Current Status and Challenges of Vaccine Development for Seasonal Human Coronaviruses
Abstract
1. Introduction
2. Pathogenesis and Epidemiology of Seasonal HCoVs
2.1. HCoV-229E
2.2. HCoV-OC43
2.3. HCoV-NL63
2.4. HCoV-HKU1
2.5. General and Distinctive Features of Seasonal HCoVs
2.6. Public Health Impact and Rationale for the Development of Seasonal HCoV Vaccine
3. Structures and Antigens of Seasonal HCoVs
3.1. Genomic Structures of Seasonal HCoVs
3.2. Major Antigens of Seasonal HCoVs
4. Vaccine Types Targeting Infectious Diseases
4.1. Inactivated Vaccine
4.2. DNA Vaccine
4.3. mRNA Vaccine
4.4. Subunit Vaccine
4.5. Viral Vector Vaccine
4.6. Virus-like Particle Vaccine
5. The Development of Vaccines Against Seasonal HCoVs
5.1. Cross-Reactivity of Natural Infection and Inactivated Vaccines to Seasonal HCoVs
5.2. Nucleic Acid Vaccines for Seasonal HCoVs
5.3. Subunit Vaccines and Virus-like Particle Vaccines for Seasonal HCoVs
5.4. Viral Vector Vaccines for Seasonal HCoVs
6. Discussion
6.1. Current Challenges in Seasonal HCoV Vaccines
6.2. Strategic Framework for the Development of Seasonal HCoV Vaccines
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamre, D.; Procknow, J.J. A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 1966, 121, 190–193. [Google Scholar] [CrossRef]
- McIntosh, K.; Becker, W.B.; Chanock, R.M. Growth in suckling-mouse brain of “IBV-like” viruses from patients with upper respiratory tract disease. Proc. Natl. Acad. Sci. USA 1967, 58, 2268–2273. [Google Scholar] [CrossRef]
- van der Hoek, L.; Pyrc, K.; Jebbink, M.F.; Vermeulen-Oost, W.; Berkhout, R.J.M.; Wolthers, K.C.; Wertheim-van Dillen, P.M.E.; Kaandorp, J.; Spaargaren, J.; Berkhout, B. Identification of a new human coronavirus. Nat. Med. 2004, 10, 368–373. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Chu, C.-m.; Chan, K.-h.; Tsoi, H.-w.; Huang, Y.; Wong, B.H.L.; Poon, R.W.S.; Cai, J.J.; Luk, W.-k.; et al. Characterization and Complete Genome Sequence of a Novel Coronavirus, Coronavirus HKU1, from Patients with Pneumonia. J. Virol. 2005, 79, 884–895. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Donnelly, C.A.; Ghani, A.C.; Leung, G.M.; Hedley, A.J.; Fraser, C.; Riley, S.; Abu-Raddad, L.J.; Ho, L.-M.; Thach, T.-Q.; Chau, P.; et al. Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet 2003, 361, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Isolation of a Novel Coronavirus from a Man with Pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016, 24, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Raoult, D.; Zumla, A.; Locatelli, F.; Ippolito, G.; Kroemer, G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress 2020, 4, 66–75. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Shi, W.; Lu, R.; Wang, W.; Zhao, Y.; Deng, Y.; Zhou, W.; Ren, H.; Wu, J.; et al. Origin and Possible Genetic Recombination of the Middle East Respiratory Syndrome Coronavirus from the First Imported Case in China: Phylogenetics and Coalescence Analysis. mBio 2015, 6, e01280-15. [Google Scholar] [CrossRef]
- Kim, K.H.; Tandi, T.E.; Choi, J.W.; Moon, J.M.; Kim, M.S. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: Epidemiology, characteristics and public health implications. J. Hosp. Infect. 2017, 95, 207–213. [Google Scholar] [CrossRef]
- Hou, Y.; Gu, T.; Ni, Z.; Shi, X.; Ranney, M.L.; Mukherjee, B. Global Prevalence of Long COVID, Its Subtypes, and Risk Factors: An Updated Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2025, 12, ofaf533. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Leighton, T.; Ostrowsky, J.T.; Anderson, C.J.; Danila, R.N.; Ulrich, A.K.; Lackritz, E.M.; Mehr, A.J.; Baric, R.S.; Baylor, N.W.; et al. A research and development (R&D) roadmap for broadly protective coronavirus vaccines: A pandemic preparedness strategy. Vaccine 2023, 41, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Grange, Z.L.; Goldstein, T.; Johnson, C.K.; Anthony, S.; Gilardi, K.; Daszak, P.; Olival, K.J.; O’Rourke, T.; Murray, S.; Olson, S.H.; et al. Ranking the risk of animal-to-human spillover for newly discovered viruses. Proc. Natl. Acad. Sci. USA 2021, 118, e2002324118. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aravena, M.; McKee, C.; Gamble, A.; Lunn, T.; Morris, A.; Snedden, C.E.; Yinda, C.K.; Port, J.R.; Buchholz, D.W.; Yeo, Y.Y.; et al. Ecology, evolution and spillover of coronaviruses from bats. Nat. Rev. Microbiol. 2022, 20, 299–314. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Pasteur, L. De l’attenuation du virus du cholera des poules. CR Acad. Sci. Paris 1880, 91, 673–680. [Google Scholar]
- Pasteur, L.; Chamberland, C.; Roux, E. Sur la Rage; Gauthier-Villars: Paris, France, 1884. [Google Scholar]
- Lobo, N.; Brooks, N.A.; Zlotta, A.R.; Cirillo, J.D.; Boorjian, S.; Black, P.C.; Meeks, J.J.; Bivalacqua, T.J.; Gontero, P.; Steinberg, G.D.; et al. 100 years of Bacillus Calmette–Guérin immunotherapy: From cattle to COVID-19. Nat. Rev. Urol. 2021, 18, 611–622. [Google Scholar] [CrossRef]
- Ryan, E.T.; Calderwood, S.B. Cholera Vaccines. Clin. Infect. Dis. 2000, 31, 561–565. [Google Scholar] [CrossRef]
- Andey, T.; Soni, S.; Modi, S. Chapter 3—Conventional vaccination methods: Inactivated and live attenuated vaccines. In Advanced Vaccination Technologies for Infectious and Chronic Diseases; Chavda, V.P., Vora, L.K., Apostolopoulos, V., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 37–50. [Google Scholar]
- Slifka, M.K.; Thomas, A.; Gao, L.; Amanna, I.J.; Orenstein, W.A. Lessons learned from successful implementation of tetanus and diphtheria vaccination programs. Clin. Microbiol. Rev. 2025, 38, e00031-00025. [Google Scholar] [CrossRef]
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef]
- Ivanoff, B.; Levine, M.M.; Lambert, P.H. Vaccination against typhoid fever: Present status. Bull. World Health Organ. 1994, 72, 957–971. [Google Scholar]
- Valenzuela, P.; Medina, A.; Rutter, W.J.; Ammerer, G.; Hall, B.D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 1982, 298, 347–350. [Google Scholar] [CrossRef]
- Burrell, C.J.; Mackay, P.; Greenaway, P.J.; Hofschneider, P.H.; Murray, K. Expression in Escherichia coli of hepatitis B virus DNA sequences cloned in plasmid pBR322. Nature 1979, 279, 43–47. [Google Scholar] [CrossRef]
- McAleer, W.J.; Buynak, E.B.; Maigetter, R.Z.; Wampler, D.E.; Miller, W.J.; Hilleman, M.R. Human hepatitis B vaccine from recombinant yeast. Nature 1984, 307, 178–180. [Google Scholar] [CrossRef]
- Stevens, C.E.; Taylor, P.E.; Tong, M.J.; Toy, P.T.; Vyas, G.N.; Nair, P.V.; Weissman, J.Y.; Krugman, S. Yeast-Recombinant Hepatitis B Vaccine: Efficacy With Hepatitis B Immune Globulin in Prevention of Perinatal Hepatitis B Virus Transmission. JAMA 1987, 257, 2612–2616. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R. Reverse vaccinology. Curr. Opin. Microbiol. 2000, 3, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.M.; Adu-Bobie, J.; Comanducci, M.; Aricò, B.; Savino, S.; Santini, L.; Brunelli, B.; Bambini, S.; Biolchi, A.; Capecchi, B.; et al. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 2006, 103, 10834–10839. [Google Scholar] [CrossRef] [PubMed]
- Dormitzer, P.R.; Ulmer, J.B.; Rappuoli, R. Structure-based antigen design: A strategy for next generation vaccines. Trends Biotechnol. 2008, 26, 659–667. [Google Scholar] [CrossRef]
- Dormitzer, P.R.; Grandi, G.; Rappuoli, R. Structural vaccinology starts to deliver. Nat. Rev. Microbiol. 2012, 10, 807–813. [Google Scholar] [CrossRef]
- Rappuoli, R.; Bottomley, M.J.; D’Oro, U.; Finco, O.; De Gregorio, E. Reverse vaccinology 2.0: Human immunology instructs vaccine antigen design. J. Exp. Med. 2016, 213, 469–481. [Google Scholar] [CrossRef]
- McLellan, J.S.; Chen, M.; Joyce, M.G.; Sastry, M.; Stewart-Jones, G.B.E.; Yang, Y.; Zhang, B.; Chen, L.; Srivatsan, S.; Zheng, A.; et al. Structure-Based Design of a Fusion Glycoprotein Vaccine for Respiratory Syncytial Virus. Science 2013, 342, 592–598. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Goldsmith, J.A.; Schaub, J.M.; DiVenere, A.M.; Kuo, H.-C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-based design of prefusion-stabilized SARS-CoV-2 spikes. Science 2020, 369, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.-H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Wilson, R.; Kovacs, D.; Crosby, M.; Ho, A. Global Epidemiology and Seasonality of Human Seasonal Coronaviruses: A Systematic Review. Open Forum Infect. Dis. 2024, 11, ofae418. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel. Med. 2020, 27, taaa021. [Google Scholar] [CrossRef]
- Alimohamadi, Y.; Tola, H.H.; Abbasi-Ghahramanloo, A.; Janani, M.; Sepandi, M. Case fatality rate of COVID-19: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2021, 62, E311–E320. [Google Scholar]
- Baj, J.; Karakuła-Juchnowicz, H.; Teresiński, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, R.; Forma, A.; Karakuła, K.; Flieger, W.; Portincasa, P.; et al. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef]
- Mackay, I.M.; Arden, K.E. MERS coronavirus: Diagnostics, epidemiology and transmission. Virol. J. 2015, 12, 222. [Google Scholar] [CrossRef]
