Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines
Abstract
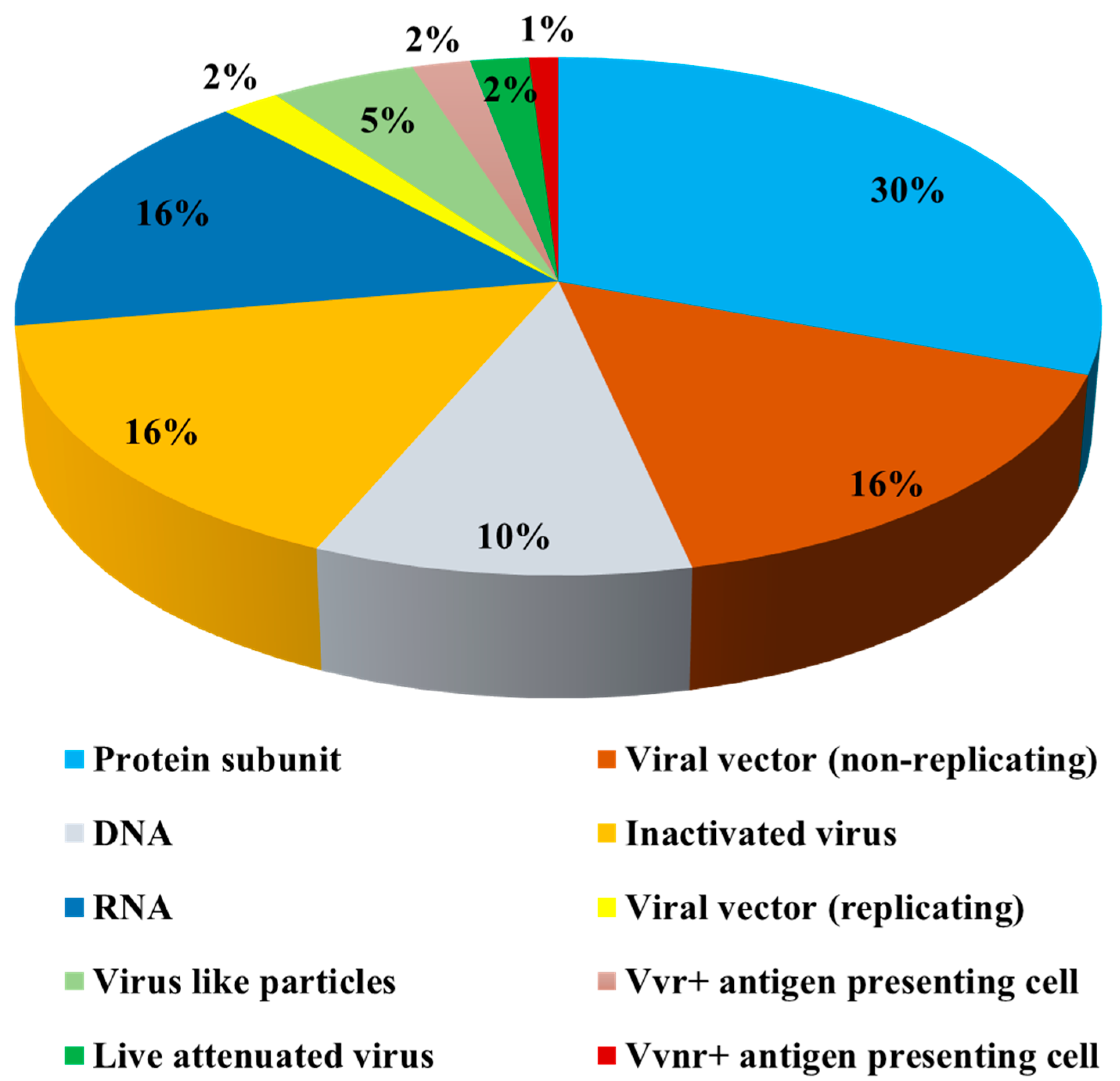
:1. Introduction
2. Plant-Based Vaccine Production for Epidemic Response
3. CoVLP: A COVID-19 VLP Vaccine in a Phase 2/3 Clinical Trial
Clinical Trial Results for CoVLP
4. KBP-201: A COVID-19 cVLP Vaccine in a Phase 1/2 Clinical Trial
5. COVID-19 Vaccines in the Preclinical Stage
6. Second-Generation COVID-19 Vaccines in the Preclinical Stage
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worldometer. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus (accessed on 19 June 2021).
- WHO. Draft Landscape and Tracker of COVID-19 Candidate Vaccines. Available online: https://www.who.int/publications/m/item/draftlandscape-of-covid-19-candidate-vaccines (accessed on 19 June 2021).
- NYT. Coronavirus Vaccine Tracker. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 19 June 2021).
