Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pd Alloy Module Preparation
2.2. Gas Permeation Measurements
2.2.1. H2 Permeation Experiments
2.2.2. H2 Permeation Experiments
2.2.3. Post-Process Characterization
3. Results and Discussion
3.1. H2 Permeation Properties of Employed Pd-Based Membranes
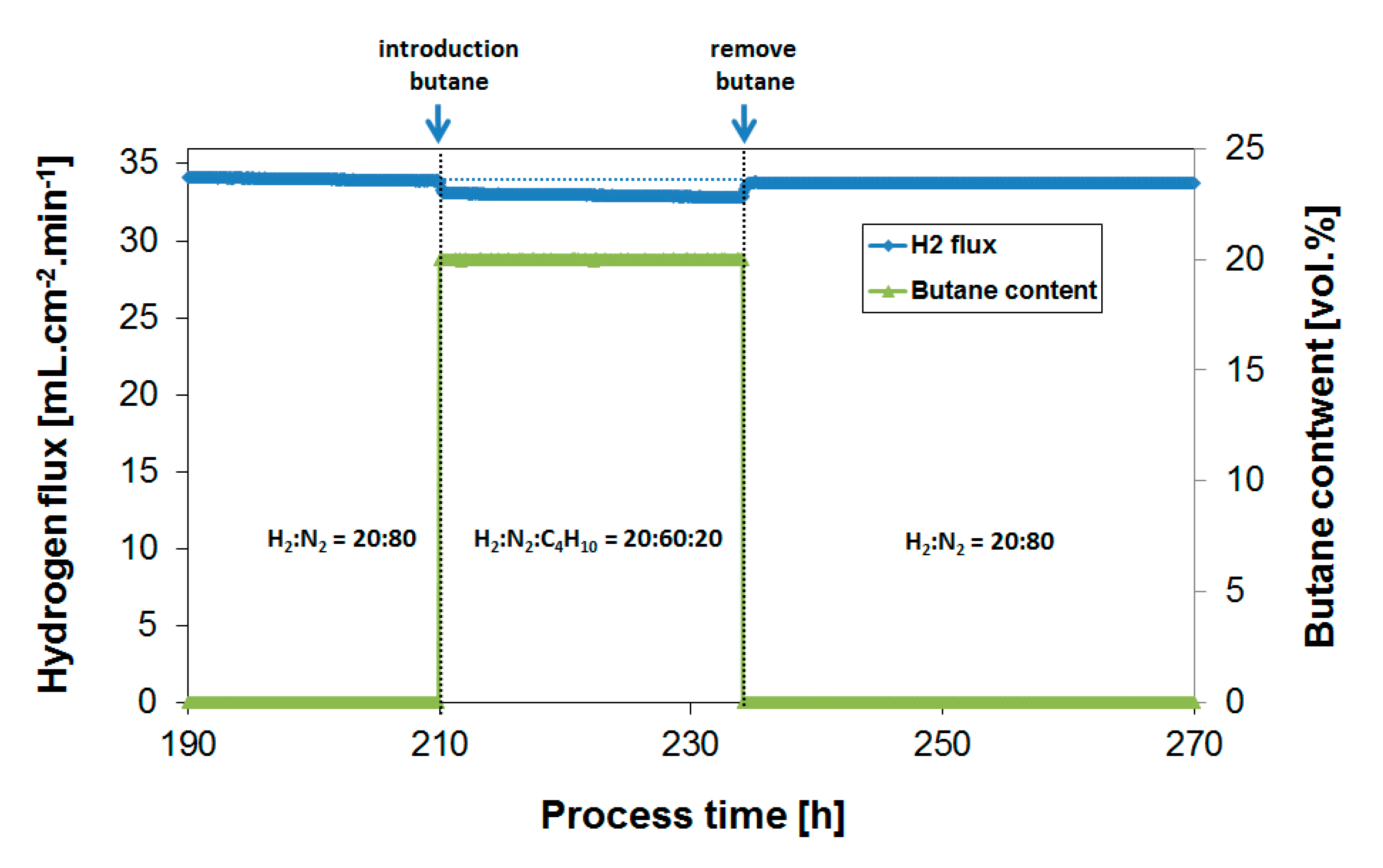
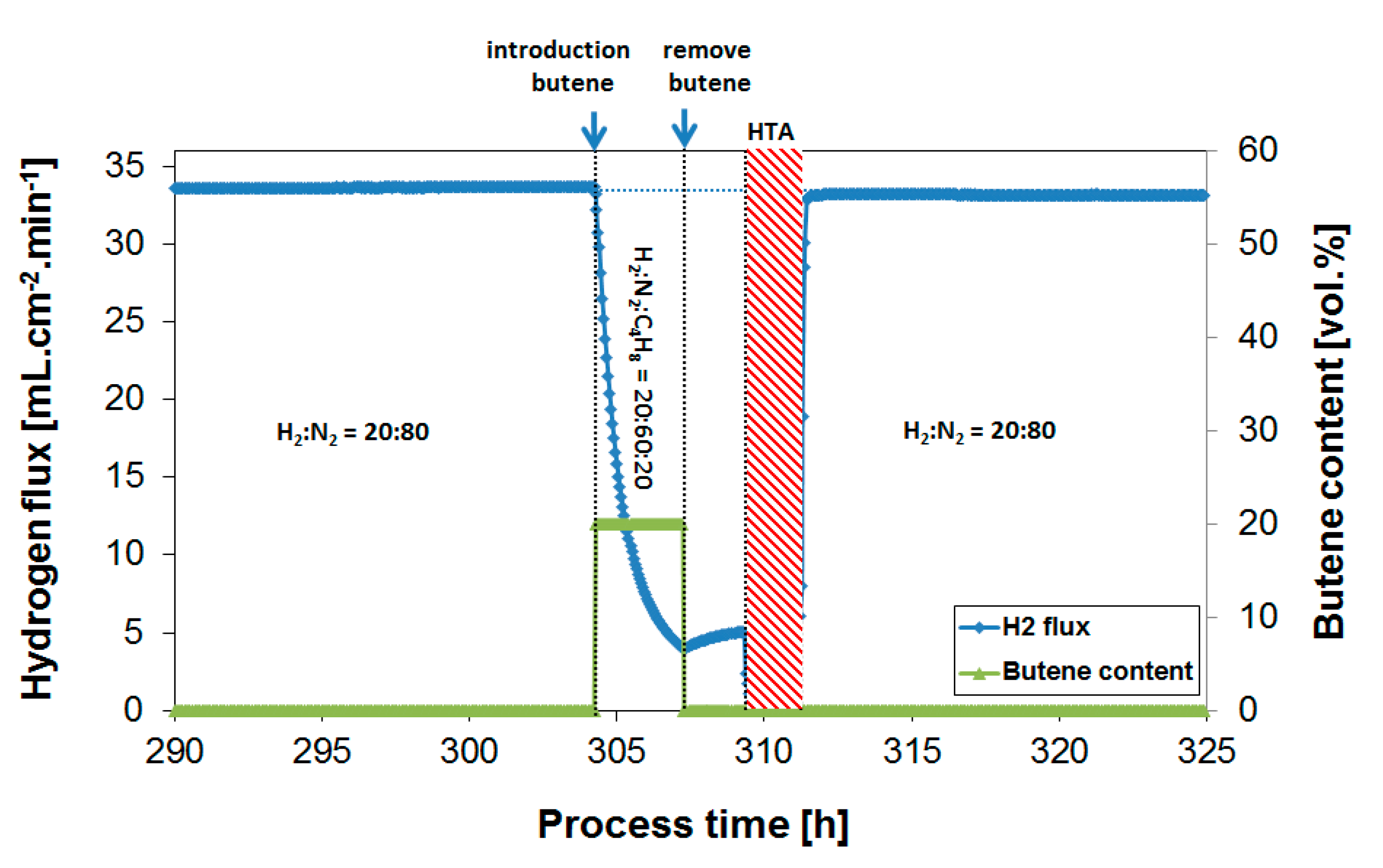
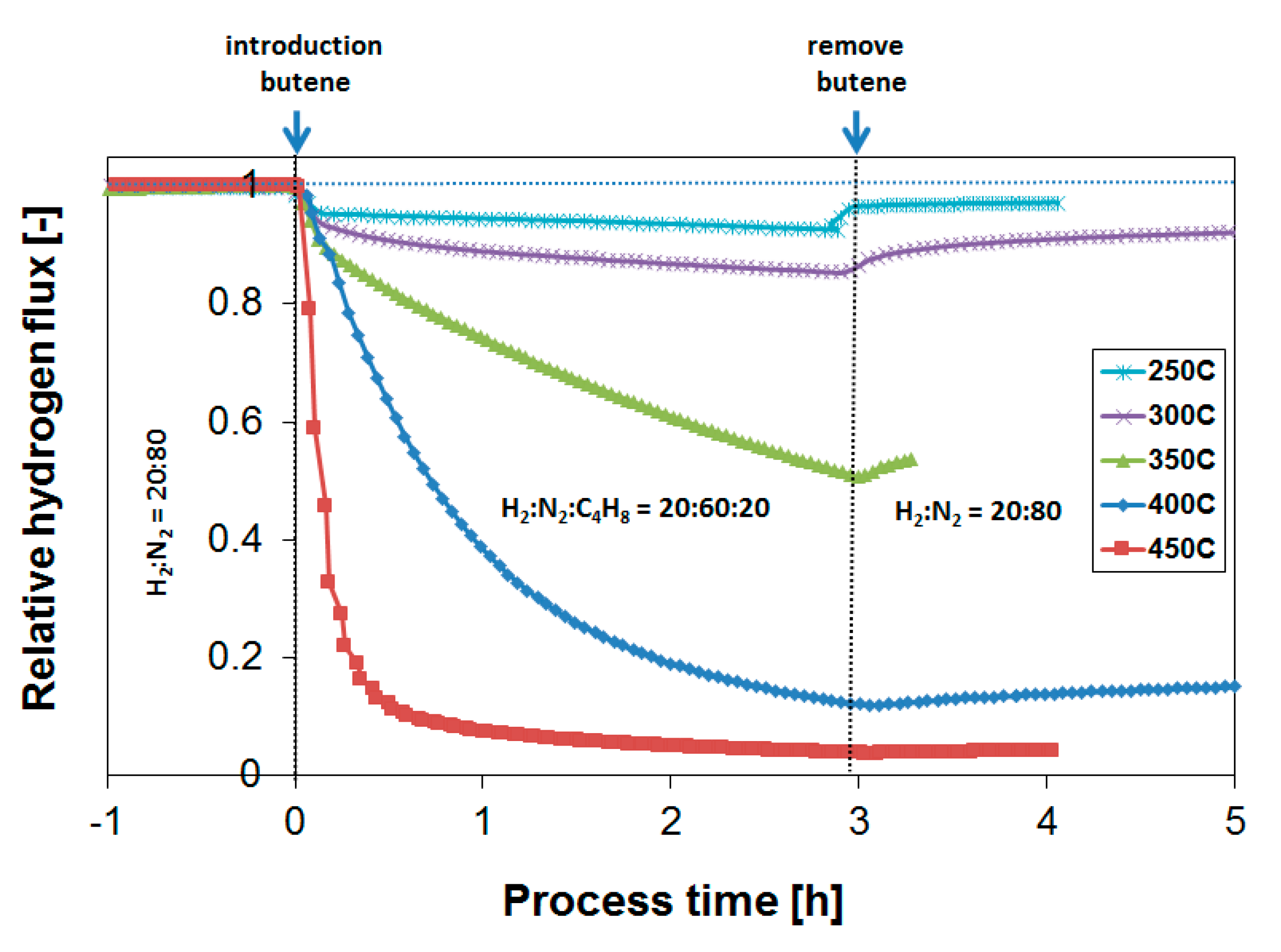
3.2. Exposure to Butane
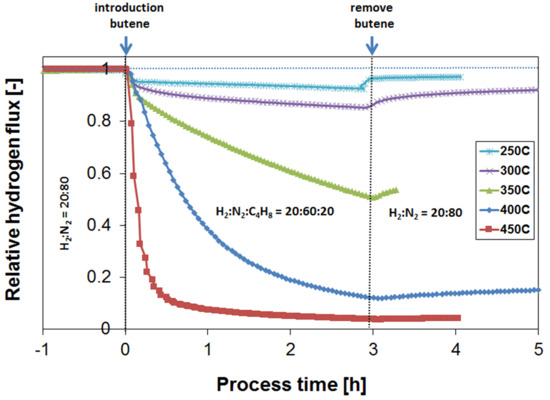
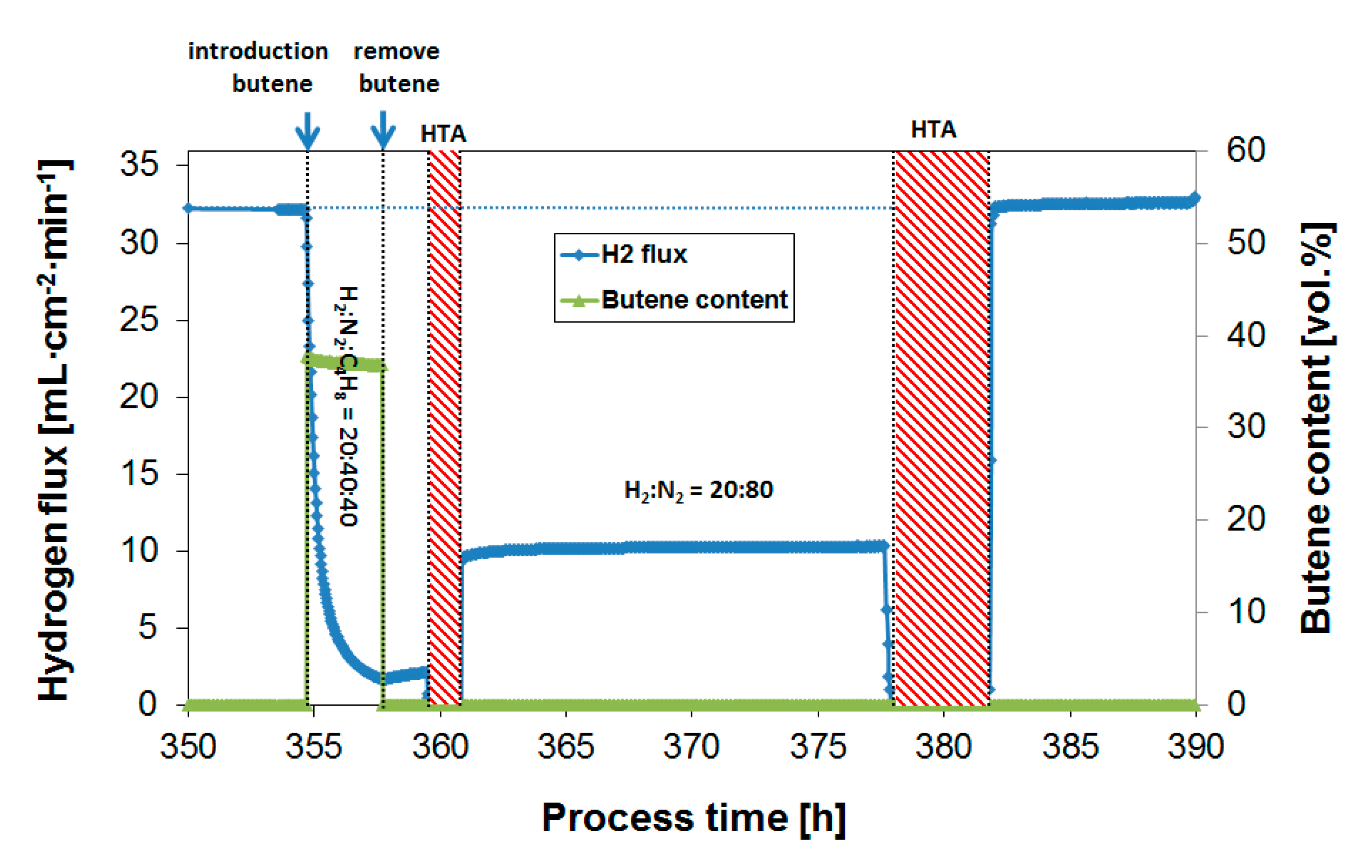
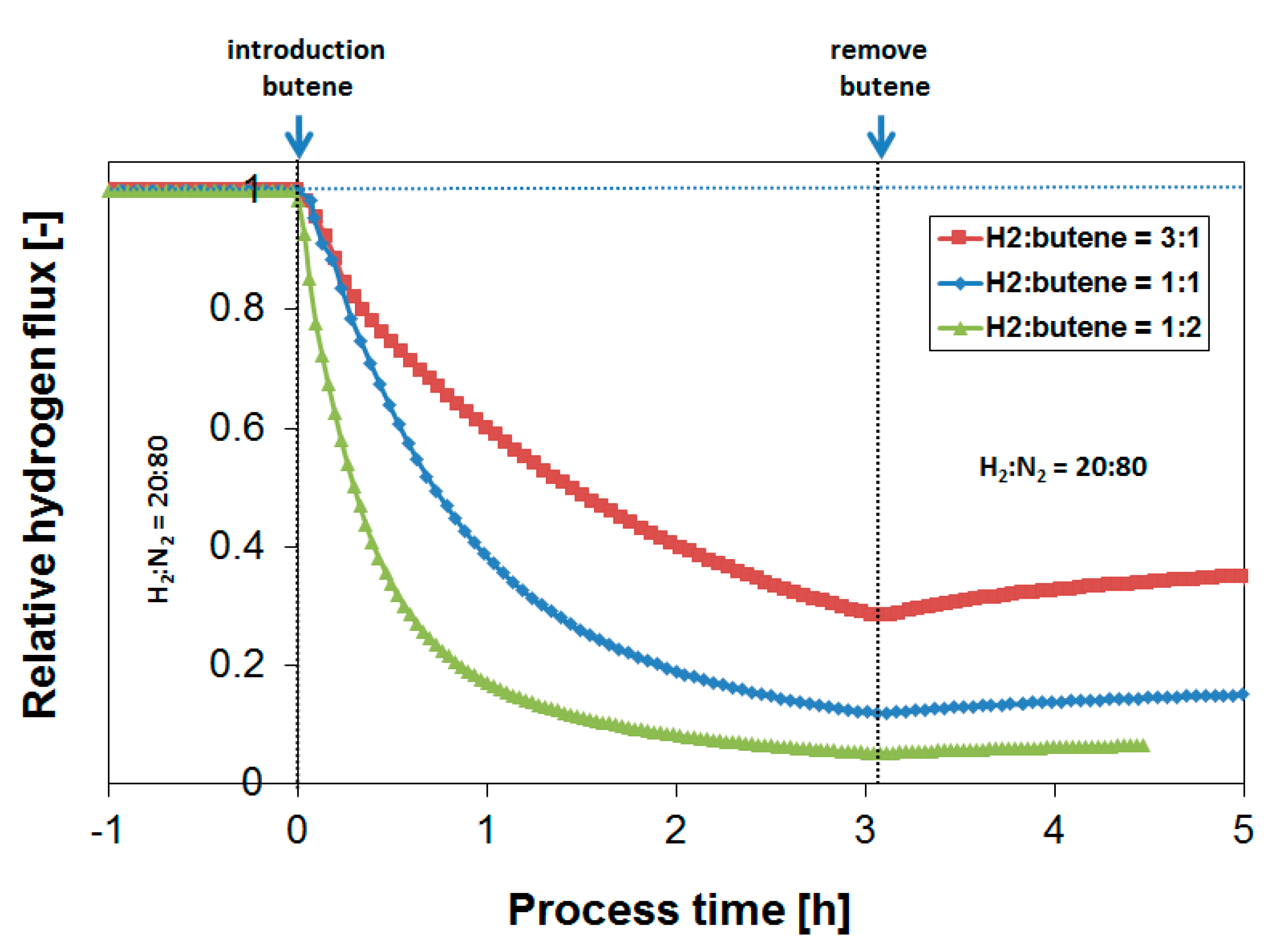
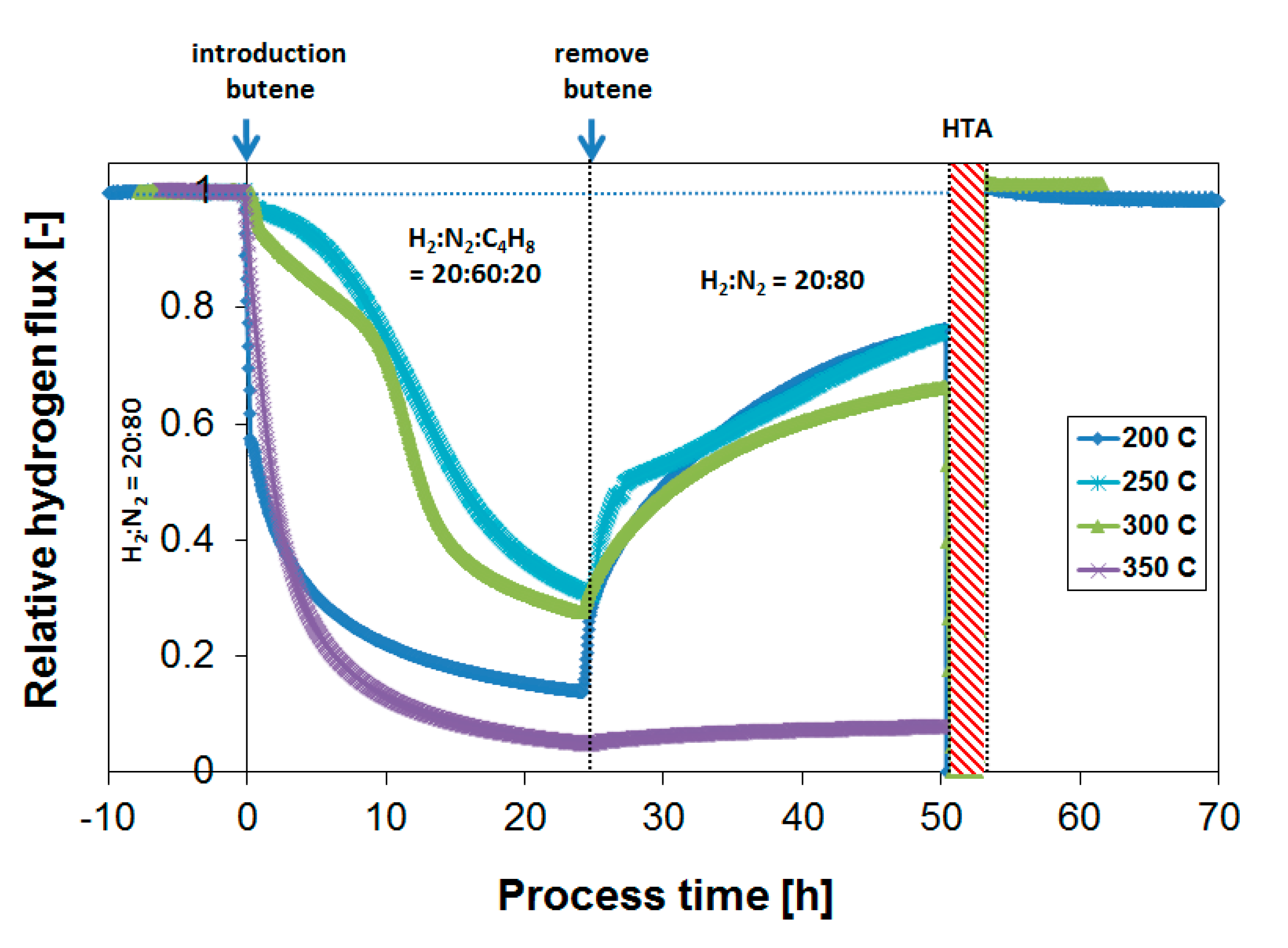
3.3. Parametric Study of H2 Flux Inhibition During Butylene Exposure
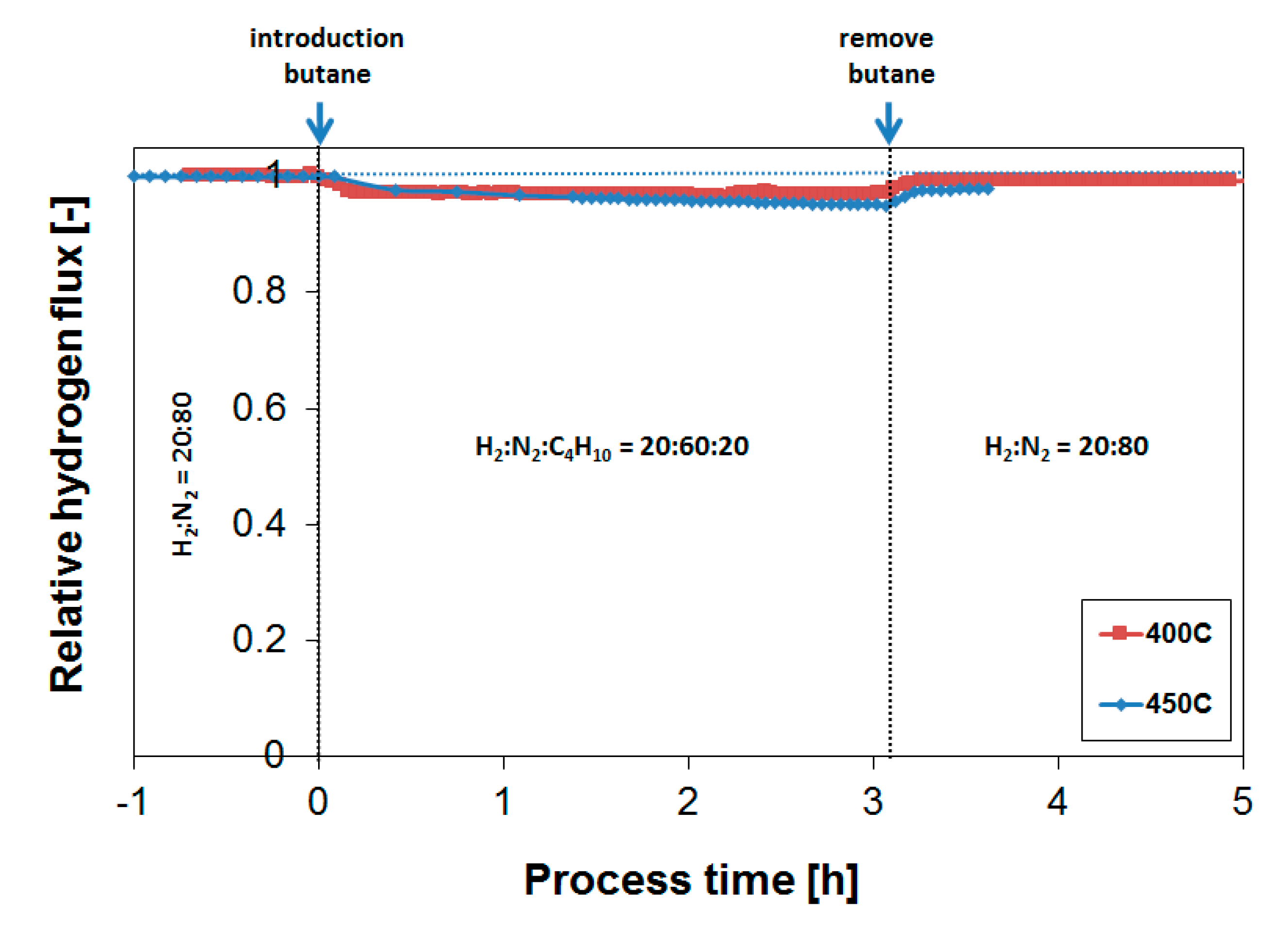
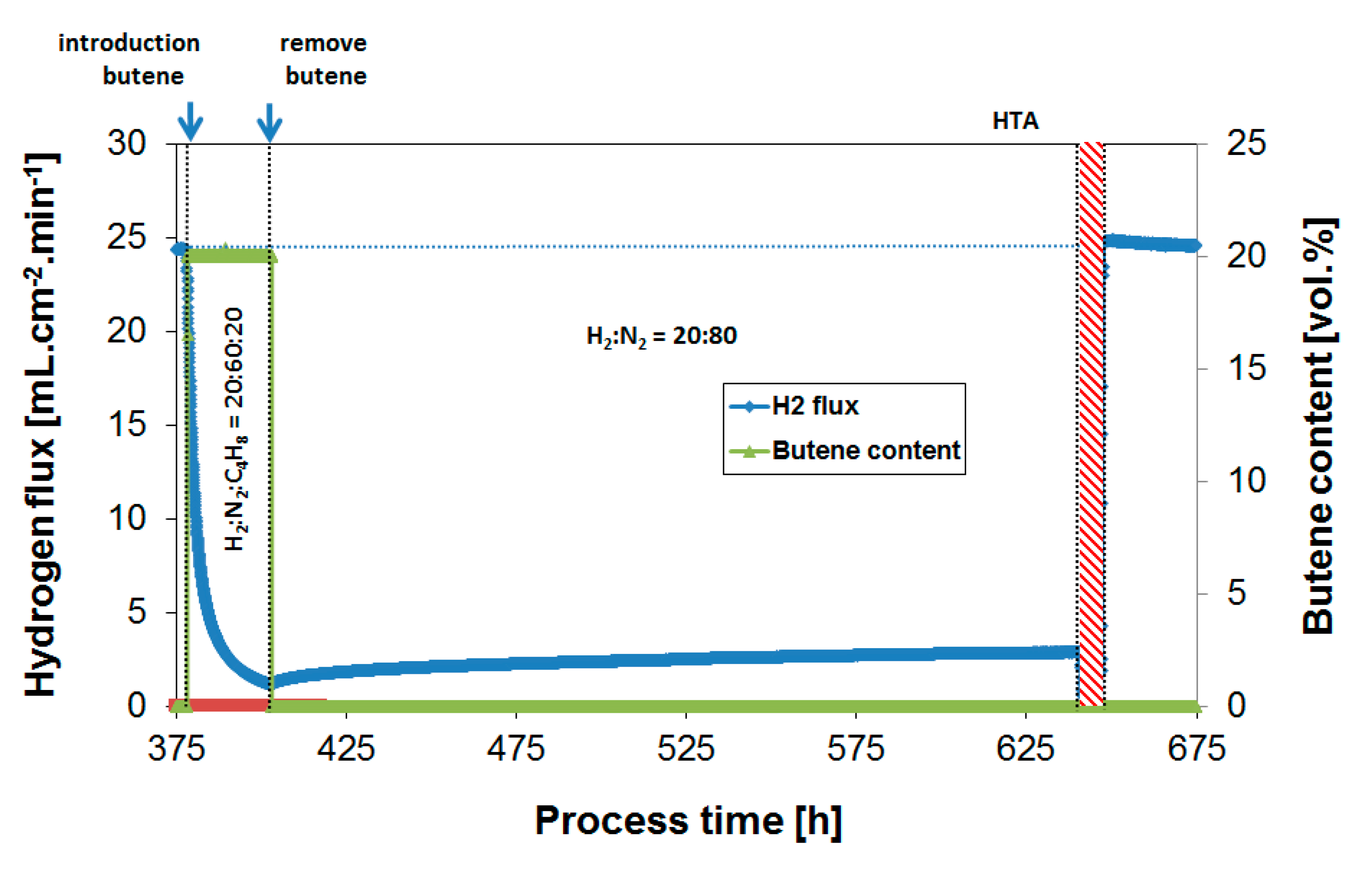
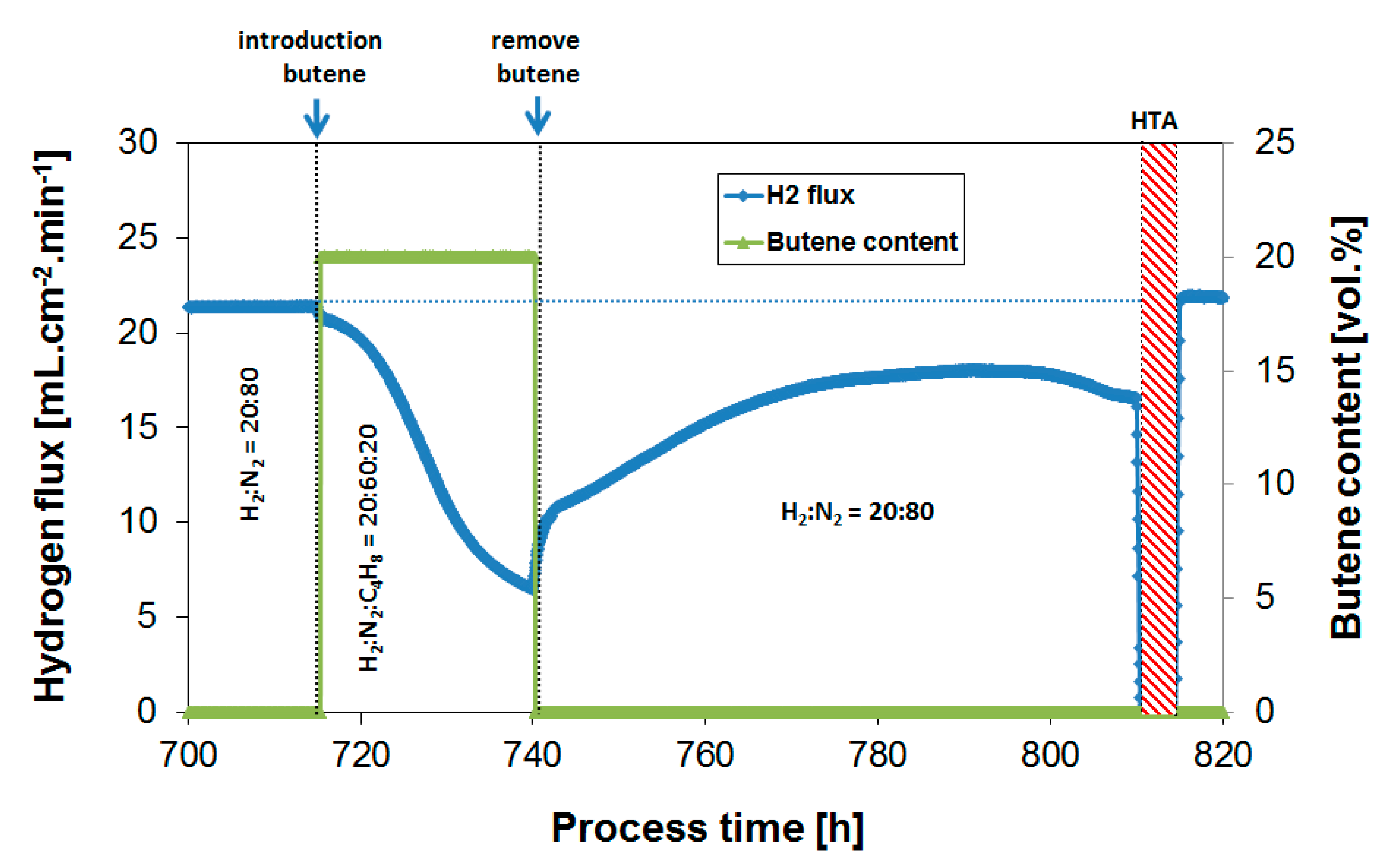
3.4. Long-Term Performance During Butylene Exposure
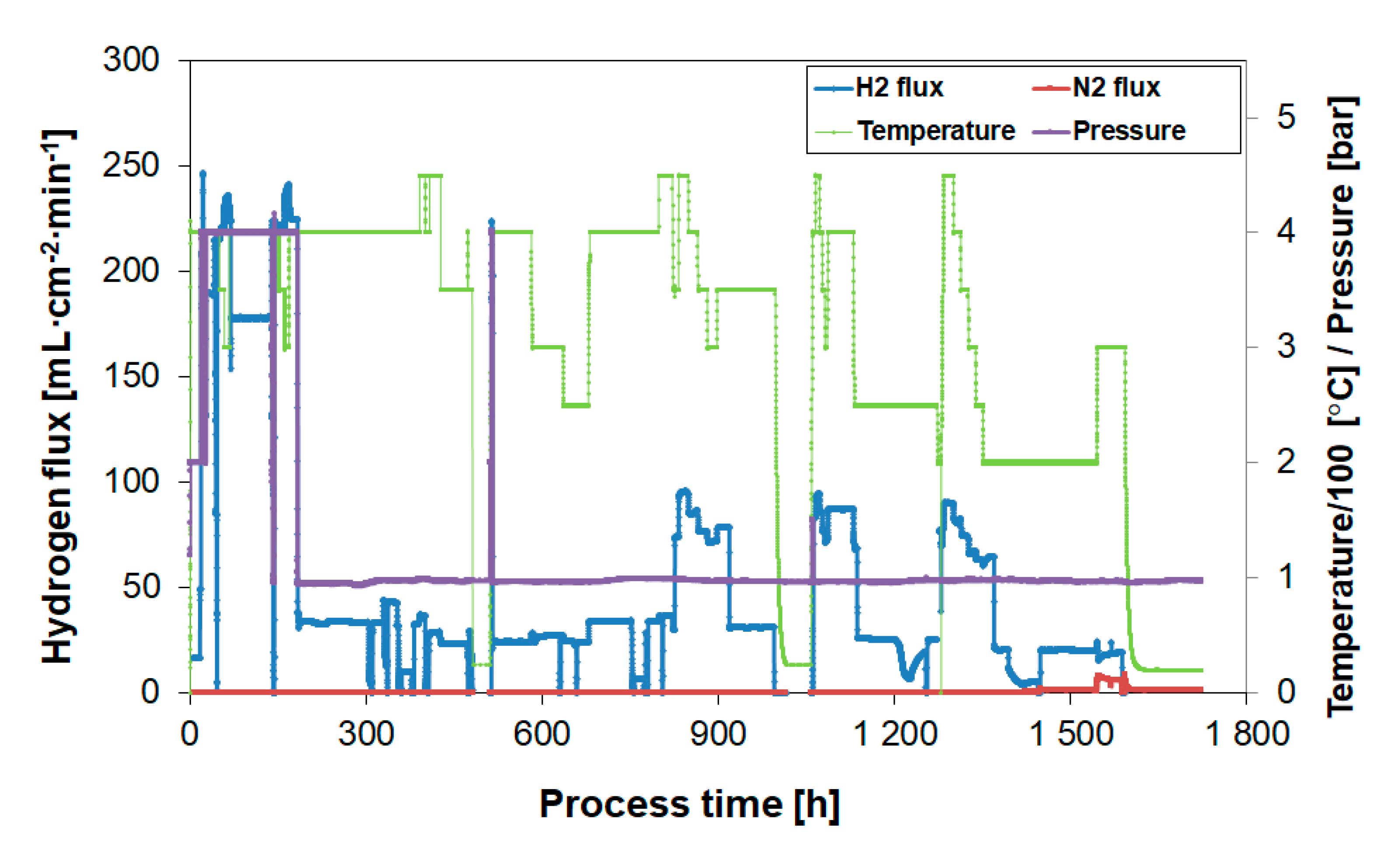
3.5. Membrane Stability
3.5.1. Separation Performance
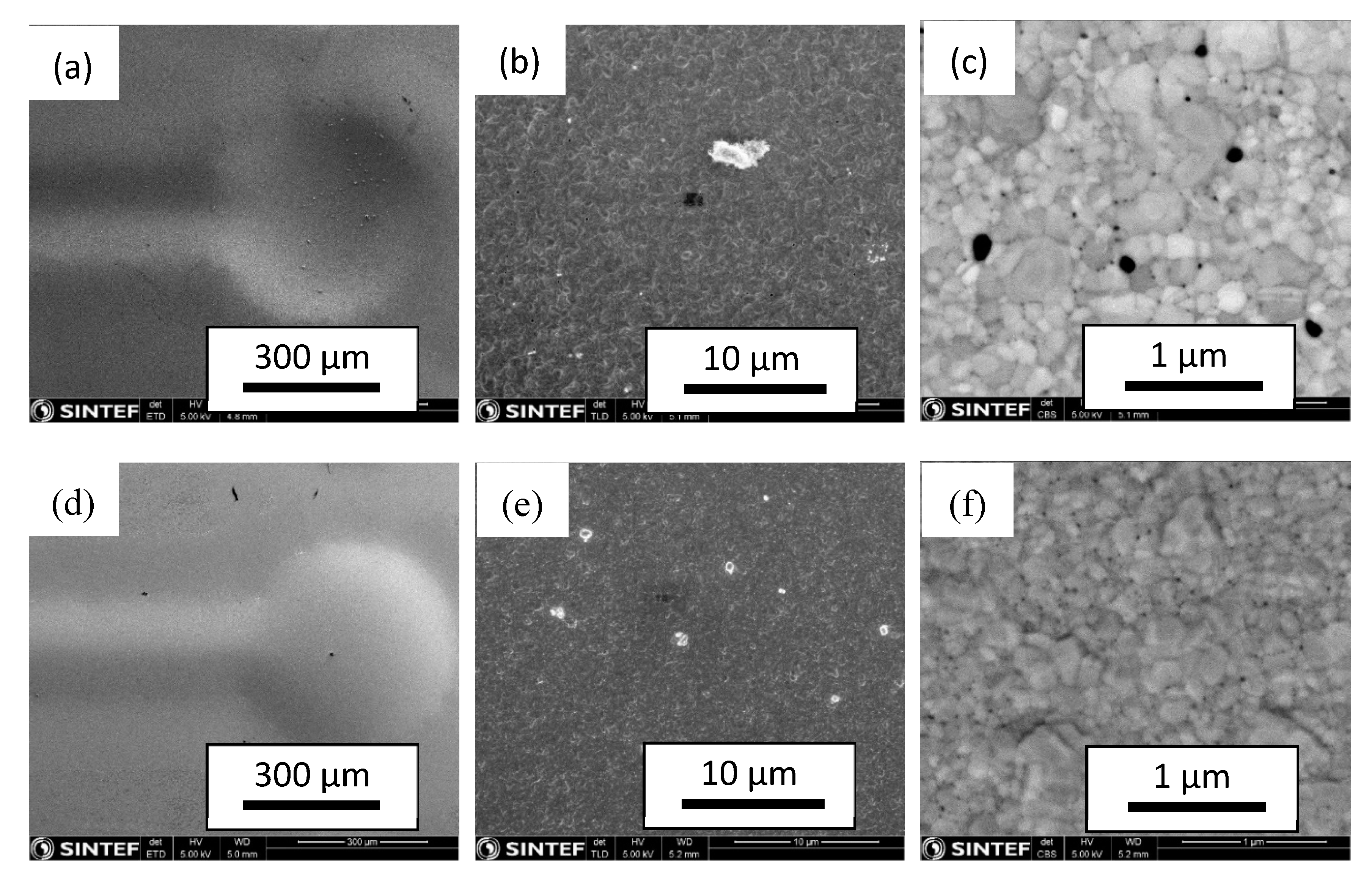
3.5.2. Post-Process Membrane Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- James, O.O.; Mandal, S.; Alele, N.; Chowdhury, B.; Maity, S. Lower alkanes dehydrogenation: Strategies and reaction routes to corresponding alkenes. Fuel Process. Technol. 2016, 149, 239–255. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Caspary, K.J.; Gehrke, H.; Heinrita-Adrian, M.; Schwefer, M. Dehydrogenation of Alkanes. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Sheintuch, M. and Simakov, D.S.A. Alkanes Dehydrogenation, in Membrane Reactors for Hydrogen Production Processes; Falco, M.D.D., Marrelli, L., Iaquaniello, G., Eds.; Springer: London, UK, 2011; pp. 183–200. [Google Scholar]
- Chang, J.S.; Roh, H.S.; Park, M.S.; Park, S.E. Propane dehydrogenation over a hydrogen permselective membrane reactor. Bull. Korean Chem. Soc. 2002, 23, 674–678. [Google Scholar]
