Adenoviral Vectors Expressing Optimized preM/E Genes of WNV Deliver Long-Term Protection Against Lethal West Nile Virus Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cell Lines
2.2. Viruses
2.3. Generation of Recombinant Plasmids Encoding Various Forms of the Native preM/E Gene Sequence
2.4. Generation of Recombinant Plasmids Encoding Various Forms of the Codon-Optimized preM/E Gene
2.5. Construction of Recombinant Adenoviral Vectors Based on Human Adenovirus Type 2
2.6. Production and Propagation of Recombinant Adenoviruses
2.7. Analysis of Antigen Expression at the mRNA Level
2.8. Evaluation of WNV E Gene Expression by Western Blotting
2.9. Laboratory Animals
2.10. Animal Immunization and Serum Collection
2.11. Determination of WNV E-Specific Antibody Titers by ELISA
2.12. Growth and Characterization of WNV
2.13. Determination of Virus-Neutralizing Antibody (NtAb) Titers Against WNV
2.14. Cloning and Expression of Tick-Borne Encephalitis Virus (TBEV) E Proteins
2.15. Determination of TBEV E-Specific Antibody Titers by ELISA
2.16. WNV Challenge
2.17. Statistical Analysis
3. Results
3.1. Generation of rAd2 Vectors Expressing Different Variants of WNV M and E Protein Genes
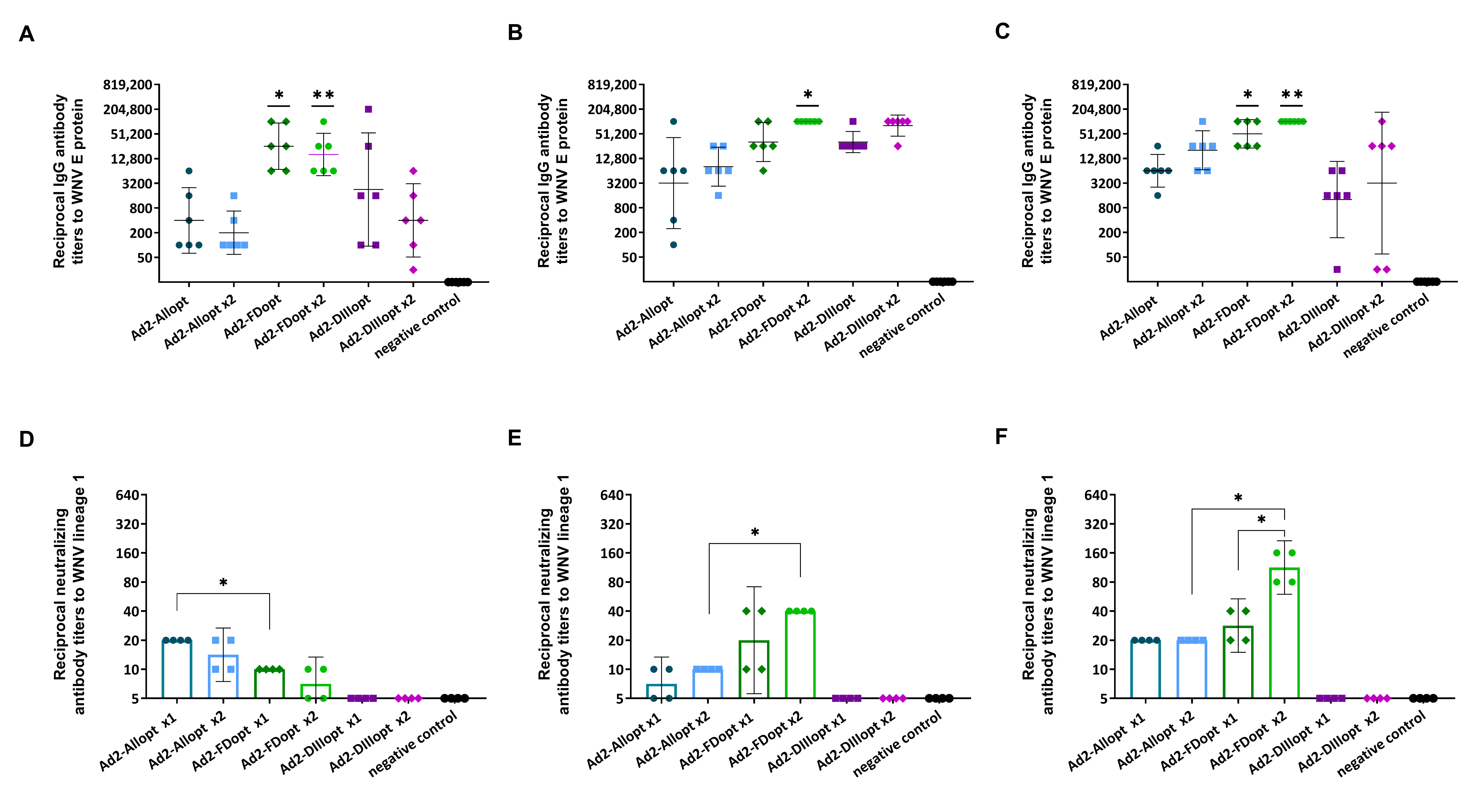
3.2. rAd2 Vectors Expressing Different WNV preM/E Gene Variants Induce a Humoral Immune Response in Mice
3.2.1. Study of the Immunogenicity of Different Variants of the Optimized WNV preM/E Protein Gene After Single and Double Administration
3.2.2. Cross-Reactivity of Neutralizing Antibodies Against Different WNV Lineages
3.2.3. rAd2s Expressing Different WNV preM/E Gene Variants Do Not Induce Cross-Reactive Immunity Against Tick-Borne Encephalitis Virus