- Chen, J.R.; Liu, Y.M.; Tseng, Y.C.; Ma, C. Better influenza vaccines: An industry perspective. J. Biomed. Sci. 2020, 27, 33. [Google Scholar] [CrossRef] [PubMed]
- Barr, I.G.; Rynehart, C.; Whitney, P.; Druce, J. SARS-CoV-2 does not replicate in embryonated hen’s eggs or in MDCK cell lines. Eurosurveillance 2020, 25, 2001122. [Google Scholar] [CrossRef]
- Li, Y.; Tenchov, R.; Smoot, J.; Liu, C.; Watkins, S.; Zhou, Q. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent. Sci. 2021, 7, 512–533. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Kumari, P.; Saha, L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharm. 2021, 892, 173751. [Google Scholar] [CrossRef]
- Capell, T.; Twyman, R.M.; Armario-Najera, V.; Ma, J.K.; Schillberg, S.; Christou, P. Potential applications of plant biotechnology against SARS-CoV-2. Trends Plant Sci. 2020, 25, 635–643. [Google Scholar] [CrossRef]
- LeBlanc, Z.; Waterhouse, P.; Bally, J. Plant-based vaccines: The way ahead? Viruses 2020, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Lico, C.; Santi, L.; Baschieri, S.; Noris, E.; Marusic, C.; Donini, M.; Pedrazzini, E.; Maga, G.; Franconi, R.; Di Bonito, P.; et al. Plant molecular farming as a strategy against COVID-19—The Italian perspective. Front. Plant Sci. 2020, 11, 609910. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.A.; Holtz, R.B. From farm to finger prick—A perspective on how plants can help in the fight against COVID-19. Front. Bioeng. Biotechnol. 2020, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S. Will plant-made biopharmaceuticals play a role in the fight against COVID-19? Expert Opin. Biol. Ther. 2020, 20, 545–548. [Google Scholar] [CrossRef] [Green Version]
- Rosales-Mendoza, S.; Márquez-Escobar, V.A.; González-Ortega, O.; Nieto-Gómez, R.; Arévalo-Villalobos, J.I. What does plant-based vaccine technology offer to the fight against COVID-19? Vaccines 2020, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Shanmugaraj, B.; Bulaon, C.J.I.; Phoolcharoen, W. Plant molecular farming: A viable platform for recombinant biopharmaceutical production. Plants 2020, 9, 842. [Google Scholar] [CrossRef]
- Shohag, M.J.I.; Khan, F.Z.; Tang, L.; Wei, Y.; He, Z.; Yang, X. COVID-19 crisis: How can plant biotechnology help? Plants 2021, 10, 352. [Google Scholar] [CrossRef]
- Kumar, A.U.; Kadiresen, K.; Gan, W.C.; Ling, A.P.K. Current updates and research on plant-based vaccines for coronavirus disease 2019. Clin. Exp. Vaccine Res. 2021, 10, 13–23. [Google Scholar] [CrossRef]
- Dhama, K.; Natesan, S.; Iqbal Yatoo, M.; Patel, S.K.; Tiwari, R.; Saxena, S.K.; Harapan, H. Plant-based vaccines and antibodies to combat COVID-19: Current status and prospects. Hum. Vaccines Immunother. 2020, 16, 2913–2920. [Google Scholar] [CrossRef]
- Tusé, D.; Nandi, S.; McDonald, K.A.; Buyel, J.F. The emergency response capacity of plant-based biopharmaceutical manufacturing—What it is and what it could be. Front. Plant. Sci. 2020, 11, 594019. [Google Scholar] [CrossRef]
- Wolfert, M.A.; Boons, G.J. Adaptive immune activation: Glycosylation does matter. Nat. Chem. Biol. 2013, 9, 776–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donini, M.; Marusic, C. Current state-of-the-art in plant-based antibody production systems. Biotechnol. Lett. 2019, 41, 335–346. [Google Scholar] [CrossRef]
- Bosch, D.; Schots, A. Plant glycans: Friend or foe in vaccine development? Expert Rev. Vaccines 2010, 9, 835–842. [Google Scholar] [CrossRef]
- Shim, B.S.; Hong, K.J.; Maharjan, P.M.; Choe, S. Plant factory: New resource for the productivity and diversity of human and veterinary vaccines. Clin. Exp. Vaccine Res. 2019, 8, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Gleba, Y.Y.; Tuse, D.; Giritch, A. Plant viral vectors for delivery by Agrobacterium. Curr. Top. Microbiol. Immunol. 2014, 375, 155–192. [Google Scholar] [CrossRef]
