BCGitis and BCGosis: Clinical Spectrum, Immunological Mechanisms, and Risk Management
Abstract
1. Introduction
2. The Clinical Spectrum of BCG-Associated Diseases
2.1. Normal Vaccination Response and Minor Local Reactions
2.2. Local Complications
2.2.1. Suppurative Lymphadenitis
2.2.2. Cutaneous Complications
2.3. Regional and Systemic Disease
2.3.1. Osteitis/Osteomyelitis
2.3.2. Disseminated BCG Disease (BCGosis)
3. Epidemiology and Risk Factors
3.1. Host-Related Risk Factors
3.1.1. Inborn Errors of Immunity (IEI)
3.1.2. Acquired Immunodeficiency
3.2. Vaccine-Related Factors
3.2.1. BCG Strain Variability
3.2.2. Dose and Viability
3.3. Technical and Operational Factors
| WHO Region/Country | BCG Strain Used | Suppurative Lymphadenitis (%) | Osteitis (Per 100,000) | Disseminated BCGosis (Per Million) | Primary Notes |
|---|---|---|---|---|---|
| European Region | |||||
| Scandinavia | Gothenburg/Danish 1331 | 0.5–2.0 | 20–40 | 0.5–1.0 | High osteitis rates linked to strain |
| United Kingdom | BCG Danish 1331 | 0.2–1.5 | <1 | 0.1–0.5 | Selective vaccination policy |
| Western Pacific | |||||
| Japan | Tokyo 172 | 0.1–0.5 | 0.1–0.5 | 0.1–0.3 | Lower reactogenicity profile |
| South Korea | Tokyo 172 | 0.2–0.8 | 0.2–0.8 | 0.2–0.6 | |
| South-East Asia | |||||
| India | Russian BCG-I, Danish 1331 | 0.5–2.0 | <1 | 0.5–2.0 | Higher in settings without NBS for SCID |
| Thailand | Tokyo 172 | 0.3–1.0 | <1 | 0.3–1.0 | |
| African Region | |||||
| South Africa | BCG Danish 1331 | 0.5–2.0 | <1 | 1.0–5.0 | Impact of HIV co-infection |
| Kenya | Russian BCG-I | 0.5–2.5 | <1 | 1.0–4.0 | |
| Region of the Americas | |||||
| Brazil | Moreau RDJ | 0.2–1.0 | <1 | 0.2–1.0 | Universal vaccination |
| Argentina | Danish 1331 | 0.3–1.2 | <1 | 0.3–1.2 |
4. Immunopathogenesis
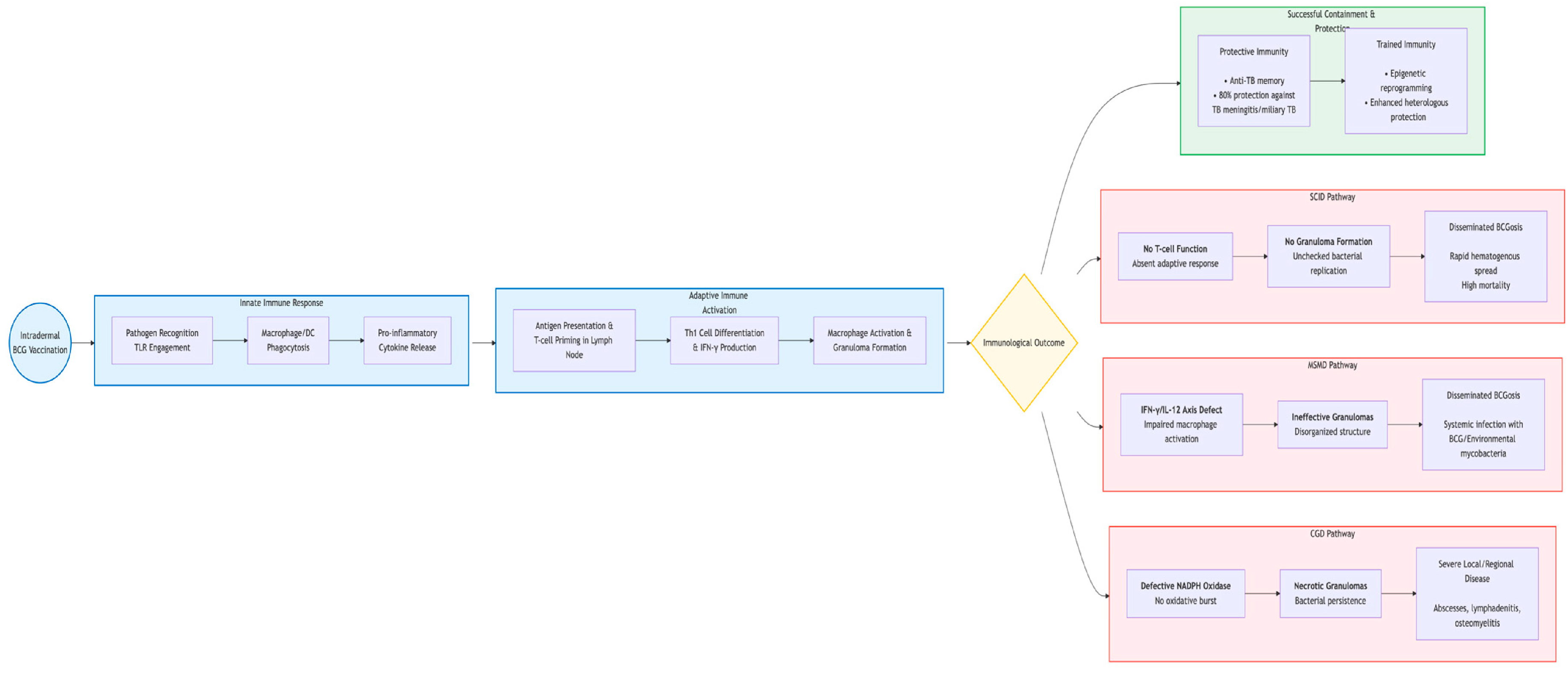
4.1. The Normal Immune Response to BCG: A Model of Successful Containment