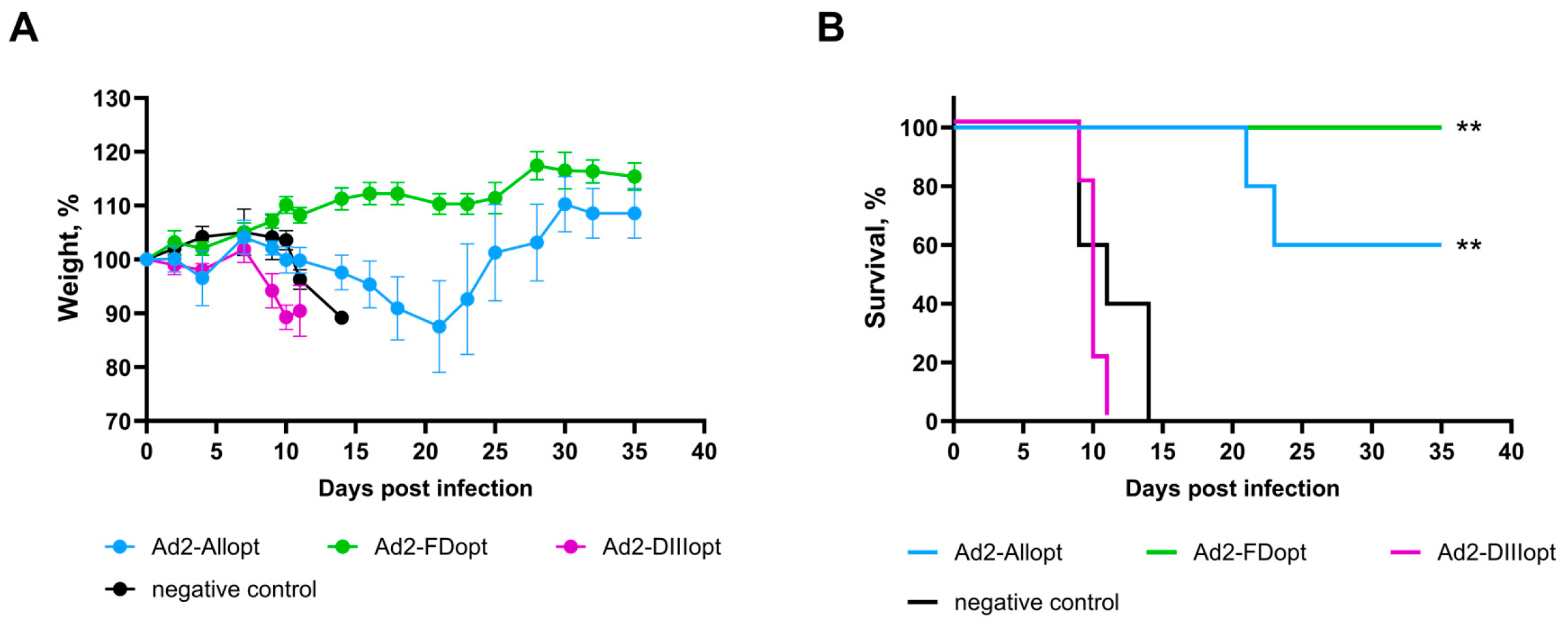
3.3. A Double Immunization with rAd2 Expressing the Optimized WNV preM/E Gene Carrying a Fusion Loop Mutation in the E Protein Protects Against Lethal WNV Infection
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ad2 | adenovirus type 2 |
| ADE | antibody-dependent enhancement of infection |
| CI | 95% confidence interval |
| DENV | Dengue virus |
| E | envelope |
| ELISA | enzyme-linked immunosorbent assay |
| FL | fusion loop |
| GMT | geometric mean titer |
| HRP | horseradish peroxidase |
| NS | nonstructural |
| NtAbs | neutralizing antibodies |
| rAd2 | recombinant adenovirus type 2 |
| TBEV | tick-borne encephalitis virus |
| TCID50 | 50% Tissue Culture Infectious Dose |
| vp | virus particles |
| WNV | West Nile Virus |
| ZIKV | Zika virus |
Appendix A
| Primer Name | 5′→3′–Sequence |
|---|---|
| WNV_L2-F1 | GTGACCCTCTCGAACTTCCAGGGCAAAG |
| WNV_L2-F2 | TCAACGAGAGCCGCGTGTCC |
| WNV_L2-F3 | GGCCGGAGCGATTCCTGTTGA |
| WNV_L2-R1 | AACGAAGGCGGGGTCAGCTC |
| WNV_L2-R2 | TCCATGGCCAGTGTCAGCGG |
| WNV_L2-R3 | AGCATGGACGTTGACCGAAAGGAAG |
| 553_Lider-F1 | GCCGCCACCATGCTCGGCCCCTGTATGCTACTGTTGCTCTTGCTTCTGGGACTGAGACTGCAGCTTTCCCTCGGA |
| 560_WNV-R | AGCRTGCACGTTCACGGAGAG |
| 561_WNV-F | GTTACCCTCTCTAACTTCCAAGGGAAGG |
| 912_anti_vector-F | CCTTTGAGTGAGCTGATACCGCTCAGATCTAAGCTTGCCGCCACCATGCTCGGCCCCT |
| 913_anti_vector-R | ACATTTCCCCGAAAAGTGCCACCCGTCTAGAAGATATCTTATCAAGCGTGCACGTTCACGGAGAG |
| 920_DIII_only_1 | ACAACCTACGGCGTCTGTTC |
| 921_DIII_only_2 | GAACAGACGCCGTAGGTTGTTCCGAGGGAAAGCTGCAGTC |
| 922_FusDam_1 | CAAAAGCTGGGTCAGCACGTTTGTCATTGTGAGCTTCTCCGCCGGCCGGGCACGCAGCTTTGGTGG |
