Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Pd Alloy Module Preparation
2.2. Gas Permeation Measurements
2.2.1. H2 Permeation Experiments
2.2.2. H2 Permeation Experiments
2.2.3. Post-Process Characterization
3. Results and Discussion
3.1. H2 Permeation Properties of Employed Pd-Based Membranes
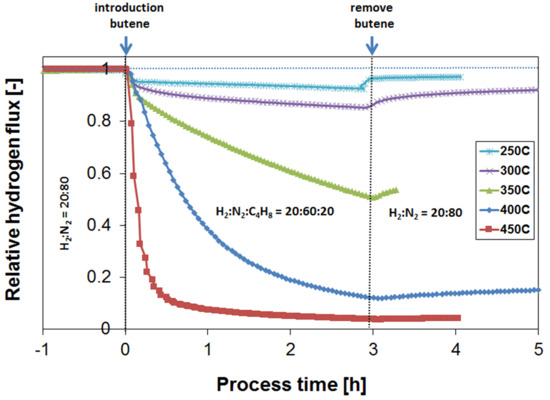
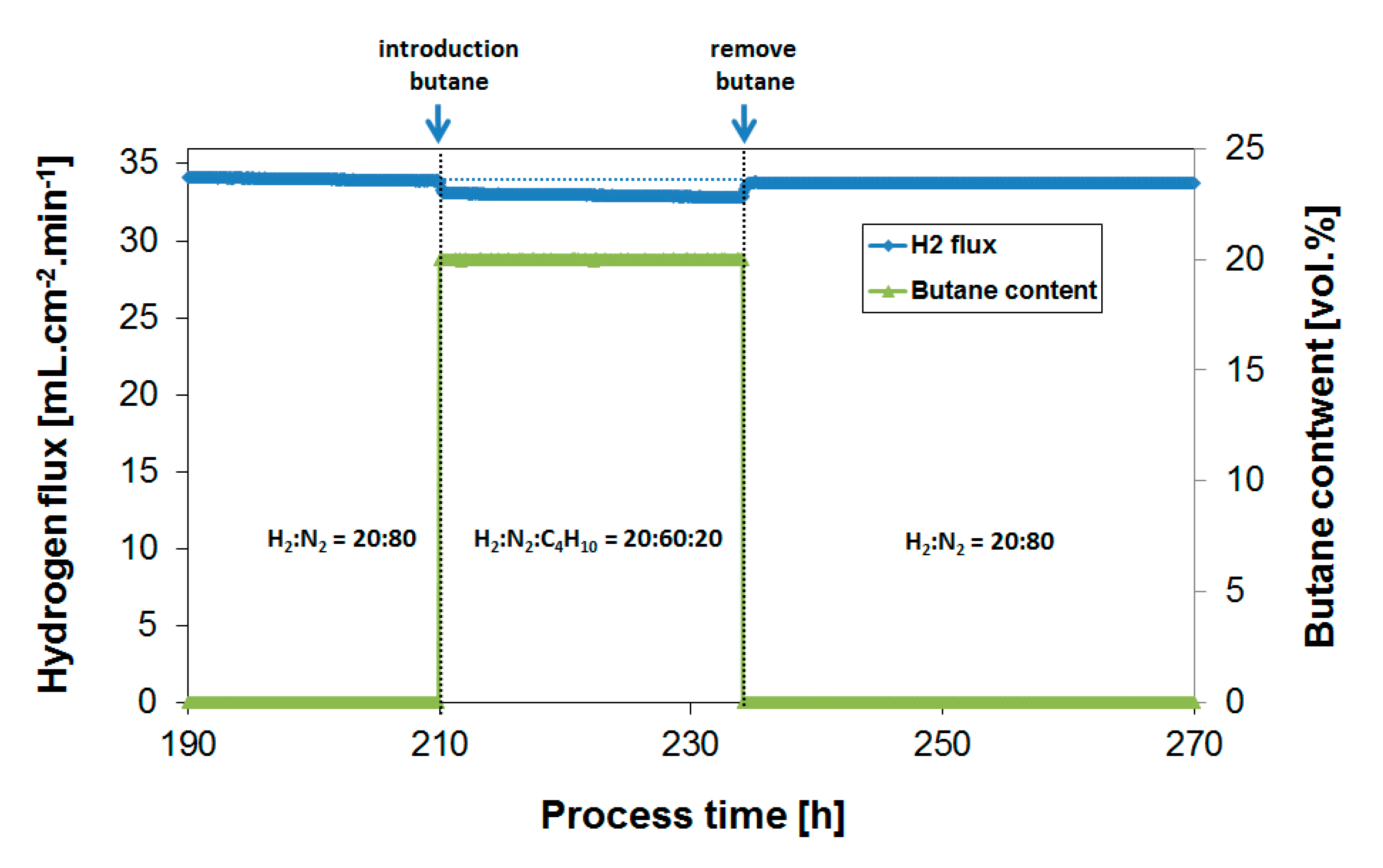
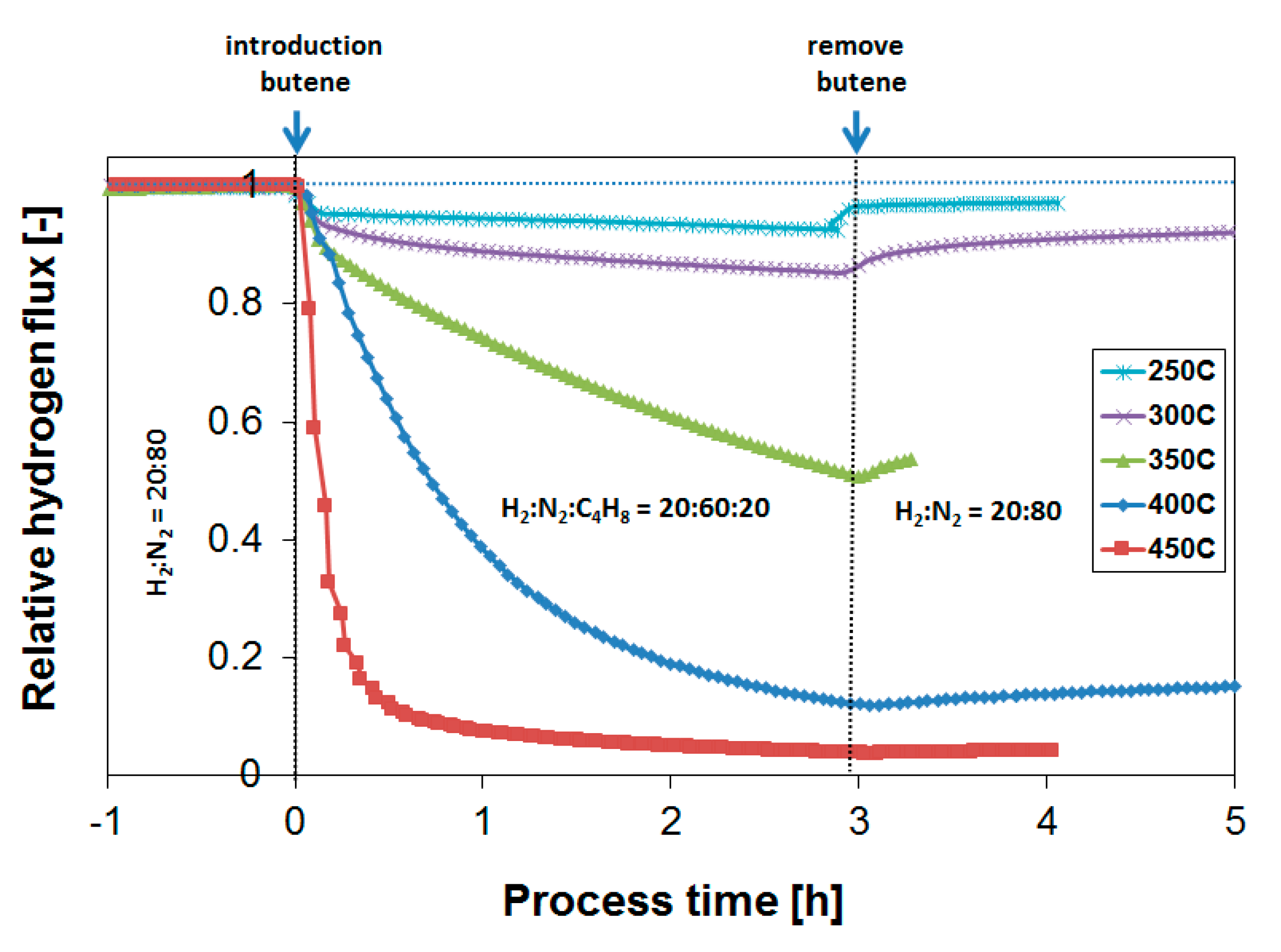
3.2. Exposure to Butane
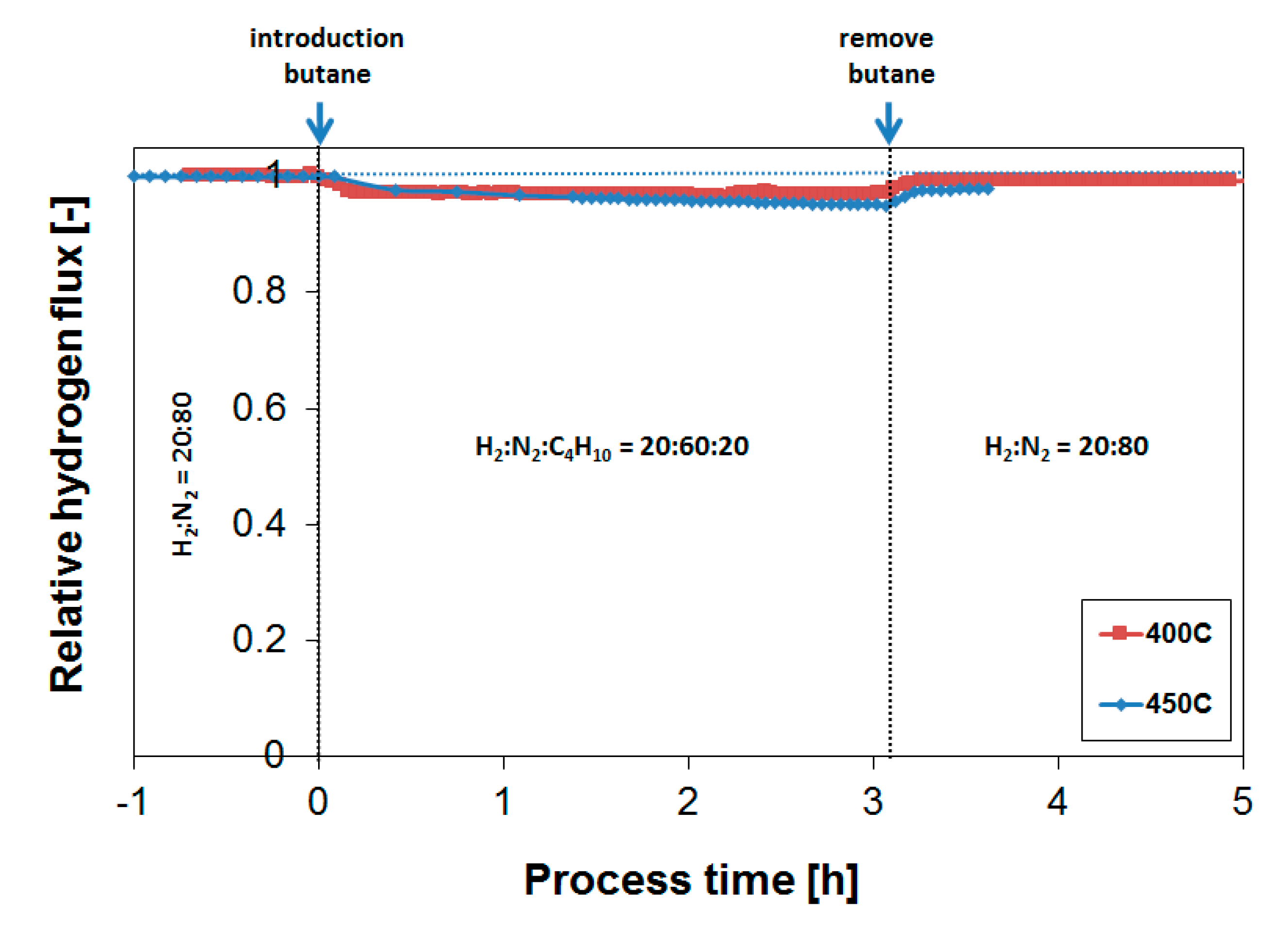
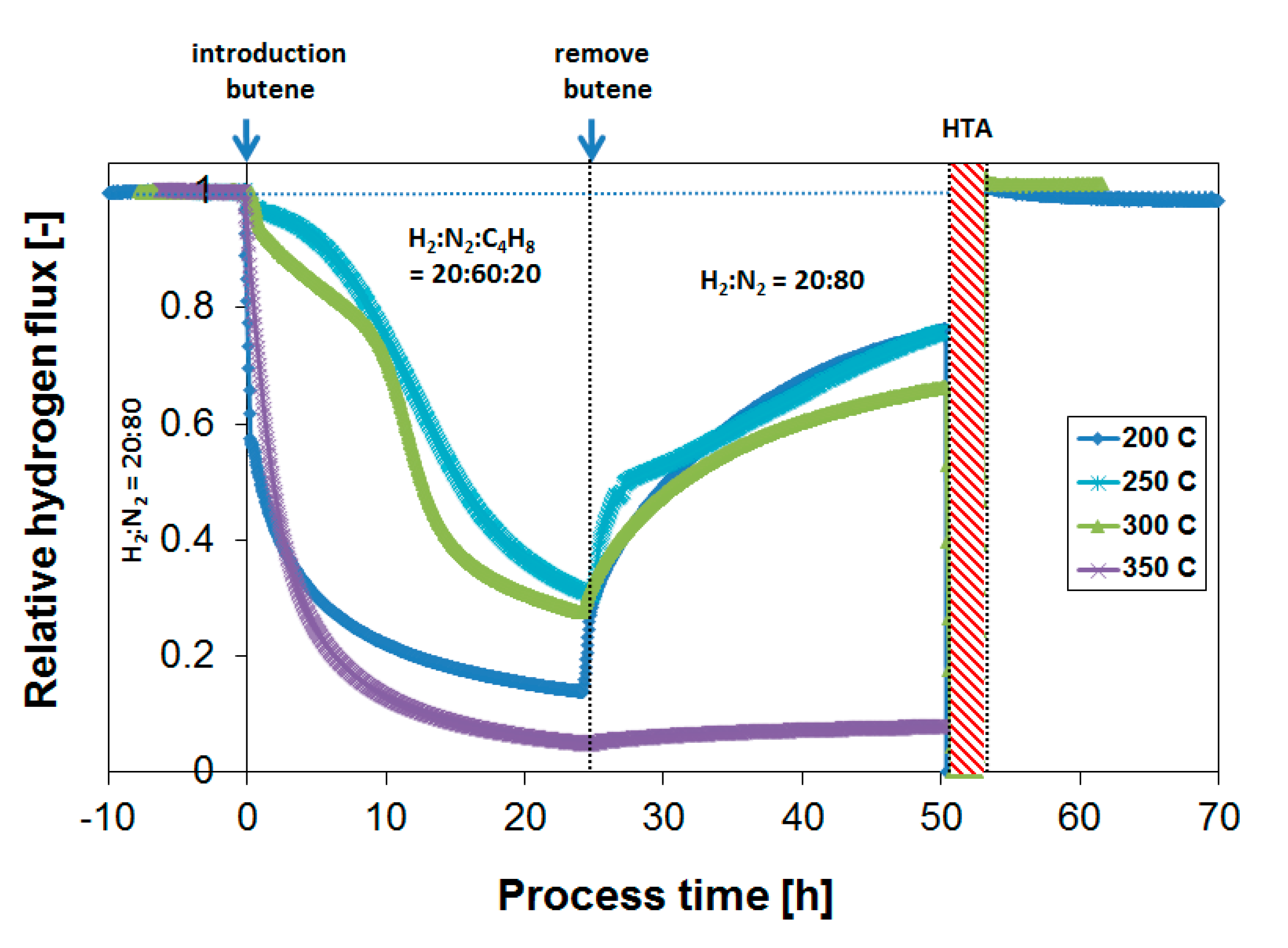
3.3. Parametric Study of H2 Flux Inhibition During Butylene Exposure
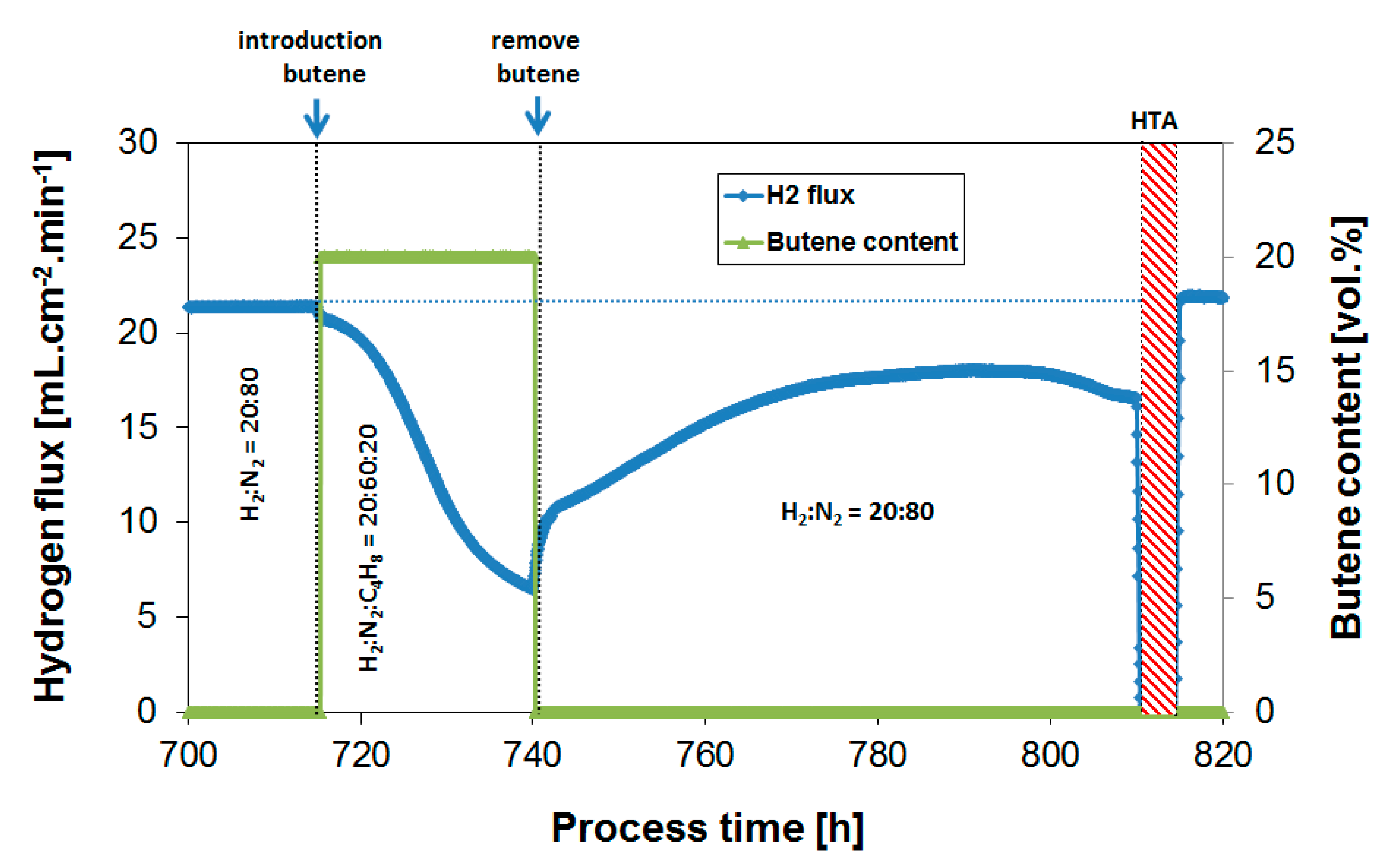
3.4. Long-Term Performance During Butylene Exposure
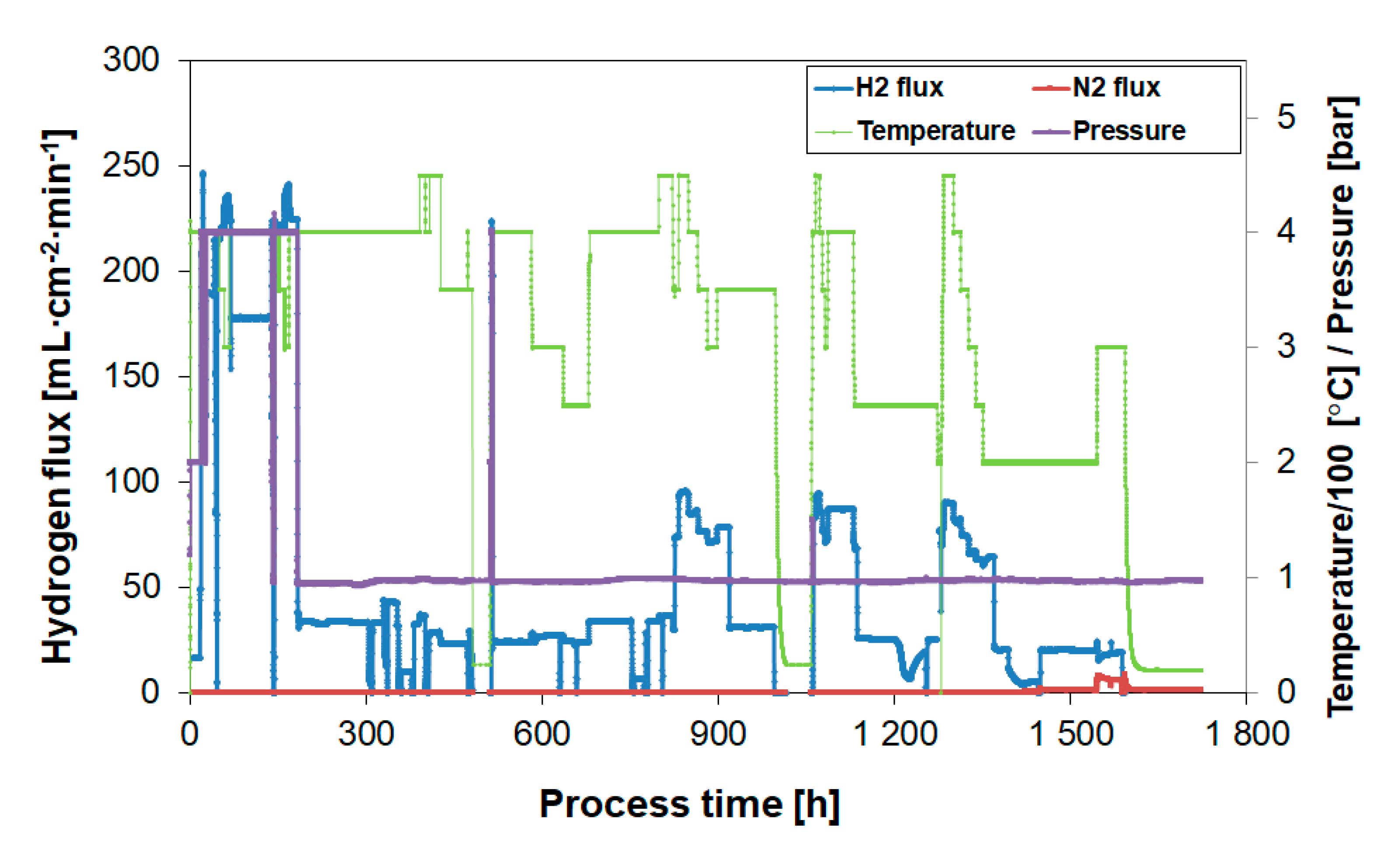
3.5. Membrane Stability
3.5.1. Separation Performance
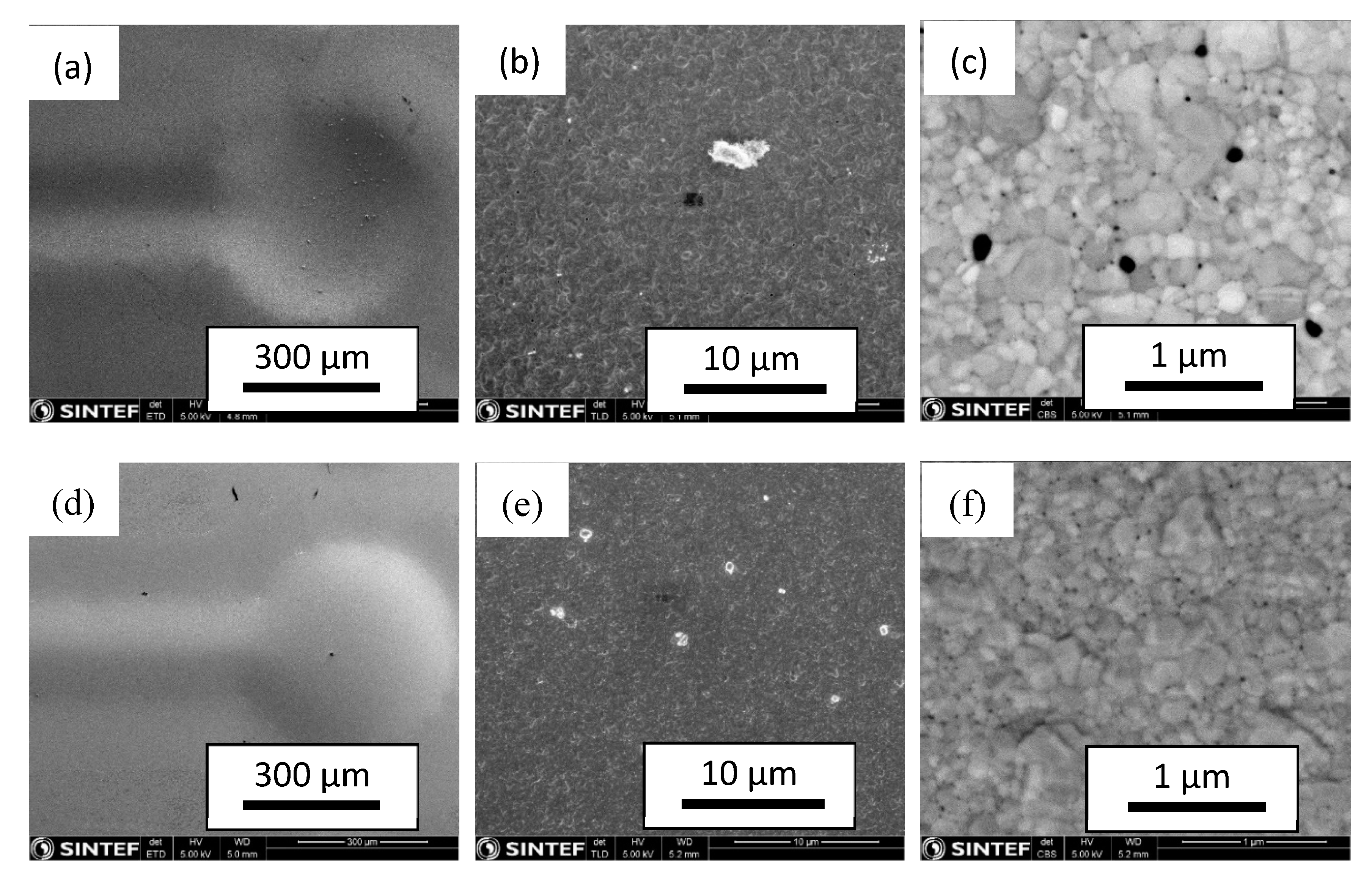
3.5.2. Post-Process Membrane Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- James, O.O.; Mandal, S.; Alele, N.; Chowdhury, B.; Maity, S. Lower alkanes dehydrogenation: Strategies and reaction routes to corresponding alkenes. Fuel Process. Technol. 2016, 149, 239–255. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Caspary, K.J.; Gehrke, H.; Heinrita-Adrian, M.; Schwefer, M. Dehydrogenation of Alkanes. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Sheintuch, M. and Simakov, D.S.A. Alkanes Dehydrogenation, in Membrane Reactors for Hydrogen Production Processes; Falco, M.D.D., Marrelli, L., Iaquaniello, G., Eds.; Springer: London, UK, 2011; pp. 183–200. [Google Scholar]
- Chang, J.S.; Roh, H.S.; Park, M.S.; Park, S.E. Propane dehydrogenation over a hydrogen permselective membrane reactor. Bull. Korean Chem. Soc. 2002, 23, 674–678. [Google Scholar]
- Ricca, A.; Montella, F.; Iaquaniello, G.; Palo, E.; Salladini, A.; Palma, V. Membrane assisted propane dehydrogenation: Experimental investigation and mathematical modelling of catalytic reactions. Catal. Today 2019, 331, 43–52. [Google Scholar] [CrossRef]