- Peiris, J.S.M.; Yuen, K.Y.; Osterhaus, A.D.M.E.; Stöhr, K. The Severe Acute Respiratory Syndrome. N. Engl. J. Med. 2003, 349, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Alter, G.; Pulendran, B. Transforming vaccinology. Cell 2024, 187, 5171–5194. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Ye, Z.-W.; Yuan, S.; Yuen, K.-S.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020, 16, 1686–1697. [Google Scholar] [CrossRef]
- Liu, D.X.; Liang, J.Q.; Fung, T.S. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). In Encyclopedia of Virology, 4th ed.; Bamford, D.H., Zuckerman, M., Eds.; Academic Press: Oxford, UK, 2021; pp. 428–440. [Google Scholar]
- Paul, D.; Gupta, A.; Bhatia, V.; Gupta, E. The neglected pathogen: Case reports of severe lower respiratory tract infection by human coronavirus 229E. Access Microbiol. 2022, 4, 000311. [Google Scholar] [CrossRef] [PubMed]
- Vassilara, F.; Spyridaki, A.; Pothitos, G.; Deliveliotou, A.; Papadopoulos, A. A Rare Case of Human Coronavirus 229E Associated with Acute Respiratory Distress Syndrome in a Healthy Adult. Case Rep. Infect. Dis. 2018, 2018, 6796839. [Google Scholar] [CrossRef]
- Zeng, Z.-Q.; Chen, D.-H.; Tan, W.-P.; Qiu, S.-Y.; Xu, D.; Liang, H.-X.; Chen, M.-X.; Li, X.; Lin, Z.-S.; Liu, W.-K.; et al. Epidemiology and clinical characteristics of human coronaviruses OC43, 229E, NL63, and HKU1: A study of hospitalized children with acute respiratory tract infection in Guangzhou, China. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 363–369. [Google Scholar] [CrossRef]
- Nicholson, K.G.; Kent, J.; Hammersley, V.; Cancio, E. Acute viral infections of upper respiratory tract in elderly people living in the community: Comparative, prospective, population based study of disease burden. BMJ 1997, 315, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, S.; Oppong, S.; Bispo de Filippis, A.M.; Gloza-Rausch, F.; Ipsen, A.; Seebens, A.; Müller, M.A.; Annan, A.; Vallo, P.; Adu-Sarkodie, Y.; et al. Distant Relatives of Severe Acute Respiratory Syndrome Coronavirus and Close Relatives of Human Coronavirus 229E in Bats, Ghana. Emerg. Infect. Dis. 2009, 15, 1377. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Baldwin, H.J.; Tateno, A.F.; Zerbinati, R.M.; Annan, A.; Owusu, M.; Nkrumah, E.E.; Maganga, G.D.; Oppong, S.; Adu-Sarkodie, Y.; et al. Evidence for an Ancestral Association of Human Coronavirus 229E with Bats. J. Virol. 2015, 89, 11858–11870. [Google Scholar] [CrossRef]
- Mah, M.G.; Zeller, M.A.; Zhang, R.; Zhuang, Y.; Maro, V.P.; Crump, J.A.; Rubach, M.P.; Ooi, E.E.; Low, J.G.; Wang, D.Y.; et al. Discordant phylodynamic and spatiotemporal transmission patterns driving the long-term persistence and evolution of human coronaviruses. npj Viruses 2024, 2, 49. [Google Scholar] [CrossRef]
- Hamre, D.; Kindig, D.A.; Mann, J. Growth and Intracellular Development of a New Respiratory Virus. J. Virol. 1967, 1, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Bradburne, A.F.; Tyrrell, D.A.J. The propagation of “coronaviruses” in tissue-culture. Arch. Gesamte Virusforsch. 1969, 28, 133–150. [Google Scholar] [CrossRef]
- Ziebuhr, J.; Herold, J.; Siddell, S.G. Characterization of a human coronavirus (strain 229E) 3C-like proteinase activity. J. Virol. 1995, 69, 4331–4338. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.S.F.; Chan, K.-h.; Cheng, V.C.C.; Woo, P.C.Y.; Lau, S.K.P.; Lam, C.C.K.; Chan, T.-l.; Wu, A.K.L.; Hung, I.F.N.; Leung, S.-y.; et al. Comparative Host Gene Transcription by Microarray Analysis Early after Infection of the Huh7 Cell Line by Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus 229E. J. Virol. 2005, 79, 6180–6193. [Google Scholar] [CrossRef]
- Bracci, N.; Pan, H.-C.; Lehman, C.; Kehn-Hall, K.; Lin, S.-C. Improved plaque assay for human coronaviruses 229E and OC43. PeerJ 2020, 8, e10639. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, C.; Chen, D.; Zhu, A.; Li, F.; Zhuang, Z.; Mok, C.K.P.; Dai, J.; Li, X.; Jin, Y.; et al. Mouse models susceptible to HCoV-229E and HCoV-NL63 and cross protection from challenge with SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2023, 120, e2202820120. [Google Scholar] [CrossRef]
- McIntosh, K.; Dees, J.H.; Becker, W.B.; Kapikian, A.Z.; Chanock, R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc. Natl. Acad. Sci. USA 1967, 57, 933–940. [Google Scholar] [CrossRef]
- Reed, S.E. The behaviour of recent isolates of human respiratory coronavirus in vitro and in volunteers: Evidence of heterogeneity among 229E-related strains. J. Med. Virol. 1984, 13, 179–192. [Google Scholar] [CrossRef]
- Brucková, M.; McIntosh, K.; Kapikian, A.Z.; Chanock, R.M. The adaptation of two human coronavirus strains (OC38 and OC43) to growth in cell monolayers. Proc. Soc. Exp. Biol. Med. 1970, 135, 431–435. [Google Scholar] [CrossRef]
- Schmidt, O.W.; Cooney, M.K.; Kenny, G.E. Plaque assay and improved yield of human coronaviruses in a human rhabdomyosarcoma cell line. J. Clin. Microbiol. 1979, 9, 722–728. [Google Scholar] [CrossRef]
- Künkel, F.; Herrler, G. Structural and Functional Analysis of the Surface Protein of Human Coronavirus OC43. Virology 1993, 195, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Vijgen, L.; Keyaerts, E.; Moës, E.; Thoelen, I.; Wollants, E.; Lemey, P.; Vandamme, A.-M.; Ranst, M.V. Complete Genomic Sequence of Human Coronavirus OC43: Molecular Clock Analysis Suggests a Relatively Recent Zoonotic Coronavirus Transmission Event. J. Virol. 2005, 79, 1595–1604. [Google Scholar] [CrossRef]
- Vijgen, L.; Keyaerts, E.; Lemey, P.; Maes, P.; Reeth, K.V.; Nauwynck, H.; Pensaert, M.; Ranst, M.V. Evolutionary History of the Closely Related Group 2 Coronaviruses: Porcine Hemagglutinating Encephalomyelitis Virus, Bovine Coronavirus, and Human Coronavirus OC43. J. Virol. 2006, 80, 7270–7274. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Woo, P.C.Y.; Li, K.S.M.; Tsang, A.K.L.; Fan, R.Y.Y.; Luk, H.K.H.; Cai, J.-P.; Chan, K.-H.; Zheng, B.-J.; Wang, M.; et al. Discovery of a Novel Coronavirus, China Rattus Coronavirus HKU24, from Norway Rats Supports the Murine Origin of Betacoronavirus 1 and Has Implications for the Ancestor of Betacoronavirus Lineage A. J. Virol. 2015, 89, 3076–3092. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Y.; Li, J.; Xiao, Y.; Zhang, J.; Wang, Y.; Chen, L.; Paranhos-Baccalà, G.; Wang, J. Genetic drift of human coronavirus OC43 spike gene during adaptive evolution. Sci. Rep. 2015, 5, 11451. [Google Scholar] [CrossRef]
- Kistler, K.E.; Bedford, T. Evidence for adaptive evolution in the receptor-binding domain of seasonal coronaviruses OC43 and 229e. eLife 2021, 10, e64509. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.; Pewe, L.; Trandem, K.; Perlman, S. Murine encephalitis caused by HCoV-OC43, a human coronavirus with broad species specificity, is partly immune-mediated. Virology 2006, 347, 410–421. [Google Scholar] [CrossRef]
- Keyaerts, E.; Li, S.; Vijgen, L.; Rysman, E.; Verbeeck, J.; Ranst, M.V.; Maes, P. Antiviral Activity of Chloroquine against Human Coronavirus OC43 Infection in Newborn Mice. Antimicrob. Agents Chemother. 2009, 53, 3416–3421. [Google Scholar] [CrossRef]
- Hirose, R.; Watanabe, N.; Bandou, R.; Yoshida, T.; Daidoji, T.; Naito, Y.; Itoh, Y.; Nakaya, T. A Cytopathic Effect-Based Tissue Culture Method for HCoV-OC43 Titration Using TMPRSS2-Expressing VeroE6 Cells. Msphere 2021, 6, e00159-21. [Google Scholar] [CrossRef]
- Savoie, C.; Lippé, R. Optimizing human coronavirus OC43 growth and titration. PeerJ 2022, 10, e13721. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Fang, Y.; Baloch, Z.; Yu, H.; Zhao, Z.; Li, R.; Zhang, T.; Li, R.; Zhao, J.; Yang, Z.; et al. A Mouse-Adapted Model of HCoV-OC43 and Its Usage to the Evaluation of Antiviral Drugs. Front. Microbiol. 2022, 13, 845269. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Lee, W.S.; Chan, W.O.Y.; He, W.; Mah, M.G.; Yong, C.L.; Deerain, J.M.; Wang, L.; Arcinas, C.; Yan, B.; et al. Betacoronaviruses SARS-CoV-2 and HCoV-OC43 infections in IGROV-1 cell line require aryl hydrocarbon receptor. Emerg. Microbes Infect. 2023, 12, 2256416. [Google Scholar] [CrossRef] [PubMed]
- Fouchier, R.A.M.; Hartwig, N.G.; Bestebroer, T.M.; Niemeyer, B.; de Jong, J.C.; Simon, J.H.; Osterhaus, A.D.M.E. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. USA 2004, 101, 6212–6216. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rasool, S.; Fielding, B.C. Understanding Human Coronavirus HCoV-NL63. Open Virol. J. 2010, 4, 76–84. [Google Scholar] [CrossRef]
- Hofmann, H.; Pyrc, K.; van der Hoek, L.; Geier, M.; Berkhout, B.; Pöhlmann, S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [Google Scholar] [CrossRef]
- Pyrc, K.; Dijkman, R.; Deng, L.; Jebbink, M.F.; Ross, H.A.; Berkhout, B.; van der Hoek, L. Mosaic Structure of Human Coronavirus NL63, One Thousand Years of Evolution. J. Mol. Biol. 2006, 364, 964–973. [Google Scholar] [CrossRef]
