Optimizing Humoral Immunity for Durable and Broad Protection in Flavivirus Vaccines
Abstract
1. Introduction
- Preserving quaternary E-dimer epitopes to promote potent and broad neutralization while minimizing off-target responses;
- Reducing prM-associated or fusion loop–biased non-neutralizing specificities that contribute to ADE risk;
- Sustaining germinal center activity and Tfh support to drive affinity maturation and long-lived plasma cell development;
- Optimizing antigen persistence and delivery platform properties to reinforce durable and high-avidity antibody responses;
- Incorporating baseline serostatus and immune imprinting patterns into vaccine design can ensure safety and consistent immunogenicity across populations.
2. Overview of Humoral Immunity in Flavivirus Vaccination
2.1. B-Cell Activation and Germinal Center Response
2.2. Antibody Affinity Maturation and Long-Lived Plasma Cell Formation
2.3. Neutralizing and Non-Neutralizing Antibody Functions
| Vaccine | Platform | Neutralizing Magnitude | Durability Tier | Breadth/Cross-Reactivity | ADE/Safe | Refs. |
|---|---|---|---|---|---|---|
| YF-17D | Live-attenuated | High titers within 2–4 weeks; polyfunctional IgG | >20 years in clinical follow up | Cross-reactive within YF genotypes; minimal heterologous activity | No ADE signal reported | [34,43] |
| JE-SA-14-14-2 | Live-attenuated | Robust titers after single dose; strong memory recall | >20 years in clinical follow up | Limited cross-neutralization to other JEV genotypes | Well-tolerated; no ADE | [13,44] |
| JE (IXIARO/inactivated) | Purified inactivated | Moderate titers; boosted by 2-dose regimen | wanes over 2–3 years | Genotype-specific; minimal heterologous response | no ADE | [44] |
| Dengue (CYD-TDV) | Chimeric yellow-fever vector | Strong neutralization; dependent on serostatus | wanes over 2–3 years | Broad but heterogeneous; partial neutralization of heterologous serotypes | Increased ADE risk in sero-negatives | [23,24,50] |
| Dengue (TAK-003) | Live-attenuated tetravalent | Balanced neutralization across serotypes | ≥4 years in clinical trials so far | Cross-neutralization with modest enhancement potential | No ADE signal to date | [51] |
| Zika (mRNA/DNA candidates) | Nucleic acid (investigational) | High titers in animals and early human trials | anticipated from platform mechanism | Strong cross-reactivity with DENV; epitope overlap | ADE potential in vitro; not confirmed in vivo | [30,52] |
3. Influence of Adjuvants on Antibody Quality and Durability
3.1. Classical Adjuvants and Their Immunomodulatory Effects
3.2. Novel Adjuvant Formulations Targeting B-Cell and T Helper Responses
3.3. Comparative Evaluation of Adjuvants Used in Flavivirus Vaccines
4. Effects of Antigen Design and Delivery Platforms
4.1. Structural Features of Flavivirus Envelope Proteins Influencing Antibody Quality
4.2. Antigen Presentation and Delivery Mechanisms in Different Vaccine Platforms
4.3. Emerging Technologies for Improving Antigen Stability and Immune Persistence
5. Cross-Reactivity and Immune Imprinting Among Flaviviruses
5.1. Cross-Reactive Antibody Responses and Their Protective or Enhancing Roles
5.2. Immune Imprinting and Its Impact on Vaccine Effectiveness
5.3. Balancing Broad Protection and Safety in Vaccine-Induced Antibody Responses
6. Strategies for Improving Antibody Maturation and Long-Term Maintenance
6.1. Optimizing Booster Intervals and Heterologous Prime-Boost Regimens
6.2. Host and Age-Related Factors Influencing Antibody Persistence
6.3. Approaches to Sustain Germinal Center Activity and Memory B-Cell Formation
7. Future Perspectives and Vaccine Design Considerations
7.1. Integration of Adjuvant and Antigen Design for Improved Humoral Immunity
7.2. Lessons from Flavivirus Vaccine Platforms for Universal Vaccine Development
7.3. Outlook for Next-Generation Flavivirus Vaccines
| Strategic Area | Emerging Concept or Approach | Expected Immunologic Impact | Development Stage or Outlook | Refs. |
|---|---|---|---|---|
| Structure-guided epitope optimization | Stabilization of E-dimer or mosaic epitopes that preserve cross-neutralizing structures | Focuses antibody response on protective epitopes and lower enhancement risk | Preclinical studies and Phase 1 early clinical testing | [98,158,196] |
| Adjuvant and antigen co-engineering | Incorporation of PRR agonists or saponin-based adjuvants into mRNA or nanoparticle platforms | Sustains germinal center activity and enhances affinity maturation | Advanced preclinical studies and Phase 1–2 clinical evaluation | [164,190,197] |
| Self-amplifying RNA and replicon platforms | Extended antigen expression with low-dose formulations | Increases magnitude and duration of antibody response | Phase 1 clinical trials | [198,199,200] |
| Pan-flavivirus vaccine design using conserved scaffolds | Use of shared E dimer and fusion loop epitope scaffolds to support broad cross protection | Enables cross serotype and cross species immunity across dengue, Zika, and JEV | Computational design with mechanistic inference | [26,101,158] |
| Systems vaccinology and immune profiling | Integration of omics signatures and antibody repertoire sequencing to define correlates of protection | Predicts antibody quality and durability for vaccine optimization | Research-stage integration with exploratory clinical programs | [201,202,203] |
| Population-tailored vaccination strategies | Adjustment of prime boost intervals and formulations based on serostatus and age | Maximizes protective efficacy while reducing enhancement risk | Program-level evaluation in endemic populations (observational evidence) | [152,157,204] |
| Combination vector or heterologous prime-boost approaches | Sequential mRNA, viral vector, or subunit vaccination | Promotes balanced B and T cell immunity with improved durability | Preclinical and translational-stage development | [205,206,207] |
| Artificial intelligence assisted immunogen design | Machine learning based prediction of stable neutralizing epitopes and escape variant targets | Accelerates design of rationally optimized immunogens | Computational modeling with mechanistic inference | [208,209,210] |
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gould, E.A.; Solomon, T. Pathogenic flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, H.M. Managing Japanese Encephalitis Virus as a Veterinary Infectious Disease Through Animal Surveillance and One Health Control Strategies. Life 2025, 15, 1260. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Molecular biology of flaviviruses. Adv. Virus Res. 2003, 59, 23–61. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, H.M.; Jun, S.H.; Kamitani, W.; Kim, O.; Shin, H.J. Insights into the Pathogenesis and Development of Recombinant Japanese Encephalitis Virus Genotype 3 as a Vaccine. Vaccines 2024, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kamitani, W.; Shin, H.J.; Lee, H.M. Japanese Encephalitis Virus Genotype 5 Infectious Clone and Reporter System for Antiviral Evaluation. J. Med. Virol. 2025, 97, e70608. [Google Scholar] [CrossRef]
- Crill, W.D.; Chang, G.J. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J. Virol. 2004, 78, 13975–13986. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- Sohn, Y.M. Japanese encephalitis immunization in South Korea: Past, present, and future. Emerg. Infect. Dis. 2000, 6, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774E. [Google Scholar] [CrossRef]
