Prevalence of Vaccine-Covered and Non-Covered HPV Genotypes Among Unvaccinated Women in Ankara: A Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Molecular Analysis
2.4. Cytological Evaluation
2.5. Statistical Analysis
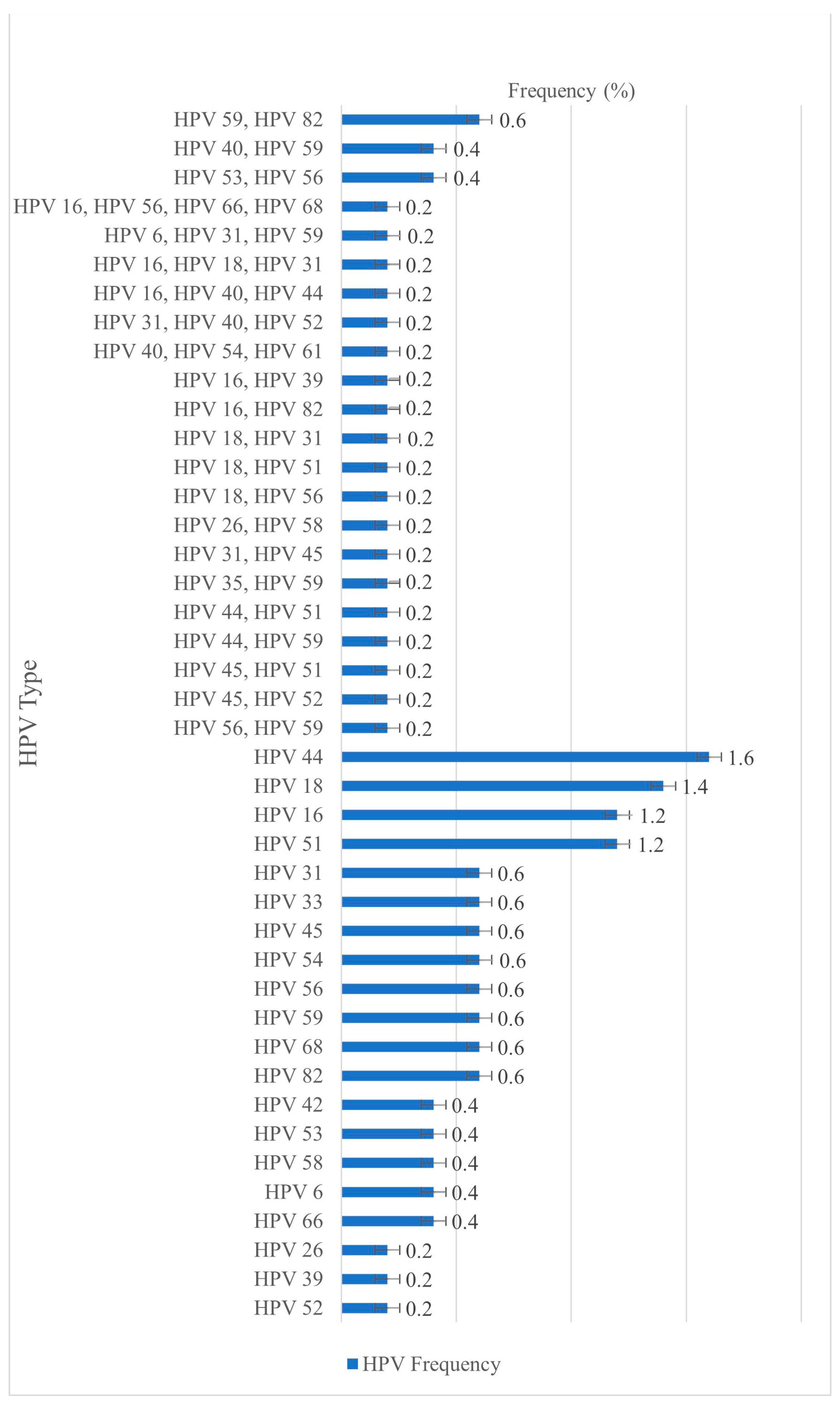
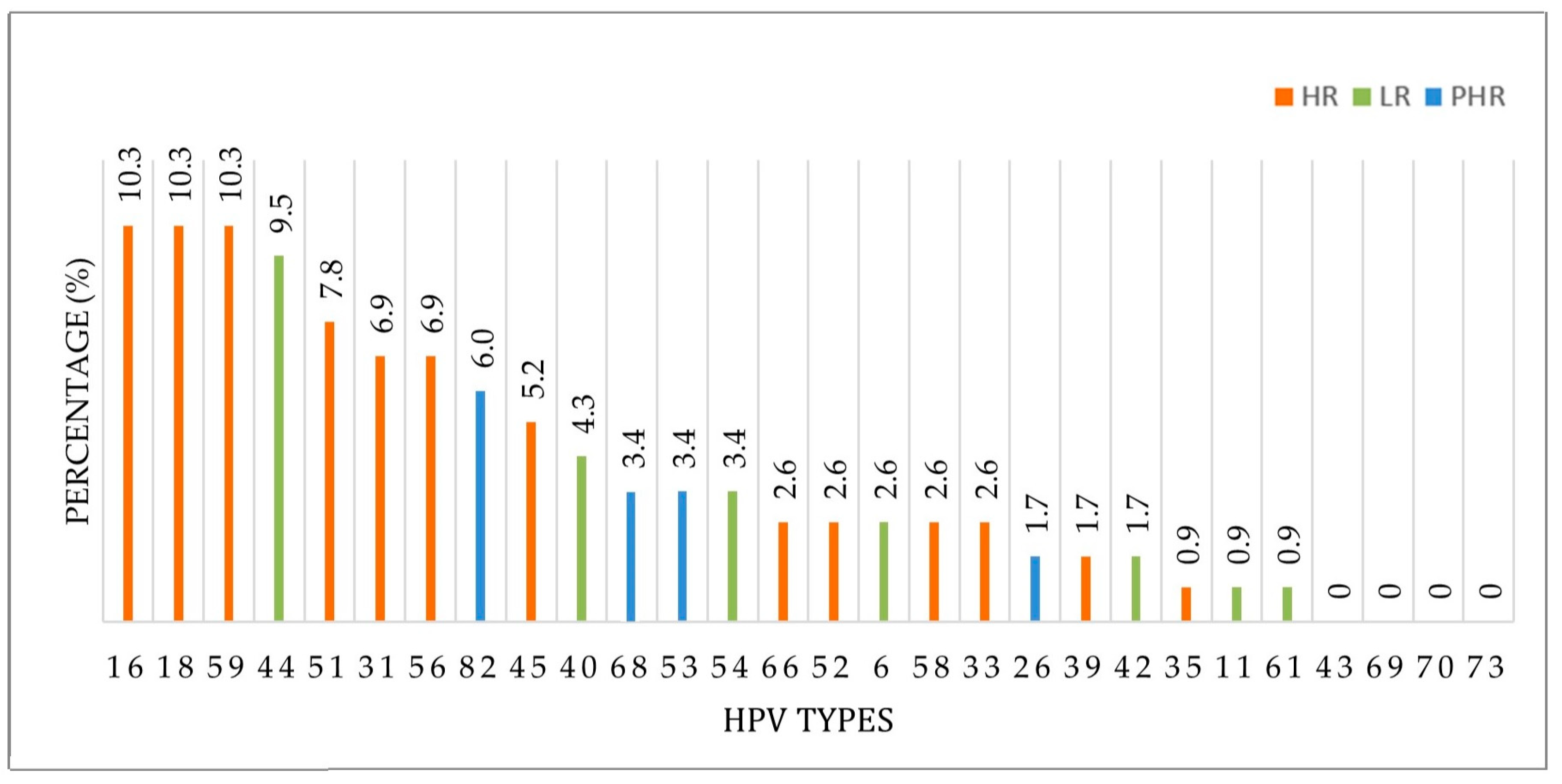
3. Results
3.1. HPV Prevalence and Most Frequently Detected Genotypes
3.2. Age-Based Distribution of HPV Infections