- Yao, J.; Weng, Y.; Dickey, A.; Wang, K.Y. Plants as factories for human pharmaceuticals: Applications and challenges. Int. J. Mol. Sci. 2015, 16, 28549–28565. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Chiang, C.H.; Li, Y.L.; Wang, N.M.; Song, P.P.; Lin, S.J.; Chen, Y.H. Oral edible plant vaccine containing hypoallergen of American cockroach major allergen per a 2 prevents roach-allergic asthma in a murine model. PLoS ONE 2018, 13, e0201281. [Google Scholar] [CrossRef]
- Moon, K.B.; Park, J.S.; Park, Y.I.; Song, I.J.; Lee, H.J.; Cho, H.S.; Jeon, J.H.; Kim, H.S. Development of systems for the production of plant-derived biopharmaceuticals. Plants 2020, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Takeyama, N.; Kiyono, H.; Yuki, Y. Plant-based vaccines for animals and humans: Recent advances in technology and clinical trials. Ther. Adv. Vaccines 2015, 3, 139–154. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant-based vaccines against viruses. Virol. J. 2014, 11, 205. [Google Scholar] [CrossRef]
- Landry, N.; Pillet, S.; Favre, D.; Poulin, J.F.; Trépanier, S.; Yassine-Diab, B.; Ward, B.J. Influenza virus-like particle vaccines made in Nicotiana benthamiana elicit durable, poly-functional and cross-reactive T cell responses to influenza HA antigens. Clin. Immunol. 2014, 154, 164–177. [Google Scholar] [CrossRef]
- Mett, V.; Musiychuk, K.; Bi, H.; Farrance, C.E.; Horsey, A.; Ugulava, N.; Shoji, Y.; De La Rosa, P.; Palmer, G.A.; Rabindran, S. A plant-produced influenza subunit vaccine protects ferrets against virus challenge. Influenza Other Respir. Viruses 2008, 2, 33–40. [Google Scholar] [CrossRef]
- Pêra, F.F.P.G.; Mutepfa, D.L.R.; Khan, A.M.; Els, J.H.; Mbewana, S.; van Dijk, A.A.A.; Rybicki, E.P.; Hitzeroth, I.I. Engineering and expression of a human rotavirus candidate vaccine in Nicotiana benthamiana. Virol. J. 2015, 12, 205. [Google Scholar] [CrossRef] [Green Version]
- Fischer, R.; Buyel, J.F. Molecular farming–The slope of enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef]
- Nochi, T.; Yuki, Y.; Katakai, Y.; Shibata, H.; Tokuhara, D.; Mejima, M.; Kurokawa, S.; Takahashi, Y.; Nakanishi, U.; Ono, F.; et al. A rice-based oral cholera vaccine induces macaque-specific systemic neutralizing antibodies but does not influence pre-existing intestinal immunity. J. Immunol. 2009, 183, 6538–6544. [Google Scholar] [CrossRef] [Green Version]
- Govea-Alonso, D.O.; Rubio-Infante, N.; García-Hernández, A.L.; Varona-Santos, J.T.; Korban, S.S.; Moreno-Fierros, L.; Rosales-Mendoza, S. Immunogenic properties of a lettuce-derived C4(V3)6 multiepitopic HIV protein. Planta 2013, 238, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, J.; Modelska, A.; Figlerowicz, M.; Pniewski, T.; Letellier, M.; Lisowa, O.; Yusibov, V.; Koprowski, H.; Plucienniczak, A.; Legocki, A.B. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 1999, 13, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Yusibov, V.; Hooper, D.C.; Spitsin, S.V.; Fleysh, N.; Kean, R.B.; Mikheeva, T.; Deka, D.; Karasev, A.; Cox, S.; Randall, J.; et al. Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 2002, 20, 3155–3164. [Google Scholar] [CrossRef]
- Chan, H.T.; Daniell, H. Plant-made oral vaccines against human infectious diseases—Are we there yet? Plant. Biotechnol. J. 2015, 13, 1056–1070. [Google Scholar] [CrossRef] [Green Version]
- Kurup, V.M.; Thomas, J. Edible vaccines: Promises and challenges. Mol. Biotechnol. 2020, 62, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Medicago. COVID-19 Vaccine Development Program. Available online: https://www.medicago.com/en/covid-19-programs (accessed on 11 May 2021).
- Marillonnet, S.; Thoeringer, C.; Kandzia, R.; Klimyuk, V.; Gleba, Y. Systemic Agrobacterium tumefaciens-mediated transfection of viral replicons for efficient transient expression in plants. Nat. Biotechnol. 2005, 23, 718–723. [Google Scholar] [CrossRef]
- RT. DARPA’s Blue Angel—Pentagon Prepares Millions of Vaccines Against Future Global Flu. Available online: https://www.rt.com/usa/future-vaccine-darpa-research-255 (accessed on 11 May 2021).