- Li, W.; Sui, J.; Huang, I.C.; Kuhn, J.H.; Radoshitzky, S.R.; Marasco, W.A.; Choe, H.; Farzan, M. The S proteins of human coronavirus NL63 and severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2. Virology 2007, 367, 367–374. [Google Scholar] [CrossRef]
- Donaldson, E.F.; Haskew, A.N.; Gates, J.E.; Huynh, J.; Moore, C.J.; Frieman, M.B. Metagenomic Analysis of the Viromes of Three North American Bat Species: Viral Diversity among Different Bat Species That Share a Common Habitat. J. Virol. 2010, 84, 13004–13018. [Google Scholar] [CrossRef]
- Huynh, J.; Li, S.; Yount, B.; Smith, A.; Sturges, L.; Olsen, J.C.; Nagel, J.; Johnson, J.B.; Agnihothram, S.; Gates, J.E.; et al. Evidence Supporting a Zoonotic Origin of Human Coronavirus Strain NL63. J. Virol. 2012, 86, 12816–12825. [Google Scholar] [CrossRef]
- Tao, Y.; Shi, M.; Chommanard, C.; Queen, K.; Zhang, J.; Markotter, W.; Kuzmin, I.V.; Holmes, E.C.; Tong, S. Surveillance of Bat Coronaviruses in Kenya Identifies Relatives of Human Coronaviruses NL63 and 229E and Their Recombination History. J. Virol. 2017, 91, e01953-16. [Google Scholar] [CrossRef]
- Schildgen, O.; Jebbink, M.F.; de Vries, M.; Pyrc, K.; Dijkman, R.; Simon, A.; Müller, A.; Kupfer, B.; van der Hoek, L. Identification of cell lines permissive for human coronavirus NL63. J. Virol. Methods 2006, 138, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Herzog, P.; Drosten, C.; Müller, M.A. Plaque assay for human coronavirus NL63 using human colon carcinoma cells. Virol. J. 2008, 5, 138. [Google Scholar] [CrossRef]
- Bentley, J.K.; Kreger, J.E.; Breckenridge, H.A.; Singh, S.; Lei, J.; Li, Y.; Baker, S.C.; Lumeng, C.N.; Hershenson, M.B. Developing a mouse model of human coronavirus NL63 infection: Comparison with rhinovirus-A1B and effects of prior rhinovirus infection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 327, L557–L573. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Woo, P.C.Y.; Yip, C.C.Y.; Tse, H.; Tsoi, H.-w.; Cheng, V.C.C.; Lee, P.; Tang, B.S.F.; Cheung, C.H.Y.; Lee, R.A.; et al. Coronavirus HKU1 and Other Coronavirus Infections in Hong Kong. J. Clin. Microbiol. 2006, 44, 2063–2071. [Google Scholar] [CrossRef]
- Pyrc, K.; Sims, A.C.; Dijkman, R.; Jebbink, M.; Long, C.; Deming, D.; Donaldson, E.; Vabret, A.; Baric, R.; van der Hoek, L.; et al. Culturing the Unculturable: Human Coronavirus HKU1 Infects, Replicates, and Produces Progeny Virions in Human Ciliated Airway Epithelial Cell Cultures. J. Virol. 2010, 84, 11255–11263. [Google Scholar] [CrossRef]
- Hulswit, R.J.G.; Lang, Y.; Bakkers, M.J.G.; Li, W.; Li, Z.; Schouten, A.; Ophorst, B.; van Kuppeveld, F.J.M.; Boons, G.-J.; Bosch, B.-J.; et al. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc. Natl. Acad. Sci. USA 2019, 116, 2681–2690. [Google Scholar] [CrossRef]
- Saunders, N.; Fernandez, I.; Planchais, C.; Michel, V.; Rajah, M.M.; Baquero Salazar, E.; Postal, J.; Porrot, F.; Guivel-Benhassine, F.; Blanc, C.; et al. TMPRSS2 is a functional receptor for human coronavirus HKU1. Nature 2023, 624, 207–214. [Google Scholar] [CrossRef]
- McCallum, M.; Park, Y.-J.; Stewart, C.; Sprouse, K.R.; Addetia, A.; Brown, J.; Tortorici, M.A.; Gibson, C.; Wong, E.; Ieven, M.; et al. Human coronavirus HKU1 recognition of the TMPRSS2 host receptor. Cell 2024, 187, 4231–4245.e4213. [Google Scholar] [CrossRef]
- Jin, M.; Hassan, Z.; Li, Z.; Liu, Y.; Marakhovskaia, A.; Wong, A.H.M.; Forman, A.; Nitz, M.; Gilbert, M.; Yu, H.; et al. Human coronavirus HKU1 spike structures reveal the basis for sialoglycan specificity and carbohydrate-promoted conformational changes. Nat. Commun. 2025, 16, 4158. [Google Scholar] [CrossRef]
- Sun, J.; Sun, D.; Yang, Q.; Wang, D.; Peng, J.; Guo, H.; Ding, X.; Chen, Z.; Yuan, B.; Ivanenkov, Y.A.; et al. A novel, covalent broad-spectrum inhibitor targeting human coronavirus Mpro. Nat. Commun. 2025, 16, 4546. [Google Scholar] [CrossRef] [PubMed]
- Yeager, C.L.; Ashmun, R.A.; Williams, R.K.; Cardellichio, C.B.; Shapiro, L.H.; Look, A.T.; Holmes, K.V. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature 1992, 357, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tomlinson, A.C.A.; Wong, A.H.M.; Zhou, D.; Desforges, M.; Talbot, P.J.; Benlekbir, S.; Rubinstein, J.L.; Rini, J.M. The human coronavirus HCoV-229E S-protein structure and receptor binding. eLife 2019, 8, e51230. [Google Scholar] [CrossRef]
- Corman, V.M.; Eckerle, I.; Memish, Z.A.; Liljander, A.M.; Dijkman, R.; Jonsdottir, H.; Juma Ngeiywa, K.J.Z.; Kamau, E.; Younan, M.; Al Masri, M.; et al. Link of a ubiquitous human coronavirus to dromedary camels. Proc. Natl. Acad. Sci. USA 2016, 113, 9864–9869. [Google Scholar] [CrossRef]
- Jacomy, H.; Talbot, P.J. Vacuolating encephalitis in mice infected by human coronavirus OC43. Virology 2003, 315, 20–33. [Google Scholar] [CrossRef]
- Jacomy, H.; Fragoso, G.; Almazan, G.; Mushynski, W.E.; Talbot, P.J. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology 2006, 349, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, O.S.; Waffa, Z. Neuropathogenic human coronaviruses: A review. Rev. Med. Virol. 2020, 30, e2118. [Google Scholar] [CrossRef]
- Morfopoulou, S.; Brown, J.R.; Davies, E.G.; Anderson, G.; Virasami, A.; Qasim, W.; Chong, W.K.; Hubank, M.; Plagnol, V.; Desforges, M.; et al. Human Coronavirus OC43 Associated with Fatal Encephalitis. N. Engl. J. Med. 2016, 375, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Edner, N.; Albert, J.; Ternhag, A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect. Dis. 2020, 52, 419–422. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Du, C.-J.; Chang, C.-C.; Chen, S.-H.; Chen, C.-J.; Chiu, C.-Y.; Chiu, C.-H. Human coronavirus OC43 infection associated pneumonia in a girl with acute lymphoblastic leukemia: A case report. Medicine 2020, 99, e21520. [Google Scholar] [CrossRef]
- El-Sahly, H.M.; Atmar, R.L.; Glezen, W.P.; Greenberg, S.B. Spectrum of Clinical Illness in Hospitalized Patients with “Common Cold” Virus Infections. Clin. Infect. Dis. 2000, 31, 96–100. [Google Scholar] [CrossRef]
- Gagneur, A.; Sizun, J.; Vallet, S.; Legr, M.C.; Picard, B.; Talbot, P.J. Coronavirus-related nosocomial viral respiratory infections in a neonatal and paediatric intensive care unit: A prospective study. J. Hosp. Infect. 2002, 51, 59–64. [Google Scholar] [CrossRef]
- Suresha, P.G.; Akhil, C.; Anjali, A.; Giselle, D.R.; Revti, B.; Arunkumar, G. Human coronaviruses in severe acute respiratory infection (SARI) cases in southwest India. J. Med. Virol. 2016, 88, 163–165. [Google Scholar] [CrossRef]
- Veiga, A.B.G.d.; Martins, L.G.; Riediger, I.; Mazetto, A.; Debur, M.d.C.; Gregianini, T.S. More than just a common cold: Endemic coronaviruses OC43, HKU1, NL63, and 229E associated with severe acute respiratory infection and fatality cases among healthy adults. J. Med. Virol. 2021, 93, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Kawataki, M.; Ito, A.; Ishida, T. Pneumonia Due to Human Coronavirus OC43 in an Immunocompetent Adult Detected by Multiplex Polymerase Chain Reaction. Intern. Med. 2021, 60, 3497–3501. [Google Scholar] [CrossRef]
- van der Hoek, L.; Sure, K.; Ihorst, G.; Stang, A.; Pyrc, K.; Jebbink, M.F.; Petersen, G.; Forster, J.; Berkhout, B.; Überla, K. Croup Is Associated with the Novel Coronavirus NL63. PLoS Med. 2005, 2, e240. [Google Scholar] [CrossRef] [PubMed]
- Moës, E.; Vijgen, L.; Keyaerts, E.; Zlateva, K.; Li, S.; Maes, P.; Pyrc, K.; Berkhout, B.; van der Hoek, L.; Van Ranst, M. A novel pancoronavirus RT-PCR assay: Frequent detection of human coronavirus NL63 in children hospitalized with respiratory tract infections in Belgium. BMC Infect. Dis. 2005, 5, 6. [Google Scholar] [CrossRef]
- Wu, P.-S.; Chang, L.-Y.; Berkhout, B.; van der Hoek, L.; Lu, C.-Y.; Kao, C.-L.; Lee, P.-I.; Shao, P.-L.; Lee, C.-Y.; Huang, F.-Y.; et al. Clinical manifestations of human coronavirus NL63 infection in children in Taiwan. Eur. J. Pediatr. 2008, 167, 75–80. [Google Scholar] [CrossRef]
- Al-Zughoul, A.B.; Elhassan, M.G. Three in One: A Case Report of Pulmonary Co-infection With a Virus, a Bacterium, and a Fungus in an Immunocompetent Adult. Cureus 2025, 17, e82025. [Google Scholar] [CrossRef] [PubMed]
- Arden, K.E.; Nissen, M.D.; Sloots, T.P.; Mackay, I.M. New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J. Med. Virol. 2005, 75, 455–462. [Google Scholar] [CrossRef]
- Wu, K.; Li, W.; Peng, G.; Li, F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc. Natl. Acad. Sci. USA 2009, 106, 19970–19974. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Tsoi, H.-w.; Huang, Y.; Poon, R.W.S.; Chu, C.-m.; Lee, R.A.; Luk, W.-k.; Wong, G.K.M.; Wong, B.H.L.; et al. Clinical and Molecular Epidemiological Features of Coronavirus HKU1–Associated Community-Acquired Pneumonia. J. Infect. Dis. 2005, 192, 1898–1907. [Google Scholar] [CrossRef]
- SUN, J.; NIE, W.; HUANG, Y. Death linked to human coronaviruses HKU1 infection in Guizhou province: A case report. Chin. J. Public Health 2020, 36, 246–249. [Google Scholar]
- Madaras, L.; Anvari, R.; Schuchardt-Peet, C.; Hoskote, A.; Kashyap, R. Co-infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and human coronavirus HKU1 (HCoV-HKU1). Eur. J. Case Rep. Intern. Med. 2025, 12, 005068. [Google Scholar]