3.3. Distribution of HPV Infections According to Vaccine Coverage
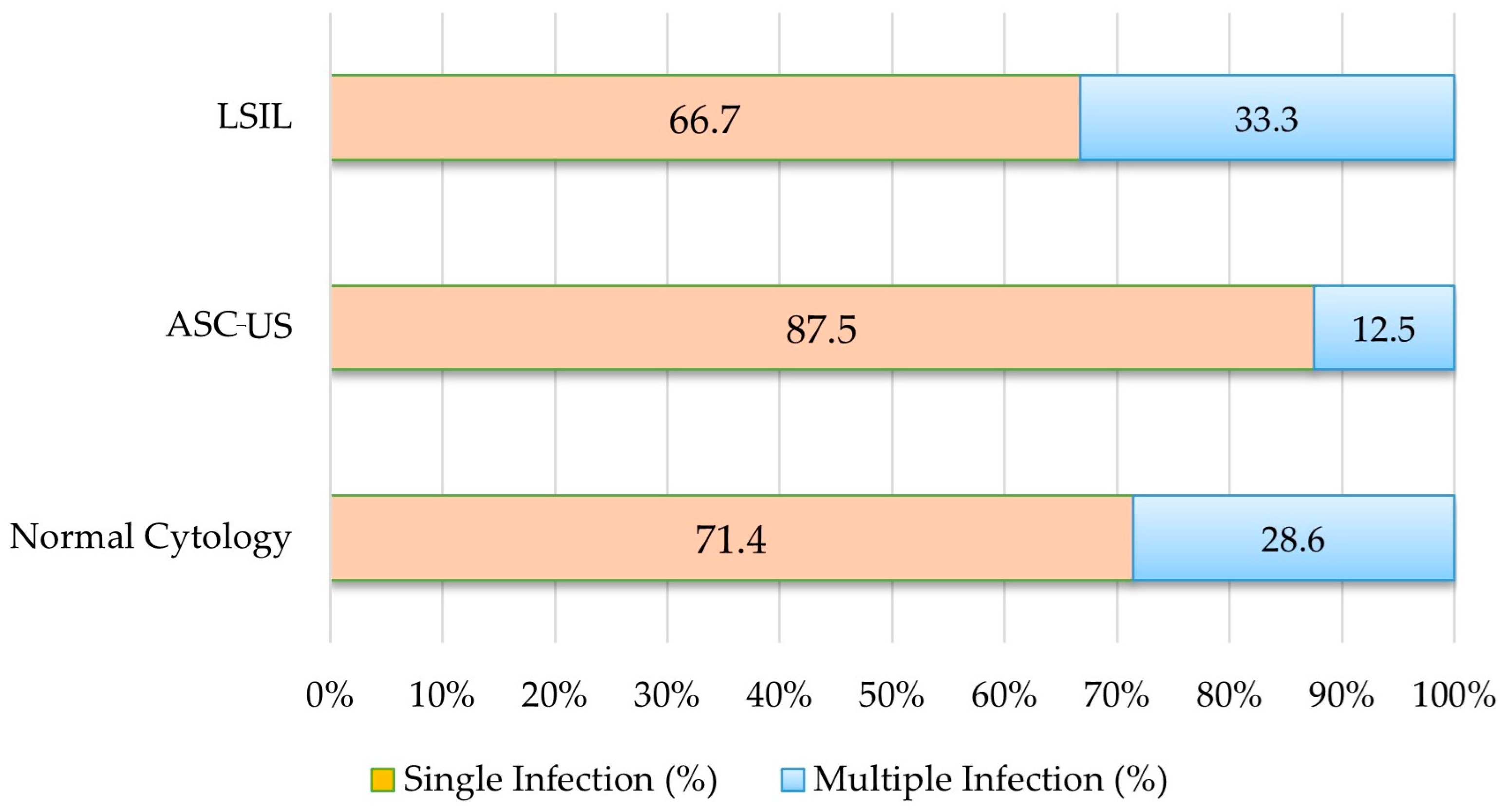
3.4. Cytological Findings and Distribution of Vaccine-Covered and Non-Covered HPV Genotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPV | human papillomavirus |
| PCR | polymerase chain reaction |
| ASC-US | atypical squamous cells of undetermined significance |
| LSIL | low-grade squamous intraepithelial lesion |
| AGC | atypical glandular cells |
| HSIL | high-grade squamous intraepithelial lesion |
| HR | high-risk |
| LR | low-risk |
| PHR | probable high-risk |
| VLP | virus-like particle |
References
- ICTV Virus Taxonomy Profile: Papillomaviridae. Available online: https://ictv.global/report/chapter/papillomaviridae/papillomaviridae (accessed on 10 April 2025).