- Rybicki, E.P. Plant-produced vaccines: Promise and reality. Drug Discov. Today 2009, 14, 16–24. [Google Scholar] [CrossRef]
- Fox, J.L. First plant-made biologic approved. Nat. Biotechnol. 2012, 30, 472. [Google Scholar] [CrossRef]
- Shaaltiel, Y.; Bartfeld, D.; Hashmueli, S.; Baum, G.; Brill-Almon, E.; Galili, G.; Dym, O.; Boldin-Adamsky, S.A.; Silman, I.; Sussman, J.L.; et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant. Biotechnol. J. 2007, 5, 579–590. [Google Scholar] [CrossRef]
- Mor, T.S. Molecular pharming’s foot in the FDA’s door: Protalix’s trailblazing story. Biotechnol. Lett. 2015, 37, 2147–2150. [Google Scholar] [CrossRef] [Green Version]
- Pogrebnyak, N.; Golovkin, M.; Andrianov, V.; Spitsin, S.; Smirnov, Y.; Egolf, R.; Koprowski, H. Severe acute respiratory syndrome (SARS) S protein production in plants: Development of recombinant vaccine. Proc. Natl. Acad. Sci. USA 2005, 102, 9062–9067. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Y.; Ramalingam, S.; Chye, M.L. Accumulation of recombinant SARS-CoV spike protein in plant cytosol and chloroplasts indicate potential for development of plant-derived oral vaccines. Exp. Biol. Med. 2006, 231, 1346–1352. [Google Scholar] [CrossRef]
- Ward, B.J.; Makarkov, A.; Séguin, A.; Pillet, S.; Trépanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (≥65 years): Two multicentre, randomised phase 3 trials. Lancet 2020, 396, 1491–1503. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Yusakul, G.; Shanmugaraj, B.; Kittirotruji, K.; Suwatsrisakul, P.; Prompetchara, E.; Taychakhoonavud, S.; Phoolcharoen, W. Plant-produced recombinant SARS-CoV-2 receptor-binding domain_ an economical, scalable biomaterial source for COVID-19 diagnosis. Bimater. Transl. 2021, 43, 43–49. [Google Scholar] [CrossRef]
- Gobeil, P.; Pillet, S.; Séguin, A.; Boulay, I.; Mahmood, A.; Vinh, D.C.; Charland, N.; Boutet, P.; Roman, F.; Van Der Most, R.; et al. Interim report of a phase 2 randomized trial of a plant-produced virus-like particle vaccine for Covid-19 in healthy adults aged 18–64 and older adults aged 65 and older. medRxiv 2021. [Google Scholar] [CrossRef]
- IBIO. Vaccines IBIO-202 COVID-19. Available online: https://www.ibioinc.com/vaccines/ibio-202 (accessed on 11 May 2021).
- KBP. Our Discovery Pipelines. Available online: https://kentuckybioprocessing.com/our-discovery-pipeline/#pandemic-preparedness (accessed on 11 May 2021).
- Siriwattananon, K.; Manopwisedjaroen, S.; Kanjanasirirat, P.; Budi Purwono, P.; Rattanapisit, K.; Shanmugaraj, B.; Smith, D.R.; Borwornpinyo, S.; Thitithanyanont, A.; Phoolcharoen, W. Development of plant-produced recombinant ACE2-Fc fusion protein as a potential therapeutic agent against SARS-CoV-2. Front. Plant. Sci. 2021, 11, 604663. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Shanmugaraj, B.; Manopwisedjaroen, S.; Purwono, P.B.; Siriwattananon, K.; Khorattanakulchai, N.; Hanittinan, O.; Boonyayothin, W.; Thitithanyanont, A.; Smith, D.R.; et al. Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci. Rep. 2020, 10, 17698. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Rattanapisit, K.; Manopwisedjaroen, S.; Thitithanyanont, A.; Phoolcharoen, W. Monoclonal antibodies B38 and H4 produced in Nicotiana benthamiana neutralize SARS-CoV-2 in vitro. Front. Plant. Sci. 2020, 11, 589995. [Google Scholar] [CrossRef]
- Makatsa, M.S.; Tincho, M.B.; Wendoh, J.M.; Ismail, S.D.; Nesamari, R.; Pera, F.; de Beer, S.; David, A.; Jugwanth, S.; Gededzha, M.P.; et al. SARS-CoV-2 antigens expressed in plants detect antibody responses in COVID-19 patients. Front. Plant. Sci. 2021, 12, 589940. [Google Scholar] [CrossRef]
- GLS. GFLAS Life Sciences Succeeds in Expressing COVID-19 Recombinant Vaccine Candidates with Plant Based Platform. Available online: http://gflas.com/about/press_view.php?idx=165 (accessed on 11 May 2021).
- Medicago. Medicago and GSK Start Phase 3 Trial of Adjuvanted COVID-19 Vaccine Candidate. Available online: https://www.medicago.com/en/media-room/medicago-and-gsk-start-phase-3-trial-of-adjuvanted-covid-19-vaccine-candidate (accessed on 11 May 2021).
- ClinicalTrial. KBP-201 COVID-19 Vaccine Trial in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04473690?term=KBP+201&draw=2&rank=1 (accessed on 11 May 2021).
- BPP. COVID-19 Vaccine Development. Available online: https://baiyaphytopharm.com/covid-19 (accessed on 11 May 2021).
- Mamedov, T.; Yuksel, D.; Ilgın, M.; Gürbüzaslan, İ.; Gulec, B.; Mammadova, G.; Say, D.; Hasanova, G. Engineering, production and characterization of spike and nucleocapsid structural proteins of SARS–CoV-2 in Nicotiana benthamiana as vaccine candidates against COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef]
- Mulangu, S.; Dodd, L.E.; Davey, R.T., Jr.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Jin, X.; Huang, Z. Fighting Ebola with ZMapp: Spotlight on plant-made antibody. Sci. China Life Sci. 2014, 57, 987–988. [Google Scholar] [CrossRef]
- Joung, Y.H.; Park, S.H.; Moon, K.B.; Jeon, J.H.; Cho, H.S.; Kim, H.S. The last ten years of advancements in plant-derived recombinant vaccines against hepatitis B. Int. J. Mol. Sci. 2016, 17, 1715. [Google Scholar] [CrossRef]
- Medicago. Medicago Pipeline. Available online: https://www.medicago.com/en/pipeline (accessed on 13 May 2021).