- Hegde, N.R.; Gore, M.M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccin. Immunother. 2017, 13, 1320–1337. [Google Scholar] [CrossRef]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef]
- Lazear, H.M.; Diamond, M.S. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J. Virol. 2016, 90, 4864–4875. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The emergence of Zika virus and its new clinical syndromes. Nature 2018, 560, 573–581. [Google Scholar] [CrossRef]
- Dowd, K.A.; Pierson, T.C. Antibody-mediated neutralization of flaviviruses: A reductionist view. Virology 2011, 411, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Montoya, M.; Gresh, L.; Balmaseda, A.; Harris, E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc. Natl. Acad. Sci. USA 2016, 113, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Kometani, K.; Ise, W. Memory B cells. Nat. Rev. Immunol. 2015, 15, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.A.; Stiasny, K.; Vaney, M.C.; Dellarole, M.; Heinz, F.X. The bright and the dark side of human antibody responses to flaviviruses: Lessons for vaccine design. EMBO Rep. 2018, 19, 206–224. [Google Scholar] [CrossRef]
- Viant, C.; Weymar, G.H.J.; Escolano, A.; Chen, S.; Hartweger, H.; Cipolla, M.; Gazumyan, A.; Nussenzweig, M.C. Antibody Affinity Shapes the Choice between Memory and Germinal Center B Cell Fates. Cell 2020, 183, 1298–1311.e11. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Coloma, J.; Harris, E. Dengue: Knowledge gaps, unmet needs, and research priorities. Lancet Infect. Dis. 2017, 17, e88–e100. [Google Scholar] [CrossRef]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rouvinski, A.; Guardado-Calvo, P.; Barba-Spaeth, G.; Duquerroy, S.; Vaney, M.C.; Kikuti, C.M.; Navarro Sanchez, M.E.; Dejnirattisai, W.; Wongwiwat, W.; Haouz, A.; et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015, 520, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, K.M.; Crotty, S. Germinal center enhancement by extended antigen availability. Curr. Opin. Immunol. 2017, 47, 64–69. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Shlomchik, M.J.; Weisel, F. Germinal center selection and the development of memory B and plasma cells. Immunol. Rev. 2012, 247, 52–63. [Google Scholar] [CrossRef]
- Akondy, R.S.; Monson, N.D.; Miller, J.D.; Edupuganti, S.; Teuwen, D.; Wu, H.; Quyyumi, F.; Garg, S.; Altman, J.D.; Del Rio, C.; et al. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J. Immunol. 2009, 183, 7919–7930. [Google Scholar] [CrossRef]
- Hou, J.; Wang, S.; Jia, M.; Li, D.; Liu, Y.; Li, Z.; Zhu, H.; Xu, H.; Sun, M.; Lu, L.; et al. A Systems Vaccinology Approach Reveals Temporal Transcriptomic Changes of Immune Responses to the Yellow Fever 17D Vaccine. J. Immunol. 2017, 199, 1476–1489. [Google Scholar] [CrossRef]
- Ghosh, D.; Basu, A. Japanese encephalitis-a pathological and clinical perspective. PLoS Negl. Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef]
- Methot, S.P.; Di Noia, J.M. Molecular Mechanisms of Somatic Hypermutation and Class Switch Recombination. Adv. Immunol. 2017, 133, 37–87. [Google Scholar] [CrossRef]
- Tas, J.M.; Mesin, L.; Pasqual, G.; Targ, S.; Jacobsen, J.T.; Mano, Y.M.; Chen, C.S.; Weill, J.C.; Reynaud, C.A.; Browne, E.P.; et al. Visualizing antibody affinity maturation in germinal centers. Science 2016, 351, 1048–1054. [Google Scholar] [CrossRef]
- De Alwis, R.; Smith, S.A.; Olivarez, N.P.; Messer, W.B.; Huynh, J.P.; Wahala, W.M.; White, L.J.; Diamond, M.S.; Baric, R.S.; Crowe, J.E., Jr.; et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc. Natl. Acad. Sci. USA 2012, 109, 7439–7444. [Google Scholar] [CrossRef] [PubMed]
- Halliley, J.L.; Tipton, C.M.; Liesveld, J.; Rosenberg, A.F.; Darce, J.; Gregoretti, I.V.; Popova, L.; Kaminiski, D.; Fucile, C.F.; Albizua, I.; et al. Long-Lived Plasma Cells Are Contained within the CD19(-)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 2015, 43, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.M.; Utley, A.; Lee, K.P. Survival of Long-Lived Plasma Cells (LLPC): Piecing Together the Puzzle. Front. Immunol. 2019, 10, 965. [Google Scholar] [CrossRef]
- Slamanig, S.A.; Nolte, M.A. The Bone Marrow as Sanctuary for Plasma Cells and Memory T-Cells: Implications for Adaptive Immunity and Vaccinology. Cells 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Wieten, R.W.; Jonker, E.F.; van Leeuwen, E.M.; Remmerswaal, E.B.; Ten Berge, I.J.; de Visser, A.W.; van Genderen, P.J.; Goorhuis, A.; Visser, L.G.; Grobusch, M.P.; et al. A Single 17D Yellow Fever Vaccination Provides Lifelong Immunity; Characterization of Yellow-Fever-Specific Neutralizing Antibody and T-Cell Responses After Vaccination. PLoS ONE 2016, 11, e0149871. [Google Scholar] [CrossRef]
- Hills, S.L.; Walter, E.B.; Atmar, R.L.; Fischer, M.; Group, A.J.E.V.W. Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2019, 68, 1–33. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. Molecular mechanisms of antibody-mediated neutralisation of flavivirus infection. Expert. Rev. Mol. Med. 2008, 10, e12. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Vogt, M.R.; Dowd, K.A.; Engle, M.; Tesh, R.B.; Johnson, S.; Pierson, T.C.; Diamond, M.S. Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcgamma receptor and complement-dependent effector mechanisms. J. Virol. 2011, 85, 11567–11580. [Google Scholar] [CrossRef]
- Wang, T.T.; Sewatanon, J.; Memoli, M.J.; Wrammert, J.; Bournazos, S.; Bhaumik, S.K.; Pinsky, B.A.; Chokephaibulkit, K.; Onlamoon, N.; Pattanapanyasat, K.; et al. IgG antibodies to dengue enhanced for FcgammaRIIIA binding determine disease severity. Science 2017, 355, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 2003, 60, 421–467. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Jackson, N. Dengue vaccine: Hypotheses to understand CYD-TDV-induced protection. Nat. Rev. Microbiol. 2016, 14, 45–54. [Google Scholar] [CrossRef]
- Biswal, S.; Reynales, H.; Saez-Llorens, X.; Lopez, P.; Borja-Tabora, C.; Kosalaraksa, P.; Sirivichayakul, C.; Watanaveeradej, V.; Rivera, L.; Espinoza, F.; et al. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N. Engl. J. Med. 2019, 381, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 169, 176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Grigoryan, L.; Feng, Y.; Bellusci, L.; Lai, L.; Wali, B.; Ellis, M.; Yuan, M.; Arunachalam, P.S.; Hu, M.; Kowli, S.; et al. AS03 adjuvant enhances the magnitude, persistence, and clonal breadth of memory B cell responses to a plant-based COVID-19 vaccine in humans. Sci. Immunol. 2024, 9, eadi8039. [Google Scholar] [CrossRef]
- Reinke, S.; Thakur, A.; Gartlan, C.; Bezbradica, J.S.; Milicic, A. Inflammasome-Mediated Immunogenicity of Clinical and Experimental Vaccine Adjuvants. Vaccines 2020, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.C.; Lin, C.Y.; Chen, C.; Cheng, H.Y.; Hsieh, E.F.; Liu, L.T.; Chiu, C.H.; Huang, L.M. Long-Term Immunogenicity Study of an Aluminum Phosphate-Adjuvanted Inactivated Enterovirus A71 Vaccine in Children: An Extension to a Phase 2 Study. Vaccines 2024, 12, 985. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lee, P.I. Safety of Japanese encephalitis vaccines. Hum. Vaccin. Immunother. 2021, 17, 4259–4264. [Google Scholar] [CrossRef]