- Della Fera, A.N.; Warburton, A.; Coursey, T.L.; Khurana, S.; McBride, A.A. Persistent Human Papillomavirus Infection. Viruses 2021, 13, 321. [Google Scholar] [CrossRef]
- Baba, S.K.; Alblooshi, S.S.E.; Yaqoob, R.; Behl, S.; Al Saleem, M.; Rakha, E.A.; Malik, F.; Singh, M.; Macha, M.A.; Akhtar, M.K.; et al. Human papilloma virus (HPV) mediated cancers: An insightful update. J. Transl. Med. 2025, 23, 483. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Hussain, A.; Fatima, N.; Altamimi, M.A. HPV pathogenesis, various types of vaccines, safety concern, prophylactic and therapeutic applications to control cervical cancer, and future perspective. Virusdisease 2023, 34, 172–190. [Google Scholar] [CrossRef]
- Debrah, O.; Agyemang-Yeboah, F.; Donkoh, E.T.; Asmah, R.H. Prevalence and distribution of human papillomavirus genotypes among Ghanaian women: A cross-sectional study. BMC Women’s Health 2021, 21, 372. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Stanley, M.; Joura, E.; Yen, G.P.; Kothari, S.; Luxembourg, A.; Saah, A.; Walia, A.; Perez, G.; Khoury, H.; Badgley, D.; et al. Systematic literature review of neutralizing antibody immune responses to non-vaccine targeted high-risk HPV types induced by the bivalent and the quadrivalent vaccines. Vaccine 2021, 39, 2214–2223. [Google Scholar] [CrossRef]
- Williamson, A.L. Recent Developments in Human Papillomavirus (HPV) Vaccinology. Viruses 2023, 15, 1440. [Google Scholar] [CrossRef]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; de Andrade, R.P.; Ault, K.A. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, H.; Yu, C.; Li, X.; Wang, Y.; Xie, L. Current status and future directions for the development of human papillomavirus vaccines. Front. Immunol. 2024, 15, 1362770. [Google Scholar] [CrossRef] [PubMed]
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M.; HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Restrepo, J.; Herrera, T.; Samakoses, R.; Reina, J.C.; Pitisuttithum, P.; Ulied, A.; Bekker, L.-G.; Moreira, E.D.; Olsson, S.-E.; Block, S.L.; et al. Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety. Pediatrics 2023, 152, e2022060993. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Strategic Advisory Group of Experts (SAGE) on Immunization: Background Document and Report on the Potential Contribution of HPV Vaccines and Immunization Towards Cervical Cancer Elimination (March 2022). Available online: https://cdn.who.int/media/docs/default-source/immunization/position_paper_documents/human-papillomavirus-(hpv)/hpv-background-document--report-march-2022.pdf (accessed on 10 June 2025).