- Ricca, A.; Montella, F.; Iaquaniello, G.; Palo, E.; Salladini, A.; Palma, V. Membrane assisted propane dehydrogenation: Experimental investigation and mathematical modelling of catalytic reactions. Catal. Today 2019, 331, 43–52. [Google Scholar] [CrossRef]
- Liang, W.; Hughes, R. The catalytic dehydrogenation of isobutane to isobutene in a palladium/silver composite membrane reactor. Catal. Today 2005, 104, 238–243. [Google Scholar] [CrossRef]
- Moparthi, A.; Uppaluri, R.; Gill, B. Economic feasibility of silica and palladium composite membranes for industrial dehydrogenation reactions. Chem. Eng. Res. Des. 2010, 88, 1088–1101. [Google Scholar] [CrossRef]
- Quicker, P.; Höllein, V.; Dittmeyer, R. Catalytic dehydrogenation of hydrocarbons in palladium composite membrane reactors. Catal. Today 2000, 56, 21–34. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Höllein, V.; Daub, K. Membrane reactors for hydrogenation and dehydrogenation processes based on supported palladium. J. Mol. Catal. A Chem. 2001, 173, 135–184. [Google Scholar] [CrossRef]
- Zhao, R.; Govind, R.; Itoh, N. Studies on Palladium Membrane Reactor for Dehydrogenation Reaction. Sep. Sci. Technol. 1990, 25, 1473–1488. [Google Scholar] [CrossRef]
- Ali, J.K.; Newson, E.; Rippin, D. Exceeding equilibrium conversion with a catalytic membrane reactor for the dehydrogenation of methylcyclohexane. Chem. Eng. Sci. 1994, 49, 2129–2134. [Google Scholar] [CrossRef]
- Ali, J.K.; Newson, E.; Rippin, D. Deactivation and regeneration of PdAg membranes for dehydrogenation reactions. J. Membr. Sci. 1994, 89, 171–184. [Google Scholar] [CrossRef]
- Itoh, N. Limiting conversions of dehydrogenation in palladium membrane reactors. Catal. Today 1995, 25, 351–356. [Google Scholar] [CrossRef]
- Collins, J.P.; Schwartz, R.W.; Sehgal, R.; Ward, T.L.; Brinker, C.J.; Hagen, G.P.; Udovich, C.A. Catalytic Dehydrogenation of Propane in Hydrogen Permselective Membrane Reactors. Ind. Eng. Chem. Res. 1996, 35, 4398–4405. [Google Scholar] [CrossRef]