- Wang, W.; Lin, X.-D.; Guo, W.-P.; Zhou, R.-H.; Wang, M.-R.; Wang, C.-Q.; Ge, S.; Mei, S.-H.; Li, M.-H.; Shi, M.; et al. Discovery, diversity and evolution of novel coronaviruses sampled from rodents in China. Virology 2015, 474, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Huang, Y.; Tsoi, H.W.; Chan, K.H.; Yuen, K.Y. Phylogenetic and recombination analysis of coronavirus HKU1, a novel coronavirus from patients with pneumonia. Arch. Virol. 2005, 150, 2299–2311. [Google Scholar] [CrossRef]
- Gunawardene, C.D.; Pandey, I.; Chatterjee, S.; Penaflor-Tellez, Y.; Odle, A.; Messyasz, A.; Rajsbaum, R.; Sariol, A.; Wong, L.-Y.R. Characterization of betacoronavirus HKU-1 and OC43 internal proteins using a prototypic coronavirus. bioRxiv 2025, bioRxiv 2025.04.23.650176. [Google Scholar] [CrossRef]
- Gartner, M.J.; Smith, M.L.; Dapat, C.; Liaw, Y.W.; Tran, T.; Suryadinata, R.; Chen, J.; Sun, G.; Shepherd, R.A.; Taiaroa, G.; et al. Contemporary seasonal human coronaviruses display differences in cellular tropism compared to laboratory-adapted reference strains. J. Virol. 2025, 99, e0068425. [Google Scholar] [CrossRef]
- Kitai, Y.; Kojima, S.; Aishajiang, A.; Kawase, M.; Watanabe, O.; Yabukami, H.; Hashimoto, R.; Akahori, Y.; Katoh, H.; Takayama, K.; et al. Changes in ORF4 of HCoV-229E under different culture conditions. J. Gen. Virol. 2025, 106, 002131. [Google Scholar] [CrossRef]
- Wratil, P.R.; Schmacke, N.A.; Karakoc, B.; Dulovic, A.; Junker, D.; Becker, M.; Rothbauer, U.; Osterman, A.; Spaeth, P.M.; Ruhle, A.; et al. Evidence for increased SARS-CoV-2 susceptibility and COVID-19 severity related to pre-existing immunity to seasonal coronaviruses. Cell Rep. 2021, 37, 110169. [Google Scholar] [CrossRef]
- Trifonova, I.; Korsun, N.; Madzharova, I.; Velikov, P.; Alexiev, I.; Grigorova, L.; Voleva, S.; Yordanova, R.; Ivanov, I.; Tcherveniakova, T.; et al. Prevalence and clinical impact of mono- and co-infections with endemic coronaviruses 229E, OC43, NL63, and HKU-1 during the COVID-19 pandemic. Heliyon 2024, 10, e29258. [Google Scholar] [CrossRef]
- Golpour, M.; Jalali, H.; Alizadeh-Navaei, R.; Talarposhti, M.R.; Mousavi, T.; Ghara, A.A.N. Co-infection of SARS-CoV-2 and influenza A/B among patients with COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2025, 25, 145. [Google Scholar] [CrossRef]
- Matsuno, A.K.; Gagliardi, T.B.; Paula, F.E.; Luna, L.K.S.; Jesus, B.L.S.; Stein, R.T.; Aragon, D.C.; Carlotti, A.P.C.P.; Arruda, E. Human coronavirus alone or in co-infection with rhinovirus C is a risk factor for severe respiratory disease and admission to the pediatric intensive care unit: A one-year study in Southeast Brazil. PLoS ONE 2019, 14, e0217744. [Google Scholar] [CrossRef]
- Pollett, S.; Conte, M.A.; Sanborn, M.; Jarman, R.G.; Lidl, G.M.; Modjarrad, K.; Maljkovic Berry, I. A comparative recombination analysis of human coronaviruses and implications for the SARS-CoV-2 pandemic. Sci. Rep. 2021, 11, 17365. [Google Scholar] [CrossRef]
- Wells, H.L.; Bonavita, C.M.; Navarrete-Macias, I.; Vilchez, B.; Rasmussen, A.L.; Anthony, S.J. The coronavirus recombination pathway. Cell Host Microbe 2023, 31, 874–889. [Google Scholar] [CrossRef]
- Focosi, D.; Maggi, F. Recombination in Coronaviruses, with a Focus on SARS-CoV-2. Viruses 2022, 14, 1239. [Google Scholar] [CrossRef] [PubMed]
- Dudas, G.; Rambaut, A. MERS-CoV recombination: Implications about the reservoir and potential for adaptation. Virus Evol. 2016, 2, vev023. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, H.; Ji, Y.; Liao, H. Varicella-Zoster Virus Infection and Varicella-Zoster Virus Vaccine-Related Ocular Complications. Vaccines 2025, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, L.; Zhang, S.; Wang, H.; Xue, W.; Li, H.; Zhang, Y.; Chen, L.; Gu, Y.; Li, T.; et al. Research Progress on Varicella-Zoster Virus Vaccines. Vaccines 2025, 13, 730. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 2022, 28, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.M.; Doster, J.M.; Landwehr, E.H.; Kumar, N.P.; White, E.J.; Beachboard, D.C.; Stobart, C.C. Evaluating the Virology and Evolution of Seasonal Human Coronaviruses Associated with the Common Cold in the COVID-19 Era. Microorganisms 2023, 11, 445. [Google Scholar] [CrossRef]
- Otieno, J.R.; Cherry, J.L.; Spiro, D.J.; Nelson, M.I.; Trovão, N.S. Origins and Evolution of Seasonal Human Coronaviruses. Viruses 2022, 14, 1551. [Google Scholar] [CrossRef] [PubMed]
- McBride, R.; Van Zyl, M.; Fielding, B.C. The Coronavirus Nucleocapsid Is a Multifunctional Protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef] [PubMed]
- Sola, I.; Almazán, F.; Zúñiga, S.; Enjuanes, L. Continuous and Discontinuous RNA Synthesis in Coronaviruses. Annu. Rev. Virol. 2015, 2, 265–288. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, K. Functional studies of the coronavirus nonstructural proteins. STEMedicine 2020, 1, e39. [Google Scholar] [CrossRef]
- Ziebuhr, J.; Schelle, B.; Karl, N.; Minskaia, E.; Bayer, S.; Siddell, S.G.; Gorbalenya, A.E.; Thiel, V. Human Coronavirus 229E Papain-Like Proteases Have Overlapping Specificities but Distinct Functions in Viral Replication. J. Virol. 2007, 81, 3922–3932. [Google Scholar] [CrossRef]
- Stobart, C.C.; Sexton, N.R.; Munjal, H.; Lu, X.; Molland, K.L.; Tomar, S.; Mesecar, A.D.; Denison, M.R. Chimeric Exchange of Coronavirus nsp5 Proteases (3CLpro) Identifies Common and Divergent Regulatory Determinants of Protease Activity. J. Virol. 2013, 87, 12611–12618. [Google Scholar] [CrossRef]
- Nemr, W.A.; Radwan, N.K. Typing of alpha and beta coronaviruses by DNA barcoding of NSP12 gene. J. Med. Virol. 2022, 94, 1926–1934. [Google Scholar] [CrossRef]
- Ivanov, K.A.; Ziebuhr, J. Human Coronavirus 229E Nonstructural Protein 13: Characterization of Duplex-Unwinding, Nucleoside Triphosphatase, and RNA 5′-Triphosphatase Activities. J. Virol. 2004, 78, 7833–7838. [Google Scholar] [CrossRef]
- Tahir, M. Coronavirus genomic nsp14-ExoN, structure, role, mechanism, and potential application as a drug target. J. Med. Virol. 2021, 93, 4258–4264. [Google Scholar] [CrossRef]
- Deng, X.; Baker, S.C. An “Old” protein with a new story: Coronavirus endoribonuclease is important for evading host antiviral defenses. Virology 2018, 517, 157–163. [Google Scholar] [CrossRef]
- Dostalik, P.; Krafcikova, P.; Silhan, J.; Kozic, J.; Chalupska, D.; Chalupsky, K.; Boura, E. Structural Analysis of the OC43 Coronavirus 2′-O-RNA Methyltransferase. J. Virol. 2021, 95, e0046321. [Google Scholar] [CrossRef]
- Kasuga, Y.; Zhu, B.; Jang, K.-J.; Yoo, J.-S. Innate immune sensing of coronavirus and viral evasion strategies. Exp. Mol. Med. 2021, 53, 723–736. [Google Scholar] [CrossRef]
- Desforges, M.; Desjardins, J.; Zhang, C.; Talbot, P.J. The Acetyl-Esterase Activity of the Hemagglutinin-Esterase Protein of Human Coronavirus OC43 Strongly Enhances the Production of Infectious Virus. J. Virol. 2013, 87, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- Herold, J.; Raabe, T.; Schelle-Prinz, B.; Siddell, S.G. Nucleotide Sequence of the Human Coronavirus 229E RNA Polymerase Locus. Virology 1993, 195, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, K.; Lv, W.; Yu, W.; Xie, S.; Xu, K.; Schwarz, W.; Xiong, S.; Sun, B. The ORF4a protein of human coronavirus 229E functions as a viroporin that regulates viral production. Biochim. Biophys. Acta 2014, 1838, 1088–1095. [Google Scholar] [CrossRef]
- Dijkman, R.; Jebbink, M.F.; Wilbrink, B.; Pyrc, K.; Zaaijer, H.L.; Minor, P.D.; Franklin, S.; Berkhout, B.; Thiel, V.; van der Hoek, L. Human coronavirus 229E encodes a single ORF4 protein between the spike and the envelope genes. Virol. J. 2006, 3, 106. [Google Scholar] [CrossRef]
- Müller, M.A.; van der Hoek, L.; Voss, D.; Bader, O.; Lehmann, D.; Schulz, A.R.; Kallies, S.; Suliman, T.; Fielding, B.C.; Drosten, C.; et al. Human Coronavirus NL63 Open Reading Frame 3 encodes a virion-incorporated N-glycosylated membrane protein. Virol. J. 2010, 7, 6. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, K.; Ping, X.; Yu, W.; Qian, Z.; Xiong, S.; Sun, B. The ns12.9 Accessory Protein of Human Coronavirus OC43 Is a Viroporin Involved in Virion Morphogenesis and Pathogenesis. J. Virol. 2015, 89, 11383–11395. [Google Scholar] [CrossRef]
- Labonté, P.; Mounir, S.; Talbot, P.J. Sequence and expression of the ns2 protein gene of human coronavirus OC43. J. Gen. Virol. 1995, 76, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.J.; Parker, M.D.; Babiuk, L.A. Bovine coronavirus nonstructural protein ns2 is a phosphoprotein. Virology 1991, 185, 509–512. [Google Scholar] [CrossRef]
- Zhao, L.; Jha, B.K.; Wu, A.; Elliott, R.; Ziebuhr, J.; Gorbalenya, A.E.; Silverman, R.H.; Weiss, S.R. Antagonism of the Interferon-Induced OAS-RNase L Pathway by Murine Coronavirus ns2 Protein Is Required for Virus Replication and Liver Pathology. Cell Host Microbe 2012, 11, 607–616. [Google Scholar] [CrossRef]