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef]
- Vinodhini, K.; Shanmughapriya, S.; Das, B.C.; Natarajaseenivasan, K. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch. Gynecol. Obstet. 2012, 285, 771–777. [Google Scholar] [CrossRef]
- Alışkan, H.E.; Öğüç Şanlı, Ö.; Aka Bolat, F.; Alkaş Yağınç, D.; Toprak, U. Determination of Human Papilloma Virus (HPV) Genotype Prevalence and Distribution in Adana: A Hospital-Based Study Between 2014–2021. Mikrobiyoloji Bul. 2023, 57, 119–133. (In Turkish) [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Song, G.; Li, Y.; Dong, Z.; Yan, X.; Wang, S.; Tian, H.; Wu, X.; Li, C.; Huo, Y. Prevalence and genotype distribution of human papillomavirus infection among women aged 30–65 years in Xi’an, China: A population-based study of 14,655 women. Hum. Vaccines Immunother. 2021, 17, 5439–5446. [Google Scholar] [CrossRef]
- Zhao, M.; Kang, P.; Zhu, L.; Zhou, D.; Cui, M.; Zhang, M.; Jia, J.; Luo, L. Global pattern of persistent human papillomavirus infection in female genital tract: An updated system review and meta-analysis. iScience 2024, 27, 110991. [Google Scholar] [CrossRef]
- Huang, W.; Xu, H.; Hu, H.; Zhang, D.; Liu, Y.; Guo, Y.; Xiao, F.; Chen, W.; Ma, Z. The prevalence of human papillomavirus among women in northern Guangdong Province of China. Sci. Rep. 2022, 12, 13353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, S.; Zhong, Z.; Hou, J.; Lin, L.; Weng, R.; Su, L.; Lei, N.; Hou, T.; Yang, H. Prevalence and genotype distribution of human papillomavirus infection among women in northeastern Guangdong Province of China. BMC Infect. Dis. 2018, 18, 204. [Google Scholar] [CrossRef]
- Zhao, F.H.; Lewkowitz, A.K.; Hu, S.Y.; Chen, F.; Li, L.Y.; Zhang, Q.M.; Wu, R.F.; Li, C.Q.; Wei, L.H.; Xu, A.D.; et al. Prevalence of human papillomavirus and cervical intraepithelial neoplasia in China: A pooled analysis of 17 population-based studies. Int. J. Cancer 2012, 131, 2929–2938. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Y.; Wei, X.; Wang, G.; Sun, R.; Wang, M.; Zhao, L. Prevalence and distribution of human papillomavirus genotypes among women attending gynecology clinics in northern Henan Province of China. Virol. J. 2022, 19, 6. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, A.I.; Benczik, M.; Moravcsik-Kornyicki, Á.; Kocsis, A.; Gyulai, A.; Kósa, Z. The prevalence of high-risk human papillomavirus in Hungary: A geographically representative, cross-sectional study. Pathol. Oncol. Res. 2022, 28, 1610424. [Google Scholar] [CrossRef]
- Rideg, O.; Dergez, T.; Farkas, K.; Kovács, K.; Kálmán, E.; Tornóczky, T.; Oszter, A. High prevalence of non-vaccinated oncogenic human papillomavirus genotypes in high-grade squamous intraepithelial lesions of the cervix: Thought-provoking results of a detailed HPV genotype analysis. Vaccines 2022, 10, 748. [Google Scholar] [CrossRef]