- Calabro, S.; Tritto, E.; Pezzotti, A.; Taccone, M.; Muzzi, A.; Bertholet, S.; De Gregorio, E.; O’Hagan, D.T.; Baudner, B.; Seubert, A. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine 2013, 31, 3363–3369. [Google Scholar] [CrossRef]
- Lofano, G.; Mancini, F.; Salvatore, G.; Cantisani, R.; Monaci, E.; Carrisi, C.; Tavarini, S.; Sammicheli, C.; Rossi Paccani, S.; Soldaini, E.; et al. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J. Immunol. 2015, 195, 1617–1627. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”—Emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines 2021, 6, 158. [Google Scholar] [CrossRef]
- Ko, E.J.; Kang, S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccin. Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef]
- Kovarik, J.; Bozzotti, P.; Tougne, C.; Davis, H.L.; Lambert, P.H.; Krieg, A.M.; Siegrist, C.A. Adjuvant effects of CpG oligodeoxynucleotides on responses against T-independent type 2 antigens. Immunology 2001, 102, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, J.; Klinman, D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine 2014, 32, 6377–6389. [Google Scholar] [CrossRef]
- Ragupathi, G.; Gardner, J.R.; Livingston, P.O.; Gin, D.Y. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert. Rev. Vaccines 2011, 10, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, N.; Zhang, X.; Wang, M.; Liu, Y.; Shi, Y. Potentials of saponins-based adjuvants for nasal vaccines. Front. Immunol. 2023, 14, 1153042. [Google Scholar] [CrossRef]
- Yoon, M.; Choi, Y.; Wi, T.; Choi, Y.S.; Choi, J. The role of cGAMP via the STING pathway in modulating germinal center responses and CD4 T cell differentiation. Front. Immunol. 2024, 15, 1340001. [Google Scholar] [CrossRef] [PubMed]
- Laczko, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castano, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H., 3rd; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses Against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e7. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Lore, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, R.; Eriksson, H. Aluminium adjuvants in vaccines—A way to modulate the immune response. Semin. Cell Dev. Biol. 2021, 115, 3–9. [Google Scholar] [CrossRef]
- Sanchez-Felipe, L.; Alpizar, Y.A.; Ma, J.; Coelmont, L.; Dallmeier, K. YF17D-based vaccines—Standing on the shoulders of a giant. Eur. J. Immunol. 2024, 54, e2250133. [Google Scholar] [CrossRef]
- Wang, P. Natural and Synthetic Saponins as Vaccine Adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef]
- Shan, C.; Xie, X.; Shi, P.Y. Zika Virus Vaccine: Progress and Challenges. Cell Host Microbe 2018, 24, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; P, S.A.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Kool, M.; Fierens, K.; Lambrecht, B.N. Alum adjuvant: Some of the tricks of the oldest adjuvant. J. Med. Microbiol. 2012, 61, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Willingham, S.B.; Ting, J.P.; Re, F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 2008, 181, 17–21. [Google Scholar] [CrossRef]
- Franchi, L.; Nunez, G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008, 38, 2085–2089. [Google Scholar] [CrossRef]
- Unger, B.; Barrett, P.N. Tick-borne encephalitis vaccines. Intern. Med. J. 2013, 43, 838–839. [Google Scholar] [CrossRef]
- Firbas, C.; Jilma, B. Product review on the JE vaccine IXIARO. Hum. Vaccin. Immunother. 2015, 11, 411–420. [Google Scholar] [CrossRef]
- Dupuis, M.; Denis-Mize, K.; LaBarbara, A.; Peters, W.; Charo, I.F.; McDonald, D.M.; Ott, G. Immunization with the adjuvant MF59 induces macrophage trafficking and apoptosis. Eur. J. Immunol. 2001, 31, 2910–2918. [Google Scholar] [CrossRef]
- Li, A.P.Y.; Cohen, C.A.; Leung, N.H.L.; Fang, V.J.; Gangappa, S.; Sambhara, S.; Levine, M.Z.; Iuliano, A.D.; Perera, R.; Ip, D.K.M.; et al. Immunogenicity of standard, high-dose, MF59-adjuvanted, and recombinant-HA seasonal influenza vaccination in older adults. NPJ Vaccines 2021, 6, 25. [Google Scholar] [CrossRef]
- Lin, L.; Lyke, K.E.; Koren, M.; Jarman, R.G.; Eckels, K.H.; Lepine, E.; McArthur, M.A.; Currier, J.R.; Friberg, H.; Moris, P.; et al. Safety and Immunogenicity of an AS03(B)-Adjuvanted Inactivated Tetravalent Dengue Virus Vaccine Administered on Varying Schedules to Healthy U.S. Adults: A Phase 1/2 Randomized Study. Am. J. Trop. Med. Hyg. 2020, 103, 132–141. [Google Scholar] [CrossRef]
- Diaz, C.; Koren, M.; Lin, L.; Martinez, L.J.; Eckels, K.H.; Campos, M.; Jarman, R.G.; De La Barrera, R.; Lepine, E.; Febo, I.; et al. Safety and Immunogenicity of Different Formulations of a Tetravalent Dengue Purified Inactivated Vaccine in Healthy Adults from Puerto Rico: Final Results after 3 Years of Follow-Up from a Randomized, Placebo-Controlled Phase I Study. Am. J. Trop. Med. Hyg. 2020, 102, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Steinhagen, F.; Kinjo, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 29, 3341–3355. [Google Scholar] [CrossRef]
- Katsenelson, N.; Kanswal, S.; Puig, M.; Mostowski, H.; Verthelyi, D.; Akkoyunlu, M. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur. J. Immunol. 2007, 37, 1785–1795. [Google Scholar] [CrossRef]
- Demento, S.L.; Bonafe, N.; Cui, W.; Kaech, S.M.; Caplan, M.J.; Fikrig, E.; Ledizet, M.; Fahmy, T.M. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J. Immunol. 2010, 185, 2989–2997. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Thotathil, N.; Zhao, Z.; Mitragotri, S. Vaccine adjuvants for infectious disease in the clinic. Bioeng. Transl. Med. 2024, 9, e10663. [Google Scholar] [CrossRef]
- Toussi, D.N.; Massari, P. Immune Adjuvant Effect of Molecularly-defined Toll-Like Receptor Ligands. Vaccines 2014, 2, 323–353. [Google Scholar] [CrossRef]
- Robert Putnak, J.; Coller, B.A.; Voss, G.; Vaughn, D.W.; Clements, D.; Peters, I.; Bignami, G.; Houng, H.S.; Chen, R.C.; Barvir, D.A.; et al. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine 2005, 23, 4442–4452. [Google Scholar] [CrossRef] [PubMed]
- Visciano, M.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Effects of adjuvants on IgG subclasses elicited by virus-like particles. J. Transl. Med. 2012, 10, 4. [Google Scholar] [CrossRef]
- Al-Osaimi, H.M.; Kanan, M.; Marghlani, L.; Al-Rowaili, B.; Albalawi, R.; Saad, A.; Alasmari, S.; Althobaiti, K.; Alhulaili, Z.; Alanzi, A.; et al. A systematic review on malaria and dengue vaccines for the effective management of these mosquito borne diseases: Improving public health. Hum. Vaccin. Immunother. 2024, 20, 2337985. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.C.; Crespo, M.P.; Abraham, W.; Moynihan, K.D.; Szeto, G.L.; Chen, S.H.; Melo, M.B.; Mueller, S.; Irvine, D.J. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J. Clin. Investig. 2015, 125, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.A.; Beatty, P.R.; Reiner, G.L.; Sivick, K.E.; Hix Glickman, L.; Dubensky, T.W., Jr.; Harris, E. Cyclic Dinucleotide-Adjuvanted Dengue Virus Nonstructural Protein 1 Induces Protective Antibody and T Cell Responses. J. Immunol. 2019, 202, 1153–1162. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, W.; Nie, J.; Zhang, L. Progress Update on STING Agonists as Vaccine Adjuvants. Vaccines 2025, 13, 371. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombacz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Wollner, C.J.; Richner, J.M. mRNA Vaccines against Flaviviruses. Vaccines 2021, 9, 148. [Google Scholar] [CrossRef]