- Ward, B.J.; Séguin, A.; Couillard, J.; Trépanier, S.; Landry, N. Phase III: Randomized observer-blind trial to evaluate lot-to-lot consistency of a new plant-derived quadrivalent virus like particle influenza vaccine in adults 18–49 years of age. Vaccine 2021, 39, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Landry, N.; Ward, B.J.; Trépanier, S.; Montomoli, E.; Dargis, M.; Lapini, G.; Vézina, L.P. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS ONE 2010, 5, e15559. [Google Scholar] [CrossRef] [Green Version]
- Ward, B.J.; Gobeil, P.; Seguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.Y.; Couture, M.; D’Aoust, M.A.; Dhaliwall, J.; Finkle, C.; et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Chichester, J.A.; Musiychuk, K.; Farrance, C.E.; Mett, V.; Lyons, J.; Mett, V.; Yusibov, V. A single component two-valent LcrV-F1 vaccine protects non-human primates against pneumonic plague. Vaccine 2009, 27, 3471–3474. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.F.; Guerrero, M.L.; Moon, J.E.; Waterman, P.; Nielsen, R.K.; Jefferson, S.; Gross, F.L.; Hancock, K.; Katz, J.M.; Yusibov, V. Safety and immunogenicity of a plant-produced recombinant monomer hemagglutinin-based influenza vaccine derived from influenza A (H1N1)pdm09 virus: A phase 1 dose-escalation study in healthy adults. Vaccine 2014, 32, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Chichester, J.A.; Mett, V.; Jaje, J.; Tottey, S.; Manceva, S.; Casta, L.J.; Gibbs, S.K.; Musiychuk, K.; Shamloul, M.; et al. A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS ONE 2013, 8, e79538. [Google Scholar] [CrossRef] [PubMed]
- Chichester, J.A.; Green, B.J.; Jones, R.M.; Shoji, Y.; Miura, K.; Long, C.A.; Lee, C.K.; Ockenhouse, C.F.; Morin, M.J.; Streatfield, S.J.; et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A phase 1 dose-escalation study in healthy adults. Vaccine 2018, 36, 5865–5871. [Google Scholar] [CrossRef]
- Chichester, J.A.; Jones, R.M.; Green, B.J.; Stow, M.; Miao, F.; Moonsammy, G.; Streatfield, S.J.; Yusibov, V. Safety and immunogenicity of a plant-produced recombinant hemagglutinin-based influenza vaccine (HAI-05) derived from A/Indonesia/05/2005 (H5N1) influenza virus: A phase 1 randomized, double-blind, placebo-controlled, dose-escalation study in healthy adults. Viruses 2012, 4, 3227–3244. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Racine, T.; Nfon, C.; Di Lenardo, T.Z.; Babiuk, S.; Ward, B.J.; Kobinger, G.P.; Landry, N. Plant-derived H7 VLP vaccine elicits protective immune response against H7N9 influenza virus in mice and ferrets. Vaccine 2015, 33, 6282–6289. [Google Scholar] [CrossRef] [PubMed]
- Kashima, K.; Yuki, Y.; Mejima, M.; Kurokawa, S.; Suzuki, Y.; Minakawa, S.; Takeyama, N.; Fukuyama, Y.; Azegami, T.; Tanimoto, T.; et al. Good manufacturing practices production of a purification-free oral cholera vaccine expressed in transgenic rice plants. Plant. Cell Rep. 2016, 35, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Thanavala, Y.; Mahoney, M.; Pal, S.; Scott, A.; Richter, L.; Natarajan, N.; Goodwin, P.; Arntzen, C.J.; Mason, H.S. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. USA 2005, 102, 3378–3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tacket, C.O.; Mason, H.S.; Losonsky, G.; Estes, M.K.; Levine, M.M.; Arntzen, C.J. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J. Infect. Dis. 2000, 182, 302–305. [Google Scholar] [CrossRef]
- IconGenetics. Icon Genetics Clinical Development of Its Novel Norovirus vaccine Reaches Milestone of Complete Dosing of the First Cohort. Available online: https://www.icongenetics.com/icon-genetics-clinical-development-of-its-novel-norovirus-vaccine-reaches-milestone-of-complete-dosing-of-the-first-cohort (accessed on 19 June 2021).
