Prevalence of High-Risk Human Papillomaviruses (HPV) in Slovenian Women Attending Organized National Cervical Cancer Screening 14 Years After Implementation of the National HPV Vaccination Program
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample and Data Collection
2.3. HPV Testing
2.4. Data Analysis
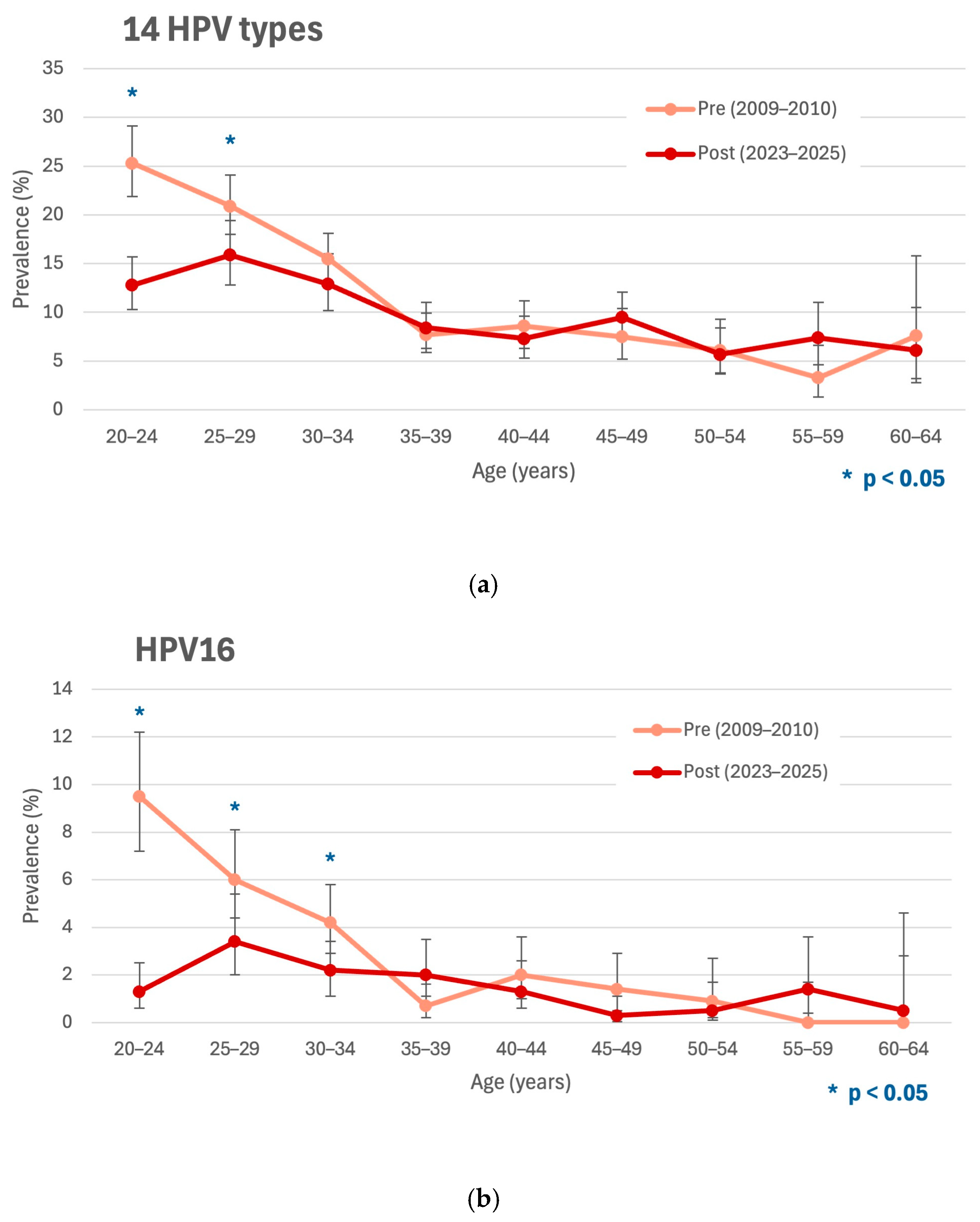
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPV | Human papillomavirus |
| hrHPV | High-risk human papillomavirus |
| NCCSP | National cervical cancer screening program |
| IARC | International Agency for Research on Cancer |
| CI | Confidence interval |
| RR | Risk ratio |
References
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Arbyn, M.; Gultekin, M.; Morice, P.; Nieminen, P.; Cruickshank, M.; Poortmans, P.; Kelly, D.; Poljak, M.; Bergeron, C.; Ritchie, D.; et al. The European response to the WHO call to eliminate cervical cancer as a public health problem. Int. J. Cancer 2021, 148, 277–284. [Google Scholar] [CrossRef]
- World Health Organization. The path to eliminating cervical cancer. In Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020; pp. 19–23. [Google Scholar]
- European Commission. European Guidelines on Cervical Cancer Screening and Diagnosis. Available online: https://cancer-screening-and-care.jrc.ec.europa.eu/en/ec-cvc/european-cervical-cancer-guidelines?topic=328&usertype=327 (accessed on 31 July 2025).