| 923_FusDam_2 | ACCCCTGTCCACTACTCCTTGTTTACACACAAAAGCTGGGTCAGCACGT |
| 924_FusDam_3 | CAAGGAGTAGTGGACAGGGGTCGGGGCAACGGCTGTGGACGGTTTGGTAAAGGAAGCATTGACAC |
| 925_preME-1 | CCTTGGAAGTTAGAGAGGGTAACTCCGAGGGAAAGCTGCAGTC |
| 926_preME-2 | GCCTGATGCAGAGCTCCCTC |
| 927_preME-3 | GAGGGAGCTCTGCATCAGGCTTTGGCTGGAGCCATTCCTGTG |
| 928_Vector_F | GGTGGCACTTTTCGGGGAAATGT |
| 929_Vector_R | GAGCGGTATCAGCTCACTCAAAGG |
| Primer Name | Target Sequence | 5′→3′–Sequence |
|---|---|---|
| Ad2-hex-F | hexon gene of Ad2 | CTCGAGATCAGGCTACTAAGA |
| Ad2-hex-R | TGTGTTTCTGCATTGTCTGAT | |
| WN-All-opt-f | codon-optimized full-length preM/E protein gene | GCCGCATGTCCCACGATG |
| WN-All-opt-r | GCCCGCACCCATTGCCC | |
| DIII opt F | codon-optimized DIII sequence | GGAACAGATGGGCCTTGC |
| DIII opt R | GCTCACCACGACCCACTA | |
| WN-FD-opt-f | codon-optimized preM/E gene with mutations in the fusion loop | CCGCATGTCCCGCTGGT |
| WN-FD-opt-r | TCCCGCACCCATTGCCTCT | |
| E-125R | E1 region of adenovirus type 5 genome | TTTCCCACCCTTAAGCCACG |
| E1b-F | GATGTGACCGAGGAGCTGAG |
References
- ICTV The ICTV Report Virus Taxonomy: The Classification and Nomenclature of Viruses. Available online: https://ictv.global/taxonomy/ (accessed on 20 October 2025).
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda. Am. J. Trop. Med. 1940, 20, 471–492. [Google Scholar] [CrossRef]
- Sule, W.F.; Oluwayelu, D.O.; Hernández-Triana, L.M.; Fooks, A.R.; Venter, M.; Johnson, N. Epidemiology and Ecology of West Nile Virus in Sub-Saharan Africa. Parasit. Vectors 2018, 11, 414. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.L.; Ross, T.M.; Evans, J.D. West Nile Virus. Clin. Lab. Med. 2010, 30, 47–65. [Google Scholar] [CrossRef]