- Liang, W.; Hughes, R. The catalytic dehydrogenation of isobutane to isobutene in a palladium/silver composite membrane reactor. Catal. Today 2005, 104, 238–243. [Google Scholar] [CrossRef]
- Moparthi, A.; Uppaluri, R.; Gill, B. Economic feasibility of silica and palladium composite membranes for industrial dehydrogenation reactions. Chem. Eng. Res. Des. 2010, 88, 1088–1101. [Google Scholar] [CrossRef]
- Quicker, P.; Höllein, V.; Dittmeyer, R. Catalytic dehydrogenation of hydrocarbons in palladium composite membrane reactors. Catal. Today 2000, 56, 21–34. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Höllein, V.; Daub, K. Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium. J. Mol. Catal. A Chem. 2001, 173, 135–184. [Google Scholar] [CrossRef]
- Zhao, R.; Govind, R.; Itoh, N. Studies on Palladium Membrane Reactor for Dehydrogenation Reaction. Sep. Sci. Technol. 1990, 25, 1473–1488. [Google Scholar] [CrossRef]
- Ali, J.K.; Newson, E.; Rippin, D. Exceeding equilibrium conversion with a catalytic membrane reactor for the dehydrogenation of methylcyclohexane. Chem. Eng. Sci. 1994, 49, 2129–2134. [Google Scholar] [CrossRef]
- Ali, J.K.; Newson, E.; Rippin, D. Deactivation and regeneration of PdAg membranes for dehydrogenation reactions. J. Membr. Sci. 1994, 89, 171–184. [Google Scholar] [CrossRef]
- Itoh, N. Limiting conversions of dehydrogenation in palladium membrane reactors. Catal. Today 1995, 25, 351–356. [Google Scholar] [CrossRef]
- Collins, J.P.; Schwartz, R.W.; Sehgal, R.; Ward, T.L.; Brinker, C.J.; Hagen, G.P.; Udovich, C.A. Catalytic Dehydrogenation of Propane in Hydrogen Permselective Membrane Reactors. Ind. Eng. Chem. Res. 1996, 35, 4398–4405. [Google Scholar] [CrossRef]