- Satchidanandam, V. Japanese Encephalitis Vaccines. Curr. Treat. Options Infect. Dis. 2020, 12, 375–386. [Google Scholar] [CrossRef]
- Kudlacek, S.T.; Metz, S.; Thiono, D.; Payne, A.M.; Phan, T.T.N.; Tian, S.; Forsberg, L.J.; Maguire, J.; Seim, I.; Zhang, S.; et al. Designed, highly expressing, thermostable dengue virus 2 envelope protein dimers elicit quaternary epitope antibodies. Sci. Adv. 2021, 7, eabg4084. [Google Scholar] [CrossRef]
- Sharma, A.; Zhang, X.; Dejnirattisai, W.; Dai, X.; Gong, D.; Wongwiwat, W.; Duquerroy, S.; Rouvinski, A.; Vaney, M.C.; Guardado-Calvo, P.; et al. The epitope arrangement on flavivirus particles contributes to Mab C10’s extraordinary neutralization breadth across Zika and dengue viruses. Cell 2021, 184, 6052–6066.e18. [Google Scholar] [CrossRef] [PubMed]
- Gallichotte, E.N.; Widman, D.G.; Yount, B.L.; Wahala, W.M.; Durbin, A.; Whitehead, S.; Sariol, C.A.; Crowe, J.E., Jr.; de Silva, A.M.; Baric, R.S. A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. mBio 2015, 6, e01461-15. [Google Scholar] [CrossRef]
- Phan, T.T.N.; Hvasta, M.G.; Kudlacek, S.T.; Thiono, D.J.; Tripathy, A.; Nicely, N.I.; de Silva, A.M.; Kuhlman, B. A conserved set of mutations for stabilizing soluble envelope protein dimers from dengue and Zika viruses to advance the development of subunit vaccines. J. Biol. Chem. 2022, 298, 102079. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Sanchez-San Martin, C.; Zheng, A.; Kielian, M. In vitro reconstitution reveals key intermediate states of trimer formation by the dengue virus membrane fusion protein. J. Virol. 2010, 84, 5730–5740. [Google Scholar] [CrossRef]
- Shen, W.F.; Galula, J.U.; Liu, J.H.; Liao, M.Y.; Huang, C.H.; Wang, Y.C.; Wu, H.C.; Liang, J.J.; Lin, Y.L.; Whitney, M.T.; et al. Epitope resurfacing on dengue virus-like particle vaccine preparation to induce broad neutralizing antibody. eLife 2018, 7, e38970. [Google Scholar] [CrossRef]
- Metz, S.W.; Gallichotte, E.N.; Brackbill, A.; Premkumar, L.; Miley, M.J.; Baric, R.; de Silva, A.M. In Vitro Assembly and Stabilization of Dengue and Zika Virus Envelope Protein Homo-Dimers. Sci. Rep. 2017, 7, 4524. [Google Scholar] [CrossRef]
- Whitehead, S.S.; Durbin, A.P.; Pierce, K.K.; Elwood, D.; McElvany, B.D.; Fraser, E.A.; Carmolli, M.P.; Tibery, C.M.; Hynes, N.A.; Jo, M.; et al. In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS Negl. Trop. Dis. 2017, 11, e0005584. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.A.; Nivarthi, U.K.; Graham, N.R.; Eisenhauer, P.; Delacruz, M.J.; Pierce, K.K.; Whitehead, S.S.; Boyson, J.E.; Botten, J.W.; Kirkpatrick, B.D.; et al. Stimulation of B Cell Immunity in Flavivirus-Naive Individuals by the Tetravalent Live Attenuated Dengue Vaccine TV003. Cell Rep. Med. 2020, 1, 100155. [Google Scholar] [CrossRef] [PubMed]
- Kadlecek, V.; Borja-Tabora, C.F.; Eder-Lingelbach, S.; Gatchalian, S.; Kiermayr, S.; Sablan, B., Jr.; Kundi, M.; Taucher, C.; Dubischar, K.L. Antibody Persistence up to 3 Years After Primary Immunization with Inactivated Japanese Encephalitis Vaccine IXIARO in Philippine Children and Effect of a Booster Dose. Pediatr. Infect. Dis. J. 2018, 37, e233–e240. [Google Scholar] [CrossRef]
- Paulke-Korinek, M.; Kollaritsch, H.; Kundi, M.; Zwazl, I.; Seidl-Friedrich, C.; Jelinek, T. Persistence of antibodies six years after booster vaccination with inactivated vaccine against Japanese encephalitis. Vaccine 2015, 33, 3600–3604. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Recombinant Protein-Based Dengue Vaccines. Front. Immunol. 2018, 9, 1919. [Google Scholar] [CrossRef]
- Georgiev, G.I.; Malonis, R.J.; Wirchnianski, A.S.; Wessel, A.W.; Jung, H.S.; Cahill, S.M.; Nyakatura, E.K.; Vergnolle, O.; Dowd, K.A.; Cowburn, D.; et al. Resurfaced ZIKV EDIII nanoparticle immunogens elicit neutralizing and protective responses in vivo. Cell Chem. Biol. 2022, 29, 811–823.e7. [Google Scholar] [CrossRef]
- Van Hoeven, N.; Wiley, S.; Gage, E.; Fiore-Gartland, A.; Granger, B.; Gray, S.; Fox, C.; Clements, D.E.; Parks, D.E.; Winram, S.; et al. A combination of TLR-4 agonist and saponin adjuvants increases antibody diversity and protective efficacy of a recombinant West Nile Virus antigen. NPJ Vaccines 2018, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified mRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 702–712. [Google Scholar] [CrossRef]
- Saunders, K.O.; Pardi, N.; Parks, R.; Santra, S.; Mu, Z.; Sutherland, L.; Scearce, R.; Barr, M.; Eaton, A.; Hernandez, G.; et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines 2021, 6, 50. [Google Scholar] [CrossRef]
- Raviprakash, K.; Wang, D.; Ewing, D.; Holman, D.H.; Block, K.; Woraratanadharm, J.; Chen, L.; Hayes, C.; Dong, J.Y.; Porter, K. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J. Virol. 2008, 82, 6927–6934. [Google Scholar] [CrossRef]
- Bullard, B.L.; Corder, B.N.; Gordon, D.N.; Pierson, T.C.; Weaver, E.A. Characterization of a Species E Adenovirus Vector as a Zika virus vaccine. Sci. Rep. 2020, 10, 3613. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.A.; Wu, K.; Kim, G.N.; Saeedian, N.; Seon, S.H.; Park, G.; Jung, D.I.; Jeong, H.W.; Kim, N.H.; Seo, S.H.; et al. Induction of protective immune responses against a lethal Zika virus challenge post-vaccination with a dual serotype of recombinant vesicular stomatitis virus carrying the genetically modified Zika virus E protein gene. J. Gen. Virol. 2021, 102, 001588. [Google Scholar] [CrossRef] [PubMed]
- Taslem Mourosi, J.; Awe, A.; Jain, S.; Batra, H. Nucleic Acid Vaccine Platform for DENGUE and ZIKA Flaviviruses. Vaccines 2022, 10, 834. [Google Scholar] [CrossRef]
- Comes, J.D.G.; Pijlman, G.P.; Hick, T.A.H. Rise of the RNA machines—Self-amplification in mRNA vaccine design. Trends Biotechnol. 2023, 41, 1417–1429. [Google Scholar] [CrossRef]
- Casmil, I.C.; Jin, J.; Won, E.J.; Huang, C.; Liao, S.; Cha-Molstad, H.; Blakney, A.K. The advent of clinical self-amplifying RNA vaccines. Mol. Ther. 2025, 33, 2565–2582. [Google Scholar] [CrossRef]
- Metz, S.W.; Thomas, A.; Brackbill, A.; Xianwen, Y.; Stone, M.; Horvath, K.; Miley, M.J.; Luft, C.; DeSimone, J.M.; Tian, S.; et al. Nanoparticle delivery of a tetravalent E protein subunit vaccine induces balanced, type-specific neutralizing antibodies to each dengue virus serotype. PLoS Negl. Trop. Dis. 2018, 12, e0006793. [Google Scholar] [CrossRef]
- Silva, E.F.; Orsi, M.; Andrade, A.L.; Domingues, R.Z.; Silva, B.M.; de Araujo, H.R.; Pimenta, P.F.; Diamond, M.S.; Rocha, E.S.; Kroon, E.G.; et al. A tetravalent dengue nanoparticle stimulates antibody production in mice. J. Nanobiotechnol. 2012, 10, 13. [Google Scholar] [CrossRef]
- Sevvana, M.; Kuhn, R.J. Mapping the diverse structural landscape of the flavivirus antibody repertoire. Curr. Opin. Virol. 2020, 45, 51–64. [Google Scholar] [CrossRef]
- Gunawardana, S.A.; Shaw, R.H. Cross-reactive dengue virus-derived monoclonal antibodies to Zika virus envelope protein: Panacea or Pandora’s box? BMC Infect. Dis. 2018, 18, 641. [Google Scholar] [CrossRef]