- Pillet, S.; Aubin, É.; Trépanier, S.; Poulin, J.-F.; Yassine-Diab, B.; Ter Meulen, J.; Ward, B.J.; Landry, N. Humoral and cell-mediated immune responses to H5N1 plant-made virus-like particle vaccine are differentially impacted by alum and GLA-SE adjuvants in a Phase 2 clinical trial. NPJ Vaccines 2018, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.L.; Cardineau, G.A. Prevention of bubonic and pneumonic plague using plant-derived vaccines. Biotechnol. Adv. 2010, 28, 184–196. [Google Scholar] [CrossRef]
- Arlen, P.A.; Singleton, M.; Adamovicz, J.J.; Ding, Y.; Davoodi-Semiromi, A.; Daniell, H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect. Immun. 2008, 76, 3640–3650. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Infante, N.; Govea-Alonso, D.O.; Romero-Maldonado, A.; García-Hernández, A.L.; Ilhuicatzi-Alvarado, D.; Salazar-González, J.A.; Korban, S.S.; Rosales-Mendoza, S.; Moreno-Fierros, L. A plant-derived multi-HIV antigen induces broad immune responses in orally immunized mice. Mol. Biotechnol. 2015, 57, 662–674. [Google Scholar] [CrossRef]
- Tottey, S.; Shoji, Y.; Jones, R.M.; Chichester, J.A.; Green, B.J.; Musiychuk, K.; Si, H.; Manceva, S.D.; Rhee, A.; Shamloul, M.; et al. Plant-produced subunit vaccine candidates against yellow fever induce virus neutralizing antibodies and confer protection against viral challenge in animal models. Am. J. Trop. Med. Hyg. 2018, 98, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, R.; Fernandes, A.; Simões, M.; Marchevsky, R.; Neves, P.; Bom, A.; Caride, E.; Freire, M. Yellow fever vaccine, recombinant envelope protein (rYFE), plant derived, for active immunization: Pre-clinical studies in mice and monkey models. In Proceedings of the Seminario Annual Cientifico e Technology de Biomanguinhos, Rio de Janeiro, Brazil, 2–4 May 2017; pp. 52–53. [Google Scholar]
- Tremouillaux-Guiller, J.; Moustafa, K.; Hefferon, K.; Gaobotse, G.; Makhzoum, A. Plant-made HIV vaccines and potential candidates. Curr. Opin. Biotechnol. 2020, 61, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Lindh, I.; Bråve, A.; Hallengärd, D.; Hadad, R.; Kalbina, I.; Strid, Å.; Andersson, S. Oral delivery of plant-derived HIV-1 p24 antigen in low doses shows a superior priming effect in mice compared to high doses. Vaccine 2014, 32, 2288–2293. [Google Scholar] [CrossRef] [PubMed]
- Phoolcharoen, W.; Bhoo, S.H.; Lai, H.; Ma, J.; Arntzen, C.J.; Chen, Q.; Mason, H.S. Expression of an immunogenic Ebola immune complex in Nicotiana Benthamiana. Plant. Biotechnol. J. 2011, 9, 807–816. [Google Scholar] [CrossRef] [Green Version]
- Van Eerde, A.; Gottschamel, J.; Bock, R.; Hansen, K.E.A.; Munang’andu, H.M.; Daniell, H.; Liu Clarke, J. Production of tetravalent dengue virus envelope protein domain III based antigens in lettuce chloroplasts and immunologic analysis for future oral vaccine development. Plant. Biotechnol. J. 2019, 17, 1408–1417. [Google Scholar] [CrossRef]
- Ponndorf, D.; Meshcheriakova, Y.; Thuenemann, E.C.; Dobon Alonso, A.; Overman, R.; Holton, N.; Dowall, S.; Kennedy, E.; Stocks, M.; Lomonossoff, G.P.; et al. Plant-made dengue virus-like particles produced by co-expression of structural and non-structural proteins induce a humoral immune response in mice. Plant. Biotechnol. J. 2021, 19, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Zhao, R.; Gao, L.J.; Gao, X.F.; Wang, D.P.; Cao, J.M. SARS-CoV-2: Structure, biology, and structure-based therapeutics development. Front. Cell Infect. Microbiol. 2020, 10, 587269. [Google Scholar] [CrossRef]
- Karamloo, F.; König, R. SARS-CoV-2 immunogenicity at the crossroads. Allergy 2020, 75, 1822–1824. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papageorgiou, A.C.; Mohsin, I. The SARS-CoV-2 spike glycoprotein as a drug and vaccine target: Structural insights into its complexes with ACE2 and antibodies. Cells 2020, 9, 2343. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, P.J.M.; Caniels, T.G.; van der Straten, K.; Snitselaar, J.L.; Aldon, Y.; Bangaru, S.; Torres, J.L.; Okba, N.M.A.; Claireaux, M.; Kerster, G.; et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 2020, 369, 643–650. [Google Scholar] [CrossRef]
- Bao, Y.; Ling, Y.; Chen, Y.Y.; Tian, D.; Zhao, G.P.; Zhang, X.H.; Hang, H.; Li, Y.; Su, B.; Lu, H.Z.; et al. Dynamic anti-spike protein antibody profiles in COVID-19 patients. Int. J. Infect. Dis. 2021, 103, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Salazar, E.; Kuchipudi, S.V.; Christensen, P.A.; Eagar, T.; Yi, X.; Zhao, P.; Jin, Z.; Long, S.W.; Olsen, R.J.; Chen, J.; et al. Convalescent plasma anti–SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization. J. Clin. Investig. 2020, 130, 6728–6738. [Google Scholar] [CrossRef]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Liu, F.; Ge, S.; Li, L.; Wu, X.; Liu, Z.; Wang, Z. Virus-like particles: Potential veterinary vaccine immunogens. Res. Vet. Sci. 2012, 93, 553–559. [Google Scholar] [CrossRef] [PubMed]