- Sheintuch, M.; Dessau, R.M. Observations, modeling and optimization of yield, selectivity and activity during dehydrogenation of isobutane and propane in a Pd membrane reactor. Chem. Eng. Sci. 1996, 51, 535–547. [Google Scholar] [CrossRef]
- Hermann, C.; Quicker, P.; Dittmeyer, R. Mathematical simulation of catalytic dehydrogenation of ethylbenzene to styrene in a composite palladium membrane reactor. J. Membr. Sci. 1997, 136, 161–172. [Google Scholar] [CrossRef]
- Yildirim, Y.; Gobina, E.; Hughes, R. An experimental evaluation of high-temperature composite membrane systems for propane dehydrogenation. J. Membr. Sci. 1997, 135, 107–115. [Google Scholar] [CrossRef]
- She, Y.; Han, J.; Ma, Y. Palladium membrane reactor for the dehydrogenation of ethylbenzene to styrene. Catal. Today 2001, 67, 43–53. [Google Scholar] [CrossRef]
- Keuler, J.N. The dehydrogenation of 2-butanol in a Pd–Ag membrane reactor. J. Membr. Sci. 2002, 202, 17–26. [Google Scholar] [CrossRef]
- Farsi, M.; Jahanmiri, A.; Rahimpour, M. Simultaneous isobutane dehydrogenation and hydrogen production in a hydrogen-permselective membrane fixed bed reactor. Theor. Found. Chem. Eng. 2014, 48, 799–805. [Google Scholar] [CrossRef]
- Wunsch, A.; Mohr, M.; Pfeifer, P. Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes. Membranes 2018, 8, 112. [Google Scholar] [CrossRef]
- Ricca, A.; Palma, V.; Iaquaniello, G.; Palo, E.; Salladini, A.A. Highly selective propylene production in a membrane assisted catalytic propane dehydrogenation. Chem. Eng. J. 2017, 330, 1119–1127. [Google Scholar] [CrossRef]
- Palo, E.; Salladini, A.A.; Morico, B.; Palma, V.; Ricca, A.; Iaquaniello, G. Application of Pd-Based Membrane Reactors: An Industrial Perspective. Membranes 2018, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, C.J. The role of heat transfer on the feasibility of a packed-bed membrane reactor for propane dehydrogenation. Chem. Eng. J. 2020, 381, 122492. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Way, J.D. INNOVATIONS IN PALLADIUM MEMBRANE RESEARCH. Sep. Purif. Methods 2002, 31, 1–169. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for Hydrogen Separation. Chem. Rev. 2010, 110, 2573–2574. [Google Scholar] [CrossRef]
