Long-Term Immunogenicity of Rabies Pre-Exposure Prophylaxis in Japanese Adult Travelers: Comparison of Dosing Regimens †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Trial Registration
2.2. Participants
2.3. Study Procedures
2.4. Measurement of Rabies Virus Neutralization Antibodies
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Characteristics of the Study Participants
3.2. Seroprotection Rate and GMT (Table 2)
| Characteristics | Seroprotection | GMT (95%CI) | ||
|---|---|---|---|---|
| n/N | % (95%CI) | |||
| Total | 84/97 | 86.6 (78.2–92.7) | 1.65 | (1.22–2.23) |
| Sex | ||||
| Male | 46/53 | 86.8 (74.7–94.5) | 1.81 | (1.10–2.98) |
| Female | 38/44 | 86.4 (72.6–94.8) | 1.47 | (1.09–1.99) |
| p = 1.000 | p = 0.951 | |||
| Age at primary vaccination (years) | ||||
| <50 | 75/85 | 88.2 (79.4–94.2) | 1.67 | (1.24–2.25) |
| ≥50 | 9/12 | 75.0 (42.8–94.5) | 1.52 | (0.39–5.92) |
| p = 0.201 | p = 0.558 | |||
| Vaccination doses | ||||
| 2 | 6/10 | 60.0 (26.2–87.8) | 0.64 | (0.31–1.33) |
| 3 | 50/58 | 86.2 (74.6–93.9) | 1.15 | (0.88–1.51) |
| 4 | 16/17 | 94.1 (71.3–99.9) | 5.58 | (1.85–16.8) |
| 5 or more | 12/12 | 100 (73.5–100) | 3.64 | (1.60–8.28) |
| p < 0.05 | p < 0.05 | |||
| Vaccine type | ||||
| PCECV-KMB | 17/33 | 51.5 (33.5–69.2) | 0.93 | (0.57–1.51) |
| Rabipur | 7/12 | 58.3 (27.7–84.8) | 3.96 | (0.92–17.1) |
| PVRV | 36/51 | 70.6 (56.2–82.5) | 1.95 | (1.40–2.72) |
| unknown | 1/1 | 1.75 | ||
| p = 0.086 | p = 0.159 | |||
| Elapsed time from last vaccination (years) | ||||
| 2–3 | 32/36 | 88.9 (73.9–96.9) | 2.23 | (1.25–3.97) |
| 4–9 | 45/53 | 84.9 (72.4–93.3) | 1.30 | (0.90–1.88) |
| 10–15 | 7/8 | 87.5 (47.3–99.7) | 2.05 | (0.78–5.41) |