- Vijgen, L.; Keyaerts, E.; Lemey, P.; Moës, E.; Li, S.; Vandamme, A.-M.; Van Ranst, M. Circulation of genetically distinct contemporary human coronavirus OC43 strains. Virology 2005, 337, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Beniac, D.R.; Andonov, A.; Grudeski, E.; Booth, T.F. Architecture of the SARS coronavirus prefusion spike. Nat. Struct. Mol. Biol. 2006, 13, 751–752. [Google Scholar] [CrossRef]
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 2020, 588, 498–502. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Liu, S.; Hu, M.; Liu, X.; Liu, X.; Chen, T.; Zhu, Y.; Liang, T.; Xiao, S.; Li, P.; Ma, X. Nanoparticles and Antiviral Vaccines. Vaccines 2024, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zou, F.; Yu, F.; Li, R.; Yuan, Y.; Zhang, Y.; Zhang, X.; Deng, J.; Chen, T.; Song, Z.; et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity 2020, 53, 1315–1330.e1319. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, J.; Wang, N.; Corbett, K.S.; Wrapp, D.; Kirchdoerfer, R.N.; Turner, H.L.; Cottrell, C.A.; Becker, M.M.; Wang, L.; Shi, W.; et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA 2017, 114, E7348–E7357. [Google Scholar] [CrossRef]
- Zhang, L.; Jackson, C.B.; Mou, H.; Ojha, A.; Peng, H.; Quinlan, B.D.; Rangarajan, E.S.; Pan, A.; Vanderheiden, A.; Suthar, M.S.; et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. 2020, 11, 6013. [Google Scholar] [CrossRef]
- Kirchdoerfer, R.N.; Cottrell, C.A.; Wang, N.; Pallesen, J.; Yassine, H.M.; Turner, H.L.; Corbett, K.S.; Graham, B.S.; McLellan, J.S.; Ward, A.B. Pre-fusion structure of a human coronavirus spike protein. Nature 2016, 531, 118–121. [Google Scholar] [CrossRef]
- White, C.L.; Richards, K.A.; Huertas, N., Jr.; Nayak, J.L.; Sant, A.J. Human HKU1-Reactive CD4 T Cells Are Enriched for Cytolytic Potential That Persists in Older Adults. J. Infect. Dis. 2025, 231, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef]
- Devaux, C.A.; Fantini, J. Unravelling Antigenic Cross-Reactions toward the World of Coronaviruses: Extent of the Stability of Shared Epitopes and SARS-CoV-2 Anti-Spike Cross-Neutralizing Antibodies. Pathogens 2023, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Artiles, A.; Nanaware, P.P.; Muneeruddin, K.; Weaver, G.C.; Shaffer, S.A.; Calvo-Calle, J.M.; Stern, L.J. Immunopeptidome profiling of human coronavirus OC43-infected cells identifies CD4 T-cell epitopes specific to seasonal coronaviruses or cross-reactive with SARS-CoV-2. PLoS Pathog. 2023, 19, e1011032. [Google Scholar] [CrossRef]
- dos Santos Alves, R.P.; Timis, J.; Miller, R.; Valentine, K.; Pinto, P.B.A.; Gonzalez, A.; Regla-Nava, J.A.; Maule, E.; Nguyen, M.N.; Shafee, N.; et al. Human coronavirus OC43-elicited CD4+ T cells protect against SARS-CoV-2 in HLA transgenic mice. Nat. Commun. 2024, 15, 787. [Google Scholar] [CrossRef]
- Sanders, B.; Koldijk, M.; Schuitemaker, H. Inactivated Viral Vaccines. In Vaccine Analysis: Strategies, Principles, and Control; Nunnally, B.K., Turula, V.E., Sitrin, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 45–80. [Google Scholar]
- Zhang, H.; Deng, X.; Dai, R.; Fu, J.; Ding, L.; Hu, X.; Sun, P.; Shu, R.; Chen, L.; Xu, X. Inadequate immune response to inactivated COVID-19 vaccine among older people living with HIV: A prospective cohort study. J. Virol. 2025, 99, e0068825. [Google Scholar] [CrossRef]
- Shi, J.; Shen, A.; Cheng, Y.; Zhang, C.; Yang, X. 30-Year Development of Inactivated Virus Vaccine in China. Pharmaceutics 2023, 15, 2721. [Google Scholar] [CrossRef]
- Wright, A.E.; Semple, D. Remarks on Vaccination against Typhoid Fever. Brit. Med. J. 1897, 1, 256–259. [Google Scholar] [CrossRef]
- Hankin, E.H. Remarks on Haffkine’s Method of Protective Inoculation Against Cholera. Brit. Med. J. 1892, 2, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Salk, J.E.; Francis, T. Immunization against influenza. Ann. Intern. Med. 1946, 25, 443–452. [Google Scholar] [CrossRef]
- Greenberg, D.P.; Robertson, C.A.; Noss, M.J.; Blatter, M.M.; Biedenbender, R.; Decker, M.D. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine 2013, 31, 770–776. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721.e719. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Rauh, L.W.; Schmidt, R. Measles Immunization With Killed Virus Vaccine: Serum Antibody Titers and Experience with Exposure to Measles Epidemic. Am. J. Dis. Child. 1965, 109, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Liao, F.-L.; Wang, H.; Tang, H.-B.; Yang, Z.-Q.; Hou, W. Evaluation of Antibody-Dependent Enhancement of SARS-CoV Infection in Rhesus Macaques Immunized with an Inactivated SARS-CoV Vaccine. Virol. Sin. 2018, 33, 201–204. [Google Scholar] [CrossRef]
- Agrawal, A.S.; Tao, X.; Algaissi, A.; Garron, T.; Narayanan, K.; Peng, B.-H.; Couch, R.B.; Tseng, C.-T.K. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum. Vaccin. Immunother. 2016, 12, 2351–2356. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct Gene Transfer into Mouse Muscle In Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Gurunathan, S. DNA Vaccines—Designer Vaccines for the 21st Century. N. Engl. J. Med. 1999, 341, 277–278. [Google Scholar] [CrossRef]
- Gurunathan, S.; Klinman, D.M.; Seder, R.A. DNA Vaccines: Immunology, Application, and Optimization. Annu. Rev. Immunol. 2000, 18, 927–974. [Google Scholar] [CrossRef]
- Davis, H.L.; Michel, M.-L.; Whalen, R.G. DNA-based immunization induces continuous secretion of hepatitis B surface antigen and high levels of circulating antibody. Hum. Mol. Genet. 1993, 2, 1847–1851. [Google Scholar] [CrossRef]
- Fynan, E.F.; Webster, R.G.; Fuller, D.H.; Haynes, J.R.; Santoro, J.C.; Robinson, H.L. DNA vaccines: Protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl. Acad. Sci. USA 1993, 90, 11478–11482. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.B.; Donnelly, J.J.; Parker, S.E.; Rhodes, G.H.; Felgner, P.L.; Dwarki, V.J.; Gromkowski, S.H.; Deck, R.R.; DeWitt, C.M.; Friedman, A.; et al. Heterologous Protection Against Influenza by Injection of DNA Encoding a Viral Protein. Science 1993, 259, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, S.; Dema, B.; Sanchez-Martinez, A.; Montalvo Zurbia-Flores, G.; Rollier, C.S. DNA Vaccines: History, Molecular Mechanisms and Future Perspectives. J. Mol. Biol. 2023, 435, 168297. [Google Scholar] [CrossRef]
- Dey, A.; Chozhavel Rajanathan, T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef] [PubMed]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Lu, Y.; Qian, C.; Huang, Y.; Ren, T.; Xie, W.; Xia, N.; Li, S. Advancing mRNA vaccines: A comprehensive review of design, delivery, and efficacy in infectious diseases. Int. J. Biol. Macromol. 2025, 319, 145501. [Google Scholar] [CrossRef]
- Leong, K.Y.; Tham, S.K.; Poh, C.L. Revolutionizing immunization: A comprehensive review of mRNA vaccine technology and applications. Virol. J. 2025, 22, 71. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of mRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Bai, Y.; Liu, J.; Wang, Y.; He, Q.; Zhang, X.; Cheng, F.; Xu, M.; Mao, Q.; Liang, Z. Research progress on the quality control of mRNA vaccines. Expert Rev. Vaccines 2024, 23, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Jirikowski, G.F.; Sanna, P.P.; Maciejewski-Lenoir, D.; Bloom, F.E. Reversal of Diabetes Insipidus in Brattleboro Rats: Intrahypothalamic Injection of Vasopressin mRNA. Science 1992, 255, 996–998. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.-J.; Stitz, L.; et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216. [Google Scholar] [CrossRef]
- Leal, L.; Guardo, A.C.; Morón-López, S.; Salgado, M.; Mothe, B.; Heirman, C.; Pannus, P.; Vanham, G.; van den Ham, H.J.; Gruters, R.; et al. Phase I clinical trial of an intranodally administered mRNA-based therapeutic vaccine against HIV-1 infection. AIDS 2018, 32, 2533–2545. [Google Scholar] [CrossRef]
- Tai, W.; Zhang, X.; Yang, Y.; Zhu, J.; Du, L. Advances in mRNA and other vaccines against MERS-CoV. Transl. Res. 2022, 242, 20–37. [Google Scholar] [CrossRef]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 2017, 168, 1114–1125.e1110. [Google Scholar] [CrossRef]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Baden, L.R.; Sahly, H.M.E.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Pollet, J.; Chen, W.-H.; Strych, U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliver. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Wang, Y. Recent advances in the production of recombinant subunit vaccines in Pichia pastoris. Bioengineered 2016, 7, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Brisse, M.; Vrba, S.M.; Kirk, N.; Liang, Y.; Ly, H. Emerging Concepts and Technologies in Vaccine Development. Front. Immunol. 2020, 11, 583077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Brady, M.I.; Furminger, I.G.S. A surface antigen influenza vaccine. 1. Purification of haemagglutinin and neuraminidase proteins. J. Hyg. 1976, 77, 161–172. [Google Scholar] [CrossRef]
- Brady, M.I.; Furminger, I.G.S. A surface antigen influenza vaccine: 2. Pyrogenicity and antigenicity. J. Hyg. 1976, 77, 173–180. [Google Scholar] [CrossRef]