- Peters, T.; Stange, M.; Klette, H.; Bredesen, R. High pressure performance of thin Pd–23%Ag/stainless steel composite membranes in water gas shift gas mixtures; influence of dilution, mass transfer and surface effects on the hydrogen flux. J. Membr. Sci. 2008, 316, 119–127. [Google Scholar] [CrossRef]
- Mejdell, A.L.; Jondahl, M.; Peters, T.A.; Bredesen, R.; Venvik, H.J. Effects of CO and CO2 on hydrogen permeation through a ~3 µm Pd/Ag 23 wt% membrane employed in a microchannel membrane configuration. Sep. Purif. Technol. 2009, 68, 178–184. [Google Scholar] [CrossRef]
- Chen, C.-H.; Ma, Y.H. The effect of H2S on the performance of Pd and Pd/Au composite membrane. J. Membr. Sci. 2010, 362, 535–544. [Google Scholar] [CrossRef]
- Caravella, A.; Scura, F.; Barbieri, G.; Drioli, E. Inhibition by CO and Polarization in Pd-Based Membranes: A Novel Permeation Reduction Coefficient. J. Phys. Chem. B 2010, 114, 12264–12276. [Google Scholar] [CrossRef]
- Miguel, C.; Mendes, A.; Tosti, S.; Madeira, L.M. Effect of CO and CO2 on H2 permeation through finger-like Pd–Ag membranes. Int. J. Hydrog. Energy 2012, 37, 12680–12687. [Google Scholar] [CrossRef]
- Kurokawa, H.; Yakabe, H.; Yasuda, I.; Peters, T.; Bredesen, R. Inhibition effect of CO on hydrogen permeability of Pd–Ag membrane applied in a microchannel module configuration. Int. J. Hydrog. Energy 2014, 39, 17201–17209. [Google Scholar] [CrossRef]
- Kulprathipanja, A.; Alptekin, G.; Falconer, J.; Way, J. Pd and Pd–Cu membranes: Inhibition of H permeation by HS. J. Membr. Sci. 2005, 254, 49–62. [Google Scholar] [CrossRef]