- Liang, L.A.; Tanaka, L.F.; Radde, K.; Bussas, U.; Ikenberg, H.; Heideman, D.A.M.; Meijer, C.J.L.M.; Blettner, M.; Klug, S.J. Population-based age- and type-specific prevalence of human papillomavirus among non-vaccinated women aged 30 years and above in Germany. BMC Infect. Dis. 2024, 24, 1008. [Google Scholar] [CrossRef]
- Agadayi, E.; Karademir, D.; Karahan, S. Knowledge, Attitudes and Behaviors of Women who have or have not had human papillomavirus vaccine in Turkey about the Virus and the vaccine. J. Community Health 2022, 47, 650–657. [Google Scholar] [CrossRef]
| HPV Type | Risk Category | Bivalent | Quadrivalent | Nonavalent |
|---|---|---|---|---|
| HPV 6 | Low risk | √ | √ | |
| HPV 11 | Low risk | √ | √ | |
| HPV 16 | High risk | √ | √ | √ |
| HPV 18 | High risk | √ | √ | √ |
| HPV 26 | Probable high risk | |||
| HPV 31 | High risk | √ | ||
| HPV 33 | High risk | √ | ||
| HPV 35 | High risk | |||
| HPV 39 | High risk | |||
| HPV 40 | Low risk | |||
| HPV 42 | Low risk | |||
| HPV 43 | Low risk | |||
| HPV 44 | Low risk | |||
| HPV 45 | High risk | √ | ||
| HPV 51 | High risk | |||
| HPV 52 | High risk | √ | ||
| HPV 53 | Probable high risk | |||
| HPV 54 | Low risk | |||
| HPV 56 | High risk | |||
| HPV 58 | High risk | √ | ||
| HPV 59 | High risk | |||
| HPV 61 | Low risk | |||
| HPV 66 | Probable high risk | |||
| HPV 68 | Probable high risk | |||
| HPV 69 | Probable high risk | |||
| HPV 70 | Low risk | |||
| HPV 73 | Probable high risk | |||
| HPV 82 | Probable high risk |
| Parameter | All Participants (N = 500) | HPV-Positive Cases (n = 91) | ||
|---|---|---|---|---|
| N (%) | 95 Cl | n (%) | 95 Cl | |
| HPV positive | 91 (18.2) | 14.9–21.5 | ||
| Single infection | 65 (13.0) | 10.2–16.0 | 65 (71.4) | 61.5–80.2 |
| Multiple infection | 26 (5.2) | 3.4–7.2 | 26 (28.6) | 19.8–38.5 |
| Low-risk HPV genotype | 24 (4.8) | 3.2–7.0 | 24 (26.4) | 18.4–36.3 |
| Probable high-risk HPV genotype | 19 (3.8) | 2.4 –5.9 | 19 (20.9) | 13.8–30.3 |
| High-risk HPV genotype | 63 (12.6) | 10.0–15.8 | 63 (69.2) | 59.1–77.8 |
| Low-risk HPV genotype | ||||
| HPV 6 | 3 (0.6) | 0.2–1.7 | 3 (3.3) | 1.1–9.2 |
| HPV 11 | 1 (0.2) | 0.0–1.1 | 1(1.1) | 0.2–6.0 |
| HPV 40 | 5 (1.0) | 0.4-2.3 | 5 (5.5) | 2.4–14.2 |
| HPV 42 | 2 (0.4) | 0.1–1.4 | 2 (2.2) | 0.6–7.7 |
| HPV 43 | 0 (0.0) | 0.0–0.8 | 0 (0.0) | 0.0–4.1 |
| HPV 44 | 11(2.2) | 1.2–3.9 | 1(1.1) | 0.2–6.0 |
| HPV 54 | 4 (0.8) | 0.3–2.0 | 4 (4.4) | 1.7–10.8 |
| HPV 61 | 1 (0.2) | 0.0–1.1 | 1(1.1) | 0.2–6.0 |
| HPV 70 | 0 (0.0) | 0.0–0.8 | 0 (0.0) | 0.0–4.1 |
| Probable high-risk HPV genotype | ||||
| HPV 26 | 2 (0.4) | 0.1–1.4 | 2 (2.2) | 0.6–7.7 |
| HPV 53 | 4 (0.8) | 0.3–2.0 | 4 (4.4) | 1.7–10.8 |
| HPV 66 | 3 (0.6) | 0.2–1.7 | 3 (3.3) | 1.1–9.2 |
| HPV 68 | 4 (0.8) | 0.3–2.0 | 4 (4.4) | 1.7–10.8 |
| HPV 69 | 0 (0.0) | 0.0–0.8 | 0 (0.0) | 0.0–4.1 |
| HPV 73 | 0 (0.0) | 0.0–0.8 | 0 (0.0) | 0.0–4.1 |
| HPV 82 | 7 (1.4) | 0.7–2.9 | 7 (7.7) | 3.8–15.0 |
| High-risk HPV genotype | ||||