| p = 0.900 | p = 0.444 | |||
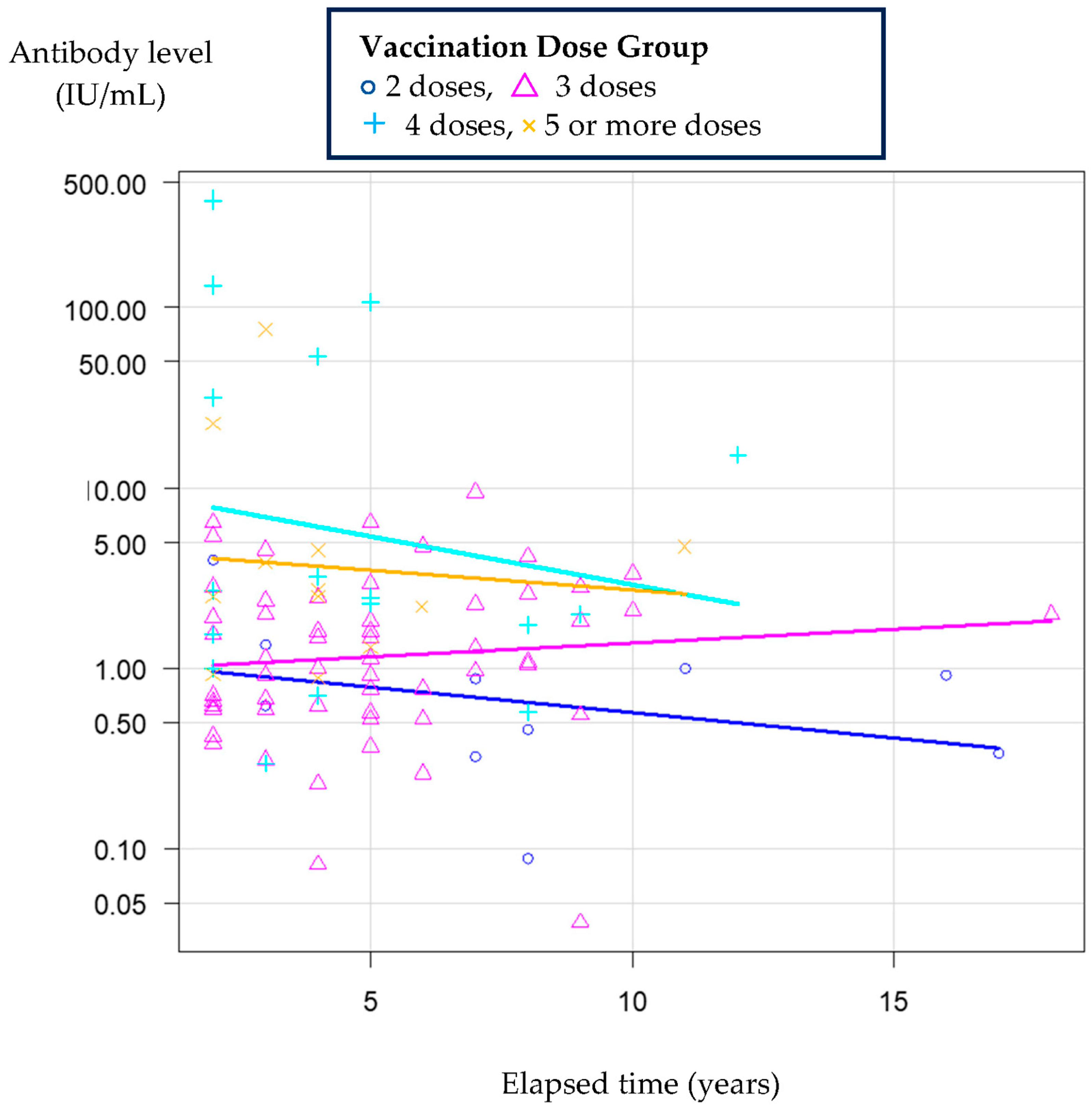
3.3. The Relationship Between the Elapsed Time and Antibody Levels
3.4. Anti-Rabies VNA Levels After Each Dose of Vaccine
3.4.1. Two-Dose Sample
3.4.2. Three-Dose Sample
3.4.3. Four-Dose Sample
3.4.4. Sample Receiving Five or More Doses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PrEP | pre-exposure prophylaxis |
| PEP | post-exposure prophylaxis |
| PVRV | purified Vero cell rabies vaccine |
| PCECV | purified chick embryo cell rabies vaccine |
| WHO | World Health Organization |
| GMT | geometric mean titer |
| CI | confidence interval |
| IQR | interquartile range |
| N | number of participants |
| VNA | viral neutralizing antibody |
| M | male |
| F | female |
| d | day |
| m | month |
| y | year |
References
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primers 2017, 3, 17091. [Google Scholar] [CrossRef] [PubMed]
- Fooks, A.R.; Banyard, A.C.; Horton, D.L.; Johnson, N.; McElhinney, L.M.; Jackson, A.C. Current status of rabies and prospects for elimination. Lancet 2014, 384, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human rabies transmitted by dogs: Current status of global data, 2015. Wkly. Epidemiol. Rec. 2016, 91, 13–20. [Google Scholar]
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015, 9, e0003786. [Google Scholar]
- World Health Organization. Human and Dog Rabies Prevention and Control: Report of the WHO/Bill and Melinda Gates Foundation Consultation, Annecy, France, 7–9 October 2009. Available online: https://iris.who.int/bitstream/handle/10665/70253/WHO_HTM_NTD_NZD_2010.1_eng.pdf?sequence=1&isAllowed=y (accessed on 7 June 2024).