4.2. Trained Immunity and Epigenetic Reprogramming
4.3. Mechanisms of Pathogenesis: The Breakdown of Containment
4.3.1. Defective Granuloma Formation and Function
4.3.2. Impaired T Cell and Macrophage Activation
4.3.3. The Role of “Atonic” or “Overactive” Immunity
5. Diagnosis, Treatment and Management
5.1. Diagnostic Challenges and Approach
5.1.1. Clinical and Histopathological Clues
5.1.2. Microbiological Confirmation: The Role of Molecular Tools
5.2. Treatment Strategies
5.3. Adverse Event Monitoring and Reporting
6. Risk–Benefit Assessment and Prevention Strategies
6.1. Global Perspective on Risk–Benefit Analysis
6.2. Core Prevention Strategy
6.2.1. Medical History Assessment
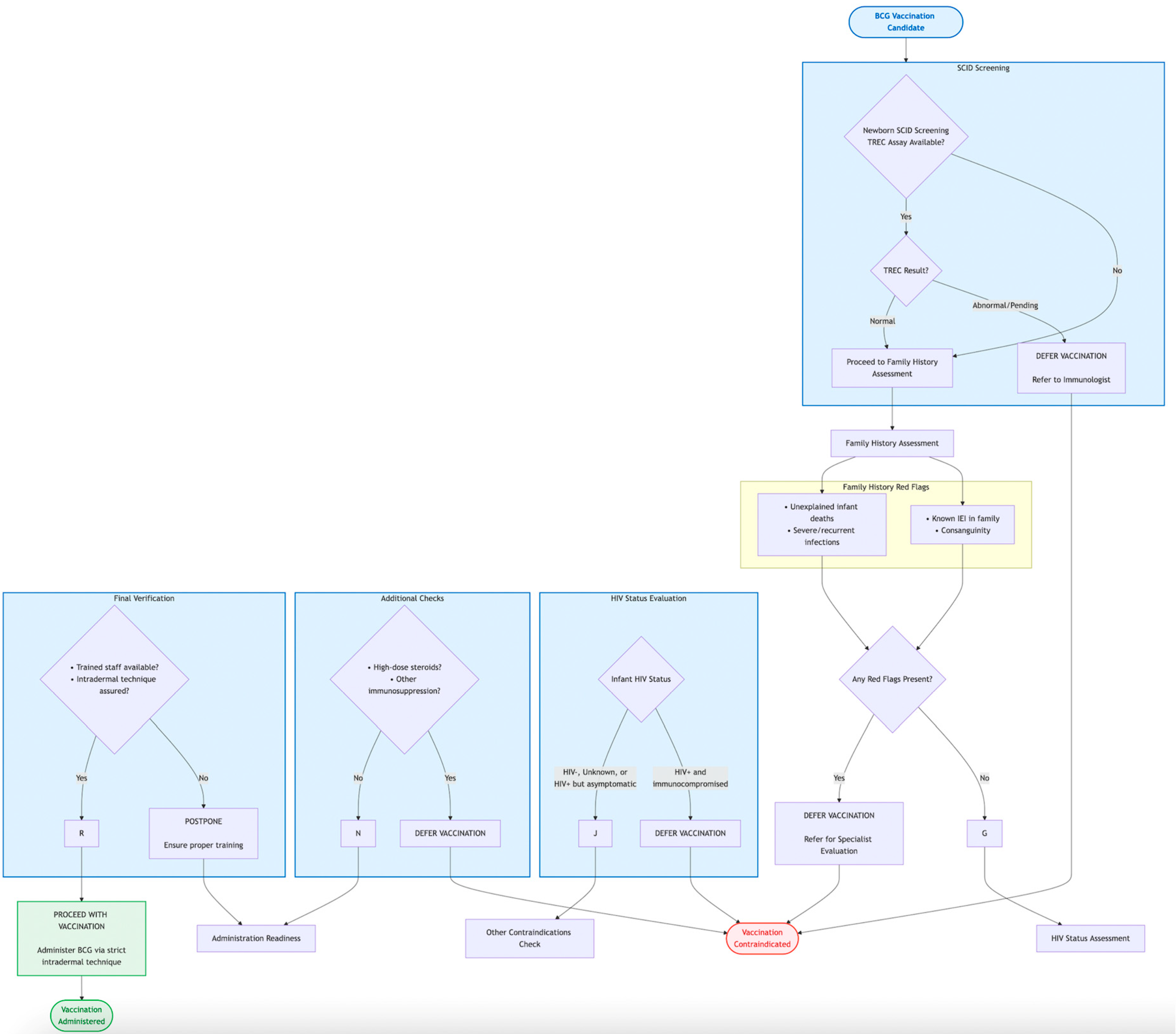
6.2.2. The Pivotal Role of Newborn Screening (NBS) for Severe Combined Immunodeficiency (SCID)
6.2.3. HIV Status Determination
6.2.4. The BCG-RAKE Checklist
6.3. Future Directions: Next-Generation Vaccines
7. Conclusions and Future Perspectives
- Global Expansion of Newborn Screening: A major public health effort is needed to make SCID screening accessible and routine in all countries that employ universal BCG vaccination. This will require overcoming significant economic and infrastructural barriers, especially in low- and middle-income countries (LMICs) but promises to virtually eliminate the deadliest complication of the vaccine. Strengthened Pre-Vaccination Screening: In the immediate term, enhancing the systematic use of detailed family history questionnaires, as operationalized by the BCG-RAKE checklist, provides a viable and critical strategy to identify at-risk infants in all resource settings.
- Advancements in Diagnostic Tools: The development and deployment of rapid, low-cost molecular point-of-care tests to differentiate BCG from Mtb complex will expedite diagnosis and ensure appropriate management of adverse events, preventing unnecessary anti-TB treatment.