- Monini, M.; Falcone, E.; Busani, L.; Romi, R.; Ruggeri, F.M. West Nile Virus: Characteristics of an African Virus Adapting to the Third Millennium World. Open Virol. J. 2010, 4, 42–51. [Google Scholar] [CrossRef][Green Version]
- Bowen, R.A.; Nemeth, N.M. Experimental Infections with West Nile Virus. Curr. Opin. Infect. Dis. 2007, 20, 293–297. [Google Scholar] [CrossRef]
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef]
- Rizzoli, A.; Jimenez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The Challenge of West Nile Virus in Europe: Knowledge Gaps and Research Priorities. Eurosurveillance 2015, 20, 21135. [Google Scholar] [CrossRef] [PubMed]
- Zeller, H.G.; Schuffenecker, I. West Nile Virus: An Overview of Its Spread in Europe and the Mediterranean Basin in Contrast to Its Spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Jeffries, C.L.; Mansfield, K.L.; Carnell, G.; Fooks, A.R.; Johnson, N. Emergence of West Nile Virus Lineage 2 in Europe: A Review on the Introduction and Spread of a Mosquito-Borne Disease. Front. Public Health 2014, 2, 271. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The Global Ecology and Epidemiology of West Nile Virus. Biomed Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control Surveillance of West Nile Virus Infections in Humans and Animals in Europe. Available online: https://www.ecdc.europa.eu/en/infectious-disease-topics/west-nile-virus-infection/surveillance-and-disease-data/monthly-updates (accessed on 20 October 2025).
- Centers for Disease Control and Prevention (CDC) West Nile Virus. Available online: https://www.cdc.gov/west-nile-virus/index.html (accessed on 20 October 2025).