- World Health Organization. Rabies vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2018, 93, 201–220. [Google Scholar]
- World Health Organization. WHO Expert Consultation on Rabies, Third Report. Available online: https://iris.who.int/bitstream/handle/10665/272364/9789241210218-eng.pdf?ua=1 (accessed on 7 June 2024).
- World Health Organization. Rabies vaccines: WHO position paper-recommendations. Vaccine 2010, 28, 7140–7142. [Google Scholar] [CrossRef]
- Kurosawa, A.; Tojinbara, K.; Kadowaki, H.; Hampson, K.; Yamada, A.; Makita, K. The rise and fall of rabies in Japan: A quantitative history of rabies epidemics in Osaka Prefecture, 1914–1933. PLoS Negl. Trop. Dis. 2017, 11, e000543511. [Google Scholar] [CrossRef]
- Yamada, A.; Makita, K.; Kadowaki, H.; Ito, N.; Sugiyama, M.; Kwan, N.C.; Sugiura, K. A comparative review of prevention of rabies incursion between Japan and other rabies-free countries or regions. Jpn. J. Infect. Dis. 2019, 72, 203–210. [Google Scholar] [CrossRef]
- Moore, S.M.; Hanlon, C.A. Rabies-specific antibodies: Measuring surrogates of protection against a fatal disease. PLoS Negl. Trop. Dis. 2010, 4, e595. [Google Scholar] [CrossRef]
- Timiryasova, T.M.; Hodge, S.A.; Zheng, L.; Singer, A.; Vincent, D.; Rahman, M.; Petit, C.; Brown, M. Preparation and qualification of internal rabies reference standards for use in the rabies rapid fluorescent focus inhibition test. Sci. Rep. 2020, 10, 9893. [Google Scholar] [CrossRef]
- Shiota, S.; Mannen, K.; Matsumoto, T.; Yamada, K.; Yasui, T.; Takayama, K.; Kobayashi, Y.; Khawplod, P.; Gotoh, K.; Ahmed, K.; et al. Development and evaluation of a rapid neutralizing antibody test for rabies. J. Virol. Methods 2009, 161, 58–62. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Kessels, J.A.; Recuenco, S.; Navarro-Vela, A.M.; Deray, R.; Vigilato, M.; Ertl, H.; Durrheim, D.; Rees, H.; Nel, L.H.; Abela-Ridder, B.; et al. Pre-exposure rabies prophylaxis: A systematic review. Bull. World Health Organ. 2017, 95, 210–219. [Google Scholar] [CrossRef]
- Parize, P.; Sommé, J.; Schaeffer, L.; Ribadeau-Dumas, F.; Benabdelkader, S.; Durand, A.; Tarantola, A.; Cailhol, J.; Goesch, J.; Kergoat, L.; et al. Systematic booster after rabies pre-exposure prophylaxis to alleviate rabies antibody monitoring in individuals at risk of occupational exposure. Vaccines 2021, 9, 309. [Google Scholar] [CrossRef]
- Guo, Y.; Mills, D.J.; Lau, C.L.; Mills, C.; Furuya-Kanamori, L. Immune response after rabies pre-exposure prophylaxis and a booster dose in Australian bat carers. Zoonoses Public Health 2023, 70, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Endy, T.P.; Keiser, P.B.; Wang, D.; Jarman, R.G.; Cibula, D.; Fang, H.; Ware, L.; Abbott, M.; Thomas, S.J.; Polhemus, M.E. Serologic response of 2 versus 3 doses and intradermal versus intramuscular administration of a licensed rabies vaccine for preexposure prophylaxis. J. Infect. Dis. 2020, 221, 1494–1498. [Google Scholar] [CrossRef] [PubMed]
- De Pijper, C.A.; Langedijk, A.C.; Terryn, S.; Van Gucht, S.; Grobusch, M.P.; Goorhuis, A.; Stijnis, C. Long-term memory response after a single intramuscular rabies booster vaccination 10–24 years after primary immunization. J. Infect. Dis. 2022, 226, 1052–1056. [Google Scholar] [CrossRef]