- Ronco, G.; Arbyn, M.; Meijer, C.J.L.M.; Snijders, P.J.F.; Cuzick, J. Screening for cervical cancer with primary testing for human papillomavirus. In European Guidelines for Quality Assurance in Cervical Cancer Screening, 2nd ed.; Anttila, A., Arbyn, M., De Vuyst, H., Eds.; Office for Official Publications of the European Communities: Luxembourg, 2015; pp. 5–8. [Google Scholar]
- Slovenian National Cervical Cancer Screening Programme and Registry. ZORA Programme Monitoring and Evaluation. Available online: https://zora.onko-i.si/en/monitoring-and-evaluation/ (accessed on 30 June 2025).
- Zadnik, V.; Žagar, T. SLORA: Slovenia and Cancer. Epidemiology and Cancer Registry. Ljubljana Institute of Oncology. Available online: www.slora.si (accessed on 1 August 2025).
- Nacionalni inštitut za javno zdravje. Analiza Izvajanja Cepljenja v Sloveniji v Letu 2019. Available online: https://nijz.si/wp-content/uploads/2023/06/POROCILO_CEPLJENJE_2019_13062023.pdf (accessed on 1 July 2025).
- Šinkovec Zorko, N.; Učakar, V.; Grgič Vitek, M. Cepljenje proti HPV v Sloveniji: Rezultati v šolskem letu 2021/22 in novosti. In Zbornik Predavanj 12. Izobraževalnega dne Programa ZORA; Ivanuš, U., Ed.; Onkološki inštitut: Ljubljana, Slovenija, 2022; Available online: https://zora.onko-i.si/fileadmin/user_upload/publikacije/izobrazevanja/2022_12ZD_zbornik/12_NadjaSZ_Cepljenje_roti_HPV_v_Sloveniji_....pdf (accessed on 1 July 2025).
- Nacionalni Inštitut za Javno Zdravje. Precepljenost Deklet 6. Razredov Osnovne Šole, Slovenija, Šolska Leta 2009/10–2022/23. Available online: https://nijz.si/wp-content/uploads/2023/08/Precepljenost-HPV-dekleta-in-fantje_avg2024.pdf (accessed on 1 July 2025).