- Sheintuch, M.; Dessau, R.M. Observations, modeling and optimization of yield, selectivity and activity during dehydrogenation of isobutane and propane in a Pd membrane reactor. Chem. Eng. Sci. 1996, 51, 535–547. [Google Scholar] [CrossRef]
- Hermann, C.; Quicker, P.; Dittmeyer, R. Mathematical simulation of catalytic dehydrogenation of ethylbenzene to styrene in a composite palladium membrane reactor. J. Membr. Sci. 1997, 136, 161–172. [Google Scholar] [CrossRef]
- Yildirim, Y.; Gobina, E.; Hughes, R. An experimental evaluation of high-temperature composite membrane systems for propane dehydrogenation. J. Membr. Sci. 1997, 135, 107–115. [Google Scholar] [CrossRef]
- She, Y.; Han, J.; Ma, Y. Palladium membrane reactor for the dehydrogenation of ethylbenzene to styrene. Catal. Today 2001, 67, 43–53. [Google Scholar] [CrossRef]
- Keuler, J.N. The dehydrogenation of 2-butanol in a Pd–Ag membrane reactor. J. Membr. Sci. 2002, 202, 17–26. [Google Scholar] [CrossRef]
- Farsi, M.; Jahanmiri, A.; Rahimpour, M. Simultaneous isobutane dehydrogenation and hydrogen production in a hydrogen-permselective membrane fixed bed reactor. Theor. Found. Chem. Eng. 2014, 48, 799–805. [Google Scholar] [CrossRef]
- Wunsch, A.; Mohr, M.; Pfeifer, P. Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes. Membranes 2018, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Ricca, A.; Palma, V.; Iaquaniello, G.; Palo, E.; Salladini, A.A. Highly selective propylene production in a membrane assisted catalytic propane dehydrogenation. Chem. Eng. J. 2017, 330, 1119–1127. [Google Scholar] [CrossRef]