- Shiota, S.; Khawplod, P.; Ahmed, K.; Mifune, K.; Nishizono, A. A pilot study on intradermal vaccination of Japanese rabies vaccine for pre-exposure immunization. Vaccine 2008, 26, 6441–6444. [Google Scholar] [CrossRef]
- Meiji Seika Pharma Co., Ltd. Inactivated Tissue Culture Rabies Vaccine: Japanese Package Insert; Meiji Seika Pharma Co., Ltd.: Tokyo, Japan, 2024. [Google Scholar]
- Kurosaki, T.; Kometani, K.; Ise, W. Memory B cells. Nat. Rev. Immunol. 2015, 15, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Overduin, L.A.; van Dongen, J.J.M.; Visser, L.G. The cellular immune response to rabies vaccination: A systematic review. Vaccines 2019, 7, 110. [Google Scholar] [CrossRef]
- Venkataswamy, M.M.; Madhusudana, S.N.; Sanyal, S.S.; Taj, S.; Belludi, A.Y.; Mani, R.S.; Hazra, N. Cellular immune response following pre-exposure and postexposure rabies vaccination by intradermal and intramuscular routes. Clin. Exp. Vaccine Res. 2015, 4, 68–74. [Google Scholar] [CrossRef]
- Strady, A.; Lang, J.; Lienard, M.; Blondeau, C.; Jaussaud, R.; Plotkin, S.A. Antibody persistence following preexposure regimens of cell-culture rabies vaccines: 10-year follow-up and proposal for a new booster policy. J. Infect. Dis. 1998, 177, 1290–1295. [Google Scholar] [CrossRef]
- Suwansrinon, K.; Wilde, H.; Benjavongkulchai, M.; Banjongkasaena, U.; Lertjarutorn, S.; Boonchang, S.; Suttisri, R.; Khowplod, P.; Daviratanasilpa, S.; Sitprija, V. Survival of neutralizing antibody in previously rabies vaccinated subjects: A prospective study showing long lasting immunity. Vaccine 2006, 24, 3878–3880. [Google Scholar] [CrossRef] [PubMed]
- Jentes, E.S.; Blanton, J.D.; Johnson, K.J.; Petersen, B.W.; Lamias, M.J.; Robertson, K.; Franka, R.; Briggs, D.; Costa, P.; Lai, I.; et al. The global availability of rabies immune globulin and rabies vaccine in clinics providing direct care to Travelers. J. Travel Med. 2013, 20, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.L.; Andrews, N.; Goharriz, H.; Goddard, T.; McElhinney, L.M.; Brown, K.E.; Fooks, A.R. Rabies pre-exposure prophylaxis elicits long-lasting immunity in humans. Vaccine 2016, 34, 5959–5967. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, A.C.; De Pijper, C.A.; Spijker, R.; Holman, R.; Grobusch, M.P.; Stijnis, C. Rabies antibody response after booster immunization: A systematic review and metaanalysis. Clin. Infect. Dis. 2018, 67, 1932–1947. [Google Scholar] [CrossRef]
- Rao, A.K.; Briggs, D.; Moore, S.M.; Whitehill, F.; Campos-Outcalt, D.; Morgan, R.L.; Wallace, R.M.; Romero, J.R.; Bahta, L.; Frey, S.E.; et al. Use of a modified preexposure prophylaxis vaccination schedule to prevent human rabies: Recommendations of the Advisory Committee on Immunization Practices-United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 619. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). ACIP Grading of Recommendations Assessment, Development, and Evaluation (GRADE) for 2-Dose Rabies Vaccination Schedule. Available online: https://www.cdc.gov/vaccines/acip/recs/grade/rabies-2-dose.html (accessed on 6 June 2024).