- Učakar, V.; Poljak, M.; Klavs, I. Pre-vaccination prevalence and distribution of high-risk human papillomavirus (HPV) types in Slovenian women: A cervical cancer screening based study. Vaccine 2012, 30, 116–120. [Google Scholar] [CrossRef]
- Oštrbenk Valenčak, A.; Bertram, A.; Gröning, A.; Poljak, M. Comparison of the clinical and analytical performance of Alinity m HR HPV and cobas 4800 HPV assays in a population-based screening setting. J. Clin. Virol. 2021, 140, 104851. [Google Scholar] [CrossRef] [PubMed]
- Oštrbenk Valenčak, A.; Kroon, K.R.; Fabjan, D.; Mlakar, J.; Seme, K.; Berkhof, J.; Poljak, M. Clinically validated HPV assays offer comparable long-term safety in primary cervical cancer screening: A 9-year follow-up of a population-based screening cohort. Int. J. Cancer 2025, 156, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.N.; Brotherton, J.M.; Kaldor, J.M.; Skinner, S.R.; Liu, B.; Bateson, D.; McNamee, K.; Garefalakis, M.; Phillips, S.; Cummins, E.; et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: A repeat cross-sectional study. Lancet Infect. Dis. 2014, 14, 958–966. [Google Scholar] [CrossRef]
- Machalek, D.A.; Garland, S.M.; Brotherton, J.M.L.; Bateson, D.; McNamee, K.; Stewart, M.; Rachel Skinner, S.; Liu, B.; Cornall, A.M.; Kaldor, J.M.; et al. Very Low Prevalence of Vaccine Human Papillomavirus Types Among 18- to 35-Year-Old Australian Women 9 Years Following Implementation of Vaccination. J. Infect. Dis. 2018, 217, 1590–1600. [Google Scholar] [CrossRef]
- Tabrizi, S.N.; Brotherton, J.M.L.; Kaldor, J.M.; Skinner, S.R.; Cummins, E.; Liu, B.; Bateson, D.; McNamee, K.; Garefalakis, M.; Garland, S.M. Fall in human papillomavirus prevalence following a national vaccination program. J. Infect. Dis. 2012, 206, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Steben, M.; Tan Thompson, M.; Rodier, C.; Mallette, N.; Racovitan, V.; DeAngelis, F.; Stutz, M.; Rampakakis, E. A review of the impact and effectiveness of the quadrivalent human papillomavirus vaccine: 10 years of clinical experience in Canada. J. Obstet. Gynaecol. Can. 2018, 40, 1635–1645. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Liu, G.; Hariri, S.; Steinau, M.; Dunne, E.F.; Unger, E.R. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatric 2016, 137, e20151968. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.L.; Kavanagh, K.; Pan, J.; Love, J.; Cuschieri, K.; Robertson, C.; Ahmed, S.; Palmer, T.; Pollock, K.G. Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg. Infect. Dis. 2016, 22, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.; Pollock, K.G.; Cuschieri, K.; Palmer, T.; Cameron, R.L.; Watt, C.; Bhatia, R.; Moore, C.; Cubie, H.; Cruickshank, M.; et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: A 7-year cross-sectional study. Lancet Infect. Dis. 2017, 17, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Checchi, M.; Mesher, D.; Panwar, K.; Anderson, A.; Beddows, S.; Soldan, K. The impact of over ten years of HPV vaccination in England: Surveillance of type-specific HPV in young sexually active females. Vaccine 2023, 41, 6734–6744. [Google Scholar] [CrossRef]
- Mesher, D.; Panwar, K.; Thomas, S.L.; Edmundson, C.; Choi, Y.H.; Beddows, S.; Soldan, K. The Impact of the National HPV Vaccination Program in England Using the Bivalent HPV Vaccine: Surveillance of Type-Specific HPV in Young Females, 2010–2016. Lancet Infect. Dis. 2018, 218, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Merckx, M.; Vanden Broeck, D.; Benoy, I.; Depuydt, C.; Weyers, S.; Arbyn, M. Early effects of human papillomavirus vaccination in Belgium. Eur. J. Cancer Prev. 2015, 24, 340–342. [Google Scholar] [CrossRef]
- Huyghe, E.; Abrams, S.; Bogers, J.P.; Verhoeven, V.; Benoy, I. Evolution of human papilloma virus prevalence in a highly vaccinated region in Belgium: A retrospective cohort study in Flemish women (2010–2019). Eur. J. Cancer Prev. 2023, 32, 48–56. [Google Scholar] [CrossRef]
- Lynge, E.; Thamsborg, L.; Larsen, L.G.; Christensen, J.; Johansen, T.; Hariri, J.; Christiansen, S.; Rygaard, C.; Andersen, B. Prevalence of high-risk human papillomavirus after HPV-vaccination in Denmark. Int. J. Cancer 2020, 147, 3446–3452. [Google Scholar] [CrossRef]
- Dillner, J.; Nygård, M.; Munk, C.; Hortlund, M.; Hansen, B.T.; Lagheden, C.; Liaw, K.L.; Kjaer, S.K. Decline of HPV infections in Scandinavian cervical screening populations after introduction of HPV vaccination programs. Vaccine 2018, 36, 3820–3829. [Google Scholar] [CrossRef]