- D’Aoust, M.A.; Couture, M.M.J.; Charland, N.; Trépanier, S.; Landry, N.; Ors, F.; Vézina, L.P. The production of hemagglutinin-based virus-like particles in plants: A rapid, efficient and safe response to pandemic influenza. Plant. Biotechnol. J. 2010, 8, 607–619. [Google Scholar] [CrossRef]
- Jennings, G.T.; Bachmann, M.F. The coming of age of virus-like particle vaccines. Biol. Chem. 2008, 389, 521–536. [Google Scholar] [CrossRef]
- Chackerian, B.; Lenz, P.; Lowy, D.R.; Schiller, J.T. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J. Immunol. 2002, 169, 6120–6126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, C.; Wagner, R. Virus-like particles-universal molecular toolboxes. Curr. Opin. Biotechnol. 2007, 18, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Roldão, A.; Mellado, M.C.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Huang, Z.; Elkin, G.; Maloney, B.J.; Beuhner, N.; Arntzen, C.J.; Thanavala, Y.; Mason, H.S. Virus-like particle expression and assembly in plants: Hepatitis B and Norwalk viruses. Vaccine 2005, 23, 1851–1858. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Gomes, A.C.; Vogel, M.; Bachmann, M.F. Interaction of viral capsid-derived virus-like particles (VLPs) with the innate immune system. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- D’Aoust, M.A.; Lavoie, P.O.; Couture, M.M.; Trépanier, S.; Guay, J.M.; Dargis, M.; Mongrand, S.; Landry, N.; Ward, B.J.; Vézina, L.P. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant. Biotechnol. J. 2008, 6, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Shoji, Y.; Farrance, C.E.; Bautista, J.; Bi, H.; Musiychuk, K.; Horsey, A.; Park, H.; Jaje, J.; Green, B.J.; Shamloul, M.; et al. A plant-based system for rapid production of influenza vaccine antigens. Influenza Other Respir. Viruses 2012, 6, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Streatfield, S.J.; Kushnir, N.; Yusibov, V. Plant-produced candidate countermeasures against emerging and reemerging infections and bioterror agents. Plant Biotechnol. J. 2015, 13, 1136–1159. [Google Scholar] [CrossRef]
- Medicago. Discovery Platforms. Available online: https://www.medicago.com/en/discovery (accessed on 11 May 2021).
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L. Adjuvants for coronavirus vaccines. Front. Immunol. 2020, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Lodaya, R.N.; Lofano, G. The continued advance of vaccine adjuvants—‘We can work it out’. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2020; p. 101426. [Google Scholar]
- Campbell, J.D. Development of the CpG adjuvant 1018: A case study. Methods Mol. Biol. 2017, 1494, 15–27. [Google Scholar] [PubMed]
- Schillie, S.; Harris, A.; Link-Gelles, R.; Romero, J.; Ward, J.; Nelson, N. Recommendations of the advisory committee on immunization practices for use of a hepatitis B vaccine with a novel adjuvant. Morb. Mortal. Wkly. Rep. 2018, 67, 455. [Google Scholar] [CrossRef] [PubMed]
- Dynavax. CpG 1018. Available online: https://www.dynavax.com/science/cpg-1018 (accessed on 11 May 2021).
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an adjuvant system containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Cohet, C.; van der Most, R.; Bauchau, V.; Bekkat-Berkani, R.; Doherty, T.M.; Schuind, A.; Tavares Da Silva, F.; Rappuoli, R.; Garçon, N.; Innis, B.L. Safety of AS03-adjuvanted influenza vaccines: A review of the evidence. Vaccine 2019, 37, 3006–3021. [Google Scholar] [CrossRef]
- Nature. How Plants Could Produce a COVID-19 Vaccine. Available online: https://www.nature.com/articles/d42473-020-00253-2 (accessed on 11 May 2021).
- Medicago. Medicago Announces Positive Phase 1 Results for Its COVID-19 Vaccine Candidate. Available online: https://www.medicago.com/en/media-room/medicago-announces-positive-phase-1-results-for-its-covid-19-vaccine-candidate (accessed on 11 May 2021).
- ClinicalTrial. Safety, Tolerability and Immunogenicinity of a Coronavirus-Like Particle COVID-19 Vaccine in Adults Aged 18–55 Years. Available online: https://clinicaltrials.gov/ct2/show/NCT04450004?term=CoVLP&draw=2 (accessed on 11 May 2021).
- Medicago. Medicago and GSK Announce Start of Phase 2/3 Clinical Trials of Adjuvanted COVID-19 Vaccine Candidate. Available online: https://www.medicago.com/en/media-room/medicago-and-gsk-announce-start-of-phase-2-3-clinical-trials-of-adjuvanted-covid-19-vaccine-candidate (accessed on 11 May 2021).
- PMLiVE. GSK and Medicago Initiate Late-Stage COVID-19 Vaccine Study. Available online: http://www.pmlive.com/pharma_news/gsk_and_medicago_initiate_late-stage_covid-19_vaccine_study_1365425 (accessed on 11 May 2021).
- Medicago. Health Canada Initiates the Review of the Rolling Submission for the First Canadian-based COVID-19 Vaccine Candidate. Available online: https://www.medicago.com/en/media-room/health-canada-initiates-the-review-of-the-rolling-submission-for-the-first-canadian-based-covid-19-vaccine-candidate (accessed on 11 May 2021).