- Walsh, E.E.; Marc, G.P.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Cox, M.M.J.; Hollister, J.R. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals 2009, 37, 182–189. [Google Scholar] [CrossRef]
- Cooper, C.L.; Davis, H.L.; Morris, M.L.; Efler, S.M.; Adhami, M.A.; Krieg, A.M.; Cameron, D.W.; Heathcote, J. CPG 7909, an Immunostimulatory TLR9 Agonist Oligodeoxynucleotide, as Adjuvant to Engerix-B® HBV Vaccine in Healthy Adults: A Double-Blind Phase I/II Study. J. Clin. Immunol. 2004, 24, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; Bouchard, C.; Mao, C.; Mehlsen, J.; Moreira, E.D.; Ngan, Y.; Petersen, L.K.; Lazcano-Ponce, E.; et al. A 9-Valent HPV Vaccine against Infection and Intraepithelial Neoplasia in Women. N. Engl. J. Med. 2015, 372, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Más-Bermejo, P.I.; Dickinson-Meneses, F.O.; Almenares-Rodríguez, K.; Sánchez-Valdés, L.; Guinovart-Díaz, R.; Vidal-Ledo, M.; Galbán-García, E.; Olivera-Nodarse, Y.; Morgado-Vega, I.; Dueñas-Carrera, S.; et al. Cuban Abdala vaccine: Effectiveness in preventing severe disease and death from COVID-19 in Havana, Cuba; A cohort study. Lancet Reg. Health Am. 2022, 16, 100366. [Google Scholar] [PubMed]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral vectored vaccines: Design, development, preventive and therapeutic applications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef]
- Tatsis, N.; Ertl, H.C.J. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef]
- Pastoret, P.P.; Vanderplasschen, A. Poxviruses as vaccine vectors. Comp. Immunol. Microbiol. Infect. Dis. 2003, 26, 343–355. [Google Scholar] [CrossRef]
- Isakova-Sivak, I.; Tretiak, T.; Rudenko, L. Cold-adapted influenza viruses as a promising platform for viral-vector vaccines. Expert Rev. Vaccines 2016, 15, 1241–1243. [Google Scholar] [CrossRef]
- Draper, S.J.; Heeney, J.L. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 2010, 8, 62–73. [Google Scholar] [CrossRef]
- Suhaimi, S.N.A.A.; Zaki, I.A.H.; Noordin, Z.M.; Hussin, N.S.M.; Ming, L.C.; Zulkifly, H.H. COVID-19 vaccine-induced immune thrombotic thrombocytopenia: A review. Clin. Exp. Vaccine Res. 2023, 12, 265–290. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, S.; Liu, J.; Wu, L.; Qiu, J.; Wang, N.; Ren, J.; Li, Z.; Guo, X.; Tao, F.; et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med. 2022, 20, 400. [Google Scholar] [CrossRef] [PubMed]
- Le Nouën, C.; Nelson, C.E.; Liu, X.; Park, H.-S.; Matsuoka, Y.; Luongo, C.; Santos, C.; Yang, L.; Herbert, R.; Castens, A.; et al. Intranasal pediatric parainfluenza virus-vectored SARS-CoV-2 vaccine is protective in monkeys. Cell 2022, 185, 4811–4825.e4817. [Google Scholar] [CrossRef]
- DiNapoli, J.M.; Kotelkin, A.; Yang, L.; Elankumaran, S.; Murphy, B.R.; Samal, S.K.; Collins, P.L.; Bukreyev, A. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc. Natl. Acad. Sci. USA 2007, 104, 9788–9793. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; et al. Safety and immunogenicity of a live-attenuated influenza virus vector-based intranasal SARS-CoV-2 vaccine in adults: Randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Lauer, K.B.; Borrow, R.; Blanchard, T.J. Multivalent and Multipathogen Viral Vector Vaccines. Clin. Vaccine Immunol. 2017, 24, e00298-16. [Google Scholar] [CrossRef]
- Beavis, A.C.; Li, Z.; Briggs, K.; Gingerich, M.C.; Wrobel, E.R.; Najera, M.; An, D.; Orr-Burks, N.; Murray, J.; Patil, P.; et al. Efficacy of parainfluenza virus 5 (PIV5)-vectored intranasal COVID-19 vaccine as a single dose primer and booster against SARS-CoV-2 variants. J. Virol. 2025, 99, e0198924. [Google Scholar] [CrossRef]
- Chackerian, B. Virus-like particles: Flexible platforms for vaccine development. Expert Rev. Vaccines 2007, 6, 381–390. [Google Scholar] [CrossRef]
- Irvine, D.J.; Read, B.J. Shaping humoral immunity to vaccines through antigen-displaying nanoparticles. Curr. Opin. Immunol. 2020, 65, 1–6. [Google Scholar] [CrossRef]
- Gupta, R.; Arora, K.; Roy, S.S.; Joseph, A.; Rastogi, R.; Arora, N.M.; Kundu, P.K. Platforms, advances, and technical challenges in virus-like particles-based vaccines. Front. Immunol. 2023, 14, 1123805. [Google Scholar] [CrossRef]
- Donaldson, B.; Lateef, Z.; Walker, G.F.; Young, S.L.; Ward, V.K. Virus-like particle vaccines: Immunology and formulation for clinical translation. Expert Rev. Vaccines 2018, 17, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Pham, M.N.; Shehata, L.; Brinkkemper, M.; Boyoglu-Barnum, S.; Sprouse, K.R.; Walls, A.C.; Cheng, S.; Murphy, M.; Pettie, D.; et al. Antigen- and scaffold-specific antibody responses to protein nanoparticle immunogens. Cell Rep. Med. 2022, 3, 100780. [Google Scholar] [CrossRef]
- Pillet, S.; Aubin, É.; Trépanier, S.; Bussière, D.; Dargis, M.; Poulin, J.-F.; Yassine-Diab, B.; Ward, B.J.; Landry, N. A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin. Immunol. 2016, 168, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Hager, K.J.; Marc, G.P.; Gobeil, P.; Diaz, R.S.; Heizer, G.; Llapur, C.; Makarkov, A.I.; Vasconcellos, E.; Pillet, S.; Riera, F.; et al. Efficacy and Safety of a Recombinant Plant-Based Adjuvanted COVID-19 Vaccine. N. Engl. J. Med. 2022, 386, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, A.-C.; Ontsouka, B.; Bozic, J.; Diress, A.; Ahmed, T.; Berthoud, T.; Tran, A.; Duque, D.; Liao, M.; McCluskie, M.; et al. An enveloped virus-like particle vaccine expressing a stabilized prefusion form of the SARS-CoV-2 spike protein elicits highly potent immunity. Vaccine 2021, 39, 4988–5001. [Google Scholar] [CrossRef]
- Cohen, A.A.; van Doremalen, N.; Greaney, A.J.; Andersen, H.; Sharma, A.; Starr, T.N.; Keeffe, J.R.; Fan, C.; Schulz, J.E.; Gnanapragasam, P.N.P.; et al. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science 2022, 377, eabq0839. [Google Scholar] [CrossRef]
- Dijkman, R.; Jebbink, M.F.; Gaunt, E.; Rossen, J.W.A.; Templeton, K.E.; Kuijpers, T.W.; van der Hoek, L. The dominance of human coronavirus OC43 and NL63 infections in infants. J. Clin. Virol. 2012, 53, 135–139. [Google Scholar] [CrossRef]
- Dijkman, R.; Jebbink, M.F.; Idrissi, N.B.E.; Pyrc, K.; Müller, M.A.; Kuijpers, T.W.; Zaaijer, H.L.; Hoek, L.v.d. Human Coronavirus NL63 and 229E Seroconversion in Children. J. Clin. Microbiol. 2008, 46, 2368–2373. [Google Scholar] [CrossRef]
- Edridge, A.W.D.; Kaczorowska, J.; Hoste, A.C.R.; Bakker, M.; Klein, M.; Loens, K.; Jebbink, M.F.; Matser, A.; Kinsella, C.M.; Rueda, P.; et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef]
- Che, X.-Y.; Qiu, L.-W.; Liao, Z.-Y.; Wang, Y.-D.; Wen, K.; Pan, Y.-X.; Hao, W.; Mei, Y.-B.; Cheng, V.C.C.; Yuen, K.-Y. Antigenic Cross-Reactivity between Severe Acute Respiratory Syndrome—Associated Coronavirus and Human Coronaviruses 229E and OC43. J. Infect. Dis. 2005, 191, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Cheng, V.C.C.; Woo, P.C.Y.; Lau, S.K.P.; Poon, L.L.M.; Guan, Y.; Seto, W.H.; Yuen, K.Y.; Peiris, J.S.M. Serological Responses in Patients with Severe Acute Respiratory Syndrome Coronavirus Infection and Cross-Reactivity with Human Coronaviruses 229E, OC43, and NL63. Clin. Vaccine Immunol. 2005, 12, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Abbad, A.; Kleiner, G.; Srivastava, K.; Gleason, C.; Carreño, J.M.; Simon, V.; Krammer, F. The post-COVID-19 population has a high prevalence of cross-reactive antibodies to spikes from all Orthocoronavirinae genera. mBio 2024, 15, e0225023. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Contant, P.; Embong, A.K.; Kanagaiah, P.; Chaves, F.A.; Yang, H.; Branche, A.R.; Topham, D.J.; Sangster, M.Y. S Protein-Reactive IgG and Memory B Cell Production after Human SARS-CoV-2 Infection Includes Broad Reactivity to the S2 Subunit. mBio 2020, 11, e01991-20. [Google Scholar] [CrossRef]
- Anderson, E.M.; Goodwin, E.C.; Verma, A.; Arevalo, C.P.; Bolton, M.J.; Weirick, M.E.; Gouma, S.; McAllister, C.M.; Christensen, S.R.; Weaver, J. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 2021, 184, 1858–1864.e1810. [Google Scholar] [CrossRef]
- Hicks, J.; Klumpp-Thomas, C.; Kalish, H.; Shunmugavel, A.; Mehalko, J.; Denson, J.-P.; Snead, K.R.; Drew, M.; Corbett, K.S.; Graham, B.S.; et al. Serologic Cross-Reactivity of SARS-CoV-2 with Endemic and Seasonal Betacoronaviruses. J. Clin. Immunol. 2021, 41, 906–913. [Google Scholar] [CrossRef]
- Bangaru, S.; Antanasijevic, A.; Kose, N.; Sewall, L.M.; Jackson, A.M.; Suryadevara, N.; Zhan, X.; Torres, J.L.; Copps, J.; de la Peña, A.T.; et al. Structural mapping of antibody landscapes to human betacoronavirus spike proteins. Sci. Adv. 2022, 8, eabn2911. [Google Scholar] [CrossRef]
- Sagar, M.; Reifler, K.; Rossi, M.; Miller, N.S.; Sinha, P.; White, L.F.; Mizgerd, J.P. Recent endemic coronavirus infection is associated with less-severe COVID-19. J. Clin. Investig. 2021, 131, e143380. [Google Scholar] [CrossRef]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.-H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020, 370, eabd4250. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wolf, J.; Brice, D.C.; Sun, Y.; Locke, M.; Cherry, S.; Castellaw, A.H.; Wehenkel, M.; Crawford, J.C.; Zarnitsyna, V.I.; et al. Pre-existing humoral immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response. Cell Host Microbe 2022, 30, 83–96.e84. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef]