- Palo, E.; Salladini, A.A.; Morico, B.; Palma, V.; Ricca, A.; Iaquaniello, G. Application of Pd-Based Membrane Reactors: An Industrial Perspective. Membranes 2018, 8, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittrich, C.J. The role of heat transfer on the feasibility of a packed-bed membrane reactor for propane dehydrogenation. Chem. Eng. J. 2020, 381, 122492. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Way, J.D. INNOVATIONS IN PALLADIUM MEMBRANE RESEARCH. Sep. Purif. Methods 2002, 31, 1–169. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Nenoff, T.M. Membranes for Hydrogen Separation. Chem. Rev. 2010, 110, 2573–2574. [Google Scholar] [CrossRef]
- Peters, T.; Stange, M.; Klette, H.; Bredesen, R. High pressure performance of thin Pd–23%Ag/stainless steel composite membranes in water gas shift gas mixtures; influence of dilution, mass transfer and surface effects on the hydrogen flux. J. Membr. Sci. 2008, 316, 119–127. [Google Scholar] [CrossRef]
- Mejdell, A.L.; Jondahl, M.; Peters, T.A.; Bredesen, R.; Venvik, H.J. Effects of CO and CO2 on hydrogen permeation through a ~3 µm Pd/Ag 23 wt% membrane employed in a microchannel membrane configuration. Sep. Purif. Technol. 2009, 68, 178–184. [Google Scholar] [CrossRef]
- Chen, C.-H.; Ma, Y.H. The effect of H2S on the performance of Pd and Pd/Au composite membrane. J. Membr. Sci. 2010, 362, 535–544. [Google Scholar] [CrossRef]
- Caravella, A.; Scura, F.; Barbieri, G.; Drioli, E. Inhibition by CO and Polarization in Pd-Based Membranes: A Novel Permeation Reduction Coefficient. J. Phys. Chem. B 2010, 114, 12264–12276. [Google Scholar] [CrossRef]
- Miguel, C.; Mendes, A.; Tosti, S.; Madeira, L.M. Effect of CO and CO2 on H2 permeation through finger-like Pd–Ag membranes. Int. J. Hydrog. Energy 2012, 37, 12680–12687. [Google Scholar] [CrossRef]
- Kurokawa, H.; Yakabe, H.; Yasuda, I.; Peters, T.; Bredesen, R. Inhibition effect of CO on hydrogen permeability of Pd–Ag membrane applied in a microchannel module configuration. Int. J. Hydrog. Energy 2014, 39, 17201–17209. [Google Scholar] [CrossRef]