| HPV 16 | 12 (2.4) | 1.4–4.1 | 12 (13.2) | 7.7–21.6 |
| HPV 18 | 12 (2.4) | 1.4–4.1 | 12 (13.2) | 7.7–21.6 |
| HPV 31 | 8 (1.6) | 0.8–3.1 | 8 (8.8) | 4.5–16.4 |
| HPV 33 | 3 (0.6) | 0.2–1.7 | 3 (3.3) | 1.1–9.2 |
| HPV 35 | 1 (0.2) | 0.0–1.1 | 1(1.1) | 0.2–6.0 |
| HPV 39 | 2 (0.4) | 0.1–1.4 | 2 (2.2) | 0.6–7.7 |
| HPV 45 | 6 (1.2) | 0.6–2.6 | 6 (6.6) | 3.1–13.6 |
| HPV 51 | 9 (1.8) | 0.9–3.4 | 9 (9.9) | 5.3–17.7 |
| HPV 52 | 3 (0.6) | 0.2–1.7 | 3 (3.3) | 1.1–9.2 |
| HPV 56 | 8 (1.6) | 0.8–3.1 | 8 (8.8) | 4.5–16.4 |
| HPV 58 | 3 (0.6) | 0.2–1.7 | 3 (3.3) | 1.1–9.2 |
| HPV 59 | 12 (2.4) | 1.4–4.1 | 12 (13.2) | 7.7–21.6 |
| Vaccine genotypes | ||||
| Bivalent | 13 (2.6) | 1.5–4.4 | 13 (14.3) | 8.5–22.9 |
| Quadrivalent | 16 (3.2) | 2.0–5.1 | 16 (17.6) | 11.1–26.7 |
| Nonavalent | 32 (6.4) | 4.6–8.9 | 32 (35.2) | 26.1–45.4 |
| HPV Types | Age Groups (Years) | ||||||
|---|---|---|---|---|---|---|---|
| 30–49 (N = 417) | ≥50 (N = 83) | p Value | |||||
| n | % | 95% CI | n | % | 95% CI | ||
| HPV 16 | 6 | 1.4 | 0.3–2.6 | 6 | 7.2 | 1.7–12.8 | 0.007 |
| HPV 18 | 11 | 2.6 | 1.1–4.2 | 1 | 1.2 | 0.0–3.6 | 0.700 |
| HPV 59 | 12 | 2.9 | 1.3–4.5 | 0 | 0.0 | 0.0–0.0 | 0.231 |
| HPV 44 | 11 | 2.6 | 1.1–4.2 | 0 | 0.0 | 0.0–0.0 | 0.225 |
| HPV 51 | 9 | 2.2 | 0.8–3.6 | 0 | 0.0 | 0.0–0.0 | 0.367 |
| HPV 31 | 7 | 1.7 | 0.4–2.9 | 1 | 1.2 | 0.0–3.6 | 1.00 |
| HPV 56 | 8 | 1.9 | 0.6–3.2 | 0 | 0.0 | 0.0–0.0 | 0.363 |
| HPV 82 | 7 | 1.7 | 0.4–2.9 | 0 | 0.0 | 0.0–0.0 | 0.607 |
| HPV 45 | 6 | 1.4 | 0.3–2.6 | 0 | 0.0 | 0.0–0.0 | 0.596 |
| HPV 40 | 5 | 1.2 | 0.2–2.2 | 0 | 0.0 | 0.0–0.0 | 0.596 |
| HPV 68 | 4 | 1.0 | 0.0–1.9 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 53 | 4 | 1.0 | 0.0–1.9 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 54 | 4 | 1.0 | 0.0–1.9 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 66 | 3 | 0.7 | 0.0–1.5 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 52 | 3 | 0.7 | 0.0–1.5 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 6 | 3 | 0.7 | 0.0–1.5 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 58 | 3 | 0.7 | 0.0–1.5 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 33 | 3 | 0.7 | 0.0–1.5 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 26 | 2 | 0.5 | 0.0–1.1 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 39 | 2 | 0.5 | 0.0–1.1 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 42 | 2 | 0.5 | 0.0–1.1 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 35 | 1 | 0.2 | 0.0–0.7 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 11 | 1 | 0.2 | 0.0–0.7 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 61 | 1 | 0.2 | 0.0–0.7 | 0 | 0.0 | 0.0–0.0 | 1.00 |
| HPV 43 | 0 | 0.0 | 0.0–0.0 | 0 | 0.0 | 0.0–0.0 | - |
| HPV 69 | 0 | 0.0 | 0.0–0.0 | 0 | 0.0 | 0.0–0.0 | - |
| HPV 70 | 0 | 0.0 | 0.0–0.0 | 0 | 0.0 | 0.0–0.0 | - |
| HPV 73 | 0 | 0.0 | 0.0–0.0 | 0 | 0.0 | 0.0–0.0 | - |
| HPV Types | HPV-Positive Case (n = 91) | |