- The Pursuit of a Safer, More Effective Successor: The long-term solution lies in the development and deployment of next-generation TB vaccines. Promising candidates, such as protein-adjuvant vaccines (e.g., M72/AS01E) or recombinant viral vectors, offer the potential for superior efficacy against pulmonary TB in adults without the risk of dissemination associated with live vaccines. The gradual replacement of BCG with a safer and more effective alternative remains the ultimate goal of TB vaccinology.
- Deepening Immunological Understanding: Ongoing research into the human genetics of mycobacterial susceptibility will continue to uncover novel immunodeficiency syndromes, further refining our understanding of risk and allowing for ever-more precise screening protocols.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lange, C.; Aaby, P.; Behr, M.A.; Donald, P.R.; Kaufmann, S.H.E.; Netea, M.G.; Mandalakas, A.M. 100 Years of Mycobacterium bovis Bacille Calmette-Guérin. Lancet Infect. Dis. 2022, 22, e2–e12. [Google Scholar] [CrossRef]
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.-C.; Croda, J.; Hill, P.C.; et al. Infant BCG Vaccination and Risk of Pulmonary and Extrapulmonary Tuberculosis throughout the Life Course: A Systematic Review and Individual Participant Data Meta-Analysis. Lancet Glob. Health 2022, 10, e1307–e1316. [Google Scholar] [CrossRef]
- World Health Organization. BCG Vaccine: WHO Position Paper, February 2018—Recommendations. Vaccine 2018, 36, 3408–3410. [Google Scholar] [CrossRef] [PubMed]
- Moorlag, S.J.C.F.M.; Folkman, L.; Ter Horst, R.; Krausgruber, T.; Barreca, D.; Schuster, L.C.; Fife, V.; Matzaraki, V.; Li, W.; Reichl, S.; et al. Multi-Omics Analysis of Innate and Adaptive Responses to BCG Vaccination Reveals Epigenetic Cell States That Predict Trained Immunity. Immunity 2024, 57, 171–187.e14. [Google Scholar] [CrossRef] [PubMed]
- Logan, N.; Gill, C.; Dolan, L.; Lynch, T.; McLaughlin, A.M. Disseminated BCGosis Following Systemic Absorption of Mycobacterium bovis. Ir. Med. J. 2022, 115, 641. [Google Scholar]
- Cocchi, N.; Jacobsen, E.-M.; Hoenig, M.; Schulz, A.; Schuetz, C. BCG Disease in SCID: Three Decades of Experience in a Pediatric Transplant Center. J. Clin. Immunol. 2022, 42, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Bravo, R.; Villaseñor, T.; Pedraza-Escalona, M.; Pérez-Martínez, L.; Hernández-Pando, R.; Pedraza-Alva, G. Negative Regulation of Autophagy during Macrophage Infection by Mycobacterium bovis BCG via Protein Kinase C Activation. Int. J. Mol. Sci. 2024, 25, 3145. [Google Scholar] [CrossRef]
- Ren, H.; He, J.; Dong, J.; Jiang, G.; Hao, J.; Han, L. Specific BCG-Related Gene Expression Levels Correlate with Immune Cell Infiltration and Prognosis in Melanoma. J. Leukoc. Biol. 2024, 117, qiae064. [Google Scholar] [CrossRef]
- Korppi, M.; Teräsjärvi, J.; Liehu-Martiskainen, M.; Barkoff, A.-M.; Lauhkonen, E.; Huhtala, H.; Pöyhönen, L.; Nuolivirta, K.; He, Q. Interleukin 17F Gene Variations Showed No Association with BCG Osteitis Risk after Newborn Vaccination. Acta Paediatr. 2021, 110, 618–623. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef]
- Khan, N.; Downey, J.; Sanz, J.; Kaufmann, E.; Blankenhaus, B.; Pacis, A.; Pernet, E.; Ahmed, E.; Cardoso, S.; Nijnik, A.; et al. M. tuberculosis Reprograms Hematopoietic Stem Cells to Limit Myelopoiesis and Impair Trained Immunity. Cell 2020, 183, 752–770.e22. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Vaseghi-Shanjani, M.; Afkhami, S.; Grondin, J.A.; Kang, A.; D’Agostino, M.R.; Yao, Y.; Jain, S.; Zganiacz, A.; Kroezen, Z.; et al. Parenteral BCG Vaccine Induces Lung-Resident Memory Macrophages and Trained Immunity via the Gut-Lung Axis. Nat. Immunol. 2022, 23, 1687–1702. [Google Scholar] [CrossRef]
- GBD 2021 Demographics Collaborators. Global Age-Sex-Specific Mortality, Life Expectancy, and Population Estimates in 204 Countries and Territories and 811 Subnational Locations, 1950–2021, and the Impact of the COVID-19 Pandemic: A Comprehensive Demographic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 1989–2056. [Google Scholar] [CrossRef]
- Laberko, A.; Yukhacheva, D.; Kan, N.; Roppelt, A.; Mukhina, A.; Rodina, Y.; Pershin, D.; Cheng, A.; Lionakis, M.S.; Solopova, G.; et al. BCG Infection in Patients With Inborn Errors of Immunity Receiving the Russian BCG Strain. J. Allergy Clin. Immunol. Pract. 2022, 10, 1797–1804.e7. [Google Scholar] [CrossRef]
- Sirak, A.; Tulu, B.; Bayissa, B.; Gumi, B.; Berg, S.; Salguero, F.J.; Ameni, G. ETHICOBOTS Consortium Cellular and Cytokine Responses in Lymph Node Granulomas of Bacillus Calmette Guérin (BCG)-Vaccinated and Non-Vaccinated Cross-Breed Calves Naturally Infected With Mycobacterium bovis. Front. Vet. Sci. 2021, 8, 698800. [Google Scholar] [CrossRef]