- Shinji, F.; Akira, N.; Takehiro, H.; Atsuo, H. Long-term immunogenicity after rabies pre-exposure prophylaxis among Japanese adults. In Proceedings of the 14th Asia Pacific Travel Health Conference (14th APTHC), Kathmandu, Nepal, 18–21 September 2024. [Google Scholar]
- World Health Organization. The Immunological Basis for Immunization Series: Module 17—Rabies; WHO: Geneva, Switzerland, 2011; ISBN 978-92-4-150108-8. Available online: https://www.who.int/publications/i/item/9789241513371 (accessed on 28 September 2025).
| Characteristics | N (%) |
|---|---|
| Sex | |
| Male | 53 (54.6) |
| Female | 44 (45.4) |
| Age at primary vaccination (years) | Median age (IQR): 35 (29–44) |
| <50 | 85 (87.6) |
| ≥50 | 12 (12.4) |
| Vaccination doses | |
| 2 | 10 (10.3) |
| 3 | 58 (59.8) |
| 4 | 17 (17.5) |
| 5 or more | 12 (12.4) |
| Vaccine type | |
| PCECV-KMB | 33 (34.0) |
| PCECV-Rabipur | 12 (12.4) |
| PVRV | 51 (52.6) |
| Unknown | 1 (1.0) |
| Elapsed time from last vaccination (years) | Median age (IQR): 4 (3–7) |
| 2–3 | 36 (37.1) |
| 4–9 | 53 (54.6) |
| 10–15 | 8 (8.3) |
| Factor | Sum of Squares | df | F Value | Pr (>F) | Significance |
|---|---|---|---|---|---|
| Intercept | 0.6848 | 1 | 2.3566 | 0.128 | |
| Age Group | 0.4398 | 1 | 1.5135 | 0.222 | |
| Vaccination Dose Group | 8.9440 | 3 | 10.2601 | <0.001 | *** |
| Interval Group | 1.6533 | 2 | 2.8449 | 0.064 | . |
| Sex | 0.0568 | 1 | 0.1954 | 0.660 | |
| Vaccine Type | 3.7207 | 3 | 4.2682 | 0.007 | ** |
| Residuals | 24.9895 | 86 |
| Vaccine Name and Schedule (d) | Sex | Age at Primary Vaccination (y) | Elapsed Time (y) | VNA Level (IU/mL) |
|---|---|---|---|---|
| PVRV (0, 7 d) | M | 32 | 2 | 4.00 |
| PVRV (0, 7 d) | F | 29 | 3 | 1.35 |
| PVRV (0, 7 d) | M | 39 | 3 | 0.62 |
| Rabipur (0, 21 d) | F | 33 | 7 | 0.88 |
| PCECV-KMB (0, 28 d) | M | 55 | 7 | 0.32 1 |
| PCECV-KMB (0, 28 d) | M | 26 | 8 | 0.09 1 |
| PCECV-KMB (0, 28 d) | M | 40 | 8 | 0.46 1 |
| PCECV-KMB (0, 28 d) | F | 26 | 11 | 1.00 |
| PCECV-KMB (0, 21 d) | F | 27 | 16 | 0.92 |
| PCECV-KMB (0, 21 d) | F | 33 | 17 | 0.34 1 |
| Vaccine Name and Schedule (d/m/y) | Elapsed Time (y) | Seroprotection | VNA Level (IU/mL) | ||
|---|---|---|---|---|---|
| <0.5 | 0.5–1.0 | >1.0 | |||
| PVRV (0, 7 d, 21–28 d) | 2–10 | 36/37 | 1 1 | 10 | 26 |
| Rabipur (0, 7 d, 21–28 d) | 2–6 | 5/5 | 0 | 2 | 3 |