|---|---|---|
| n | % | |
| HPV 59 | 12 | 9.5 |
| HPV 44 | 11 | 8.7 |
| HPV 51 | 9 | 7.1 |
| HPV 56 | 8 | 6.3 |
| HPV 82 | 7 | 5.6 |
| HPV 40 | 5 | 4.0 |
| HPV 68 | 4 | 3.2 |
| HPV 53 | 4 | 3.2 |
| HPV 54 | 4 | 3.2 |
| HPV 66 | 3 | 2.4 |
| HPV 39 | 2 | 1.6 |
| HPV 26 | 2 | 1.6 |
| HPV 42 | 2 | 1.6 |
| HPV 35 | 1 | 0.8 |
| HPV Type | Normal (n = 106) | ASC-US (n = 9) | LSIL (n = 11) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Vaccine type | ||||||
| HPV 6 | 3 | 2.8 | 0 | 0.0 | 0 | 0.0 |
| HPV 11 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 |
| HPV 16 | 10 | 9.4 | 0 | 0.0 | 2 | 18.2 |
| HPV 18 | 9 | 8.5 | 2 | 22.2 | 1 | 9.1 |
| HPV 31 | 7 | 6.6 | 0 | 0.0 | 1 | 9.1 |
| HPV 33 | 3 | 2.8 | 0 | 0.0 | 0 | 0.0 |
| HPV 45 | 3 | 2.8 | 1 | 11.1 | 2 | 18.2 |
| HPV 52 | 2 | 1.9 | 0 | 0.0 | 1 | 9.1 |
| HPV 58 | 2 | 1.9 | 1 | 11.1 | 0 | 0.0 |
| Non-vaccine type | ||||||
| HPV 26 | 1 | 0.9 | 1 | 11.1 | 0 | 0.0 |
| HPV 35 | 0 | 0.0 | 0 | 0.0 | 1 | 9.1 |
| HPV 39 | 2 | 1.9 | 0 | 0.0 | 0 | 0.0 |
| HPV 40 | 5 | 4.7 | 0 | 0.0 | 0 | 0.0 |
| HPV 42 | 2 | 1.9 | 0 | 0.0 | 0 | 0.0 |
| HPV 43 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| HPV 44 | 10 | 9.4 | 0 | 0.0 | 1 | 9.1 |
| HPV 51 | 8 | 7.5 | 0 | 0.0 | 1 | 9.1 |
| HPV 53 | 4 | 3.8 | 0 | 0.0 | 0 | 0.0 |
| HPV 54 | 3 | 2.8 | 1 | 11.1 | 0 | 0.0 |
| HPV 56 | 6 | 5.7 | 2 | 22.2 | 0 | 0.0 |
| HPV 59 | 11 | 10.4 | 0 | 0.0 | 1 | 9.1 |
| HPV 61 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 |
| HPV 66 | 3 | 2.8 | 0 | 0.0 | 0 | 0.0 |
| HPV 68 | 3 | 2.8 | 1 | 11.1 | 0 | 0.0 |
| HPV 69 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| HPV 70 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| HPV 73 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| HPV 82 | 7 | 6.6 | 0 | 0.0 | 0 | 0.0 |
| Vaccine genotype coverage | ||||||
| Bivalent | 10 | 13.0 | 2 | 25.0 | 1 | 17.0 |
| Quadrivalent | 12 | 16.0 | 3 | 38.0 | 1 | 17.0 |
| Nonavalent | 25 | 33.0 | 3 | 38.0 | 4 | 67.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakır, A.; Sapmaz, M.A. Prevalence of Vaccine-Covered and Non-Covered HPV Genotypes Among Unvaccinated Women in Ankara: A Single-Center Study. Vaccines 2025, 13, 640. https://doi.org/10.3390/vaccines13060640
Bakır A, Sapmaz MA. Prevalence of Vaccine-Covered and Non-Covered HPV Genotypes Among Unvaccinated Women in Ankara: A Single-Center Study. Vaccines. 2025; 13(6):640. https://doi.org/10.3390/vaccines13060640
Chicago/Turabian StyleBakır, Ayfer, and Mehmet Alican Sapmaz. 2025. "Prevalence of Vaccine-Covered and Non-Covered HPV Genotypes Among Unvaccinated Women in Ankara: A Single-Center Study" Vaccines 13, no. 6: 640. https://doi.org/10.3390/vaccines13060640
APA StyleBakır, A., & Sapmaz, M. A. (2025). Prevalence of Vaccine-Covered and Non-Covered HPV Genotypes Among Unvaccinated Women in Ankara: A Single-Center Study. Vaccines, 13(6), 640. https://doi.org/10.3390/vaccines13060640