- Le Naour, S.; Boyer, J.; Malard, O.; Guillouzouic, A.; Aubry, A.; Launay, E.; Barbarot, S. Cervicofacial nontuberculous mycobacteria in children: Clinical, microbiological and therapeutic features. A retrospective study and literature review. Ann. Dermatol. Venereol. 2020, 147, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Jakhar, D.; Mehta, S.; Singal, A. Cutaneous Tuberculosis. Part II: Complications, Diagnostic Workup, Histopathologic Features, and Treatment. J. Am. Acad. Dermatol. 2023, 89, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Dolezalova, K.; Strachan, T.; Matej, R.; Ricna, D.; Bloomfield, M. Manifestations of Cutaneous Mycobacterial Infections in Patients with Inborn Errors of IL-12/IL-23-IFNγ Immunity. Eur. J. Dermatol. 2022, 32, 495–504. [Google Scholar] [CrossRef]
- Korppi, M. Bacille Calmette-Guérin Osteitis After Newborn Vaccination. Pediatr. Infect. Dis. J. 2021, 40, e170. [Google Scholar] [CrossRef]
- Korppi, M. The Sixty-Year Story of Finnish Bacillus Calmette-Guérin (BCG) Osteitis. Acta Paediatr. 2021, 110, 1119–1124. [Google Scholar] [CrossRef]
- Okuno, H.; Satoh, H.; Morino, S.; Arai, S.; Ochiai, M.; Fujita, K.; Naito, S.; Kato, A.; Ishii, K.; Oishi, K.; et al. Characteristics and Incidence of Vaccine Adverse Events after Bacille Calmette-Guérin Vaccination: A National Surveillance Study in Japan from 2013 to 2017. Vaccine 2022, 40, 4922–4928. [Google Scholar] [CrossRef]
- Yang, H.; Hu, Z.; Zhang, J.; Lowrie, D.B.; Liu, T.-F.; Fan, X.-Y.; Lu, S.-H. Disseminated BCG Disease with Defective Immune Metabolism Caused by Protein Kinase C-Delta Deficiency. J. Allergy Clin. Immunol. Pract. 2022, 10, 3333–3335.e1. [Google Scholar] [CrossRef]
- Urdinez, L.; Goris, V.; Danielian, S.; Oleastro, M. Garrahan Hospital Consortium Disseminated BCG Disease in a Patient with Hyper IgE Syndrome Due to Dominant-Negative STAT3 Mutation-Case Report. J. Clin. Immunol. 2023, 43, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Mahdaviani, S.A.; Fallahi, M.; Jamee, M.; Marjani, M.; Tabarsi, P.; Moniri, A.; Farnia, P.; Daneshmandi, Z.; Parvaneh, N.; Casanova, J.-L.; et al. Effective Anti-Mycobacterial Treatment for BCG Disease in Patients with Mendelian Susceptibility to Mycobacterial Disease (MSMD): A Case Series. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 8. [Google Scholar] [CrossRef]
- Villanueva, P.; Wadia, U.; Crawford, N.; Messina, N.L.; Kollmann, T.R.; Lucas, M.; Manning, L.; Richmond, P.; Pittet, L.F.; Curtis, N. Revaccination with Bacille Calmette-Guérin (BCG) Is Associated with an Increased Risk of Abscess and Lymphadenopathy. NPJ Vaccines 2022, 7, 6. [Google Scholar] [CrossRef]
- Grabe-Heyne, K.; Henne, C.; Odeyemi, I.; Pöhlmann, J.; Ahmed, W.; Pollock, R.F. Evaluating the Cost-Utility of Intravesical Bacillus Calmette-Guérin versus Radical Cystectomy in Patients with High-Risk Non-Muscle-Invasive Bladder Cancer in the UK. J. Med. Econ. 2023, 26, 411–421. [Google Scholar] [CrossRef]
- Li, W.; Moorlag, S.J.C.F.M.; Koeken, V.A.C.M.; Röring, R.J.; de Bree, L.C.J.; Mourits, V.P.; Gupta, M.K.; Zhang, B.; Fu, J.; Zhang, Z.; et al. A Single-Cell View on Host Immune Transcriptional Response to in Vivo BCG-Induced Trained Immunity. Cell Rep. 2023, 42, 112487. [Google Scholar] [CrossRef] [PubMed]
- Lyra, P.T.; Souza, E.; Moura, A.C.A.; Matta, M.C.; Torres, L.C.; Coelho, A.V.C.; Rocha, M.Â.W.; Arraes, L.; Oliveira, J.B. Inborn Errors of Immunity in Patients with Adverse Events Following BCG Vaccination in Brazil. J. Clin. Immunol. 2022, 42, 1708–1720. [Google Scholar] [CrossRef]
- Yadav, R.M.; Dalvi, A.; Gupta, M.; Bargir, U.A.; Shabrish, S.; Aluri, J.; Kulkarni, M.; Hule, G.; Kambli, P.; Setia, P.; et al. Spectrum of Inborn Errors of Immunity in a Cohort of 90 Patients Presenting with Complications to BCG Vaccination in India. Scand. J. Immunol. 2021, 93, e13010. [Google Scholar] [CrossRef] [PubMed]
- Vicuña, A.K.P.; Nakashimada, M.Y.; Lara, X.L.; Flores, E.M.; Núñez, M.E.N.; Lona-Reyes, J.C.; Nieto, L.H.; Vázquez, M.G.R.; Santos, J.B.; Iñiguez, Á.L.; et al. Mendelian Susceptibility to Mycobacterial Disease: Retrospective Clinical and Genetic Study in Mexico. J. Clin. Immunol. 2023, 43, 123–135. [Google Scholar] [CrossRef]
- Yao, Q.; Zhou, Q.-H.; Shen, Q.-L.; Wang, X.-C.; Hu, X.-H. Imaging Characteristics of Pulmonary BCG/TB Infection in Patients with Chronic Granulomatous Disease. Sci. Rep. 2022, 12, 11765. [Google Scholar] [CrossRef]
- León-Lara, X.; Pérez-Blanco, U.; Yamazaki-Nakashimada, M.A.; Bustamante-Ogando, J.C.; Aguilar-Gómez, N.; Cristerna-Tarrasa, H.; Staines-Boone, A.-T.; Saucedo-Ramírez, O.J.; Fregoso-Zuñiga, E.; Macías-Robles, A.-P.; et al. Description of BCG and Tuberculosis Disease in a Cohort of 79 Patients with Chronic Granulomatous Disease. J. Clin. Immunol. 2024, 44, 171. [Google Scholar] [CrossRef]