- Kulprathipanja, A.; Alptekin, G.; Falconer, J.; Way, J. Pd and Pd–Cu membranes: Inhibition of H permeation by HS. J. Membr. Sci. 2005, 254, 49–62. [Google Scholar] [CrossRef]
- Gao, H.; Lin, Y.; Li, Y.; Zhang, B. Chemical Stability and Its Improvement of Palladium-Based Metallic Membranes. Ind. Eng. Chem. Res. 2004, 43, 6920–6930. [Google Scholar] [CrossRef]
- Fernandez, E.E.; Helmi, A.; Coenen, K.K.; Rey, J.M.; Viviente, J.L.; Tanaka, D.A.P.; Annaland, M.M.V.S.; Gallucci, F.F. Development of thin Pd–Ag supported membranes for fluidized bed membrane reactors including WGS related gases. Int. J. Hydrog. Energy 2015, 40, 3506–3519. [Google Scholar] [CrossRef] [Green Version]
- De Nooijer, N.; Sanchez, J.D.; Melendez, J.; Fernandez, E.; Tanaka, D.A.P.; Annaland, M.V.S.; Gallucci, F. Influence of H2S on the hydrogen flux of thin-film PdAgAu membranes. Int. J. Hydrog. Energy 2020, 45, 7303–7312. [Google Scholar] [CrossRef]
- Montesinos, H.; Julián, I.; Herguido, J.; Menéndez, M. Effect of the presence of light hydrocarbon mixtures on hydrogen permeance through Pd–Ag alloyed membranes. Int. J. Hydrog. Energy 2015, 40, 3462–3471. [Google Scholar] [CrossRef]
- Peters, T.; Liron, O.; Tschentscher, R.; Sheintuch, M.; Bredesen, R. Investigation of Pd-based membranes in propane dehydrogenation (PDH) processes. Chem. Eng. J. 2016, 305, 191–200. [Google Scholar] [CrossRef]
- Easa, J.; Jin, R.; O’Brien, C.P. Evolution of surface and bulk carbon species derived from propylene and their influence on the interaction of hydrogen with palladium. J. Membr. Sci. 2020, 596, 117738. [Google Scholar] [CrossRef]
- Palo, E.; Iaquaniello, G. Method for olefins production. U.S. Patent 9776935B2, 28 March 2012. [Google Scholar]
- Boeltken, T.; Belimov, M.; Pfeifer, P.; Peters, T.; Bredesen, R.; Dittmeyer, R. Fabrication and testing of a planar microstructured concept module with integrated palladium membranes. Chem. Eng. Process. Process. Intensif. 2013, 67, 136–147. [Google Scholar] [CrossRef]