- L’vov, D.K.; Savchenko, S.T.; Alekseev, V.V.; Lipnitsky, A.V.; Pashanina, T.P. Epidemiological Situation and Prognostication of the West Nile Fever Morbidity in the Territory of the Russian Federation. Probl. Part. Danger. Infect. 2008, 1, 10–12. [Google Scholar] [CrossRef][Green Version]
- Putintseva, E.V.; Udovichenko, S.K.; Nikitin, D.N.; Boroday, N.V.; Antonov, A.S.; Toporkov, A.V. West Nile Fever: Analysis of the Epidemiological Situation in the Russian Federation in 2023, Forecast for 2024. Probl. Part. Danger. Infect. 2024, 10, 89–101. [Google Scholar] [CrossRef]
- Putintseva, E.V.; Udovichenko, S.K.; Nikitin, D.N.; Boroday, N.V.; Koloskova, A.Y.; Antonov, A.S.; Bondareva, O.S.; Toporkov, A.V. West Nile Fever in the Russian Federation in 2024, Forecast for 2025. Probl. Part. Danger. Infect. 2025, 1, 84–95. [Google Scholar] [CrossRef]
- The Federal Service for Surveillance on Consumer Rights Protection and Human Welfare (Rospotrebnadzor) Continues to Monitor the West Nile Fever Situation. Available online: https://rospotrebnadzor.ru/region/rss/rss.php?ELEMENT_ID=28278 (accessed on 20 October 2025).
- Sampathkumar, P. West Nile Virus: Epidemiology, Clinical Presentation, Diagnosis, and Prevention. Mayo Clin. Proc. 2003, 78, 1137–1143, quiz 1144. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, C.; Lourens, C.; Mendes, A.; de Villiers, M.; Avenant, T.; du Plessis, N.M.; Leendertz, F.H.; Venter, M. West Nile Virus, an Underdiagnosed Cause of Acute Fever of Unknown Origin and Neurological Disease among Hospitalized Patients in South Africa. Viruses 2023, 15, 2207. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Kramer, I.M.; Reuss, F.; Müller, R.; Groneberg, D.A.; Brüggmann, D. A Virus Becomes a Global Concern: Research Activities on West-Nile Virus. Emerg. Microbes Infect. 2023, 12, 2256424. [Google Scholar] [CrossRef]
- García-Carrasco, J.-M.; Muñoz, A.-R.; Olivero, J.; Segura, M.; Real, R. An African West Nile Virus Risk Map for Travellers and Clinicians. Travel Med. Infect. Dis. 2023, 52, 102529. [Google Scholar] [CrossRef]
- Karim, S.-U.; Bai, F. Introduction to West Nile Virus. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2023; Volume 2585, pp. 1–7. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kim, B.-S.; Chipman, P.R.; Rossmann, M.G.; Kuhn, R.J. Structure of West Nile Virus. Science 2003, 302, 248. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. Flaviviruses and Their Antigenic Structure. J. Clin. Virol. 2012, 55, 289–295. [Google Scholar] [CrossRef]
- Zhang, Y. Structures of Immature Flavivirus Particles. EMBO J. 2003, 22, 2604–2613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, R.; Shen, H.; Wang, M.; Yin, Z.; Cheng, A. Structures and Functions of the Envelope Glycoprotein in Flavivirus Infections. Viruses 2017, 9, 338. [Google Scholar] [CrossRef]
- Brinton, M.A. Host Factors Involved in West Nile Virus Replication. Ann. N. Y. Acad. Sci. 2001, 951, 207–219. [Google Scholar] [CrossRef]