| PCECV-KMB (0, 7 d, 21–28 d) | 4–9 | 4/7 | 3 1 | 1 | 3 |
| PCECV-KMB (0, 1 m, 6–12 m) | 2–9 | 4/8 | 4 1 | 1 | 3 |
| PCECV-KMB (0, 4 y, 6 y) | 18 | 1/1 | 0 | 0 | 1 |
| Vaccine Name and Schedule (d/m/y) | Sex | Age at Primary Vaccination (y) | Elapsed Time (y) | VNA Level (IU/mL) |
|---|---|---|---|---|
| PVRV (0, 7 d, 28 d, 2 y) | M | 50 | 2 | 133.96 |
| PVRV (0, 7 d, 28 d, 4 y) | M | 55 | 2 | 32.00 |
| PVRV (0, 7 d, 28 d, 7 y) | M | 21 | 2 | 1.54 |
| PVRV (0, 7 d, 28 d, 6 y) | M | 49 | 4 | 3.22 |
| PVRV (0, 7 d, 28 d, 1 y) | F | 40 | 5 | 2.48 |
| PVRV (0, 7 d, 28 d, 1 y) | M | 55 | 5 | 2.28 |
| PVRV (0, 14 d, 28 d, 8 m) | F | 38 | 8 | 0.57 |
| PVRV (0, 14 d, 28 d) Rabipur (1 y) | M | 41 | 4 | 53.85 |
| Rabipur (0, 7 d, 28 d, 1 y) | M | 47 | 4 | 0.71 |
| Rabipur (0, 7 d, 28 d, 1 y) | M | 36 | 5 | 108.49 |
| Rabipur (0, 7 d, 28 d, 3 y) | M | 50 | 9 | 2.00 |
| Rabipur (0, 7 d, 28 d) Chirorab (1 y) | M | 29 | 2 | 400 |
| PCECV-KMB (0, 1 m, 6 m, 3 y) | F | 48 | 2 | 2.71 |
| PCECV-KMB (0, 7 d, 8 m, 9 y) | M | 46 | 2 | 1.00 |
| PCECV-KMB (0, 1 m, 7 m, 4 y) | F | 58 | 3 | 0.30 1 |
| PCECV-KMB (0, 1 m, 9 m) Pasteur (7 y) | F | 30 | 12 | 15.32 |
| Unknown (0, 1 m, 10 m) PCECV-KMB (7 y) | F | 34 | 8 | 1.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushima, S.; Nishizono, A.; Hashimoto, T.; Hamada, A. Long-Term Immunogenicity of Rabies Pre-Exposure Prophylaxis in Japanese Adult Travelers: Comparison of Dosing Regimens. Vaccines 2025, 13, 1169. https://doi.org/10.3390/vaccines13111169
Fukushima S, Nishizono A, Hashimoto T, Hamada A. Long-Term Immunogenicity of Rabies Pre-Exposure Prophylaxis in Japanese Adult Travelers: Comparison of Dosing Regimens. Vaccines. 2025; 13(11):1169. https://doi.org/10.3390/vaccines13111169
Chicago/Turabian StyleFukushima, Shinji, Akira Nishizono, Takehiro Hashimoto, and Atsuo Hamada. 2025. "Long-Term Immunogenicity of Rabies Pre-Exposure Prophylaxis in Japanese Adult Travelers: Comparison of Dosing Regimens" Vaccines 13, no. 11: 1169. https://doi.org/10.3390/vaccines13111169
APA StyleFukushima, S., Nishizono, A., Hashimoto, T., & Hamada, A. (2025). Long-Term Immunogenicity of Rabies Pre-Exposure Prophylaxis in Japanese Adult Travelers: Comparison of Dosing Regimens. Vaccines, 13(11), 1169. https://doi.org/10.3390/vaccines13111169