- Rodenhuis-Zybert, I.A.; van der Schaar, H.M.; da Silva Voorham, J.M.; van der Ende-Metselaar, H.; Lei, H.Y.; Wilschut, J.; Smit, J.M. Immature dengue virus: A veiled pathogen? PLoS Pathog. 2010, 6, e1000718. [Google Scholar] [CrossRef]
- Wirawan, M.; Fibriansah, G.; Marzinek, J.K.; Lim, X.X.; Ng, T.S.; Sim, A.Y.L.; Zhang, Q.; Kostyuchenko, V.A.; Shi, J.; Smith, S.A.; et al. Mechanism of Enhanced Immature Dengue Virus Attachment to Endosomal Membrane Induced by prM Antibody. Structure 2019, 27, 253–267.e8. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, M.; Cao, J.; Shen, J.; Zhou, X.; Wang, D.; Cao, J. Flavivirus: From Structure to Therapeutics Development. Life 2021, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Langenburg, T. A Perspective on Current Flavivirus Vaccine Development: A Brief Review. Viruses 2023, 15, 860. [Google Scholar] [CrossRef] [PubMed]
- Larena, M.; Prow, N.A.; Hall, R.A.; Petrovsky, N.; Lobigs, M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8(+) T cells and pre-exposure neutralizing antibody. J. Virol. 2013, 87, 4395–4402. [Google Scholar] [CrossRef]
- Araujo, S.C.; Pereira, L.R.; Alves, R.P.S.; Andreata-Santos, R.; Kanno, A.I.; Ferreira, L.C.S.; Goncalves, V.M. Anti-Flavivirus Vaccines: Review of the Present Situation and Perspectives of Subunit Vaccines Produced in Escherichia coli. Vaccines 2020, 8, 492. [Google Scholar] [CrossRef]
- Martin, J.; Hermida, L. Dengue vaccine: An update on recombinant subunit strategies. Acta Virol. 2016, 60, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Fahimi, H.; Mohammadipour, M.; Haddad Kashani, H.; Parvini, F.; Sadeghizadeh, M. Dengue viruses and promising envelope protein domain III-based vaccines. Appl. Microbiol. Biotechnol. 2018, 102, 2977–2996. [Google Scholar] [CrossRef]
- Wong, S.H.; Jassey, A.; Wang, J.Y.; Wang, W.C.; Liu, C.H.; Lin, L.T. Virus-Like Particle Systems for Vaccine Development against Viruses in the Flaviviridae Family. Vaccines 2019, 7, 123. [Google Scholar] [CrossRef]
- Unali, G.; Douam, F. Orthoflavivirus Vaccine Platforms: Current Strategies and Challenges. Vaccines 2025, 13, 1015. [Google Scholar] [CrossRef] [PubMed]
- Ebel, H.; Benecke, T.; Vollmer, B. Stabilisation of Viral Membrane Fusion Proteins in Pre-fusion Conformation by Structure-Based Design for Structure Determination and Vaccine Development. Viruses 2022, 14, 1816. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, F.; Lin, S.; Yang, F.; Cheng, Y.; Cao, Y.; Chen, Z.; Lu, G. Crystal structure of Usutu virus envelope protein in the pre-fusion state. Virol. J. 2018, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.H.; Klein, D.E.; Schmidt, A.G.; Pena, J.M.; Harrison, S.C. Sequential conformational rearrangements in flavivirus membrane fusion. eLife 2014, 3, e04389. [Google Scholar] [CrossRef]
- He, J.; Ding, X.; Zhao, J.; Zeng, J.; Zhou, Y.; Xiao, W.; Hua, D.; Liu, M.; Guo, H.; Zhang, Y.; et al. A novel pan-epitope based nanovaccine self-assembled with CpG enhances immune responses against flavivirus. J. Nanobiotechnol. 2024, 22, 738. [Google Scholar] [CrossRef]
- Ripoll, D.R.; Khavrutskii, I.; Wallqvist, A.; Chaudhury, S. Modeling the Role of Epitope Arrangement on Antibody Binding Stoichiometry in Flaviviruses. Biophys. J. 2016, 111, 1641–1654. [Google Scholar] [CrossRef]
- Blakney, A.K.; Ip, S.; Geall, A.J. An Update on Self-Amplifying mRNA Vaccine Development. Vaccines 2021, 9, 97. [Google Scholar] [CrossRef]
- Silva-Pilipich, N.; Beloki, U.; Salaberry, L.; Smerdou, C. Self-Amplifying RNA: A Second Revolution of mRNA Vaccines against COVID-19. Vaccines 2024, 12, 318. [Google Scholar] [CrossRef]
- Battisti, P.; Ykema, M.R.; Kasal, D.N.; Jennewein, M.F.; Beaver, S.; Weight, A.E.; Hanson, D.; Singh, J.; Bakken, J.; Cross, N.; et al. A bivalent self-amplifying RNA vaccine against yellow fever and Zika viruses. Front. Immunol. 2025, 16, 1569454. [Google Scholar] [CrossRef]
- Rathore, A.P.S.; St John, A.L. Cross-Reactive Immunity Among Flaviviruses. Front. Immunol. 2020, 11, 334. [Google Scholar] [CrossRef]
- Maciejewski, S.; Pierson, T.C. Cross-Reactive Flavivirus Antibody: Friend and Foe? Cell Host Microbe 2018, 24, 622–624. [Google Scholar] [CrossRef]
- Swanstrom, J.A.; Plante, J.A.; Plante, K.S.; Young, E.F.; McGowan, E.; Gallichotte, E.N.; Widman, D.G.; Heise, M.T.; de Silva, A.M.; Baric, R.S. Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. mBio 2016, 7, e01123-16. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Stuart, J.B.; Levoir, L.M.; Belmont, L.; Goo, L. Defining the impact of flavivirus envelope protein glycosylation site mutations on sensitivity to broadly neutralizing antibodies. mBio 2024, 15, e03048-23. [Google Scholar] [CrossRef] [PubMed]
- Fibriansah, G.; Ibarra, K.D.; Ng, T.S.; Smith, S.A.; Tan, J.L.; Lim, X.N.; Ooi, J.S.; Kostyuchenko, V.A.; Wang, J.; de Silva, A.M.; et al. DENGUE VIRUS. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 2015, 349, 88–91. [Google Scholar] [CrossRef]
- Fernandez, E.; Dejnirattisai, W.; Cao, B.; Scheaffer, S.M.; Supasa, P.; Wongwiwat, W.; Esakky, P.; Drury, A.; Mongkolsapaya, J.; Moley, K.H.; et al. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat. Immunol. 2017, 18, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef]
- Sun, J.; Du, S.; Zheng, Z.; Cheng, G.; Jin, X. Defeat Dengue and Zika Viruses with a One-Two Punch of Vaccine and Vector Blockade. Front. Microbiol. 2020, 11, 362. [Google Scholar] [CrossRef]
- Lin, H.H.; Yang, S.P.; Tsai, M.J.; Lin, G.C.; Wu, H.C.; Wu, S.C. Dengue and Zika Virus Domain III-Flagellin Fusion and Glycan-Masking E Antigen for Prime-Boost Immunization. Theranostics 2019, 9, 4811–4826. [Google Scholar] [CrossRef]
- Midgley, C.M.; Bajwa-Joseph, M.; Vasanawathana, S.; Limpitikul, W.; Wills, B.; Flanagan, A.; Waiyaiya, E.; Tran, H.B.; Cowper, A.E.; Chotiyarnwong, P.; et al. An in-depth analysis of original antigenic sin in dengue virus infection. J. Virol. 2011, 85, 410–421. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika virus infection enhances future risk of severe dengue disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.E.; Kalimuddin, S. Insights into dengue immunity from vaccine trials. Sci. Transl. Med. 2023, 15, eadh3067. [Google Scholar] [CrossRef] [PubMed]
- Dussupt, V.; Modjarrad, K.; Krebs, S.J. Landscape of Monoclonal Antibodies Targeting Zika and Dengue: Therapeutic Solutions and Critical Insights for Vaccine Development. Front. Immunol. 2020, 11, 621043. [Google Scholar] [CrossRef]
- St John, A.L.; Rathore, A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019, 19, 218–230. [Google Scholar] [CrossRef]
- World Health Organization. Dengue vaccine: WHO position paper, September 2018—Recommendations. Vaccine 2019, 37, 4848–4849. [Google Scholar] [CrossRef]
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvinski, A.; Vaney, M.C.; Medits, I.; Sharma, A.; Simon-Loriere, E.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Haouz, A.; et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016, 536, 48–53. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Katzelnick, L.C.; Burger-Calderon, R.; Gallini, J.; Moore, R.H.; Kuan, G.; Balmaseda, A.; Pinsky, B.A.; Harris, E. Antibody-Dependent Enhancement of Severe Disease Is Mediated by Serum Viral Load in Pediatric Dengue Virus Infections. J. Infect. Dis. 2020, 221, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Hadinegoro, S.R.; Arredondo-Garcia, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef]