- Lieberman, M.M.; Clements, D.E.; Ogata, S.; Wang, G.; Corpuz, G.; Wong, T.; Martyak, T.; Gilson, L.; Coller, B.-A.; Leung, J.; et al. Preparation and Immunogenic Properties of a Recombinant West Nile Subunit Vaccine. Vaccine 2007, 25, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.H.; Sui, J.; Foellmer, H.; Oliphant, T.; Wang, T.; Ledizet, M.; Murakami, A.; Noonan, K.; Lambeth, C.; Kar, K.; et al. Protective and Therapeutic Capacity of Human Single-Chain Fv-Fc Fusion Proteins against West Nile Virus. J. Virol. 2005, 79, 14606–14613. [Google Scholar] [CrossRef]
- Beasley, D.W.C.; Barrett, A.D.T. Identification of Neutralizing Epitopes within Structural Domain III of the West Nile Virus Envelope Protein. J. Virol. 2002, 76, 13097–13100. [Google Scholar] [CrossRef] [PubMed]
- Kaaijk, P.; Luytjes, W. Are We Prepared for Emerging Flaviviruses in Europe? Challenges for Vaccination. Hum. Vaccin. Immunother. 2018, 14, 337–344. [Google Scholar] [CrossRef]
- Gould, C.V.; Staples, J.E.; Huang, C.Y.-H.; Brault, A.C.; Nett, R.J. Combating West Nile Virus Disease—Time to Revisit Vaccination. N. Engl. J. Med. 2023, 388, 1633–1636. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Liu, J.; Kanesa-Thasan, N.; Myers, G.A.; Nichols, R.; Deary, A.; McCarthy, K.; Johnson, C.; Ermak, T.; Shin, S.; et al. A Live, Attenuated Recombinant West Nile Virus Vaccine. Proc. Natl. Acad. Sci. USA 2006, 103, 6694–6699. [Google Scholar] [CrossRef]
- Ozharovskaia, T.; Popova, O.; Zubkova, O.; Vavilova, I.; Pochtovyy, A.; Shcheblyakov, D.; Gushchin, V.; Logunov, D.y.; Gintsburg, A. Development and Characterization of a Vector System Based on the Simian Adenovirus Type 25. Bull. Russ. State Med. Univ. 2023, 1, 4–11. [Google Scholar] [CrossRef]
- Kanegae, Y.; Makimura, M.; Saito, I. A Simple and Efficient Method for Purification of Infectious Recombinant Adenovirus. Jpn. J. Med. Sci. Biol. 1994, 47, 157–166. [Google Scholar] [CrossRef]
- Maizel, J.V.; White, D.O.; Scharff, M.D. The Polypeptides of Adenovirus. I. Evidence for Multiple Protein Components in the Virion and a Comparison of Types 2, 7A, and 12. Virology 1968, 36, 115–125. [Google Scholar] [CrossRef] [PubMed]
- GOST R 53434-2009; Principles of Good Laboratory Practice. Federal Agency on Technical Regulating and Metrology: Moscow, Russia, 2009.
- Setoh, Y.X.; Prow, N.A.; Hobson-Peters, J.; Lobigs, M.; Young, P.R.; Khromykh, A.A.; Hall, R.A. Identification of Residues in West Nile Virus Pre-Membrane Protein That Influence Viral Particle Secretion and Virulence. J. Gen. Virol. 2012, 93, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-P.; Huo, H.; Wang, X.-L.; Bu, Z.-G.; Hua, R.-H. Generation and Characterization of a Monoclonal Antibody against PrM Protein of West Nile Virus. Monoclon. Antib. Immunodiagn. Immunother. 2014, 33, 438–443. [Google Scholar] [CrossRef]
- Kovalev, S.Y.; Mukhacheva, T.A.; Kokorev, V.S.; Belyaeva, I.V. Tick-Borne Encephalitis Virus: Reference Strain Sofjin and Problem of Its Authenticity. Virus Genes 2012, 44, 217–224. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; Botikov, A.G.; et al. An Open, Non-Randomised, Phase 1/2 Trial on the Safety, Tolerability, and Immunogenicity of Single-Dose Vaccine “Sputnik Light” for Prevention of Coronavirus Infection in Healthy Adults. Lancet Reg. Health-Eur. 2021, 11, 100241. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and Efficacy of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, F.; Oude Munnink, B.B.; Munger, E.; Sikkema, R.S.; Pappa, S.; Tsioka, K.; Sinigaglia, A.; Dal Molin, E.; Shih, B.B.; et al. West Nile Virus Spread in Europe: Phylogeographic Pattern Analysis and Key Drivers. PLoS Pathog. 2024, 20, e1011880. [Google Scholar] [CrossRef]