- Mejdell, A.; Peters, T.; Stange, M.; Venvik, H.; Bredesen, R. Performance and application of thin Pd-alloy hydrogen separation membranes in different configurations. J. Taiwan Inst. Chem. Eng. 2009, 40, 253–259. [Google Scholar] [CrossRef]
- Peters, T.; Polfus, J.M.; Van Berkel, F.; Bredesen, R. Interplay between propylene and H2S co-adsorption on the H2 flux characteristics of Pd-alloy membranes employed in propane dehydrogenation (PDH) processes. Chem. Eng. J. 2016, 304, 134–140. [Google Scholar] [CrossRef]
- Peters, T.; Stange, M.; Veenstra, P.; Nijmeijer, A.; Bredesen, R. The performance of Pd–Ag alloy membrane films under exposure to trace amounts of H2S. J. Membr. Sci. 2016, 499, 105–115. [Google Scholar] [CrossRef]
- Peters, T.; Polfus, J.M.; Stange, M.; Veenstra, P.; Nijmeijer, A.; Bredesen, R. H 2 flux inhibition and stability of Pd-Ag membranes under exposure to trace amounts of NH 3. Fuel Process. Technol. 2016, 152, 259–265. [Google Scholar] [CrossRef]
- Tucho, W.M.; Venvik, H.; Stange, M.; Walmsley, J.C.; Holmestad, R.; Bredesen, R. Effects of thermal activation on hydrogen permeation properties of thin, self-supported Pd/Ag membranes. Sep. Purif. Technol. 2009, 68, 403–410. [Google Scholar] [CrossRef]
- Mejdell, A.; Klette, H.; Ramachandran, A.; Borg, A.L.; Bredesen, R. Hydrogen permeation of thin, free-standing Pd/Ag23% membranes before and after heat treatment in air. J. Membr. Sci. 2008, 307, 96–104. [Google Scholar] [CrossRef]
- Reyerson, L.H.; Cines, M.R. Adsorption of Propane and Propylene by Silica Gel and Metallized Silica Gel. J. Phys. Chem. 1942, 46, 1060–1068. [Google Scholar] [CrossRef]
- Abir, H.; Sheintuch, M. Modeling H2 transport through a Pd or Pd/Ag membrane, and its inhibition by co-adsorbates, from first principles. J. Membr. Sci. 2014, 466, 58–69. [Google Scholar] [CrossRef]