- Gao, H.; Lin, Y.; Li, Y.; Zhang, B. Chemical Stability and Its Improvement of Palladium-Based Metallic Membranes. Ind. Eng. Chem. Res. 2004, 43, 6920–6930. [Google Scholar] [CrossRef]
- Fernandez, E.E.; Helmi, A.; Coenen, K.K.; Rey, J.M.; Viviente, J.L.; Tanaka, D.A.P.; Annaland, M.M.V.S.; Gallucci, F.F. Development of thin Pd–Ag supported membranes for fluidized bed membrane reactors including WGS related gases. Int. J. Hydrog. Energy 2015, 40, 3506–3519. [Google Scholar] [CrossRef]
- De Nooijer, N.; Sanchez, J.D.; Melendez, J.; Fernandez, E.; Tanaka, D.A.P.; Annaland, M.V.S.; Gallucci, F. Influence of H2S on the hydrogen flux of thin-film PdAgAu membranes. Int. J. Hydrog. Energy 2020, 45, 7303–7312. [Google Scholar] [CrossRef]
- Montesinos, H.; Julián, I.; Herguido, J.; Menéndez, M. Effect of the presence of light hydrocarbon mixtures on hydrogen permeance through Pd–Ag alloyed membranes. Int. J. Hydrog. Energy 2015, 40, 3462–3471. [Google Scholar] [CrossRef]
- Peters, T.; Liron, O.; Tschentscher, R.; Sheintuch, M.; Bredesen, R. Investigation of Pd-based membranes in propane dehydrogenation (PDH) processes. Chem. Eng. J. 2016, 305, 191–200. [Google Scholar] [CrossRef]
- Easa, J.; Jin, R.; O’Brien, C.P. Evolution of surface and bulk carbon species derived from propylene and their influence on the interaction of hydrogen with palladium. J. Membr. Sci. 2020, 596, 117738. [Google Scholar] [CrossRef]
- Palo, E.; Iaquaniello, G. Method for olefins production. U.S. Patent 9776935B2, 28 March 2012. [Google Scholar]
- Boeltken, T.; Belimov, M.; Pfeifer, P.; Peters, T.; Bredesen, R.; Dittmeyer, R. Fabrication and testing of a planar microstructured concept module with integrated palladium membranes. Chem. Eng. Process. Process. Intensif. 2013, 67, 136–147. [Google Scholar] [CrossRef]
