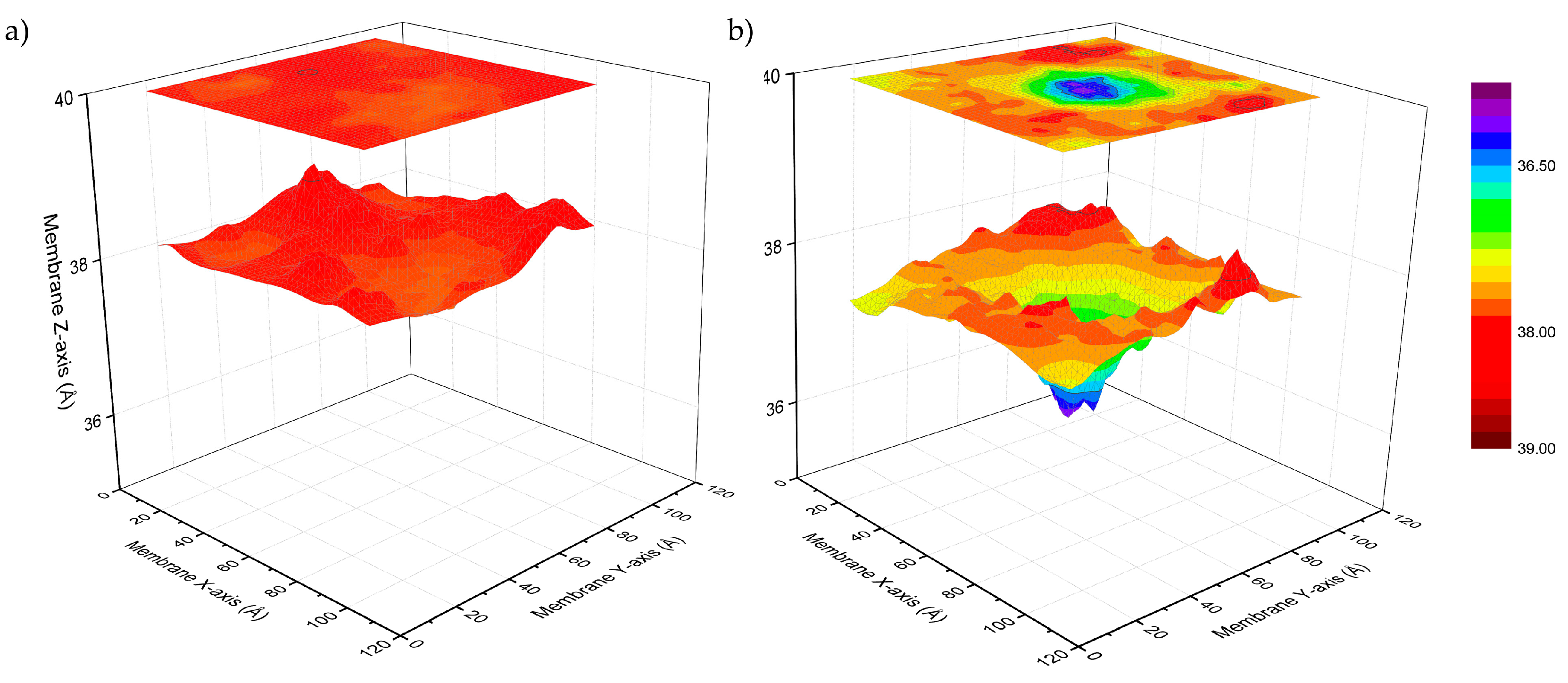
3.1. Peptide Localization within Lipid Bilayers: a MD Simulation Analysis
3.2. Experimental Membrane Permeability Measurements
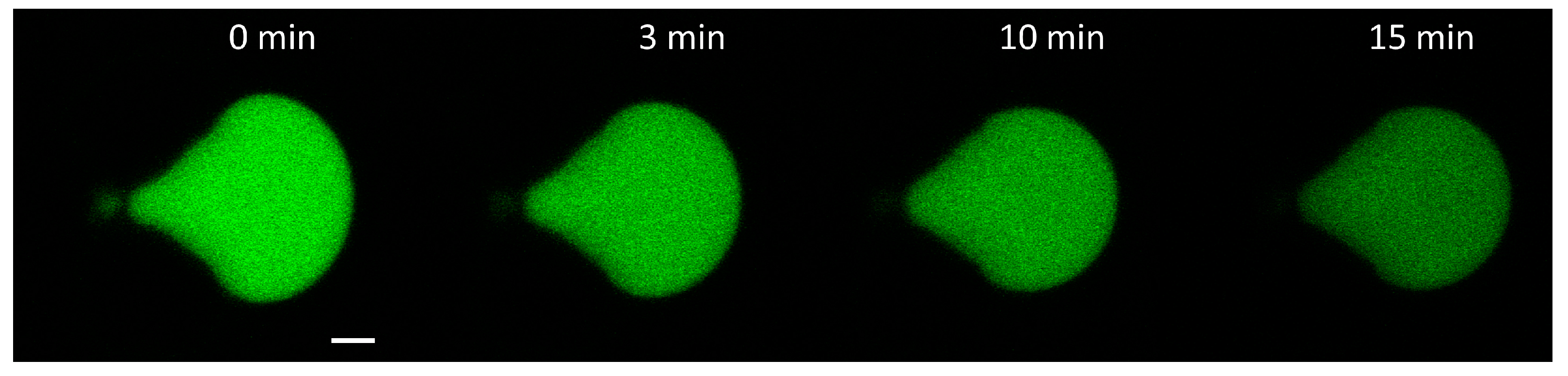
3.2.1. Calcein Leakage from GUVs
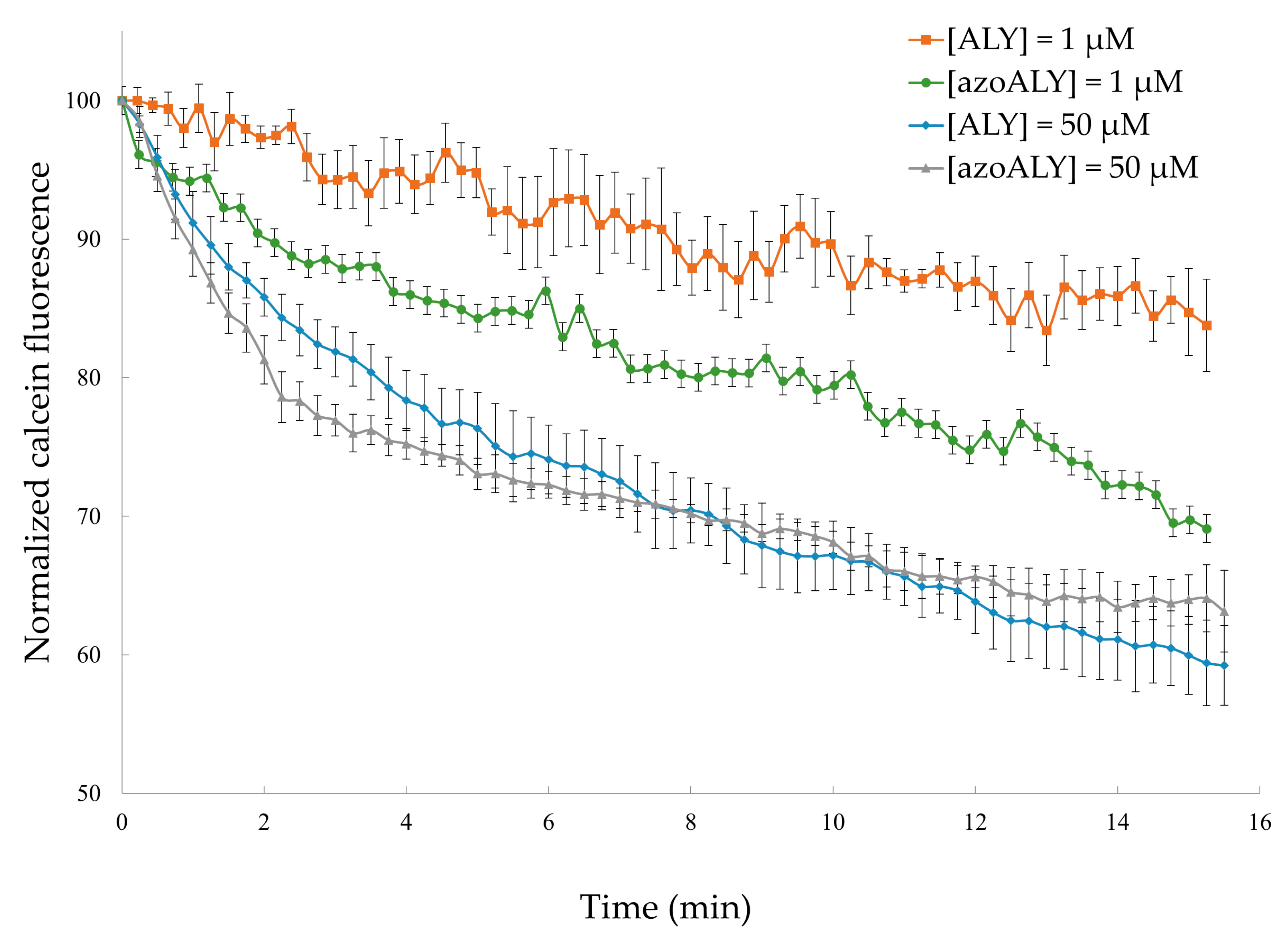
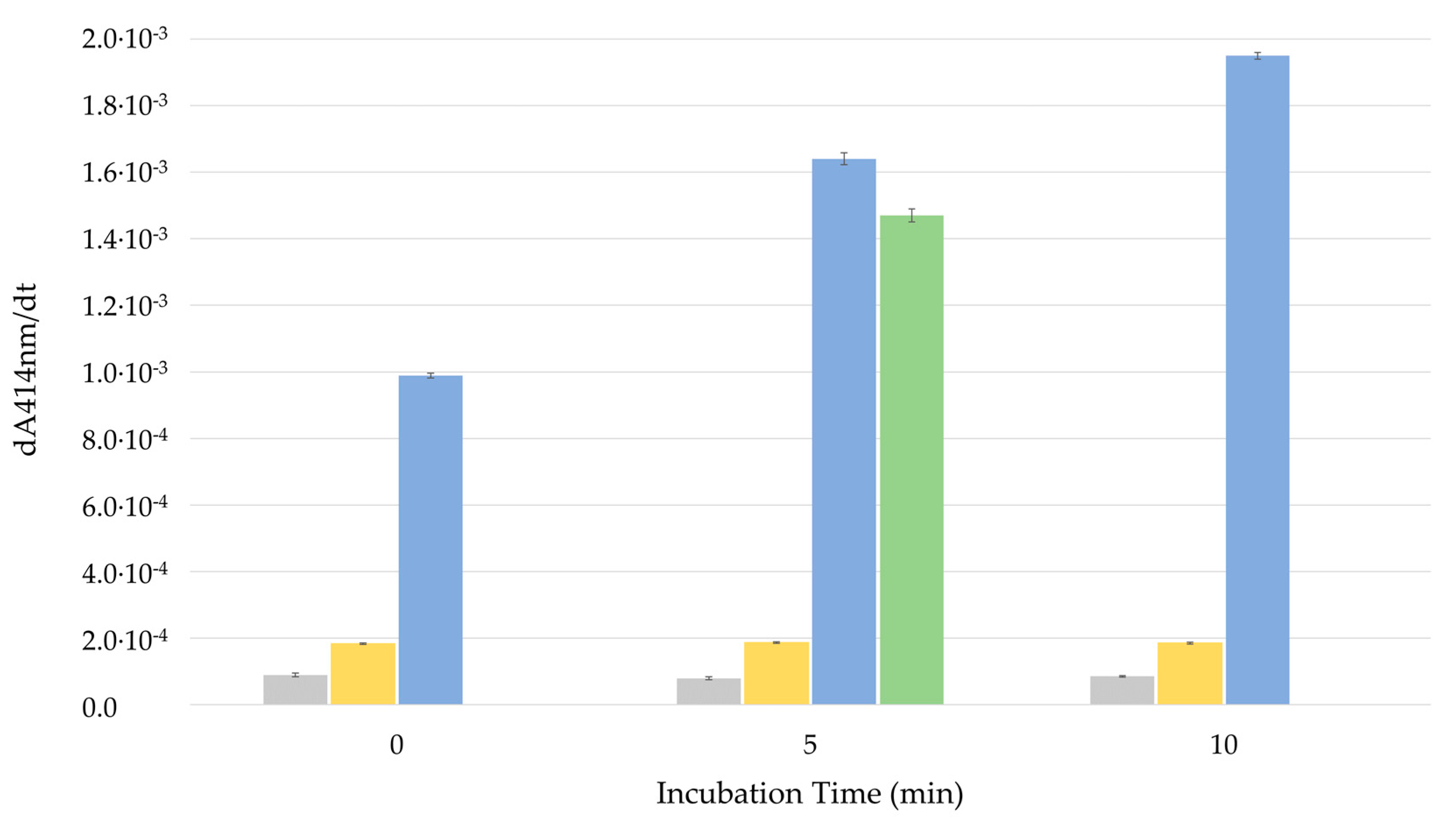
3.2.2. Permeabilization of LUVs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guha, S.; Ghimire, J.; Wu, E.; Wimley, W.C. Mechanistic Landscape of Membrane-Permeabilizing Peptides. Chem. Rev. 2019, 119, 6040–6085. [Google Scholar] [CrossRef]
- Hasan, M.; Yamazaki, M. Elementary Processes and Mechanisms of Interactions of Antimicrobial Peptides with Membranes—Single Giant Unilamellar Vesicle Studies. In Antimicrobial Peptides. Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1117, pp. 17–32. [Google Scholar]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta (BBA) Biomembr. 1999, 1462, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef] [Green Version]
- Galdiero, S.; Falanga, A.; Cantisani, M.; Vitiello, M.; Morelli, G.; Galdiero, M. Peptide-Lipid Interactions: Experiments and Applications. Int. J. Mol. Sci. 2013, 14, 18758–18789. [Google Scholar] [CrossRef] [Green Version]
- Hall, B.A.; Chetwynd, A.P.; Sansom, M.S.P. Exploring Peptide-Membrane Interactions with Coarse-Grained MD Simulations. Biophys. J. 2011, 100, 1940–1948. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Feix, J.B. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 1245–1256. [Google Scholar] [CrossRef] [Green Version]
- Hollmann, A.; Martinez, M.; Maturana, P.; Semorile, L.C.; Maffia, P.C. Antimicrobial Peptides: Interaction With Model and Biological Membranes and Synergism With Chemical Antibiotics. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The interaction of antimicrobial peptides with membranes. Adv. Colloid Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef]