- Roa, F.; Way, J. The effect of air exposure on palladium–copper composite membranes. Appl. Surf. Sci. 2005, 240, 85–104. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Tam, S.Y. Hydrogen selective protective coating, coated article and method. U.S. Patent 13/581,587, 21 March 2011. [Google Scholar]
- Yu, J.; Qi, C.; Zhang, J.; Bao, C.; Xu, H. Synthesis of a zeolite membrane as a protective layer on a metallic Pd composite membrane for hydrogen purification. J. Mater. Chem. A 2015, 3, 5000–5006. [Google Scholar] [CrossRef]
- Akporiaye, D.; Jensen, S.F.; Olsbye, U.; Rohr, F.; Rytter, E.; Rønnekleiv, A.M.; Spjelkavik, A.I. A Novel, Highly Efficient Catalyst for Propane Dehydrogenation. Ind. Eng. Chem. Res. 2001, 40, 4741–4748. [Google Scholar] [CrossRef]
- Peters, T.; Stange, M.; Sunding, M.; Bredesen, R. Stability investigation of micro-configured Pd–Ag membrane modules – Effect of operating temperature and pressure. Int. J. Hydrog. Energy 2015, 40, 3497–3505. [Google Scholar] [CrossRef]
- Peters, T.; Tucho, W.M.; Ramachandran, A.; Stange, M.; Walmsley, J.C.; Holmestad, R.; Borg, A.L.; Bredesen, R. Thin Pd–23%Ag/stainless steel composite membranes: Long-term stability, life-time estimation and post-process characterisation. J. Membr. Sci. 2009, 326, 572–581. [Google Scholar] [CrossRef]
- Mardilovich, P.P.; She, Y.; Ma, Y.H.; Rei, M.-H. Defect-free palladium membranes on porous stainless-steel support. AIChE J. 1998, 44, 310–322. [Google Scholar] [CrossRef]
- Peters, T.; Carvalho, P.; Van Wees, J.; Overbeek, J.; Sagvolden, E.; Van Berkel, F.; Løvvik, O.M.; Bredesen, R. Leakage evolution and atomic-scale changes in Pd-based membranes induced by long-term hydrogen permeation. J. Membr. Sci. 2018, 563, 398–404. [Google Scholar] [CrossRef]
- Peters, T.; Carvalho, P.; Stange, M.; Bredesen, R. Formation of hydrogen bubbles in Pd-Ag membranes during H2 permeation. Int. J. Hydrog. Energy 2020, 45, 7488–7496. [Google Scholar] [CrossRef]