- Mejdell, A.; Peters, T.; Stange, M.; Venvik, H.; Bredesen, R. Performance and application of thin Pd-alloy hydrogen separation membranes in different configurations. J. Taiwan Inst. Chem. Eng. 2009, 40, 253–259. [Google Scholar] [CrossRef]
- Peters, T.; Polfus, J.M.; Van Berkel, F.; Bredesen, R. Interplay between propylene and H2S co-adsorption on the H2 flux characteristics of Pd-alloy membranes employed in propane dehydrogenation (PDH) processes. Chem. Eng. J. 2016, 304, 134–140. [Google Scholar] [CrossRef]
- Peters, T.; Stange, M.; Veenstra, P.; Nijmeijer, A.; Bredesen, R. The performance of Pd–Ag alloy membrane films under exposure to trace amounts of H2S. J. Membr. Sci. 2016, 499, 105–115. [Google Scholar] [CrossRef]
- Peters, T.; Polfus, J.M.; Stange, M.; Veenstra, P.; Nijmeijer, A.; Bredesen, R. H 2 flux inhibition and stability of Pd-Ag membranes under exposure to trace amounts of NH 3. Fuel Process. Technol. 2016, 152, 259–265. [Google Scholar] [CrossRef]
- Tucho, W.M.; Venvik, H.; Stange, M.; Walmsley, J.C.; Holmestad, R.; Bredesen, R. Effects of thermal activation on hydrogen permeation properties of thin, self-supported Pd/Ag membranes. Sep. Purif. Technol. 2009, 68, 403–410. [Google Scholar] [CrossRef]
- Mejdell, A.; Klette, H.; Ramachandran, A.; Borg, A.L.; Bredesen, R. Hydrogen permeation of thin, free-standing Pd/Ag23% membranes before and after heat treatment in air. J. Membr. Sci. 2008, 307, 96–104. [Google Scholar] [CrossRef]
- Reyerson, L.H.; Cines, M.R. Adsorption of Propane and Propylene by Silica Gel and Metallized Silica Gel. J. Phys. Chem. 1942, 46, 1060–1068. [Google Scholar] [CrossRef]
- Abir, H.; Sheintuch, M. Modeling H2 transport through a Pd or Pd/Ag membrane, and its inhibition by co-adsorbates, from first principles. J. Membr. Sci. 2014, 466, 58–69. [Google Scholar] [CrossRef]