- Loenenbach, A.; Schönfeld, V.; Takla, A.; Wiese-Posselt, M.; Marquis, A.; Thies, S.; Sand, M.; Kaufmann, A.M.; Wichmann, O.; Harder, T. Human papillomavirus prevalence and vaccine effectiveness in young women in Germany, 2017/2018: Results from a nationwide study. Front. Public Health 2023, 11, 1204101. [Google Scholar] [CrossRef]
- Deleré, Y.; Remschmidt, C.; Leuschner, J.; Schuster, M.; Fesenfeld, M.; Schneider, A.; Wichmann, O.; Kaufmann, A.M. Human Papillomavirus prevalence and probable first effects of vaccination in 20 to 25 year-old women in Germany: A population-based cross-sectional study via home-based self-sampling. BMC Infect Dis. 2014, 14, 87. [Google Scholar] [CrossRef]
- Heard, I.; Tondeur, L.; Arowas, L.; Demazoin, M.; Falguières, M.; Parent Du Chatelet, I. Effectiveness of human papillomavirus vaccination on prevalence of vaccine genotypes in young sexually active women in France. J. Infect. Dis. 2017, 215, 757–763. [Google Scholar] [CrossRef]
- Bretagne, C.H.; Jooste, V.; Guenat, D.; Riethmuller, D.; Bouvier, A.M.; Bedgedjian, I.; Prétet, J.L.; Valmary-Degano, S.; Mougin, C. Prevalence and distribution of HPV genotypes and cervical-associated lesions in sexually active young French women following HPV vaccine. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Woestenberg, P.J.; King, A.J.; van Benthem, B.H.B.; Donken, R.; Leussink, S.; van der Klis, F.R.M.; de Melker, H.E.; van der Sande, M.A.B.; Hoebe, C.J.P.A.; Bogaards, J.A.; et al. Bivalent vaccine effectiveness against type-specific HPV positivity: Evidence for cross-protection against oncogenic types among Dutch STI clinic visitors. J. Infect. Dis. 2018, 217, 213–222. [Google Scholar] [CrossRef]
- Kusters, J.M.A.; Schim van der Loeff, M.F.; Heijne, J.C.M.; King, A.J.; de Melker, H.E.; Heijman, T.; Bogaards, J.A.; van Benthem, B.H.B.; Papillomavirus Surveillance among STI Clinic Youngsters in the Netherlands (PASSYON) Study Group. Changes in genital human papillomavirus (HPV) prevalence during 12 years of girls-only bivalent HPV vaccination: Results from a biennial repeated cross-sectional study. J. Infect. Dis. 2025, 231, e165–e176. [Google Scholar] [CrossRef]
- Donken, R.; King, A.J.; Bogaards, J.A.; Woestenberg, P.J.; Meijer, C.J.L.M.; de Melker, H.E. High Effectiveness of the Bivalent Human Papillomavirus (HPV) Vaccine Against Incident and Persistent HPV Infections up to 6 Years After Vaccination in Young Dutch Women. J. Infect. Dis. 2018, 217, 1579–1589. [Google Scholar] [CrossRef]
- Carozzi, F.; Puliti, D.; Ocello, C.; Anastasio, P.S.; Moliterni, E.A.; Perinetti, E.; Serradell, L.; Burroni, E.; Confortini, M.; Mantellini, P.; et al. Monitoring vaccine and non-vaccine HPV type prevalence in the post-vaccination era in women living in the Basilicata region, Italy. BMC Infect. Dis. 2018, 18, 38. [Google Scholar] [CrossRef]
- Purriños-Hermida, M.J.; Santiago-Pérez, M.I.; Treviño, M.; Dopazo, R.; Cañizares, A.; Bonacho, I.; Trigo, M.; Fernández, M.E.; Cid, A.; Gómez, D.; et al. Direct, indirect and total effectiveness of bivalent HPV vaccine in women in Galicia, Spain. PLoS ONE 2018, 13, e0201653. [Google Scholar] [CrossRef]
- Freire-Salinas, J.; Benito, R.; Azueta, A.; Gil, J.; Mendoza, C.; Nicolás, M.; García-Berbel, P.; Algarate, S.; Gómez-Román, J. Genotype Distribution change after human papillomavirus vaccination in two autonomous communities in Spain. Front. Cell. Infect. Microbiol. 2021, 11, 633162. [Google Scholar] [CrossRef]
- Saldanha, C.; Vieira-Baptista, P.; Costa, M.; Silva, A.R.; Picão, M.; Sousa, C. Impact of a High Coverage Vaccination Rate on Human Papillomavirus Infection Prevalence in Young Women: A Cross-sectional Study. J. Low. Genit. Tract Dis. 2020, 24, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Oštrbenk Valenčak, A.; Šterbenc, A.; Seme, K.; Poljak, M. Alinity m HR HPV Assay fulfills criteria for human papillomavirus test requirements in cervical cancer screening settings. J. Clin. Microbiol. 2019, 58, e01120-19. [Google Scholar] [CrossRef] [PubMed]
- Oštrbenk Valenčak, A.; Cuschieri, K.; Connor, L.; Zore, A.; Smrkolj, Š.; Poljak, M. Allplex HPV HR Detection assay fulfils all clinical performance and reproducibility validation requirements for primary cervical cancer screening. J. Clin. Virol. 2024, 170, 105638. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. List of Classifications by Cancer Sites with Sufficient or Limited Evidence in Humans, IARC Monographs Volumes 1–139. Available online: https://monographs.iarc.who.int/wp-content/uploads/2019/07/Classifications_by_cancer_site.pdf (accessed on 25 August 2025).