- Rey, F.A.; Stiasny, K.; Vaney, M.; Dellarole, M.; Heinz, F.X. The Bright and the Dark Side of Human Antibody Responses to Flaviviruses: Lessons for Vaccine Design. EMBO Rep. 2018, 19, 206–224. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Narvaez, C.; Arguello, S.; Lopez Mercado, B.; Collado, D.; Ampie, O.; Elizondo, D.; Miranda, T.; Bustos Carillo, F.; Mercado, J.C.; et al. Zika Virus Infection Enhances Future Risk of Severe Dengue Disease. Science 2020, 369, 1123–1128. [Google Scholar] [CrossRef]
- Weiß, R.; Issmail, L.; Rockstroh, A.; Grunwald, T.; Fertey, J.; Ulbert, S. Immunization with Different Recombinant West Nile Virus Envelope Proteins Induces Varying Levels of Serological Cross-Reactivity and Protection from Infection. Front. Cell. Infect. Microbiol. 2023, 13, 1279147. [Google Scholar] [CrossRef]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika Virus Pathogenesis by Preexisting Antiflavivirus Immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified MRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e10. [Google Scholar] [CrossRef]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like Particles That Display Zika Virus Envelope Protein Domain III Induce Potent Neutralizing Immune Responses in Mice. Sci. Rep. 2017, 7, 7679. [Google Scholar] [CrossRef]
- Treangen, T.J.; Abraham, A.-L.; Touchon, M.; Rocha, E.P.C. Genesis, Effects and Fates of Repeats in Prokaryotic Genomes. FEMS Microbiol. Rev. 2009, 33, 539–571. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.B.; Alsaadi, A.; Naeem, A.; Almahboub, S.A.; Bosaeed, M.; Aljedani, S.S. Development of Nucleic Acid-Based Vaccines against Dengue and Other Mosquito-Borne Flaviviruses: The Past, Present, and Future. Front. Immunol. 2024, 15, 1475886. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qu, L.; Ye, X.; Yi, C.; Zheng, X.; Hao, M.; Su, W.; Yao, Z.; Chen, P.; Zhang, S.; et al. Incorporation of NS1 and PrM/M Are Important to Confer Effective Protection of Adenovirus-Vectored Zika Virus Vaccine Carrying E Protein. npj Vaccines 2018, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Guo, X.; Han, X.; Li, M.; Zhai, C.; Bing, J.; Jin, L.; Jiang, Y.; Li, J.; Wang, T.; et al. Humoral and Cellular Immune Response to a Single Dose of a Novel Bivalent Recombinant Adenovirus-Vector Vaccine against West Nile Virus and Chikungunya Virus in Mice. Virol. J. 2025, 22, 256. [Google Scholar] [CrossRef]
- Chung, K.M.; Liszewski, M.K.; Nybakken, G.; Davis, A.E.; Townsend, R.R.; Fremont, D.H.; Atkinson, J.P.; Diamond, M.S. West Nile Virus Nonstructural Protein NS1 Inhibits Complement Activation by Binding the Regulatory Protein Factor H. Proc. Natl. Acad. Sci. USA 2006, 103, 19111–19116. [Google Scholar] [CrossRef]
- Samuel, M.A.; Diamond, M.S. Pathogenesis of West Nile Virus Infection: A Balance between Virulence, Innate and Adaptive Immunity, and Viral Evasion. J. Virol. 2006, 80, 9349–9360. [Google Scholar] [CrossRef]