- Pillong, M.; Hiss, J.A.; Schneider, P.; Lin, Y.-C.; Posselt, G.; Pfeiffer, B.; Blatter, M.; Müller, A.T.; Bachler, S.; Neuhaus, C.S.; et al. Rational Design of Membrane-Pore-Forming Peptides. Small 2017, 13, 1701316. [Google Scholar] [CrossRef]
- Friedman, R.; Khalid, S.; Aponte-Santamaría, C.; Arutyunova, E.; Becker, M.; Boyd, K.J.; Christensen, M.; Coimbra, J.T.S.; Concilio, S.; Daday, C.; et al. Understanding Conformational Dynamics of Complex Lipid Mixtures Relevant to Biology. J. Membr. Biol. 2018, 251, 609–631. [Google Scholar] [CrossRef] [Green Version]
- Ishibashi, J.; Saido-Sakanaka, H.; Yang, J.; Sagisaka, A.; Yamakawa, M. Purification, cDNA cloning and modification of a defensin from the coconut rhinoceros beetle, Oryctes rhinoceros. Eur. J. Biochem. 1999, 266, 616–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotto, S.P.; Sessa, L.; Concilio, S.; Iannelli, P. YADAMP: Yet another database of antimicrobial peptides. Int. J. Antimicrob. Agents 2012, 39, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Piotto, S.; Di Biasi, L.; Sessa, L.; Concilio, S. Transmembrane Peptides as Sensors of the Membrane Physical State. Front. Phys. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Scrima, M.; Di Marino, S.; Grimaldi, M.; Campana, F.; Vitiello, G.; Piotto, S.P.; D’Errico, G.; D’Ursi, A.M. Structural features of the C8 antiviral peptide in a membrane-mimicking environment. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 1010–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Melo, M.C.; Berglund, N.; Khan, A.; de la Fuente-Nunez, C.; Ulmschneider, J.P.; Ulmschneider, M.B. Understanding and modelling the interactions of peptides with membranes: From partitioning to self-assembly. Curr. Opin. Struct. Biol. 2020, 61, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Khelashvili, G.; Shan, J.; Andersen, O.S.; Weinstein, H. Quantitative modeling of membrane deformations by multihelical membrane proteins: Application to G-protein coupled receptors. Biophys. J. 2011, 101, 2092–2101. [Google Scholar] [CrossRef] [Green Version]