- Prakash, S.; Srivastava, R.; Coulon, P.-G.; Dhanushkodi, N.R.; Chentoufi, A.A.; Tifrea, D.F.; Edwards, R.A.; Figueroa, C.J.; Schubl, S.D.; Hsieh, L.; et al. Genome-Wide B Cell, CD4+, and CD8+ T Cell Epitopes That Are Highly Conserved between Human and Animal Coronaviruses, Identified from SARS-CoV-2 as Targets for Preemptive Pan-Coronavirus Vaccines. J. Immunol. 2021, 206, 2566–2582. [Google Scholar] [CrossRef]
- Dangi, T.; Palacio, N.; Sanchez, S.; Park, M.; Class, J.; Visvabharathy, L.; Ciucci, T.; Koralnik, I.J.; Richner, J.M.; Penaloza-MacMaster, P. Cross-protective immunity following coronavirus vaccination and coronavirus infection. J. Clin. Investig. 2021, 131, e151969. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Z.; Ren, L.; Hao, Y.; Zhu, M.; Jiang, H.; Wang, S.; Li, D.; Shao, Y. Pre-existing anti-HCoV-OC43 immunity influences the durability and cross-reactivity of humoral response to SARS-CoV-2 vaccination. Front. Cell. Infect. Microbiol. 2022, 12, 978440. [Google Scholar] [CrossRef]
- Hasan, Z.; Masood, K.I.; Veldhoen, M.; Qaiser, S.; Alenquer, M.; Akhtar, M.; Balouch, S.; Iqbal, J.; Wassan, Y.; Hussain, S.; et al. Pre-existing IgG antibodies to HCoVs NL63 and OC43 Spike increased during the pandemic and after COVID-19 vaccination. Heliyon 2025, 11, e42171. [Google Scholar] [CrossRef]
- Lavell, A.H.A.; Sikkens, J.J.; Edridge, A.W.D.; van der Straten, K.; Sechan, F.; Oomen, M.; Buis, D.T.P.; Schinkel, M.; Burger, J.A.; Poniman, M.; et al. Recent infection with HCoV-OC43 may be associated with protection against SARS-CoV-2 infection. iScience 2022, 25, 105105. [Google Scholar] [CrossRef]
- Yu, J.; Tostanoski, L.H.; Peter, L.; Mercado, N.B.; McMahan, K.; Mahrokhian, S.H.; Nkolola, J.P.; Liu, J.; Li, Z.; Chandrashekar, A.; et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020, 369, 806–811. [Google Scholar] [CrossRef]
- Jacob-Dolan, C.; Feldman, J.; McMahan, K.; Yu, J.; Zahn, R.; Wegmann, F.; Schuitemaker, H.; Schmidt, A.G.; Barouch, D.H. Coronavirus-Specific Antibody Cross Reactivity in Rhesus Macaques following SARS-CoV-2 Vaccination and Infection. J. Virol. 2021, 95, e00117-21. [Google Scholar] [CrossRef]
- Dolgin, E. Pan-coronavirus vaccine pipeline takes form. Nat. Rev. Drug Discov. 2022, 21, 324–326. [Google Scholar] [CrossRef]
- Dangi, T.; Li, S.; Penaloza-MacMaster, P. Development of a cross-protective common cold coronavirus vaccine. bioRxiv 2025, bioRxiv 2025.05.12.653567. [Google Scholar] [CrossRef]
- Cantoni, D.; Siracusano, G.; Mayora-Neto, M.; Pastori, C.; Fantoni, T.; Lytras, S.; Di Genova, C.; Hughes, J.; The Ambulatorio Medico San Luca Villanuova Group; Lopalco, L.; et al. Analysis of Antibody Neutralisation Activity against SARS-CoV-2 Variants and Seasonal Human Coronaviruses NL63, HKU1, and 229E Induced by Three Different COVID-19 Vaccine Platforms. Vaccines 2023, 11, 58. [Google Scholar] [CrossRef]
- Tang, W.; Chang, Z.W.; Goh, Y.S.; Tan, Y.J.; Hor, P.X.; Loh, C.Y.; Lye, D.C.; Young, B.E.; Ng, L.F.P.; Tay, M.Z.; et al. SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses. Vaccines 2025, 13, 268. [Google Scholar] [CrossRef]
- Amanat, F.; Thapa, M.; Lei, T.; Ahmed, S.M.S.; Adelsberg, D.C.; Carreño, J.M.; Strohmeier, S.; Schmitz, A.J.; Zafar, S.; Zhou, J.Q.; et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 2021, 184, 3936–3948.e3910. [Google Scholar] [CrossRef]
- Grobben, M.; van der Straten, K.; Brouwer, P.J.M.; Brinkkemper, M.; Maisonnasse, P.; Dereuddre-Bosquet, N.; Appelman, B.; Lavell, A.H.A.; van Vught, L.A.; Burger, J.A.; et al. Cross-reactive antibodies after SARS-CoV-2 infection and vaccination. eLife 2021, 10, e70330. [Google Scholar] [CrossRef]
- Angyal, A.; Longet, S.; Moore, S.C.; Payne, R.P.; Harding, A.; Tipton, T.; Rongkard, P.; Ali, M.; Hering, L.M.; Meardon, N.; et al. T-cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: A multicentre prospective cohort study. Lancet Microbe 2022, 3, e21–e31. [Google Scholar] [CrossRef]
- Amanat, F.; Clark, J.; Carreño, J.M.; Strohmeier, S.; Yellin, T.; Meade, P.S.; Bhavsar, D.; Muramatsu, H.; Sun, W.; Coughlan, L.; et al. Immunity to Seasonal Coronavirus Spike Proteins Does Not Protect from SARS-CoV-2 Challenge in a Mouse Model but Has No Detrimental Effect on Protection Mediated by COVID-19 mRNA Vaccination. J. Virol. 2023, 97, e0166422. [Google Scholar] [CrossRef]
- Sasaki, H.; Ishikawa, H.; Shigenaga, A.; Suzuki, Y.; Iyoda, M. Construction and Evaluation of a HCoV-OC43 S2 Subunit Vaccine Fused with Nasal Immuno-Inducible Sequence Against Coronavirus Infection. Curr. Issues Mol. Biol. 2025, 47, 355. [Google Scholar] [CrossRef]
- Zoest, V.; Lee, W.S.; Murdiyarso, L.; Burmas, L.; Pymm, P.; Esterbauer, R.; Kelly, A.; Kelly, H.G.; Barber-Axthelm, I.; Cooney, J.P.; et al. A novel chimeric coronavirus spike vaccine combining SARS-CoV-2 RBD and scaffold domains from HKU-1 elicits potent neutralising antibody responses. bioRxiv 2025, bioRxiv 2025.07.16.665240. [Google Scholar]
- Hutchinson, G.B.; Abiona, O.M.; Ziwawo, C.T.; Werner, A.P.; Ellis, D.; Tsybovsky, Y.; Leist, S.R.; Palandjian, C.; West, A.; Fritch, E.J.; et al. Nanoparticle display of prefusion coronavirus spike elicits S1-focused cross-reactive antibody response against diverse coronavirus subgenera. Nat. Commun. 2023, 14, 6195. [Google Scholar] [CrossRef]
- Kapingidza, A.B.; Marston, D.J.; Harris, C.; Wrapp, D.; Winters, K.; Mielke, D.; Xiaozhi, L.; Yin, Q.; Foulger, A.; Parks, R.; et al. Engineered immunogens to elicit antibodies against conserved coronavirus epitopes. Nat. Commun. 2023, 14, 7897. [Google Scholar] [CrossRef]
- Deming, D.; Sheahan, T.; Heise, M.; Yount, B.; Davis, N.; Sims, A.; Suthar, M.; Harkema, J.; Whitmore, A.; Pickles, R.; et al. Vaccine Efficacy in Senescent Mice Challenged with Recombinant SARS-CoV Bearing Epidemic and Zoonotic Spike Variants. PLoS Med. 2006, 3, e525. [Google Scholar] [CrossRef]
- Halfmann, P.J.; Patel, R.S.; Loeffler, K.; Yasuhara, A.; Van De Velde, L.-A.; Yang, J.E.; Chervin, J.; Troxell, C.; Huang, M.; Zheng, N.; et al. Multivalent S2 subunit vaccines provide broad protection against Clade 1 sarbecoviruses in female mice. Nat. Commun. 2025, 16, 462. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Corredor, J.C.; Pei, Y.; Nagy, É. Fowl Adenovirus-Based Vaccine Platform. Methods Mol. Biol. 2017, 1581, 29–54. [Google Scholar]
- Naghibosadat, M.; Babuadze, G.G.; Pei, Y.; Hurst, J.; Salvant, E.; Gaete, K.; Biondi, M.; Moloo, B.; Goldstein, A.; Avery, S.; et al. Vaccination against SARS-CoV-2 provides low-level cross-protection against common cold coronaviruses in mouse and non-human primate animal models. J. Virol. 2025, 99, e0139024. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.-B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and efficacy of heterologous ChAdOx1–BNT162b2 vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef]
- Lawrenz, J.; Xie, Q.; Zech, F.; Weil, T.; Seidel, A.; Krnavek, D.; van der Hoek, L.; Münch, J.; Müller, J.A.; Kirchhoff, F. Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination Boosts Neutralizing Activity Against Seasonal Human Coronaviruses. Clin. Infect. Dis. 2022, 75, e653–e661. [Google Scholar] [CrossRef]
- Garziano, M.; Cano Fiestas, M.; Vanetti, C.; Strizzi, S.; Murno, M.L.; Clerici, M.; Biasin, M. SARS-CoV-2 natural infection, but not vaccine-induced immunity, elicits cross-reactive immunity to OC43. Heliyon 2024, 10, e37928. [Google Scholar] [CrossRef]
- Abbink, P.; Lemckert, A.A.C.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; et al. Comparative Seroprevalence and Immunogenicity of Six Rare Serotype Recombinant Adenovirus Vaccine Vectors from Subgroups B and D. J. Virol. 2007, 81, 4654–4663. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Lu, S. Heterologous prime–boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 439. [Google Scholar] [CrossRef]
- Li, K.; Wohlford-Lenane, C.L.; Channappanavar, R.; Park, J.-E.; Earnest, J.T.; Bair, T.B.; Bates, A.M.; Brogden, K.A.; Flaherty, H.A.; Gallagher, T.; et al. Mouse-adapted MERS coronavirus causes lethal lung disease in human DPP4 knockin mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3119–E3128. [Google Scholar] [CrossRef]
- Dinnon, K.H.; Leist, S.R.; Schäfer, A.; Edwards, C.E.; Martinez, D.R.; Montgomery, S.A.; West, A.; Yount, B.L.; Hou, Y.J.; Adams, L.E.; et al. A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures. Nature 2020, 586, 560–566. [Google Scholar] [CrossRef]