- Jauro, S.; Larson, E.C.; Gleim, J.L.; Wahlberg, B.M.; Rodgers, M.A.; Chehab, J.C.; Lopez-Velazques, A.E.; Ameel, C.L.; Tomko, J.A.; Sakal, J.L.; et al. Intravenous Bacillus Calmette-Guérin (BCG) Induces a More Potent Airway and Lung Immune Response than Intradermal BCG in Simian Immunodeficiency Virus-Infected Macaques. J. Immunol. 2024, 213, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Gies, V.; Dieudonné, Y.; Morel, F.; Sougakoff, W.; Carapito, R.; Martin, A.; Weingertner, N.; Jacquel, L.; Hubele, F.; Kuhnert, C.; et al. Case Report: Acquired Disseminated BCG in the Context of a Delayed Immune Reconstitution After Hematological Malignancy. Front. Immunol. 2021, 12, 696268. [Google Scholar] [CrossRef]
- Colomba, C.; Rubino, R.; Mantia, G.; Guida Marascia, F.; Abbott, M.; Gizzi, A.; Anastasia, A.; Palermo, G.; Tolomeo, M.; Cascio, A. Clinical Use of BCG and Its Complications: A Case Series. Infez. Med. 2021, 29, 123–129. [Google Scholar]
- Kato, S.; Shida, H.; Okamura, T.; Zhang, X.; Miura, T.; Mukai, T.; Inoue, M.; Shu, T.; Naruse, T.K.; Kimura, A.; et al. CD8 T Cells Show Protection against Highly Pathogenic Simian Immunodeficiency Virus (SIV) after Vaccination with SIV Gene-Expressing BCG Prime and Vaccinia Virus/Sendai Virus Vector Boosts. J. Virol. 2021, 95, e01718-20. [Google Scholar] [CrossRef] [PubMed]
- Nadolinskaia, N.I.; Kotliarova, M.S.; Goncharenko, A.V. Fighting Tuberculosis: In Search of a BCG Replacement. Microorganisms 2022, 11, 51. [Google Scholar] [CrossRef]
- Satti, I.; Marshall, J.L.; Harris, S.A.; Wittenberg, R.; Tanner, R.; Lopez Ramon, R.; Wilkie, M.; Ramos Lopez, F.; Riste, M.; Wright, D.; et al. Safety of a Controlled Human Infection Model of Tuberculosis with Aerosolised, Live-Attenuated Mycobacterium bovis BCG versus Intradermal BCG in BCG-Naive Adults in the UK: A Dose-Escalation, Randomised, Controlled, Phase 1 Trial. Lancet Infect. Dis. 2024, 24, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Subbian, S.; Singh, P.; Kolloli, A.; Nemes, E.; Scriba, T.; Hanekom, W.A.; Kaplan, G. BCG Vaccination of Infants Confers Mycobacterium tuberculosis Strain-Specific Immune Responses by Leukocytes. ACS Infect. Dis. 2020, 6, 3141–3146. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; Bivalacqua, T.J.; Montgomery, J.S.; Lerner, S.P.; Busby, J.E.; et al. Intravesical Nadofaragene Firadenovec Gene Therapy for BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer: A Single-Arm, Open-Label, Repeat-Dose Clinical Trial. Lancet Oncol. 2021, 22, 107–117. [Google Scholar] [CrossRef]
- Schmidt, A.C.; Fairlie, L.; Hellström, E.; Luabeya Kany Kany, A.; Middelkoop, K.; Naidoo, K.; Nair, G.; Gela, A.; Nemes, E.; Scriba, T.J.; et al. BCG Revaccination for the Prevention of Mycobacterium tuberculosis Infection. N. Engl. J. Med. 2025, 392, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Ying, W.; Wang, W.; Hou, J.; Liu, L.; Sun, B.; Hui, X.; Gu, Y.; Song, X.; Wang, X.; et al. Clinical and Genetic Characteristics of BCG Disease in Chinese Children: A Retrospective Study. J. Clin. Immunol. 2023, 43, 756–768. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, A.M. Immunopathogenesis of Pulmonary Tuberculosis. Hosp. Pract. (Off. Ed.) 1993, 28, 51–58. [Google Scholar] [CrossRef]
- Sulavik, S.B. Bronchocentric Granulomatosis and Allergic Bronchopulmonary Aspergillosis. Clin. Chest Med. 1988, 9, 609–621. [Google Scholar] [CrossRef]
- Collins, F.M. Cellular Antimicrobial Immunity. CRC Crit. Rev. Microbiol. 1978, 7, 27–91. [Google Scholar] [CrossRef]
- Shariq, M.; Quadir, N.; Alam, A.; Zarin, S.; Sheikh, J.A.; Sharma, N.; Samal, J.; Ahmad, U.; Kumari, I.; Hasnain, S.E.; et al. The Exploitation of Host Autophagy and Ubiquitin Machinery by Mycobacterium Tuberculosis in Shaping Immune Responses and Host Defense during Infection. Autophagy 2023, 19, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Strandgaard, T.; Nordentoft, I.; Birkenkamp-Demtröder, K.; Salminen, L.; Prip, F.; Rasmussen, J.; Andreasen, T.G.; Lindskrog, S.V.; Christensen, E.; Lamy, P.; et al. Field Cancerization Is Associated with Tumor Development, T-Cell Exhaustion, and Clinical Outcomes in Bladder Cancer. Eur. Urol. 2024, 85, 82–92. [Google Scholar] [CrossRef]
- Sartono, E.; Lisse, I.M.; Terveer, E.M.; van de Sande, P.J.M.; Whittle, H.; Fisker, A.B.; Roth, A.; Aaby, P.; Yazdanbakhsh, M.; Benn, C.S. Oral Polio Vaccine Influences the Immune Response to BCG Vaccination. A Natural Experiment. PLoS ONE 2010, 5, e10328. [Google Scholar] [CrossRef] [PubMed]
- Scordo, J.M.; Piergallini, T.J.; Reuter, N.; Headley, C.A.; Hodara, V.L.; Gonzalez, O.; Giavedoni, L.D.; Papin, J.F.; Turner, J. Local Immune Responses to Tuberculin Skin Challenge in Mycobacterium bovis BCG-Vaccinated Baboons: A Pilot Study of Younger and Older Animals. Immun. Ageing 2021, 18, 16. [Google Scholar] [CrossRef]