- Piotto, S.; Concilio, S.; Sessa, L.; Diana, R.; Torrens, G.; Juan, C.; Caruso, U.; Iannelli, P. Synthesis and antimicrobial studies of new antibacterial azo-compounds active against staphylococcus aureus and listeria monocytogenes. Molecules 2017, 22, 1372. [Google Scholar] [CrossRef] [Green Version]
- Concilio, S.; Sessa, L.; Petrone, A.M.; Porta, A.; Diana, R.; Iannelli, P.; Piotto, S. Structure modification of an active azo-compound as a route to new antimicrobial compounds. Molecules 2017, 22, 875. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.H.M.; Boxer, S.G. Model membrane systems and their applications. Curr. Opin. Chem. Biol. 2007, 11, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, P.S.; Sousa, V.K.; Donato, M.; Itri, R.; Beltramini, L.M.; Araujo, A.P.; Buerck, J.; Wallace, B.; Lopes, J.L. Unveiling the binding and orientation of the antimicrobial peptide Plantaricin 149 in zwitterionic and negatively charged membranes. Eur. Biophys. J. 2019, 48, 621–633. [Google Scholar] [CrossRef]
- Ning, L.; Mu, Y. Aggregation of PrP106–126 on surfaces of neutral and negatively charged membranes studied by molecular dynamics simulations. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 1936–1948. [Google Scholar] [CrossRef]
- Leber, R.; Pachler, M.; Kabelka, I.; Svoboda, I.; Enkoller, D.; Vácha, R.; Lohner, K.; Pabst, G. Synergism of antimicrobial frog peptides couples to membrane intrinsic curvature strain. Biophys. J. 2018, 114, 1945–1954. [Google Scholar] [CrossRef] [Green Version]
- Salnikov, E.S.; Bechinger, B. Lipid-controlled peptide topology and interactions in bilayers: Structural insights into the synergistic enhancement of the antimicrobial activities of PGLa and magainin 2. Biophys. J. 2011, 100, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Tzong-Hsien, L.; Kristopher, N.H.; Marie-Isabel, A. Antimicrobial Peptide Structure and Mechanism of Action: A Focus on the Role of Membrane Structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Harada, M.; Handa, T.; Funakoshi, S.; Fujii, N.; Yajima, H.; Miyajima, K. Magainin 1-Induced Leakage of Entrapped Calcein out of Negatively-Charged Lipid Vesicles. Biochim. Biophys. Acta 1989, 981, 130–134. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Fukui, M.; Fujii, N.; Miyajima, K. Interactions of an antimicrobial peptide, tachyplesin I, with lipid membranes. Biochim. Biophys. Acta 1991, 1070, 259–264. [Google Scholar] [CrossRef]
- King, R.D.; Sternberg, M.J. Identification and application of the concepts important for accurate and reliable protein secondary structure prediction. Protein Sci. 1996, 5, 2298–2310. [Google Scholar] [CrossRef] [PubMed]
- Holton, T.A.; Pollastri, G.; Shields, D.C.; Mooney, C. CPPpred: Prediction of cell penetrating peptides. Bioinformatics 2013, 29, 3094–3096. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI Membrane Builder for Mixed Bilayers and Its Application to Yeast Membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Krieger, E.; Vriend, G. YASARA View-molecular graphics for all devices-from smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guixà-González, R.; Rodriguez-Espigares, I.; Ramírez-Anguita, J.M.; Carrió-Gaspar, P.; Martinez-Seara, H.; Giorgino, T.; Selent, J. MEMBPLUGIN: Studying membrane complexity in VMD. Bioinformatics 2014, 30, 1478–1480. [Google Scholar] [CrossRef] [Green Version]
- Concilio, S.; Ferrentino, I.; Sessa, L.; Massa, A.; Iannelli, P.; Diana, R.; Panunzi, B.; Rella, A.; Piotto, S. A novel fluorescent solvatochromic probe for lipid bilayers. Supramol. Chem. 2017, 29, 887–895. [Google Scholar] [CrossRef]
- Maget-Dana, R.; Bonmatin, J.M.; Hetru, C.; Ptak, M.; Maurizot, J.C. The secondary structure of the insect defensin A depends on its environment. A circular dichroism study. Biochimie 1995, 77, 240–244. [Google Scholar] [CrossRef]