- Roa, F.; Way, J. The effect of air exposure on palladium–copper composite membranes. Appl. Surf. Sci. 2005, 240, 85–104. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Tam, S.Y. Hydrogen selective protective coating, coated article and method. U.S. Patent 13/581,587, 21 March 2011. [Google Scholar]
- Yu, J.; Qi, C.; Zhang, J.; Bao, C.; Xu, H. Synthesis of a zeolite membrane as a protective layer on a metallic Pd composite membrane for hydrogen purification. J. Mater. Chem. A 2015, 3, 5000–5006. [Google Scholar] [CrossRef]
- Akporiaye, D.; Jensen, S.F.; Olsbye, U.; Rohr, F.; Rytter, E.; Rønnekleiv, A.M.; Spjelkavik, A.I. A Novel, Highly Efficient Catalyst for Propane Dehydrogenation. Ind. Eng. Chem. Res. 2001, 40, 4741–4748. [Google Scholar] [CrossRef]
- Peters, T.; Stange, M.; Sunding, M.; Bredesen, R. Stability investigation of micro-configured Pd–Ag membrane modules – Effect of operating temperature and pressure. Int. J. Hydrog. Energy 2015, 40, 3497–3505. [Google Scholar] [CrossRef]
- Peters, T.; Tucho, W.M.; Ramachandran, A.; Stange, M.; Walmsley, J.C.; Holmestad, R.; Borg, A.L.; Bredesen, R. Thin Pd–23%Ag/stainless steel composite membranes: Long-term stability, life-time estimation and post-process characterisation. J. Membr. Sci. 2009, 326, 572–581. [Google Scholar] [CrossRef]
- Mardilovich, P.P.; She, Y.; Ma, Y.H.; Rei, M.-H. Defect-free palladium membranes on porous stainless-steel support. AIChE J. 1998, 44, 310–322. [Google Scholar] [CrossRef]
- Peters, T.; Carvalho, P.; Van Wees, J.; Overbeek, J.; Sagvolden, E.; Van Berkel, F.; Løvvik, O.M.; Bredesen, R. Leakage evolution and atomic-scale changes in Pd-based membranes induced by long-term hydrogen permeation. J. Membr. Sci. 2018, 563, 398–404. [Google Scholar] [CrossRef]
- Peters, T.; Carvalho, P.; Stange, M.; Bredesen, R. Formation of hydrogen bubbles in Pd-Ag membranes during H2 permeation. Int. J. Hydrog. Energy 2020, 45, 7488–7496. [Google Scholar] [CrossRef]