- de Freitas Costa, E.; Streng, K.; Avelino de Souza Santos, M.; Counotte, M.J. The Effect of Temperature on the Boundary Conditions of West Nile Virus Circulation in Europe. PLoS Negl. Trop. Dis. 2024, 18, e0012162. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, I.; Betášová, L.; Blažejová, H.; Venclíková, K.; Straková, P.; Šebesta, O.; Mendel, J.; Bakonyi, T.; Schaffner, F.; Nowotny, N.; et al. West Nile Virus in Overwintering Mosquitoes, Central Europe. Parasit. Vectors 2017, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Kampen, H.; Tews, B.A.; Werner, D. First Evidence of West Nile Virus Overwintering in Mosquitoes in Germany. Viruses 2021, 13, 2463. [Google Scholar] [CrossRef] [PubMed]
- Blom, R.; Schrama, M.J.J.; Spitzen, J.; Weller, B.F.M.; van der Linden, A.; Sikkema, R.S.; Koopmans, M.P.G.; Koenraadt, C.J.M. Arbovirus Persistence in North-Western Europe: Are Mosquitoes the Only Overwintering Pathway? One Health 2023, 16, 100467. [Google Scholar] [CrossRef]
- Chu, J.-H.J.; Chiang, C.-C.S.; Ng, M.-L. Immunization of Flavivirus West Nile Recombinant Envelope Domain III Protein Induced Specific Immune Response and Protection against West Nile Virus Infection. J. Immunol. 2007, 178, 2699–2705. [Google Scholar] [CrossRef]
- Martina, B.E.; Koraka, P.; van den Doel, P.; van Amerongen, G.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E. Immunization with West Nile Virus Envelope Domain III Protects Mice against Lethal Infection with Homologous and Heterologous Virus. Vaccine 2008, 26, 153–157. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozharovskaia, T.A.; Zubkova, O.V.; Korobova, E.V.; Dolzhikova, I.V.; Zrelkin, D.I.; Popova, O.; Goldovskaya, P.P.; Kovyrshina, A.V.; Korobkova, A.I.; Favorskaya, I.A.; et al. Adenoviral Vectors Expressing Optimized preM/E Genes of WNV Deliver Long-Term Protection Against Lethal West Nile Virus Challenge. Vaccines 2025, 13, 1177. https://doi.org/10.3390/vaccines13121177
Ozharovskaia TA, Zubkova OV, Korobova EV, Dolzhikova IV, Zrelkin DI, Popova O, Goldovskaya PP, Kovyrshina AV, Korobkova AI, Favorskaya IA, et al. Adenoviral Vectors Expressing Optimized preM/E Genes of WNV Deliver Long-Term Protection Against Lethal West Nile Virus Challenge. Vaccines. 2025; 13(12):1177. https://doi.org/10.3390/vaccines13121177
Chicago/Turabian StyleOzharovskaia, Tatiana A., Olga V. Zubkova, Elizaveta V. Korobova, Inna V. Dolzhikova, Denis I. Zrelkin, Olga Popova, Polina P. Goldovskaya, Anna V. Kovyrshina, Anastasia I. Korobkova, Irina A. Favorskaya, and et al. 2025. "Adenoviral Vectors Expressing Optimized preM/E Genes of WNV Deliver Long-Term Protection Against Lethal West Nile Virus Challenge" Vaccines 13, no. 12: 1177. https://doi.org/10.3390/vaccines13121177
APA StyleOzharovskaia, T. A., Zubkova, O. V., Korobova, E. V., Dolzhikova, I. V., Zrelkin, D. I., Popova, O., Goldovskaya, P. P., Kovyrshina, A. V., Korobkova, A. I., Favorskaya, I. A., Vavilova, I. V., Grousova, D. M., Zorkov, I. D., Iliukhina, A. A., Ermolova, I. A., Tukhvatulin, A. I., Shcherbinin, D. N., Ermolova, E. I., Kunda, M. S., ... Gintsburg, A. L. (2025). Adenoviral Vectors Expressing Optimized preM/E Genes of WNV Deliver Long-Term Protection Against Lethal West Nile Virus Challenge. Vaccines, 13(12), 1177. https://doi.org/10.3390/vaccines13121177