- Polfus, J.M.; Løwik, O.M.; Bredesen, R.; Peters, T. Hydrogen Induced Vacancy Clustering and Void Formation Mechanisms at Grain Boundaries in Palladium. Acta Mater. 2020. In press. [Google Scholar]
- Guazzone, F.; Engwall, E.E.; Ma, Y.H. Effects of surface activity, defects and mass transfer on hydrogen permeance and n-value in composite palladium-porous stainless steel membranes. Catal. Today 2006, 118, 24–31. [Google Scholar] [CrossRef]
- Tucho, W.M.; Venvik, H.J.; Walmsley, J.C.; Stange, M.; Ramachandran, A.; Mathiesen, R.H.; Borg, A.; Bredesen, R. and Holmesstad, R. Microstructural studies of self-supported (1.5–10 µm) Pd/23 wt %Ag hydrogen separation membranes subjected to different heat treatments. J. Mater. Sci. 2009, 44, 4429–4442. [Google Scholar] [CrossRef]
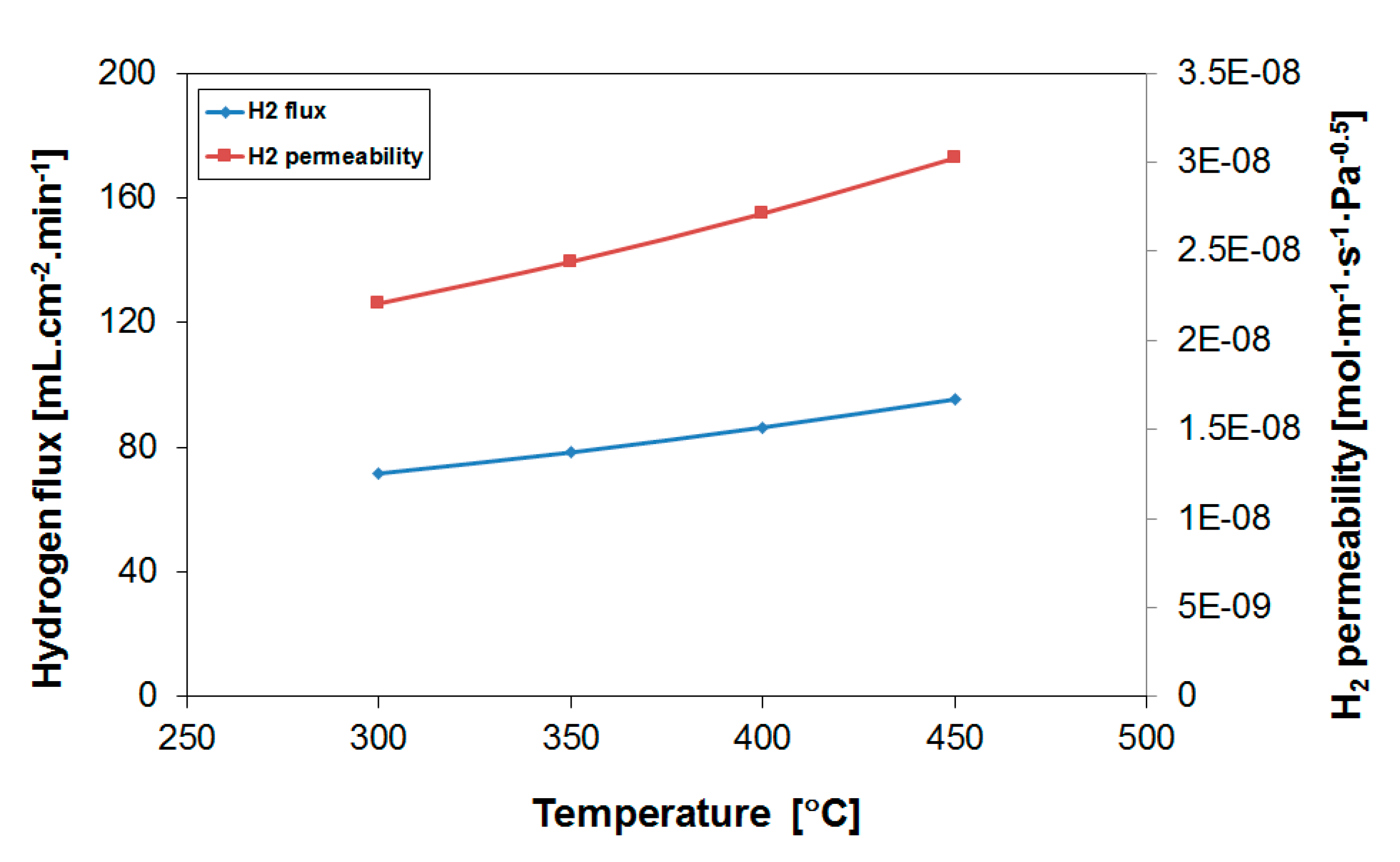
| Operation Temperature [°C] | H2 Flux [mL·cm−2·min−1] | H2 Permeability [mol·m−1·s−1·Pa−0.5] |
|---|---|---|
| 450 | 95.4 | 3.0·10−8 |
| 400 | 86.4 | 2.7·10−8 |
| 350 | 78.4 | 2.4·10−8 |
| 300 | 71.7 | 2.2·10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, T.A.; Stange, M.; Bredesen, R. Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes. Membranes 2020, 10, 291. https://doi.org/10.3390/membranes10100291
Peters TA, Stange M, Bredesen R. Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes. Membranes. 2020; 10(10):291. https://doi.org/10.3390/membranes10100291
Chicago/Turabian StylePeters, Thijs A., Marit Stange, and Rune Bredesen. 2020. "Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes" Membranes 10, no. 10: 291. https://doi.org/10.3390/membranes10100291
APA StylePeters, T. A., Stange, M., & Bredesen, R. (2020). Flux-Reducing Tendency of Pd-Based Membranes Employed in Butane Dehydrogenation Processes. Membranes, 10(10), 291. https://doi.org/10.3390/membranes10100291