- Polfus, J.M.; Løwik, O.M.; Bredesen, R.; Peters, T. Hydrogen Induced Vacancy Clustering and Void Formation Mechanisms at Grain Boundaries in Palladium. Acta Mater. 2020. In press. [Google Scholar]
- Guazzone, F.; Engwall, E.E.; Ma, Y.H. Effects of surface activity, defects and mass transfer on hydrogen permeance and n-value in composite palladium-porous stainless steel membranes. Catal. Today 2006, 118, 24–31. [Google Scholar] [CrossRef]
- Tucho, W.M.; Venvik, H.J.; Walmsley, J.C.; Stange, M.; Ramachandran, A.; Mathiesen, R.H.; Borg, A.; Bredesen, R. and Holmesstad, R. Microstructural studies of self-supported (1.5–10 µm) Pd/23 wt %Ag hydrogen separation membranes subjected to different heat treatments. J. Mater. Sci. 2009, 44, 4429–4442. [Google Scholar] [CrossRef]
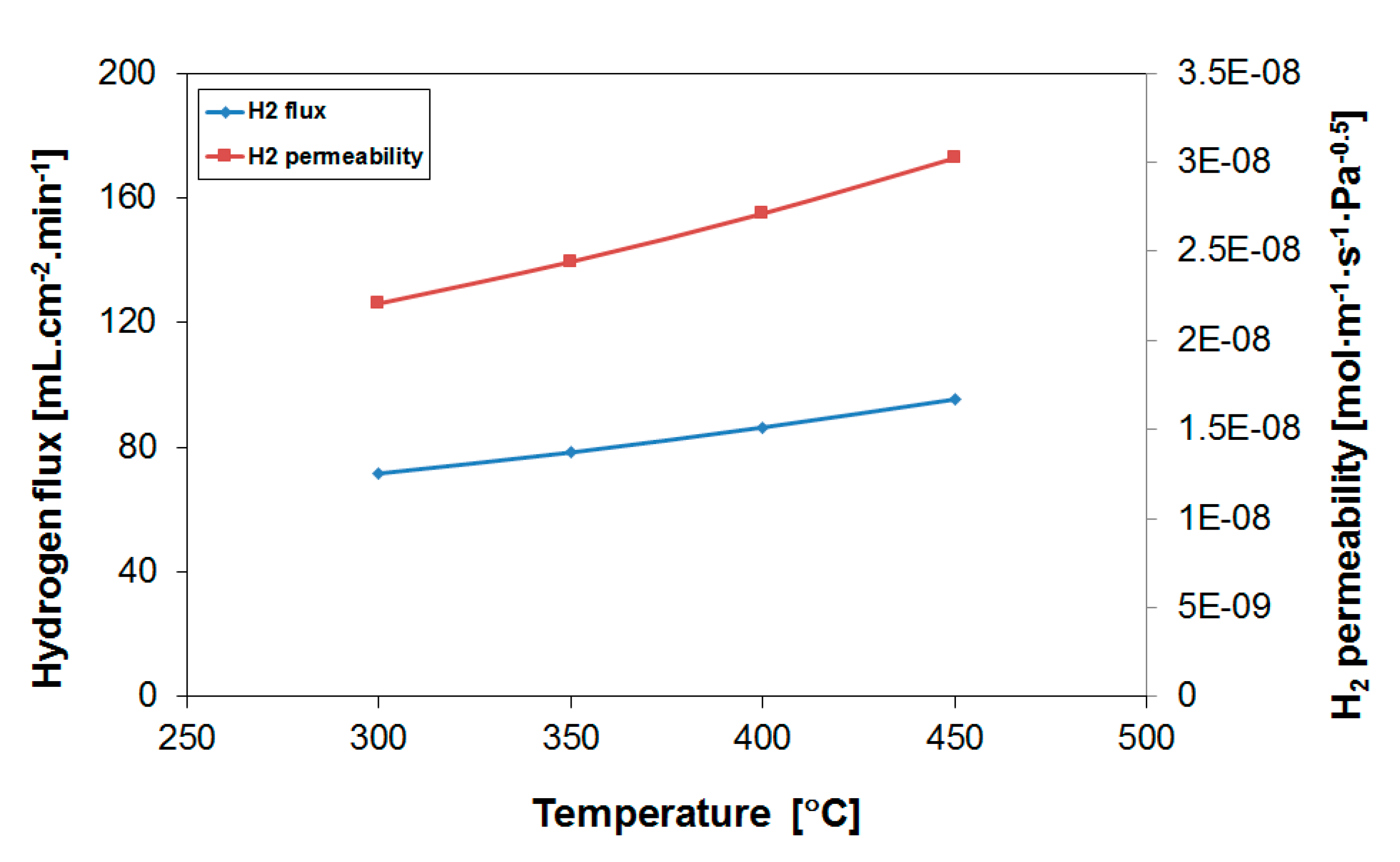
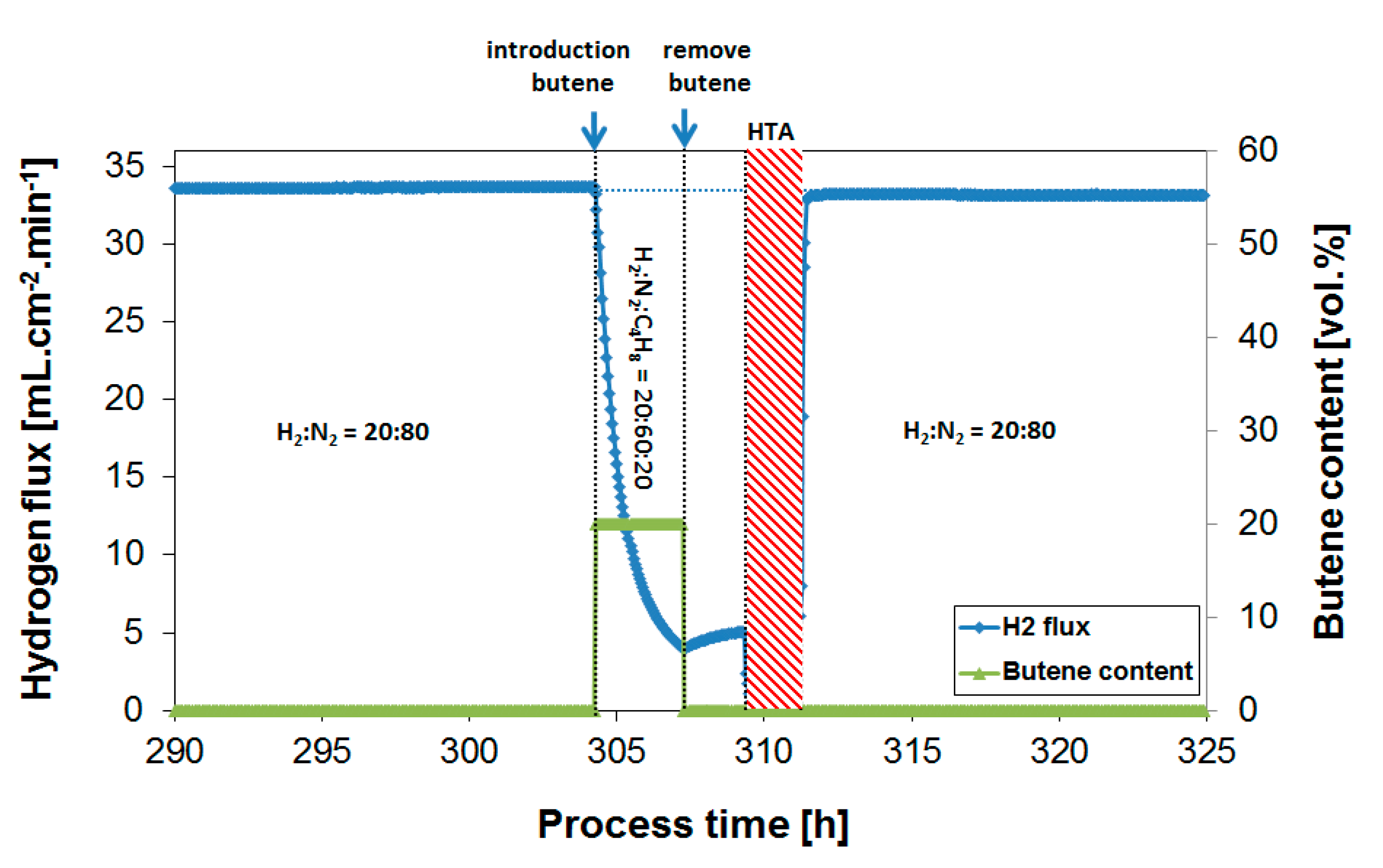
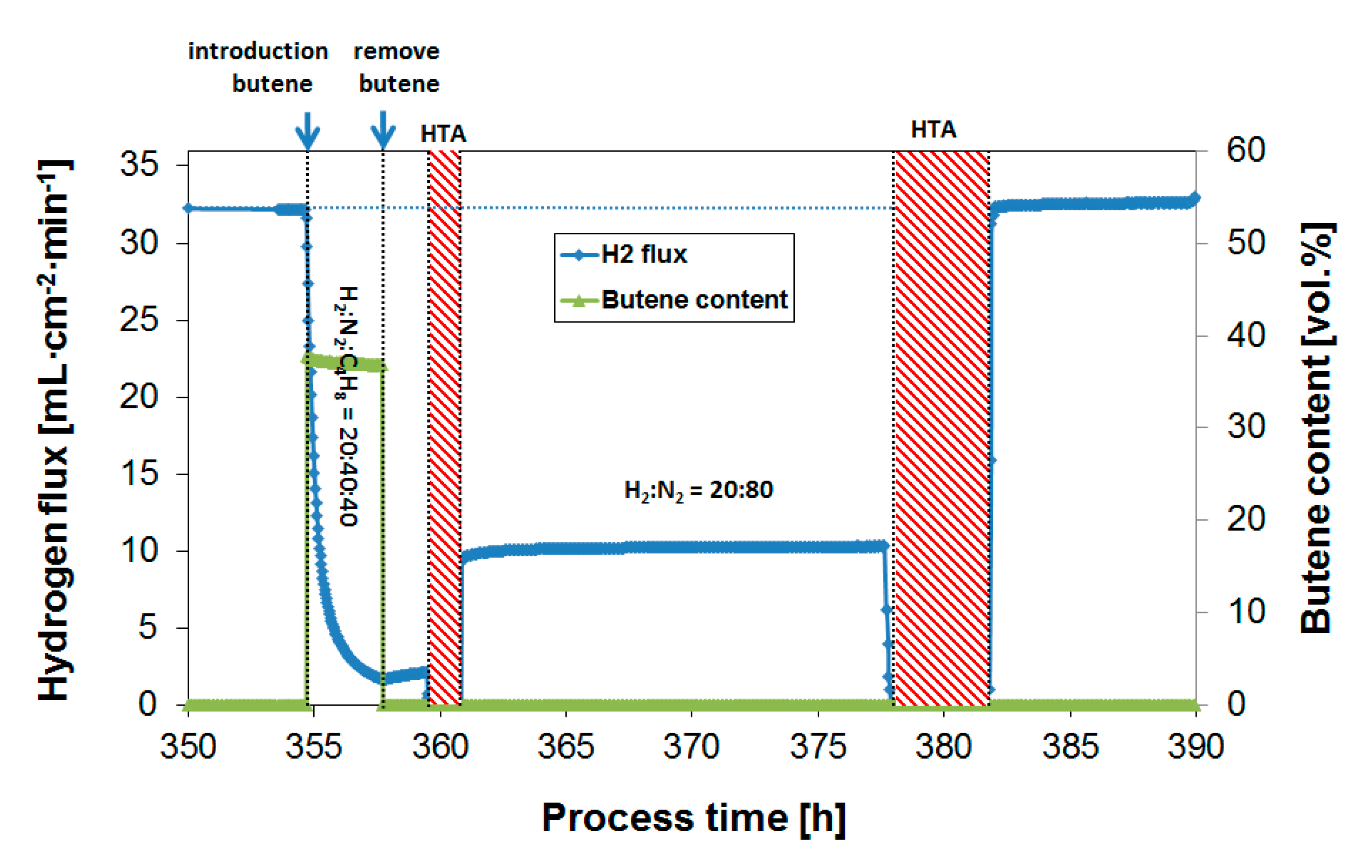
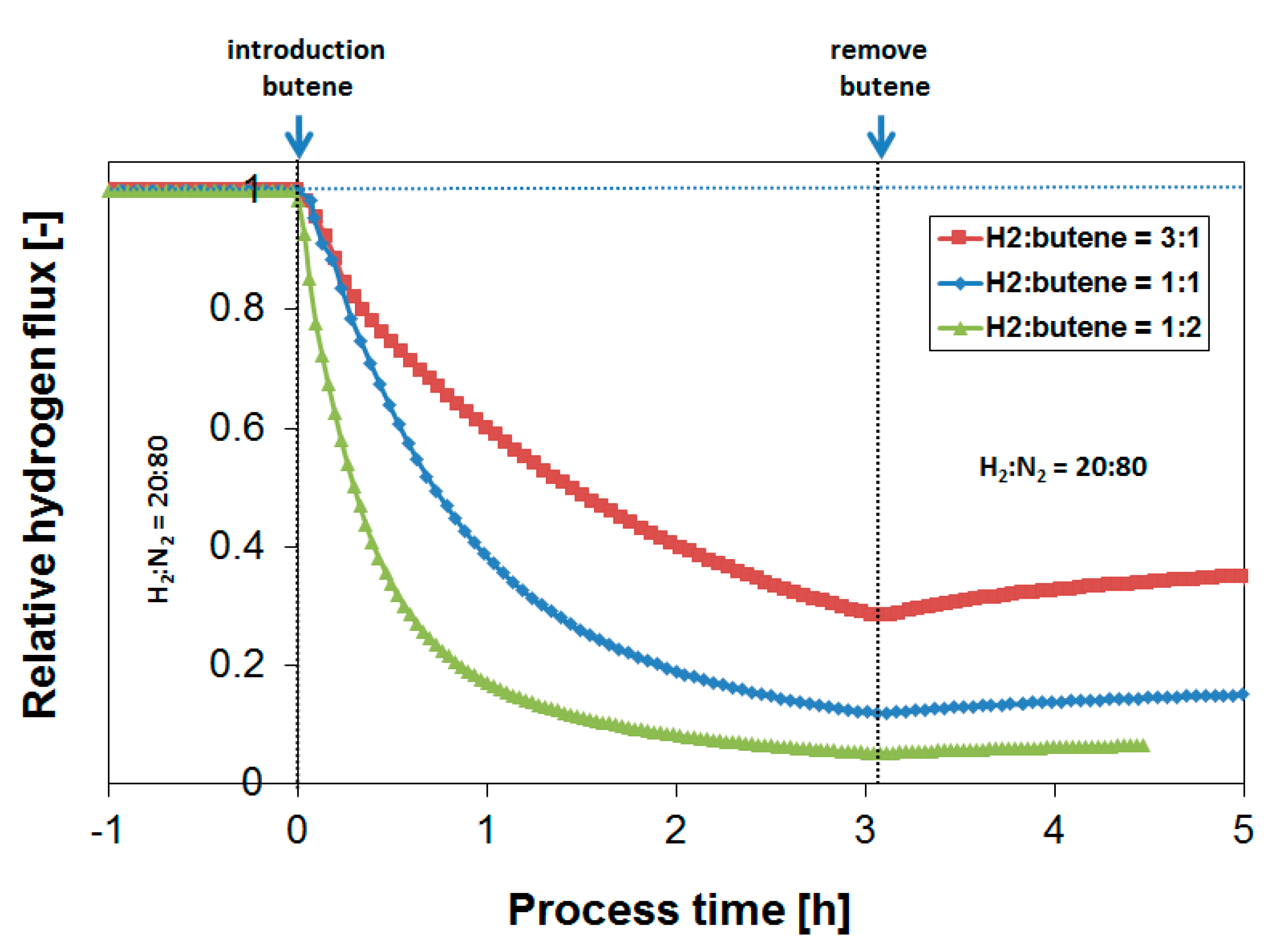
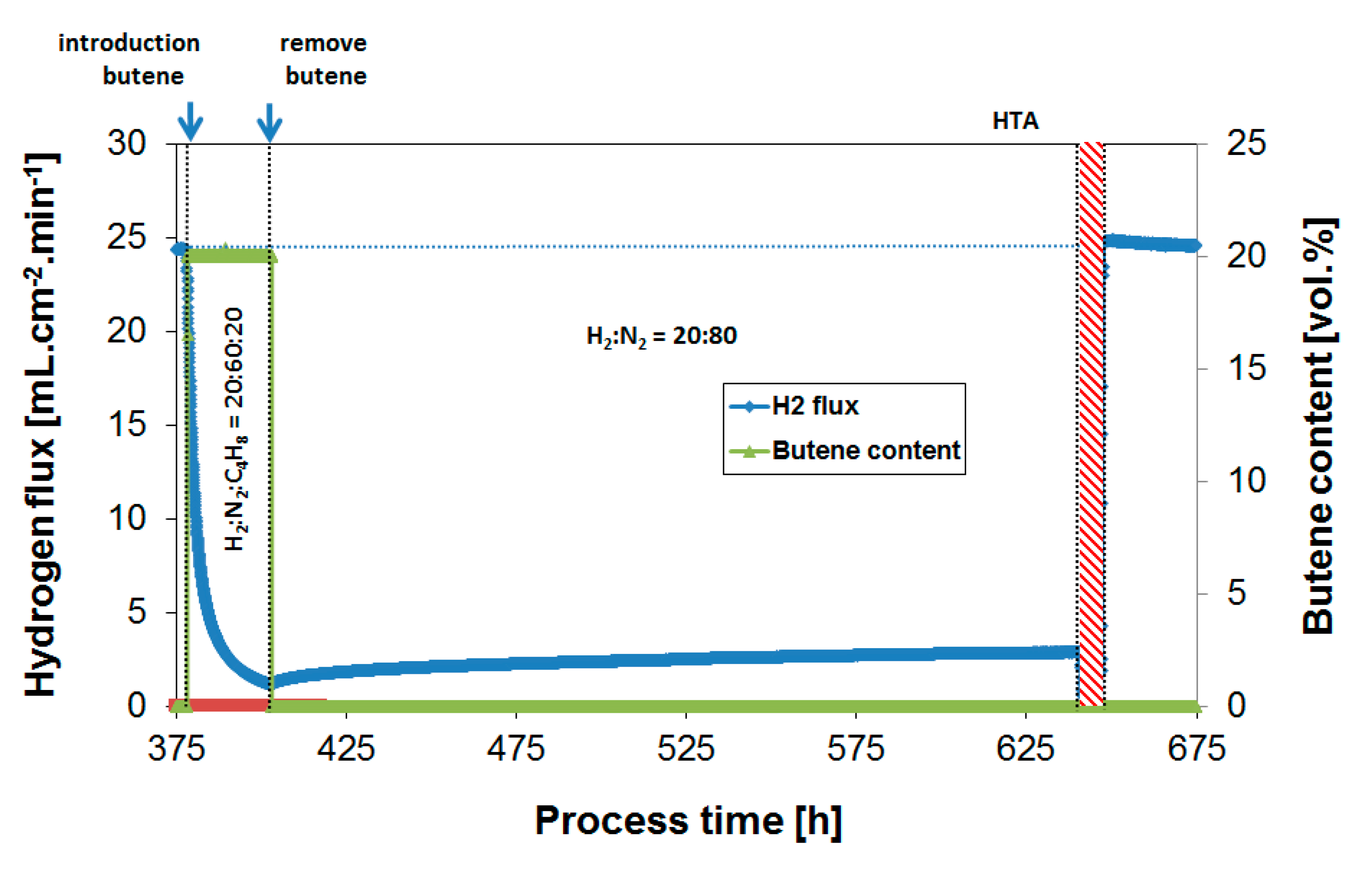
| Operation Temperature [°C] | H2 Flux [mL·cm−2·min−1] | H2 Permeability [mol·m−1·s−1·Pa−0.5] |
|---|---|---|
| 450 | 95.4 | 3.0·10−8 |
| 400 | 86.4 | 2.7·10−8 |
| 350 | 78.4 | 2.4·10−8 |
| 300 | 71.7 | 2.2·10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, T.A.; Stange, M.; Bredesen, R. Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes. Membranes 2020, 10, 291. https://doi.org/10.3390/membranes10100291
Peters TA, Stange M, Bredesen R. Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes. Membranes. 2020; 10(10):291. https://doi.org/10.3390/membranes10100291
Chicago/Turabian StylePeters, Thijs A., Marit Stange, and Rune Bredesen. 2020. "Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes" Membranes 10, no. 10: 291. https://doi.org/10.3390/membranes10100291
APA StylePeters, T. A., Stange, M., & Bredesen, R. (2020). Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes. Membranes, 10(10), 291. https://doi.org/10.3390/membranes10100291