- Pieri, E.; Ledentu, V.; Huix-Rotllant, M.; Ferré, N. Sampling the protonation states: The pH-dependent UV absorption spectrum of a polypeptide dyad. Phys. Chem. Chem. Phys. 2018, 20, 23252–23261. [Google Scholar] [CrossRef] [Green Version]
- Angelova, M.I.; Soleau, S.; Meleard, P.; Faucon, J.F.; Bothorel, P. Preparation of Giant Vesicles by External Ac Electric-Fields-Kinetics and Applications. Prog. Coll. Pol. Sci. 1992, 89, 127–131. [Google Scholar] [CrossRef]
- Robinson, T.; Dittrich, P.S. Observations of membrane domain reorganization in mechanically compressed artificial cells. ChemBioChem 2019, 20, 2666–2673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, T.; Kuhn, P.; Eyer, K.; Dittrich, P.S. Microfluidic trapping of giant unilamellar vesicles to study transport through a membrane pore. Biomicrofluidics 2013, 7, 044105. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Schmid, Y.R.F.; Luginbühl, S.; Wang, Q.; Dittrich, P.S.; Walde, P. Spectrophotometric Quantification of Peroxidase with p-Phenylene-diamine for Analyzing Peroxidase-Encapsulating Lipid Vesicles. Anal. Chem. 2017, 89, 5484–5493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, R.E.; Bardsley, W.G. The steady-state kinetics of peroxidase with 2,2’-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem. J. 1975, 145, 93–103. [Google Scholar] [CrossRef]
- Welinder, K.G.; Smillie, L.B. Amino acid sequence studies of horseradish peroxidase. II. Thermolytic peptides. Can. J. Biochem. 1972, 50, 63–90. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Porstmann, B.; Porstmann, T.; Gaede, D.; Nugel, E.; Egger, E. Temperature dependent rise in activity of horseradish peroxidase caused by non-ionic detergents and its use in enzyme-immunoassay. Clin. Chim. Acta 1981, 109, 175–181. [Google Scholar] [CrossRef]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant vesicles: Preparations and applications. ChemBioChem 2010, 11, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R. Giant vesicles and their use in assays for assessing membrane phase state, curvature, mechanics, and electrical properties. Annu. Rev. Biophys. 