- Gunasena, M.; Shukla, R.K.; Yao, N.; Rosas Mejia, O.; Powell, M.D.; Oestreich, K.J.; Aceves-Sánchez, M.d.J.; Flores-Valdez, M.A.; Liyanage, N.P.M.; Robinson, R.T. Evaluation of Early Innate and Adaptive Immune Responses to the TB Vaccine Mycobacterium bovis BCG and Vaccine Candidate BCGΔBCG1419c. Sci. Rep. 2022, 12, 12377. [Google Scholar] [CrossRef]
- Rytkönen, J.; Karttunen, T.J.; Karttunen, R.; Valkonen, K.H.; Björkstén, B.; Kokkonen, J. BCG Vaccine Modulates Intestinal and Systemic Response to Beta-Lactoglobulin. Pediatr. Allergy Immunol. 2004, 15, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The Mechanism of Action of BCG Therapy for Bladder Cancer—A Current Perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gennery, A.R.; Worth, A. Severe Combined Immunodeficiency: Newborn Screening and the BCG Vaccination. Arch. Dis. Child. 2022, 107, 955. [Google Scholar] [CrossRef]
- Ben Belgacem, H.; Bouguila, J.; Tej, A.; Tilouche, S.; Kebaili, R.; Kahloul, N.; Barbouche, M.R.; Soyah, N.; Boughammoura, L. Disseminated BCG Infection Revealing a Severe Combined Immunodeficiency: A Case Report. Tunis. Med. 2022, 100, 881–886. [Google Scholar] [PubMed]
- Laberko, A.; Yukhacheva, D.; Rodina, Y.; Abramov, D.; Konovalov, D.; Radygina, S.; Shelikhova, L.; Pershin, D.; Kadnikova, O.; Maschan, M.; et al. BCG-Related Inflammatory Syndromes in Severe Combined Immunodeficiency After TCRαβ+/CD19+ Depleted HSCT. J. Clin. Immunol. 2020, 40, 625–636. [Google Scholar] [CrossRef]
- Sotomayor, F.C.; Palma, B.J.; Comité Asesor en Vacunas y Estrategias de Vacunación. BCG Vaccine, Primary Immunodeficiencies and Severe Combined Immunodeficiency. Rev. Chil. Pediatr. 2020, 91, 648. [Google Scholar]
- Barkai, G.; Somech, R.; Stauber, T.; Barziali, A.; Greenberger, S. Bacille Calmette-Guerin (BCG) Complications in Children with Severe Combined Immunodeficiency (SCID). Infect. Dis. 2019, 51, 585–592. [Google Scholar] [CrossRef]
- Marciano, B.E.; Huang, C.-Y.; Joshi, G.; Rezaei, N.; Carvalho, B.C.; Allwood, Z.; Ikinciogullari, A.; Reda, S.M.; Gennery, A.; Thon, V.; et al. BCG Vaccination in Patients with Severe Combined Immunodeficiency: Complications, Risks, and Vaccination Policies. J. Allergy Clin. Immunol. 2014, 133, 1134–1141. [Google Scholar] [CrossRef]
- Zou, T.-T.; Liao, Q.; Liu, Y.; Guo, Q.; Zhu, Y.; Wan, C.-M. Rapid Diagnosis of Hemophagocytic Lymphohistiocytosis Triggered by Disseminated BCG Infection in Infants With Severe Combined Immunodeficiency: Case Report. Open Forum Infect. Dis. 2023, 10, ofad548. [Google Scholar] [CrossRef]
- Botaro, M.H.; E Silva, J.M.; Jamra, S.R.A.; Geraldino, S.Z.; Roxo-Junior, P. BCG Vaccination in Children with Severe Combined Immunodeficiency in a Tertiary Center: Evaluation of Complications and Risks. J. Pediatr. 2025, 101, 224–230. [Google Scholar] [CrossRef]
- Dutt, T.S.; Karger, B.R.; Fox, A.; Youssef, N.; Dadhwal, R.; Ali, M.Z.; Patterson, J.; Creissen, E.; Rampacci, E.; Cooper, S.K.; et al. Mucosal Exposure to Non-Tuberculous Mycobacteria Elicits B Cell-Mediated Immunity against Pulmonary Tuberculosis. Cell Rep. 2022, 41, 111783. [Google Scholar] [CrossRef]
- Shah, J.A.; Lindestam Arlehamn, C.S.; Horne, D.J.; Sette, A.; Hawn, T.R. Nontuberculous mycobacteria and Heterologous Immunity to Tuberculosis. J. Infect. Dis. 2019, 220, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Kumari, A.; Das, G.; Dwivedi, V.P. Tuberculosis Vaccine: A Journey from BCG to Present. Life Sci. 2020, 252, 117594. [Google Scholar] [CrossRef]
- Bonenberger, T.E.; Ihrke, P.J.; Naydan, D.K.; Affolter, V.K. Rapid Identification of Tissue Micro-Organisms in Skin Biopsy Specimens from Domestic Animals Using Polyclonal BCG Antibody. Vet. Dermatol. 2001, 12, 41–47. [Google Scholar] [CrossRef] [PubMed]
- de Jong, F.C.; Laajala, T.D.; Hoedemaeker, R.F.; Jordan, K.R.; van der Made, A.C.J.; Boevé, E.R.; van der Schoot, D.K.E.; Nieuwkamer, B.; Janssen, E.A.M.; Mahmoudi, T.; et al. Non-Muscle-Invasive Bladder Cancer Molecular Subtypes Predict Differential Response to Intravesical Bacillus Calmette-Guérin. Sci. Transl. Med. 2023, 15, eabn4118. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Simmons, A.M.; Rowneki, M.; Parmar, H.; Cao, Y.; Ryan, J.; Banada, P.P.; Deshpande, S.; Shenai, S.; Gall, A.; et al. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. mBio 2017, 8, e00812-17. [Google Scholar] [CrossRef]
- Kowalewicz-Kulbat, M.; Locht, C. BCG and Protection against Inflammatory and Auto-Immune Diseases. Expert Rev. Vaccines 2017, 16, 699–708. [Google Scholar] [CrossRef]