- Kovachev, S.; Slavov, V. Prevalence of human papillomavirus infection in women in Bulgaria: A 2017 update. J. Med. Virol. 2018, 90, 1142–1149. [Google Scholar] [CrossRef]
- Pešut, E.; Šimić, I.; Fureš, R.; Milutin Gašperov, N.; Lež, C.; Feratović, F.; Kukina Žvigač, T.; Grce, M.; Erceg Ivkošić, I.; Sabol, I. Monitoring HPV prevalence and risk cofactors for abnormal cytology in the post-vaccination period among Croatian women. Viruses 2024, 16, 642. [Google Scholar] [CrossRef]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Kesic, V.; Poljak, M.; Rogovskaya, S. Cervical cancer burden and prevention activities in Europe. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Schlecht, N.F.; Diaz, A.; Nucci-Sack, A.; Shankar, V.; Guillot, M.; Hollman, D.; Strickler, H.D.; Burk, R.D. Incidence and types of human papillomavirus infections in adolescent girls and young women immunized with the human papillomavirus vaccine. JAMA Netw. Open 2021, 4, e2121893. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data. Which Countries Include Human Papillomavirus (HPV) Vaccines in Their National Vaccination Programs? Available online: https://ourworldindata.org/grapher/human-papillomavirus-vaccine-immunization-schedule?time=2022&mapSelect=~BIH (accessed on 1 July 2025).

| HPV Type | Pre-Vaccination Period (2009–2010), Women Age 20–64 (n = 4425) | After Implementation of HPV Vaccination (2023–2025), Women Age 20–64 (n = 4419) | p-Value * | Relative Reduction (%) | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Prevalence (%) | 95% CI | n | Prevalence (%) | 95% CI | ||||
| HPV positive a | 589 | 13.3 | 12.3–14.3 | 443 | 10.0 | 9.2–10.9 | <0.001 | 24.7 | 15.4–32.9 |
| Multiple HPV types b | 134 | 3.0 | 2.6–3.6 | 76 | 1.7 | 1.4–2.1 | <0.001 | 43.2 | 25.0–57.0 |
| HPV 16/18 c | 199 | 4.5 | 3.9–5.1 | 87 | 2.0 | 1.6–2.4 | <0.001 | 56.2 | 43.9–65.9 |
| HPV16 | 157 | 3.5 | 3.0–4.1 | 66 | 1.5 | 1.2–1.9 | <0.001 | 57.9 | 44.1–68.3 |
| HPV18 | 48 | 1.1 | 0.8–1.4 | 24 | 0.5 | 0.4–0.8 | 0.005 | 49.9 | 18.4–69.3 |
| HPV31 | 114 | 2.6 | 2.1–3.1 | 63 | 1.4 | 1.1–1.8 | <0.001 | 44.7 | 24.9–59.2 |
| HPV33 | 32 | 0.7 | 0.5–1.0 | 23 | 0.5 | 0.3–0.8 | 0.225 | 28.0 | −22.8–57.8 |
| HPV35 | 9 | 0.2 | 0.1–0.4 | 19 | 0.4 | 0.3–0.7 | 0.058 | −111.4 | −366.7–4.3 |
| HPV39 | 50 | 1.1 | 0.9–1.5 | 39 | 0.9 | 0.6–1.2 | 0.244 | 21.9 | −18.5–48.5 |
| HPV45 | 42 | 0.9 | 0.7–1.3 | 24 | 0.5 | 0.4–0.8 | 0.027 | 42.8 | 5.7–65.3 |