- Laydon, D.J.; Dorigatti, I.; Hinsley, W.R.; Nedjati-Gilani, G.; Coudeville, L.; Ferguson, N.M. Efficacy profile of the CYD-TDV dengue vaccine revealed by Bayesian survival analysis of individual-level phase III data. eLife 2021, 10, e65131. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Zambrana, J.V.; Elizondo, D.; Collado, D.; Garcia, N.; Arguello, S.; Mercado, J.C.; Miranda, T.; Ampie, O.; Mercado, B.L.; et al. Dengue and Zika virus infections in children elicit cross-reactive protective and enhancing antibodies that persist long term. Sci. Transl. Med. 2021, 13, eabg9478. [Google Scholar] [CrossRef]
- Slon-Campos, J.L.; Dejnirattisai, W.; Jagger, B.W.; Lopez-Camacho, C.; Wongwiwat, W.; Durnell, L.A.; Winkler, E.S.; Chen, R.E.; Reyes-Sandoval, A.; Rey, F.A.; et al. A protective Zika virus E-dimer-based subunit vaccine engineered to abrogate antibody-dependent enhancement of dengue infection. Nat. Immunol. 2019, 20, 1291–1298. [Google Scholar] [CrossRef]
- Silva, M.; Kato, Y.; Melo, M.B.; Phung, I.; Freeman, B.L.; Li, Z.; Roh, K.; Van Wijnbergen, J.W.; Watkins, H.; Enemuo, C.A.; et al. A particulate saponin/TLR agonist vaccine adjuvant alters lymph flow and modulates adaptive immunity. Sci. Immunol. 2021, 6, eabf1152. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Zaman, K.; Yunus, M.; Aziz, A.B.; Feser, J.; Mooney, J.; Tang, Y.; Ellison, D.W.; Thaisomboonsuk, B.; Zhang, L.; Neuzil, K.M.; et al. Antibody persistence and immune memory response following primary vaccination and boosting with live attenuated SA 14-14-2 Japanese encephalitis vaccine (CD-JEV) in Bangladesh: A phase 4 open-label clinical trial. Vaccine X 2022, 10, 100143. [Google Scholar] [CrossRef]
- Wec, A.Z.; Haslwanter, D.; Abdiche, Y.N.; Shehata, L.; Pedreno-Lopez, N.; Moyer, C.L.; Bornholdt, Z.A.; Lilov, A.; Nett, J.H.; Jangra, R.K.; et al. Longitudinal dynamics of the human B cell response to the yellow fever 17D vaccine. Proc. Natl. Acad. Sci. USA 2020, 117, 6675–6685. [Google Scholar] [CrossRef] [PubMed]
- Bouckenooghe, A.; Bailleux, F.; Feroldi, E. Modeling the long-term persistence of neutralizing antibody in children and toddlers after vaccination with live attenuated Japanese encephalitis chimeric virus vaccine. Hum. Vaccin. Immunother. 2019, 15, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Kling, K.; Domingo, C.; Bogdan, C.; Duffy, S.; Harder, T.; Howick, J.; Kleijnen, J.; McDermott, K.; Wichmann, O.; Wilder-Smith, A.; et al. Duration of Protection After Vaccination Against Yellow Fever: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022, 75, 2266–2274. [Google Scholar] [CrossRef]
- Keelapang, P.; Ketloy, C.; Puttikhunt, C.; Sriburi, R.; Prompetchara, E.; Sae-Lim, M.; Siridechadilok, B.; Duangchinda, T.; Noisakran, S.; Charoensri, N.; et al. Heterologous prime-boost immunization induces protection against dengue virus infection in cynomolgus macaques. J. Virol. 2023, 97, e0096323. [Google Scholar] [CrossRef]
- George, J.A.; Eo, S.K. Distinct Humoral and Cellular Immunity Induced by Alternating Prime-boost Vaccination Using Plasmid DNA and Live Viral Vector Vaccines Expressing the E Protein of Dengue Virus Type 2. Immune Netw. 2011, 11, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Salem, G.M.; Galula, J.U.; Wu, S.R.; Liu, J.H.; Chen, Y.H.; Wang, W.H.; Wang, S.F.; Song, C.S.; Chen, F.C.; Abarientos, A.B.; et al. Antibodies from dengue patients with prior exposure to Japanese encephalitis virus are broadly neutralizing against Zika virus. Commun. Biol. 2024, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.S.; Marcial-Juarez, E.; Linterman, M.A. The cellular factors that impair the germinal center in advanced age. J. Immunol. 2025, 214, 862–871. [Google Scholar] [CrossRef]
- Lee, J.L.; Linterman, M.A. Mechanisms underpinning poor antibody responses to vaccines in ageing. Immunol. Lett. 2022, 241, 1–14. [Google Scholar] [CrossRef]
- Siegrist, C.A.; Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef]
- Rodriguez-Barraquer, I.; Costa, F.; Nascimento, E.J.M.; Nery, N.J.; Castanha, P.M.S.; Sacramento, G.A.; Cruz, J.; Carvalho, M.; De Olivera, D.; Hagan, J.E.; et al. Impact of pre-existing dengue immunity on Zika virus emergence in a dengue endemic region. Science 2019, 363, 607–610. [Google Scholar] [CrossRef]
- Palacios-Pedrero, M.A.; Osterhaus, A.; Becker, T.; Elbahesh, H.; Rimmelzwaan, G.F.; Saletti, G. Aging and Options to Halt Declining Immunity to Virus Infections. Front. Immunol. 2021, 12, 681449. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Expert. Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Bian, Y.; Hu, Y.; Chuan, J.; Zhong, L.; Zhu, Y.; Tong, R. Insights into vaccines for elderly individuals: From the impacts of immunosenescence to delivery strategies. NPJ Vaccines 2024, 9, 77. [Google Scholar] [CrossRef]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef]
- Lotspeich-Cole, L.; Parvathaneni, S.; Sakai, J.; Liu, L.; Takeda, K.; Lee, R.C.; Akkoyunlu, M. Sustained antigen delivery improves germinal center reaction and increases antibody responses in neonatal mice. NPJ Vaccines 2024, 9, 92. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Liu, Y.; Li, Q.; Xue, Y.; Liu, L.; Liang, R.; Xiong, Y.; Zhao, J.; Chen, J.; et al. BAFF promotes follicular helper T cell development and germinal center formation through BR3 signal. JCI Insight 2024, 9, e183400. [Google Scholar] [CrossRef]
- Zhang, Y.; Tech, L.; George, L.A.; Acs, A.; Durrett, R.E.; Hess, H.; Walker, L.S.K.; Tarlinton, D.M.; Fletcher, A.L.; Hauser, A.E.; et al. Plasma cell output from germinal centers is regulated by signals from Tfh and stromal cells. J. Exp. Med. 2018, 215, 1227–1243. [Google Scholar] [CrossRef]
- Vassilieva, E.V.; Taylor, D.W.; Compans, R.W. Combination of STING Pathway Agonist with Saponin Is an Effective Adjuvant in Immunosenescent Mice. Front. Immunol. 2019, 10, 3006. [Google Scholar] [CrossRef]
- Anasir, M.I.; Poh, C.L. Structural Vaccinology for Viral Vaccine Design. Front. Microbiol. 2019, 10, 738. [Google Scholar] [CrossRef]
- Leong, K.Y.; Tham, S.K.; Poh, C.L. Revolutionizing immunization: A comprehensive review of mRNA vaccine technology and applications. Virol. J. 2025, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Flores, H.E.; Pinzon Burgos, E.F.; Camacho Ortega, S.; Heredia, A.; Chua, J.V. From Antibodies to Immunity: Assessing Correlates of Flavivirus Protection and Cross-Reactivity. Vaccines 2025, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, E.C.; McEntee, C.P. Vaccine adjuvants: Tailoring innate recognition to send the right message. Immunity 2024, 57, 772–789. [Google Scholar] [CrossRef]
- Yang, C.; Gong, R.; de Val, N. Development of Neutralizing Antibodies against Zika Virus Based on Its Envelope Protein Structure. Virol. Sin. 2019, 34, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.S.; Baillet, J.; Filsinger Interrante, M.V.; Adamska, J.Z.; Zhou, X.; Saouaf, O.M.; Yan, J.; Klich, J.H.; Jons, C.K.; Meany, E.L.; et al. Saponin nanoparticle adjuvants incorporating Toll-like receptor agonists drive distinct immune signatures and potent vaccine responses. Sci. Adv. 2024, 10, eadn7187. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.Q.; Yang, X.; Wei, Y.; Chen, J.T.; Wang, X.J.; Peng, H.J. A Review on Dengue Vaccine Development. Vaccines 2020, 8, 63. [Google Scholar] [CrossRef]