- Messina, N.L.; Pittet, L.F.; McDonald, E.; Moore, C.; Barry, S.; Bonten, M.; Byrne, A.; Campbell, J.; Croda, J.; Croda, M.G.; et al. BCG Vaccination of Healthcare Workers for Protection against COVID-19: 12-Month Outcomes from an International Randomised Controlled Trial. J. Infect. 2024, 89, 106245. [Google Scholar] [CrossRef]
- Dutto, D.; Livoti, S.; Soria, F.; Gontero, P. Developments in Conservative Treatment for BCG-Unresponsive Non-Muscle Invasive Bladder Cancer. Expert Opin. Pharmacother. 2024, 25, 1335–1348. [Google Scholar] [CrossRef]
- Lidagoster, S.; Ben-David, R.; De Leon, B.; Sfakianos, J.P. BCG and Alternative Therapies to BCG Therapy for Non-Muscle-Invasive Bladder Cancer. Curr. Oncol. 2024, 31, 1063–1078. [Google Scholar] [CrossRef]
- Savchenko, E.; Rosenfeld, A.; Bunimovich-Mendrazitsky, S. Mathematical Modeling of BCG-Based Bladder Cancer Treatment Using Socio-Demographics. Sci. Rep. 2023, 13, 18754. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.S.; Zafar, A.; Magdamo, C.; Chung, S.Y.; Chou, W.H.; Nayan, M.; Deodhar, M.; Frendl, D.M.; Feldman, A.S.; Faustman, D.L.; et al. Association of BCG Vaccine Treatment With Death and Dementia in Patients with Non-Muscle-Invasive Bladder Cancer. JAMA Netw. Open 2023, 6, e2314336. [Google Scholar] [CrossRef]
- Bonetto, C.; Trotta, F.; Felicetti, P.; Alarcón, G.S.; Santuccio, C.; Bachtiar, N.S.; Brauchli Pernus, Y.; Chandler, R.; Girolomoni, G.; Hadden, R.D.M.; et al. Vasculitis as an Adverse Event Following Immunization—Systematic Literature Review. Vaccine 2016, 34, 6641–6651. [Google Scholar] [CrossRef]
- Pérez-Jacoiste Asín, M.A.; Fernández-Ruiz, M.; López-Medrano, F.; Lumbreras, C.; Tejido, Á.; San Juan, R.; Arrebola-Pajares, A.; Lizasoain, M.; Prieto, S.; Aguado, J.M. Bacillus Calmette-Guérin (BCG) Infection Following Intravesical BCG Administration as Adjunctive Therapy for Bladder Cancer: Incidence, Risk Factors, and Outcome in a Single-Institution Series and Review of the Literature. Medicine 2014, 93, 236–254. [Google Scholar] [CrossRef]
- Martinez, L.; Cords, O.; Horsburgh, C.R.; Andrews, J.R.; Pediatric TB Contact Studies Consortium. The Risk of Tuberculosis in Children after Close Exposure: A Systematic Review and Individual-Participant Meta-Analysis. Lancet 2020, 395, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Cicione, A.; Lombardo, R.; Nacchia, A.; Franco, A.; Simone, G.; Pastore, A.; Leonardo, C.; Franco, G.; Tubaro, A.; DE Nunzio, C. No Clinical Benefit from Sequential Combination of Mitomycin C plus Bacillus Calmette-Guérin (BCG) than BCG Alone in the Adjuvant Treatment of High Risk Non Muscle Invasive Bladder Cancer: Result of a Planned Interim Analysis of a Prospective Randomized Trial. Minerva Urol. Nephrol. 2024, 76, 458–466. [Google Scholar] [CrossRef]
- Faust, L.; Schreiber, Y.; Bocking, N. A Systematic Review of BCG Vaccination Policies among High-Risk Groups in Low TB-Burden Countries: Implications for Vaccination Strategy in Canadian Indigenous Communities. BMC Public Health 2019, 19, 1504. [Google Scholar] [CrossRef]
- Brett, K.; Severn, M. Bacille Calmette-Guérin Vaccination: A Review of Clinical Effectiveness and Guidelines. In CADTH Rapid Response Reports; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. [Google Scholar]
- GBD 2023 Vaccine Coverage Collaborators. Global, Regional, and National Trends in Routine Childhood Vaccination Coverage from 1980 to 2023 with Forecasts to 2030: A Systematic Analysis for the Global Burden of Disease Study 2023. Lancet 2025, 406, 235–260. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Xu, Q.; Zhang, F.; Buckland, K.F.; Gao, Y.; Du, W.; Ding, Y.; Zhou, L.; Sun, X.; Ma, L.; et al. Preclinical Ex Vivo IL2RG Gene Therapy Using Autologous Hematopoietic Stem Cells as an Effective and Safe Treatment for X-Linked Severe Combined Immunodeficiency Disease. Genes Dis. 2025, 12, 101445. [Google Scholar] [CrossRef]
- Huda, M.N.; Ahmad, S.M.; Alam, M.J.; Khanam, A.; Afsar, M.N.A.; Wagatsuma, Y.; Raqib, R.; Stephensen, C.B.; Laugero, K.D. Infant Cortisol Stress-Response Is Associated with Thymic Function and Vaccine Response. Stress 2019, 22, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Faurholt-Jepsen, D.; Range, N.; Praygod, G.; Jeremiah, K.; Faurholt-Jepsen, M.; Aabye, M.G.; Grewal, H.M.S.; Changalucha, J.; Witte, D.R.; Andersen, A.B.; et al. BCG Protects against Tuberculosis Irrespective of HIV Status: A Matched Case-Control Study in Mwanza, Tanzania. Thorax 2013, 68, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, A.; Martinez, L.; Gupta, R.K.; Hamada, Y.; Ness, T.; Kay, A.; Bonnet, M.; Sester, M.; Kaufmann, S.H.E.; Lange, C.; et al. Tuberculosis Prevention: Current Strategies and Future Directions. Clin. Microbiol. Infect. 2024, 30, 1123–1130. [Google Scholar] [CrossRef]