| HPV51 | 81 | 1.8 | 1.5–2.3 | 48 | 1.1 | 0.8–1.4 | 0.004 | 40.7 | 15.4–58.4 |
| HPV52 | 77 | 1.7 | 1.4–2.2 | 52 | 1.2 | 0.9–1.5 | 0.027 | 32.4 | 4.1–52.3 |
| HPV56 | 31 | 0.7 | 0.5–1.0 | 57 | 1.3 | 1.0–1.7 | 0.005 | −84.1 | −184.6–19.1 |
| HPV58 | 29 | 0.7 | 0.5–0.9 | 24 | 0.5 | 0.4–0.8 | 0.494 | 17.1 | −42.1–51.7 |
| HPV59 | 48 | 1.1 | 0.8–1.4 | 45 | 1.0 | 0.8–1.4 | 0.759 | 6.1 | −40.7–37.4 |
| HPV Type | Pre-Vaccination Period (2009–2010), Women Age 20–24 (n = 580) | After Implementation of HPV Vaccination (2023–2025), Women Age 20–24 (n = 632) | p-Value * | Relative Reduction (%) | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Prevalence (%) | 95% CI | n | Prevalence (%) | 95% CI | ||||
| HPV positive a | 147 | 25.3 | 22.0–29.0 | 81 | 12.8 | 10.4–15.6 | <0.001 | 49.4 | 35.3–60.5 |
| Multiple HPV types b | 52 | 9.0 | 6.9–11.6 | 21 | 3.3 | 2.2–5.0 | <0.001 | 62.9 | 39.3–77.4 |
| HPV 16/18 c | 64 | 11.0 | 8.7–13.8 | 12 | 1.9 | 1.1–3.3 | <0.001 | 82.8 | 68.5–90.6 |
| HPV16 | 55 | 9.5 | 7.4–12.1 | 8 | 1.3 | 0.6–2.5 | <0.001 | 86.7 | 72.2–93.6 |
| HPV18 | 12 | 2.1 | 1.2–3.6 | 4 | 0.6 | 0.2–1.6 | 0.041 | 69.4 | 5.7–90.1 |
| HPV31 | 28 | 4.8 | 3.4–6.9 | 8 | 1.3 | 0.6–2.5 | <0.001 | 73.8 | 42.9–88.0 |
| HPV33 | 7 | 1.2 | 0.6–2.5 | 6 | 0.9 | 0.4–2.1 | 0.783 | 21.3 | −132.7–73.4 |
| HPV35 | 2 | 0.3 | 0.1–1.2 | 3 | 0.5 | 0.2–1.4 | 1.000 | −37.7 | −720.9–76.9 |
| HPV39 | 16 | 2.8 | 1.7–4.4 | 9 | 1.4 | 0.8–2.7 | 0.110 | 48.4 | −15.9–77.0 |
| HPV45 | 13 | 2.2 | 1.3–3.8 | 2 | 0.3 | 0.1–1.1 | 0.003 | 85.9 | 37.7–96.8 |
| HPV51 | 25 | 4.3 | 2.9–6.3 | 15 | 2.4 | 1.4–3.9 | 0.059 | 44.9 | −3.4–70.7 |
| HPV52 | 25 | 4.3 | 2.9–6.3 | 11 | 1.7 | 1.0–3.1 | 0.008 | 59.6 | 18.7–80.0 |
| HPV56 | 13 | 2.2 | 1.3–3.8 | 15 | 2.4 | 1.4–3.9 | 0.879 | −5.9 | −120.6–49.2 |
| HPV58 | 12 | 2.1 | 1.2–3.6 | 4 | 0.6 | 0.2–1.6 | 0.041 | 69.4 | 5.7–90.1 |
| HPV59 | 15 | 2.6 | 1.6–4.2 | 15 | 2.4 | 1.4–3.9 | 0.812 | 8.2 | −86.1–54.7 |
| HPV Type | Vaccinated (n = 253) | Unvaccinated (n = 379) | p-Value * | Risk Ratio (RR) ** | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | Prevalence (%) | 95% CI | n | Prevalence (%) | 95% CI | ||||
| 12hr HPV positive | 31 | 12.3 | 8.8–16.9 | 41 | 10.8 | 8.1–14.3 | 0.578 | 1.13 | 0.7–1.8 |
| Multiple 12 hrHPV a | 8 | 3.2 | 1.6–6.1 | 13 | 3.4 | 2.0–5.8 | 0.854 | 0.92 | 0.4–2.2 |
| HPV16/18 b | 0 | 0.0 | 0.0–1.5 | 12 | 3.3 | 1.8–5.5 | 0.002 | 0.06 | 0.0–1.0 |