- Bakkers, M.J.G.; Lang, Y.; Feitsma, L.J.; Hulswit, R.J.G.; de Poot, S.A.H.; van Vliet, A.L.W.; Margine, I.; de Groot-Mijnes, J.D.F.; van Kuppeveld, F.J.M.; Langereis, M.A.; et al. Betacoronavirus Adaptation to Humans Involved Progressive Loss of Hemagglutinin-Esterase Lectin Activity. Cell Host Microbe 2017, 21, 356–366. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Yip, C.C.Y.; Huang, Y.; Tsoi, H.-W.; Chan, K.-H.; Yuen, K.-Y. Comparative Analysis of 22 Coronavirus HKU1 Genomes Reveals a Novel Genotype and Evidence of Natural Recombination in Coronavirus HKU1. J. Virol. 2006, 80, 7136–7145. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I.; et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e1014. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Ellebedy, A.H. The germinal centre B cell response to SARS-CoV-2. Nat. Rev. Immunol. 2022, 22, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.D.; Narowski, T.M.; Wang, E.; Garrigan, E.; Mateus, J.; Frazier, A.; Weiskopf, D.; Grifoni, A.; Premkumar, L.; da Silva Antunes, R.; et al. Immunological memory to common cold coronaviruses assessed longitudinally over a three-year period pre-COVID-19 pandemic. Cell Host Microbe 2022, 30, 1269–1278.e1264. [Google Scholar] [CrossRef]
- Chen, T.; Lai, J.; Hu, M.; Ma, X. Revolution in vaccine development led by protein optimization design and de novo synthesis. Syn. Biol. J. 2025, 6, 1–25. [Google Scholar]
- Jardine, J.G.; Ota, T.; Sok, D.; Pauthner, M.; Kulp, D.W.; Kalyuzhniy, O.; Skog, P.D.; Thinnes, T.C.; Bhullar, D.; Briney, B.; et al. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science 2015, 349, 156–161. [Google Scholar] [CrossRef]
- Yassine, H.M.; Boyington, J.C.; McTamney, P.M.; Wei, C.-J.; Kanekiyo, M.; Kong, W.-P.; Gallagher, J.R.; Wang, L.; Zhang, Y.; Joyce, M.G.; et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 2015, 21, 1065–1070. [Google Scholar] [CrossRef]
- Tahir ul Qamar, M.; Shokat, Z.; Muneer, I.; Ashfaq, U.A.; Javed, H.; Anwar, F.; Bari, A.; Zahid, B.; Saari, N. Multiepitope-Based Subunit Vaccine Design and Evaluation against Respiratory Syncytial Virus Using Reverse Vaccinology Approach. Vaccines 2020, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Zhang, Y.; Methot, N.; Narowski, T.M.; Phillips, E.; Mallal, S.; Frazier, A.; Filaci, G.; Weiskopf, D.; Dan, J.M.; et al. Targets and cross-reactivity of human T cell recognition of common cold coronaviruses. Cell Rep. Med. 2023, 4, 101088. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, J.; Li, R.; Wen, Z.; Cai, Y.; Wallin, J.; Shu, Y.; Du, X.; Sun, C. Rational Design of a Pan-Coronavirus Vaccine Based on Conserved CTL Epitopes. Viruses 2021, 13, 333. [Google Scholar] [CrossRef]
- Shin, H.; Iwasaki, A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 2012, 491, 463–467. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Cardin, R.D.; Bravo, F.J.; Awasthi, S.; Lu, P.; Pullum, D.A.; Dixon, D.A.; Iwasaki, A.; Friedman, H.M. Successful application of prime and pull strategy for a therapeutic HSV vaccine. npj Vaccines 2019, 4, 33. [Google Scholar] [CrossRef]
- Mao, T.; Israelow, B.; Peña-Hernández, M.A.; Suberi, A.; Zhou, L.; Luyten, S.; Reschke, M.; Dong, H.; Homer, R.J.; Saltzman, W.M.; et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 2022, 378, eabo2523. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Rodrigues, G.; Wang, J.; Jayewickreme, R.; Hudak, A.; Dong, H.; Homer, R.J.; Ma, S.; Iwasaki, A. Intranasal hemagglutinin protein boosters induce protective mucosal immunity against influenza A viruses in mice. Proc. Natl. Acad. Sci. USA 2025, 122, e2422171122. [Google Scholar] [CrossRef] [PubMed]


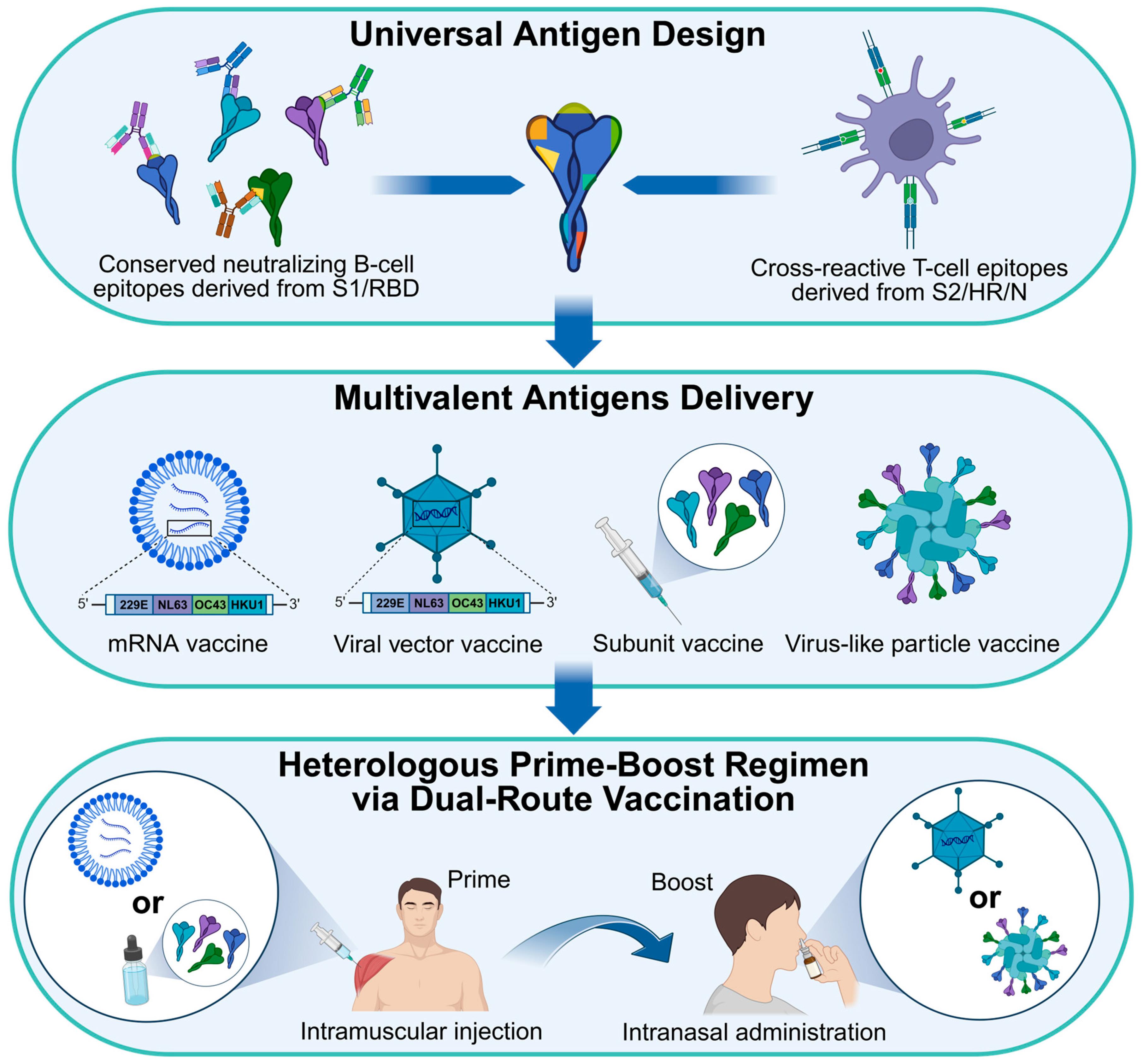
| Coronavirus | HCoV-229E | HCoV-OC43 | HCoV-NL63 | HCoV-HKU1 |
|---|---|---|---|---|
| Discovery (Year/Country) | 1966/United States | 1967/United States | 2004/The Netherlands | 2005/Hong Kong, China |
| Classification | Alphacoronavirus | Betacoronavirus | Alphacoronavirus | Betacoronavirus |
| Symptom | Coryza, rhinorrhea, nasal congestion, sore throat, cough, sneezing, malaise, headache, low-grade fever, myalgia | Sore throat, rhinorrhea, nasal congestion, cough, sneezing, malaise, headache, low-grade fever, myalgia | Rhinorrhea, fever, dry cough, wheezing, respiratory distress, coryza, croup | Rhinorrhea, fever, sore throat, cough, sputum production, dyspnea, chills, rigors, myalgia, headaches, febrile seizures |
| Incubation time (Day) | 2–5 | 2–5 | 2–4 | 2–4 |
| Epidemiology | Globally in winter | Globally in winter | Globally in winter | Globally in winter |
| Natural host | Bats | Rodents | Bats | Rodents |
| Intermediate host | Camels | Bovines | Unknown | Unknown |
| Evolutionary rate of Spike (substitutions/site/year) | 7.34 × 10−4 | 8 × 10−4 | 3-6 × 10−4 | 4.39 × 10−4 (HKU1A) 1.46 × 10−4 (HKU1B) |
| Receptor | hAPN | 9-O-Ac-Sia | hACE2 | 9-O-Ac-Sia, TMPRSS2 |
| Tissue culture (Cell/Organoid) | WI-38, L-132, MRC-5, Huh7, Mv1Lu | BS-C-1, FT, RD, Mv1Lu, MRC-5, Huh7.5, HCT-8, HRT-18, IGROV-1 | hACE2-overexpressing HEK293T, LLC-MK2, Vero-B4, Caco-2 | HAE, 2D airway organoid |
| Mouse model | Ad5-hAPN-transduced Ifnar1−/− mice | BALB/c mice, C57BL/6 mice | K18-hACE2 mice, Ad5-hACE2-transduced Ifnar1−/− mice | Not available |
| Reference | [1,46,51,52,53,54,55,56,57,58,59] | [2,46,53,58,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75] | [3,53,59,76,77,78,79,80,81,82,83,84,85,86] | [4,8,53,67,87,88,89,90,91,92,93] |
| Vaccine Type | Vaccine Name | Target Virus | Reactivity to HCoV | Study Host | Reference | |||
|---|---|---|---|---|---|---|---|---|
| 229E | NL63 | OC43 | HKU1 | |||||
| Inactivated Vaccine | γ-irradiated SARS-CoV-2 | SARS-CoV-2 | ND 1 | ND | Yes * | ND | Mouse | [258] |
| BBIBP-CorV | SARS-CoV-2 | Yes | Yes | Yes | Yes | Human | [259] | |
| SinoVac | SARS-CoV-2 | ND | Yes | No | ND | Human | [260] | |
| DNA Vaccine | S/S.dCT/S.dTM/S1/RBD/S.dTM.PP 2 | SARS-CoV-2 | Yes | Yes | Yes | Yes | Rhesus Macaque | [262,263] |
| SARS-CoV-2 S, M, or N | SARS-CoV-2 | ND | ND | Yes | ND | Mouse | [168] | |
| mRNA Vaccine | mRNA-1287 3 | HCoV-229E HCoV-NL63 HCoV-OC43 HCoV-HKU1 | ND | ND | ND | ND | Human | [264] |
| mRNA-OC43 | HCoV-OC43 | ND | ND | Yes * | ND | Mouse | [265] | |
| mRNA-1273 | SARS-CoV-2 | No | Yes * | Yes | Yes | Human, Mouse | [248,258,266,267] | |
| BNT162b2 | SARS-CoV-2 | Yes | Yes | Yes | Yes | Human, Mouse | [260,267,268,269,270] | |
| Subunit Vaccine | HCoV-229E S | HCoV-229E | Yes | Yes | No | No | Mouse | [271] |
| HCoV-NL63 S | HCoV-NL63 | Yes | Yes | No | No | Mouse | [271] | |
| HCoV-OC43 S | HCoV-OC43 | No | No | Yes | Yes | Mouse | [271] | |
| HCoV-HKU1 S | HCoV-HKU1 | No | No | Yes | Yes | Mouse | [271] | |
| SARS-CoV-2 S | SARS-CoV-2 | No | No | Yes | Yes | Mouse | [271] | |
| SARS-CoV-2 RBD or S | SARS-CoV-2 | ND | ND | Yes | ND | Mouse | [258] | |
| NAIS-ag | HCoV-OC43 | ND | ND | Yes * | ND | Rabbit | [272] | |
| CTR | SARS-CoV-2 | ND | ND | ND | Yes | Mouse, Pigtail Macaque | [273] | |
| VLP Vaccine | β-CoV mosaic_I53_dn5 | β-CoVs | Yes | No | Yes | Yes | Mouse | [274] |
| Ex_mosaic-NP | β-CoVs | ND | ND | Yes | Yes | Mouse | [275] | |
| VRP-229E-S | HCoV-229E | Yes * | ND | ND | ND | Mouse | [59,276] | |
| VRP-NL63-S | HCoV-NL63 | ND | Yes * | ND | ND | Mouse | [59,276] | |
| RBD/HR_Ferritin | SARS-CoV-2 | Yes * | ND | Yes * | ND | Mouse | [160] | |
| VLP-CoV S2 | SARS-CoV | ND | No | Yes | Yes | Mouse | [277] | |
| VLP-CoV-2 S2 | SARS-CoV-2 | ND | Yes | Yes | Yes | Mouse | [277] | |
| S-I53-50 NP | SARS-CoV-2 | Yes | Yes | Yes | Yes | Cynomolgus Macaque | [269] | |
| Vector Vaccine | ChAdOx1-S-nCoV-19 | SARS-CoV-2 | Yes * | No | ND | No | Human | [266,278] |
| FAdV-9-S19 | SARS-CoV-2 | Yes * | Yes * | Yes * | ND | Mouse, Cynomolgus Macaque | [279,280] | |
| Ad26.COV2.S | SARS-CoV-2 | Yes | Yes | Yes | Yes | Rhesus Macaque | [263] | |
| Ad5-SARS-CoV-2 S | SARS-CoV-2 | ND | ND | Yes | ND | Mouse | [258] | |
| Ad5-SARS-CoV-2 N | SARS-CoV-2 | ND | ND | Yes | ND | Mouse | [258] | |
| VSV-SARS-CoV-2 S | SARS-CoV-2 | ND | ND | Yes | ND | Mouse | [258] | |
| MVA-SARS-CoV S | SARS-CoV | ND | ND | Yes | ND | Mouse | [258] | |
| ChAd-BNT 4 | SARS-CoV-2 | Yes * | Yes * | Yes * | ND | Human | [281,282] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Liu, Y.; Chen, T.; Lai, J.; Liu, S.; Liu, X.; Zhu, Y.; Rao, H.; Peng, H.; Ma, X. Current Status and Challenges of Vaccine Development for Seasonal Human Coronaviruses. Vaccines 2025, 13, 1168. https://doi.org/10.3390/vaccines13111168
Zhang B, Liu Y, Chen T, Lai J, Liu S, Liu X, Zhu Y, Rao H, Peng H, Ma X. Current Status and Challenges of Vaccine Development for Seasonal Human Coronaviruses. Vaccines. 2025; 13(11):1168. https://doi.org/10.3390/vaccines13111168
Chicago/Turabian StyleZhang, Bin, Yaoming Liu, Tao Chen, Jintao Lai, Sen Liu, Xiaoqing Liu, Yiqiang Zhu, Haiyue Rao, Haojie Peng, and Xiancai Ma. 2025. "Current Status and Challenges of Vaccine Development for Seasonal Human Coronaviruses" Vaccines 13, no. 11: 1168. https://doi.org/10.3390/vaccines13111168
APA StyleZhang, B., Liu, Y., Chen, T., Lai, J., Liu, S., Liu, X., Zhu, Y., Rao, H., Peng, H., & Ma, X. (2025). Current Status and Challenges of Vaccine Development for Seasonal Human Coronaviruses. Vaccines, 13(11), 1168. https://doi.org/10.3390/vaccines13111168