- Cui, J.Z.; Xiong, X.H.; Wang, Q.Y.; Dong, H.L.; Liu, G.; Chen, H.P. Insect-Specific Flaviviruses Have Potential Applications as a Scaffold for Pathogenic Flavivirus Vaccines. Vaccines 2025, 13, 769. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, J.A.; Duffy, D. Systems vaccinology studies—Achievements and future potential. Microbes Infect. 2024, 26, 105318. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Recent updates on correlates of vaccine-induced protection. Front. Immunol. 2022, 13, 1081107. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.B.; Yang, Q.W.; Huang, H.W.; Chiu, K.C.; Su, S.L.; Liao, C.L.; Yen, L.C. A novel immunogen comprising a bc loop and mutant fusion loop epitopes generates potent neutralization and protective abilities against flaviviruses without risk of disease enhancement. Vaccine 2025, 57, 127219. [Google Scholar] [CrossRef]
- Thomas, A.; Thiono, D.J.; Kudlacek, S.T.; Forsberg, J.; Premkumar, L.; Tian, S.; Kuhlman, B.; de Silva, A.M.; Metz, S.W. Dimerization of Dengue Virus E Subunits Impacts Antibody Function and Domain Focus. J. Virol. 2020, 94, e00745-20. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Rasheed, M.A.U.; Havenar-Daughton, C.; Pham, M.; Legere, T.; Sher, Z.J.; Kovalenkov, Y.; Gumber, S.; Huang, J.Y.; Gottardo, R.; et al. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope-specific plasma cells and humoral immunity in nonhuman primates. Sci. Immunol. 2020, 5, eabb1025. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef]
- Luisi, K.; Morabito, K.M.; Burgomaster, K.E.; Sharma, M.; Kong, W.P.; Foreman, B.M.; Patel, S.; Fisher, B.; Aleshnick, M.A.; Laliberte, J.; et al. Development of a potent Zika virus vaccine using self-amplifying messenger RNA. Sci. Adv. 2020, 6, eaba5068. [Google Scholar] [CrossRef]
- Essink, B.; Chu, L.; Seger, W.; Barranco, E.; Le Cam, N.; Bennett, H.; Faughnan, V.; Pajon, R.; Paila, Y.D.; Bollman, B.; et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: The results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 2023, 23, 621–633. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Gerritsen, B.; Tomalin, L.E.; Fourati, S.; Mule, M.P.; Chawla, D.G.; Rychkov, D.; Henrich, E.; Miller, H.E.R.; Diray-Arce, J.; et al. Transcriptional atlas of the human immune response to 13 vaccines reveals a common predictor of vaccine-induced antibody responses. Nat. Immunol. 2022, 23, 1788–1798. [Google Scholar] [CrossRef]
- Gonzalez-Dias, P.; Lee, E.K.; Sorgi, S.; de Lima, D.S.; Urbanski, A.H.; Silveira, E.L.; Nakaya, H.I. Methods for predicting vaccine immunogenicity and reactogenicity. Hum. Vaccin. Immunother. 2020, 16, 269–276. [Google Scholar] [CrossRef]
- Tricou, V.; Yu, D.; Reynales, H.; Biswal, S.; Saez-Llorens, X.; Sirivichayakul, C.; Lopez, P.; Borja-Tabora, C.; Bravo, L.; Kosalaraksa, P.; et al. Long-term efficacy and safety of a tetravalent dengue vaccine (TAK-003): 4.5-year results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2024, 12, e257–e270. [Google Scholar] [CrossRef]
- Valdes, I.; Izquierdo, A.; Cobas, K.; Thao, P.; Anh Duc, H.; Duc Loc, H.; Dung, L.T.; Lazo, L.; Suzarte, E.; Perez, Y.; et al. A heterologous prime-boost strategy for immunization against Dengue virus combining the Tetra DIIIC subunit vaccine candidate with the TV005 live-attenuated tetravalent vaccine. J. Gen. Virol. 2019, 100, 975–984. [Google Scholar] [CrossRef]
- Sun, P.; Jani, V.; Johnson, A.; Cheng, Y.; Nagabhushana, N.; Williams, M.; Morrison, B.J.; Defang, G. T cell and memory B cell responses in tetravalent DNA, tetravalent inactivated and tetravalent live-attenuated prime-boost dengue vaccines in rhesus macaques. Vaccine 2021, 39, 7510–7520. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, G.; Tandavanitj, R.; Preciado-Llanes, L.; Sanchez-Velazquez, R.; Prado Rocha, R.; Kim, Y.C.; Reyes-Sandoval, A.; Patel, A.H. Heterologous prime-boost Zika virus vaccination induces comprehensive humoral and cellular immunity in mouse models. Front. Immunol. 2025, 16, 1578427. [Google Scholar] [CrossRef]
- Natsrita, P.; Charoenkwan, P.; Shoombuatong, W.; Mahalapbutr, P.; Faksri, K.; Chareonsudjai, S.; Rungrotmongkol, T.; Pipattanaboon, C. Machine-learning-assisted high-throughput identification of potent and stable neutralizing antibodies against all four dengue virus serotypes. Sci. Rep. 2024, 14, 17165. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, G.; Fassi, E.M.A.; Romanato, A.; D’Annessa, I.; Odinolfi, M.T.; Brambilla, D.; Damin, F.; Chiari, M.; Gori, A.; Colombo, G.; et al. Computational Analysis of Dengue Virus Envelope Protein (E) Reveals an Epitope with Flavivirus Immunodiagnostic Potential in Peptide Microarrays. Int. J. Mol. Sci. 2019, 20, 1921. [Google Scholar] [CrossRef]
- Deepthi, V.; Sasikumar, A.; Mohanakumar, K.P.; Rajamma, U. Computationally designed multi-epitope vaccine construct targeting the SARS-CoV-2 spike protein elicits robust immune responses in silico. Sci. Rep. 2025, 15, 9562. [Google Scholar] [CrossRef]
- Schnyder, J.L.; de Jong, H.K.; Bache, B.E.; Schaumburg, F.; Grobusch, M.P. Long-term immunity following yellow fever vaccination: A systematic review and meta-analysis. Lancet Glob. Health 2024, 12, e445–e456. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Dhama, N.; Gupta, R.D. Dengue virus neutralizing antibody: A review of targets, cross-reactivity, and antibody-dependent enhancement. Front. Immunol. 2023, 14, 1200195. [Google Scholar] [CrossRef]
- Shukla, R.; Ramasamy, V.; Shanmugam, R.K.; Ahuja, R.; Khanna, N. Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine. Front. Cell Infect. Microbiol. 2020, 10, 572681. [Google Scholar] [CrossRef]
- Chan, K.R.; Ismail, A.A.; Thergarajan, G.; Raju, C.S.; Yam, H.C.; Rishya, M.; Sekaran, S.D. Serological cross-reactivity among common flaviviruses. Front. Cell Infect. Microbiol. 2022, 12, 975398. [Google Scholar] [CrossRef]
- Parra-Gonzalez, M.; Najera-Maldonado, L.; Peralta-Cuevas, E.; Gutierrez-Onofre, A.J.; Garcia-Atutxa, I.; Villanueva-Flores, F. Overcoming dengue vaccine challenges through next-generation virus-like particle immunization strategies. Front. Cell Infect. Microbiol. 2025, 15, 1614805. [Google Scholar] [CrossRef] [PubMed]
- Santos-Peral, A.; Zaucha, M.; Nikolova, E.; Yaman, E.; Puzek, B.; Winheim, E.; Goresch, S.; Scheck, M.K.; Lehmann, L.; Dahlstroem, F.; et al. Basal T cell activation predicts yellow fever vaccine response independently of cytomegalovirus infection and sex-related immune variations. Cell Rep. Med. 2025, 6, 101946. [Google Scholar] [CrossRef] [PubMed]
| Adjuvant Class | Mechanism Target | Immune Profile | Effects on Antibody Quality | Durability Titer | Representative Flavivirus Context | Refs. |
|---|---|---|---|---|---|---|
| Alum | NLRP3 inflammasome activation; antigen depot | Predominantly Th2; limited Tfh | Increases antibody magnitude but limited affinity maturation | wanes over 2–3 years | Inactivated JE, TBEV, WNV vaccines | [75,76,77,78,79] |
| MF59 (oil-in-water emulsion) | Enhanced APC recruitment and cytokine induction | Balanced Th1/Th2; improved Tfh | Enhances avidity and broad subclass distribution | up to 5 years in available studies | WNV and JE experimental vaccines | [61,80,81] |