- Ofori-Anyinam, B.; Kanuteh, F.; Agbla, S.C.; Adetifa, I.; Okoi, C.; Dolganov, G.; Schoolnik, G.; Secka, O.; Antonio, M.; de Jong, B.C.; et al. Impact of the Mycobaterium africanum West Africa 2 Lineage on TB Diagnostics in West Africa: Decreased Sensitivity of Rapid Identification Tests in The Gambia. PLoS Negl. Trop. Dis. 2016, 10, e0004801. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.E.; Kulkarni, P.S.; Shaligram, U.; Cotton, M.F.; Rentsch, C.A.; Eisele, B.; Grode, L.; Kaufmann, S.H.E. The Recombinant Bacille Calmette-Guérin Vaccine VPM1002: Ready for Clinical Efficacy Testing. Front. Immunol. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
| Clinical Entity | Typical Onset | Key Features | Estimated Incidence | Primary Risk Factor |
|---|---|---|---|---|
| Suppurative Lymphadenitis | 2–6 months | Fluctuant, ipsilateral lymph node (>1.5 cm), may fistulize | 0.1–1% | Infant age, vaccine strain, injection technique |
| Osteitis/Osteomyelitis | 6–24 months | Insidious onset, metaphysis of long bones, limp | 0.1–40/100,000 | Specific BCG strain (e.g., Gothenburg) |
| Disseminated BCGosis | 3–12 months | Systemic illness (fever, hepatosplenomegaly, pancytopenia) | 0.1–1.0/1,000,000 | Underlying severe immunodeficiency |
| Risk Factor Category | Specific Factor | Associated Adverse Event(s) | Preventive Action |
|---|---|---|---|
| Host-Related | SCID, MSMD, CGD | BCGosis | Absolute contraindication. Pre-vaccination screening (family history, NBS for SCID). |
| HIV/AIDS (symptomatic, immunocompromised) | Disseminated BCGosis, localized complications | Absolute contraindication. HIV testing of infant/mother prior to vaccination. | |
| Vaccine-Related | High-reactogenicity strain | Suppurative lymphadenitis | National program strain selection based on benefit-risk profile. |
| Overdose/High potency | Local and systemic complications | Adherence to good manufacturing and distribution practices. | |
| Technical | Subcutaneous/intramuscular injection | Abscess, severe lymphadenitis | Strict intradermal technique. Training of healthcare workers. |
| Young infant age (<3 months) | Suppurative lymphadenitis | Adherence to recommended immunization schedule |
| Clinical Condition | First-Line Treatment | Alternative/Adjunctive Options | Duration | Key Considerations |
|---|---|---|---|---|
| Suppurative Lymphadenitis | Aspiration; Observation | INH + RIF | 3–6 months (if medical therapy used) | Avoid incision & drainage. |
| Osteitis/Osteomyelitis | INH + RIF + (FQ or EMB) | Surgery for debridement/diagnosis | 9–12 months | Ensure PZA is NOT included. |
| Disseminated BCGosis | INH + RIF + FQ + (EMB or Macrolide) | Adjunctive IFN-γ (for MSMD); G-CSF (for CGD); HSCT (for SCID) | ≥9–12 months | Treat underlying immunodeficiency |
| Strategy | Target Group | Action | Expected Outcome |
|---|---|---|---|
| Newborn Screening (TREC assay) | All newborns in BCG-vaccinating countries | Defer BCG vaccination until SCID result is available. If positive, absolute contraindication. | Near-elimination of BCGosis in SCID patients. |
| HIV Testing | Infants born to HIV-positive mothers | Determine infant’s HIV status. Withhold BCG if infant is HIV+ and immunocompromised. | Prevention of BCGosis in HIV-infected infants. |
| Family History Review | All infants prior to vaccination | Defer vaccination if history suggests inherited immunodeficiency; refer for specialist evaluation. | Identification of at-risk infants beyond SCID (e.g., CGD, MSMD). |
| Healthcare Worker Training | Vaccinators | Education on: strict intradermal technique; recognizing contraindications; managing minor reactions. | Reduction in technical errors (e.g., abscesses) and inappropriate administration. |
| Screening Domain | Key Screening Questions/Criteria | Action Required |
|---|---|---|
| 1. Newborn Screening (NBS) Status |
|
|
| 2. Family History |
|
|
| 3. HIV Status |
|
|
| 4. Clinical Status & Other Contraindications |
|
|
| 5. Administration Readiness |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Dai, X.; Wei, S. BCGitis and BCGosis: Clinical Spectrum, Immunological Mechanisms, and Risk Management. Vaccines 2025, 13, 1179. https://doi.org/10.3390/vaccines13121179
Liu Q, Dai X, Wei S. BCGitis and BCGosis: Clinical Spectrum, Immunological Mechanisms, and Risk Management. Vaccines. 2025; 13(12):1179. https://doi.org/10.3390/vaccines13121179
Chicago/Turabian StyleLiu, Qibin, Xiyong Dai, and Shuang Wei. 2025. "BCGitis and BCGosis: Clinical Spectrum, Immunological Mechanisms, and Risk Management" Vaccines 13, no. 12: 1179. https://doi.org/10.3390/vaccines13121179
APA StyleLiu, Q., Dai, X., & Wei, S. (2025). BCGitis and BCGosis: Clinical Spectrum, Immunological Mechanisms, and Risk Management. Vaccines, 13(12), 1179. https://doi.org/10.3390/vaccines13121179