2019, 48, 93–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimoto, M.; Higa, M. A kinetic analysis of catalytic production of oxygen in catalase-containing liposome dispersions for controlled transfer of oxygen in a bioreactor. J. Chem. Technol. Biotechnol. 2014, 89, 1388–1395. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Walde, P.; Gardiner, J.; Mahajan, Y.R.; Seebach, D.; Thomae, A.; Krämer, S.D.; Voser, M.; Kuboi, R. Permeation of a β-heptapeptide derivative across phospholipid bilayers. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 2726–2736. [Google Scholar] [CrossRef]
- Zepik, H.H.; Walde, P.; Kostoryz, E.L.; Code, J.; Yourtee, D.M. Lipid vesicles as membrane models for toxicological assessment of xenobiotics. Crit. Rev. Toxicol. 2008, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sessa, L.; Concilio, S.; Walde, P.; Robinson, T.; Dittrich, P.S.; Porta, A.; Panunzi, B.; Caruso, U.; Piotto, S. Study of the Interaction of a Novel Semi-Synthetic Peptide with Model Lipid Membranes. Membranes 2020, 10, 294. https://doi.org/10.3390/membranes10100294
Sessa L, Concilio S, Walde P, Robinson T, Dittrich PS, Porta A, Panunzi B, Caruso U, Piotto S. Study of the Interaction of a Novel Semi-Synthetic Peptide with Model Lipid Membranes. Membranes. 2020; 10(10):294. https://doi.org/10.3390/membranes10100294
Chicago/Turabian StyleSessa, Lucia, Simona Concilio, Peter Walde, Tom Robinson, Petra S. Dittrich, Amalia Porta, Barbara Panunzi, Ugo Caruso, and Stefano Piotto. 2020. "Study of the Interaction of a Novel Semi-Synthetic Peptide with Model Lipid Membranes" Membranes 10, no. 10: 294. https://doi.org/10.3390/membranes10100294
APA StyleSessa, L., Concilio, S., Walde, P., Robinson, T., Dittrich, P. S., Porta, A., Panunzi, B., Caruso, U., & Piotto, S. (2020). Study of the Interaction of a Novel Semi-Synthetic Peptide with Model Lipid Membranes. Membranes, 10(10), 294. https://doi.org/10.3390/membranes10100294