| AS03 | Innate sensor activation, IL-6 and cytokine induction | Balanced Th1/th2 with robust Tfh | Improves avidity and functional diversity | ≥3 years in clinical follow up | DENV and ZIKV subunit studies | [82,83] |
| CpG ODN (TLR9 agonist) | TLR9 activation of plasmacytoid DCs, IL-12 and IFN induction | Strong Th1 and Tfh | Enhances class switching & affinity maturation | anticipated from platform mechanism | JEV and WNV DNA vaccine studies | [84,85,86,87] |
| MPLA (TLR4 agonist) | NF-κB and TRIF signaling | Th1-skewed, synergizes with alum | High-avidity antibodies; enhanced Fc-mediated functions | wanes over 2–3 years | DENV and YF envelope antigen studies | [87,88,89] |
| QS-21 (saponin) | ISCOM formation; antigen cross-presentation | Balanced Th1/Th2; expanded Tfh | High-affinity, polyfunctional antibodies | Sustained long-term titers (≥3 years) | DENV and Matrix-M flavivirus platforms | [89,90,91] |
| STING agonists | Cytosolic DNA sensing; STING–IRF3 pathway | Strong Th1/Tfh; DC activation | Enhances affinity maturation & cross-neutralization | anticipated from platform mechanism | DENV and ZIKV preclinical vaccines | [92,93,94] |
| Lipid nanoparticle (LNP) | RNA sensing (TLR7/8); antigen expression | Th1-skewed; strong Tfh and GC | High-avidity antibodies; balanced subclass | anticipated from platform mechanism | mRNA ZIKV vaccines | [30,52,95,96] |
| Live-attenuated platform (self-adjuvanted) | Multifaceted innate sensing (RIG-I, TLR3, TLR7) | Balanced Th1/Tfh; strong GC | High-avidity, polyclonal antibodies | >20 years in clinical follow up | YF-17D, JE SA 14-14-2 | [43,97] |
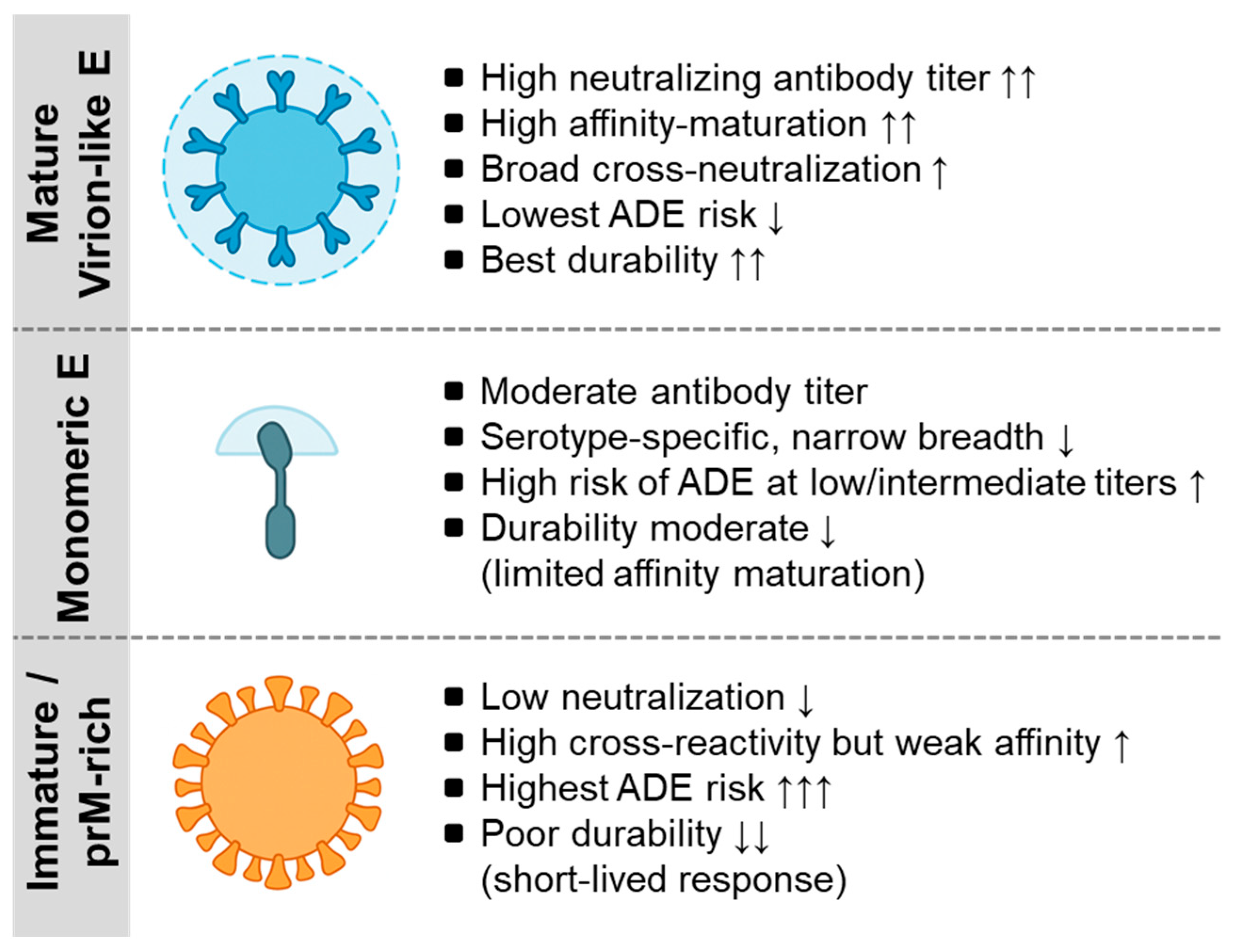
| Platform or Antigen Type | Structural or Mechanistic Features | Effect on Antibody Quality (Affinity/Breadth) | Durability Tier | Representative Flavivirus Context | Refs. |
|---|---|---|---|---|---|
| Native virion-like E (quaternary epitope preserving) | E dimers maintain authentic virion-like quaternary organization | Induces high-affinity, cross-neutralizing antibodies recognizing EDE and domain III | >20 years in clinical follow up | Dengue and Zika native E dimer constructs | [98,99,100] |
| Prefusion-stabilized E protein mutants | Engineered mutations lock E in prefusion conformation | Focuses B-cell responses on neutralizing epitopes; reduces non-neutralizing reactivity | ≥4 years in clinical trials so far | Stabilized DENV and JEV E proteins | [98,101,102] |
| VLPs | Multivalent epitope displays mimicking mature virion surface | Enhances BCR cross-linking and Tfh activation; increases antibody avidity | anticipated from platform mechanism | Zika and DENV VLP vaccine candidates | [103,104] |
| Live-attenuated platform | Limited replication provides prolonged antigen exposure & innate activation | Generates high-avidity polyclonal antibodies with road diversity | >20 years in clinical follow up | YF-17D, SA14-14-2 JE | [105,106] |
| Inactivated whole-virus vaccines | Chemical inactivation may distort E conformation | Produces serotype-specific antibodies with limited breadth | wanes over 2–3 years | IXIARO (JE), TBEV vaccines | [13,107,108] |
| Recombinant subunit (E monomer or domain III) | Soluble antigens present only monomeric epitopes | Elicits moderate titers; limited affinity maturation unless adjuvanted | anticipated from platform mechanism | Dengue E and domain III vaccines | [109,110,111] |
| mRNA platform | Sustained intracellular antigen expression in LNPs; innate RNA sensing | Drives robust GC reactions and high-avidity antibody production | ≥4 years in clinical trials so far | mRNA Zika and dengue candidates | [52,112,113] |
| Viral vector platform (adenovirus, measles, VSV) | Intracellular expression of E proteins mimicking infection | Balanced humoral and T-cell responses; strong neutralization | ≥4 years in clinical trials so far | Adenoviral DENV and measles-ZIKV constructs | [114,115,116] |
| saRNA/replicon systems | RNA replication prolongs antigen expression at low doses | Enhances GC responses and cross-serotype breadth | anticipated from platform mechanism | saRNA dengue and Zika vaccine candidates | [117,118,119] |
| Nanoparticle scaffolds or polymer-encapsulated antigens | Multimerized epitope display; sustained release | Enhances BCR cross-linking and Tfh responses | anticipated from platform mechanism | Flavivirus VLP or polymeric constructs | [120,121] |
| Phenomenon | Prior Exposure or Baseline State | Dominant Antibody Features | Observed Outcome | Vaccine or Epidemiologic Context | Refs. |
|---|---|---|---|---|---|
| Cross neutralization through quaternary epitope recognition | Prior dengue infection or vaccination with antigens that preserve E dimer geometry | Broadly neutralizing antibodies targeting the envelope dimer epitope with high avidity | Protection across dengue serotypes and partial Zika cross protection | Human monoclonal antibody and structural studies (mechanistic inference) | [26,27,158] |
| Enhancement at intermediate antibody titers | Previous dengue infection with waning or incomplete neutralizing titers | Cross reactive antibodies with strong Fc gamma receptor binding but low neutralization potency | Increased risk of severe dengue during reinfection | Prospective Nicaraguan pediatric cohort (observational serology) | [23,159] |
| Dengue to Zika cross reactivity | Prior dengue exposure before Zika introduction | Antibodies recognizing Zika envelope epitopes with variable neutralization | In vitro enhancement and partial protection in vivo | Human sera from 2016 Zika epidemic (observational serology) | [148,158] |
| Immune imprinting shaping vaccine outcomes | Seronegative or monotypic dengue infection at baseline | Recall of original epitope hierarchy with limited breadth after vaccination | Reduced efficacy and higher hospitalization risk in seronegative vaccinees | CYD-TDV Phase 3 trials (Phase 3 clinical data) | [152,160,161] |
| Post Zika increase in dengue severity | Zika infection followed by dengue exposure | Cross reactive antibodies with altered Fc function | Higher incidence of severe dengue following Zika epidemics | Population-level analyses in Central America (observational epidemiologic evidence) | [153,162] |
| Mitigation through antigen focus and enhanced maturation | Use of structurally stabilized E antigens and potent adjuvants | High avidity neutralizing antibodies with reduced non neutralizing responses | Broader and safer protection in preclinical and early clinical studies | Dengue and Zika subunit or mRNA vaccine development (preclinical and early phase clinical data) | [98,163,164,165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-Y.; Lee, H.-M. Optimizing Humoral Immunity for Durable and Broad Protection in Flavivirus Vaccines. Vaccines 2025, 13, 1182. https://doi.org/10.3390/vaccines13121182
Park J-Y, Lee H-M. Optimizing Humoral Immunity for Durable and Broad Protection in Flavivirus Vaccines. Vaccines. 2025; 13(12):1182. https://doi.org/10.3390/vaccines13121182
Chicago/Turabian StylePark, Jae-Yeon, and Hye-Mi Lee. 2025. "Optimizing Humoral Immunity for Durable and Broad Protection in Flavivirus Vaccines" Vaccines 13, no. 12: 1182. https://doi.org/10.3390/vaccines13121182
APA StylePark, J.-Y., & Lee, H.-M. (2025). Optimizing Humoral Immunity for Durable and Broad Protection in Flavivirus Vaccines. Vaccines, 13(12), 1182. https://doi.org/10.3390/vaccines13121182