| HPV16 | 0 | 0.0 | 0.0–1.5 | 8 | 2.1 | 1.1–4.1 | 0.024 | 0.09 | 0.0–1.5 |
| HPV18 | 0 | 0.0 | 0.0–1.5 | 4 | 1.1 | 0.4–2.7 | 0.154 | 0.17 | 0.0–3.1 |
| HPV31 | 3 | 1.2 | 0.4–3.4 | 5 | 1.3 | 0.6–3.1 | 1.000 | 0.90 | 0.2–3.7 |
| HPV33 | 2 | 0.8 | 0.2–2.8 | 4 | 1.1 | 0.4–2.7 | 1.000 | 0.75 | 0.1–4.1 |
| HPV35 | 1 | 0.4 | 0.1–2.2 | 2 | 0.5 | 0.1–1.9 | 1.000 | 0.75 | 0.1–8.2 |
| HPV39 | 6 | 2.4 | 1.1–5.1 | 3 | 0.8 | 0.3–2.3 | 0.167 | 3.00 | 0.8–11.9 |
| HPV45 | 0 | 0.0 | 0.0–1.5 | 2 | 0.5 | 0.1–1.9 | 0.519 | 0.30 | 0.1–6.2 |
| HPV51 | 7 | 2.8 | 1.3–5.6 | 8 | 2.1 | 1.1–4.1 | 0.604 | 1.31 | 0.5–3.6 |
| HPV52 | 5 | 2.0 | 0.8–4.5 | 6 | 1.6 | 0.7–3.4 | 0.762 | 1.25 | 0.4–4.0 |
| HPV56 | 6 | 2.4 | 1.1–5.1 | 9 | 2.4 | 1.3–4.5 | 1.000 | 1.00 | 0.4–2.8 |
| HPV58 | 2 | 0.8 | 0.2–2.8 | 2 | 0.5 | 0.1–1.9 | 1.000 | 1.50 | 0.2–10.6 |
| HPV59 | 8 | 3.2 | 1.6–6.1 | 7 | 1.8 | 0.9–3.8 | 0.287 | 1.71 | 0.6–4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasič, M.; Oštrbenk, A.; Smrkolj, Š.; Bohinc, K.B.; Pflaum, A.; Poljak, M. Prevalence of High-Risk Human Papillomaviruses (HPV) in Slovenian Women Attending Organized National Cervical Cancer Screening 14 Years After Implementation of the National HPV Vaccination Program. Vaccines 2025, 13, 1050. https://doi.org/10.3390/vaccines13101050
Lasič M, Oštrbenk A, Smrkolj Š, Bohinc KB, Pflaum A, Poljak M. Prevalence of High-Risk Human Papillomaviruses (HPV) in Slovenian Women Attending Organized National Cervical Cancer Screening 14 Years After Implementation of the National HPV Vaccination Program. Vaccines. 2025; 13(10):1050. https://doi.org/10.3390/vaccines13101050
Chicago/Turabian StyleLasič, Mateja, Anja Oštrbenk, Špela Smrkolj, Klara B. Bohinc, Ana Pflaum, and Mario Poljak. 2025. "Prevalence of High-Risk Human Papillomaviruses (HPV) in Slovenian Women Attending Organized National Cervical Cancer Screening 14 Years After Implementation of the National HPV Vaccination Program" Vaccines 13, no. 10: 1050. https://doi.org/10.3390/vaccines13101050
APA StyleLasič, M., Oštrbenk, A., Smrkolj, Š., Bohinc, K. B., Pflaum, A., & Poljak, M. (2025). Prevalence of High-Risk Human Papillomaviruses (HPV) in Slovenian Women Attending Organized National Cervical Cancer Screening 14 Years After Implementation of the National HPV Vaccination Program. Vaccines, 13(10), 1050. https://doi.org/10.3390/vaccines13101050