- Li, F.; Li, W.; Farzan, M.; Harrison, S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005, 309, 1864–1868. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292.e286. [Google Scholar] [CrossRef] [PubMed]
- NPR. Tobacco Plants Contribute Key Ingredient for COVID-19 Vaccine. Available online: https://www.npr.org/sections/health-shots/2020/10/15/923210562/tobacco-plants-contribute-key-ingredient-for-covid-19-vaccine (accessed on 11 May 2021).
- Chen, Q.; Lai, H. Plant-derived virus-like particles as vaccines. Hum. Vaccines Immunother. 2013, 9, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Emini, E.A.; Jameson, B.A.; Wimmer, E. Priming for and induction of anti-poliovirus neutralizing antibodies by synthetic peptides. Nature 1983, 304, 699–703. [Google Scholar] [CrossRef]
- Butler, P.J.G. The current picture of the structure and assembly of tobacco mosaic virus. J. Gen. Virol. 1984, 65, 253–279. [Google Scholar] [CrossRef]
- Haynes, J.R.; Cunningham, J.; von Seefried, A.; Lennick, M.; Garvin, R.T.; Shen, S.H. Development of a genetically–engineered, candidate polio vaccine employing the self–assembling properties of the tobacco mosaic virus coat protein. Bio/Technology 1986, 4, 637–641. [Google Scholar] [CrossRef] [PubMed]
- BPN. BAT Biotech Using Tobacco to Make COVID-19 Vaccine Candidate. Available online: https://bioprocessintl.com/bioprocess-insider/therapeutic-class/bat-biotech-using-tobacco-to-make-covid-19-vaccine-candidate (accessed on 11 May 2021).
- BAT. BAT Progresses COVID-19 Candidate Vaccine into Phase I Human Clinical Trials. Available online: https://www.bat-science.com/GROUPMS/SITES/BAT_B9JBW3.NSF/vwPagesWebLive/DOBVULEJ (accessed on 11 May 2021).
- ClinicalTrial. Study to Evaluate the Safety and Immunogenicity of KBP-V001 Quadrivalent Influenza Vaccine in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04439695?term=Kentucky+bioprocessing&draw=2&rank=2 (accessed on 12 May 2021).
- Wintjens, R.; Bifani, A.M.; Bifani, P. Impact of glycan cloud on the B-cell epitope prediction of SARS-CoV-2 Spike protein. NPJ Vaccines 2020, 5, 81. [Google Scholar] [CrossRef]
- Ozdilek, A.; Paschall, A.V.; Dookwah, M.; Tiemeyer, M.; Avci, F.Y. Host protein glycosylation in nucleic acid vaccines as a potential hurdle in vaccine design for nonviral pathogens. Proc. Natl. Acad. Sci. USA 2020, 117, 1280–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BS. G+Flas, Setting a Development of Plant-Based ‘COVID-19′ Vaccine. Available online: http://www.biospectator.com/view/news_view.php?varAtcId=9750 (accessed on 11 May 2021).
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333. [Google Scholar] [CrossRef]
- Marusic, C.; Pioli, C.; Stelter, S.; Novelli, F.; Lonoce, C.; Morrocchi, E.; Benvenuto, E.; Salzano, A.M.; Scaloni, A.; Donini, M. N-glycan engineering of a plant-produced anti-CD20-hIL-2 immunocytokine significantly enhances its effector functions. Biotechnol. Bioeng. 2018, 115, 565–576. [Google Scholar] [CrossRef]
- Maharjan, P.M.; Cheon, J.; Jung, J.; Kim, H.; Lee, J.; Song, M.; Jeong, G.U.; Kwon, Y.; Shim, B.; Choe, S. Plant-Expressed Receptor Binding Domain of the SARS-CoV-2 Spike Protein Elicits Humoral Immunity in Mice. Vaccines 2021, 9, 978. [Google Scholar] [CrossRef]
- Pulse. G+FLAS Life Sciences Readying Animal Testing of Coronavirus Vaccine Candidate. Available online: https://pulsenews.co.kr/view.php?year=2020&no=236363 (accessed on 11 May 2021).
- CNN. iBio Announces Advancement of COVID-19 Vaccine Program. Available online: https://edition.cnn.com/business/newsfeeds/globenewswire/7874773.html (accessed on 25 April 2021).
- Musiychuk, K.; Stephenson, N.; Bi, H.; Farrance, C.E.; Orozovic, G.; Brodelius, M.; Brodelius, P.; Horsey, A.; Ugulava, N.; Shamloul, A.M. A launch vector for the production of vaccine antigens in plants. Influenza Other Respir. Viruses 2007, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Franconi, R.; Brandi, R.; Muller, A.; Mett, V.; Yusibov, V.; Venuti, A. Anti-cancer activity of plant-produced HPV16 E7 vaccine. Vaccine 2007, 25, 3018–3021. [Google Scholar] [CrossRef]
- Buyel, J.F.; Bautista, J.A.; Fischer, R.; Yusibov, V.M. Extraction, purification and characterization of the plant-produced HPV16 subunit vaccine candidate E7 GGG. J. Chromatogr B Anal. Technol. Biomed. Life Sci. 2012, 880, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Venuti, A.; Curzio, G.; Mariani, L.; Paolini, F. Immunotherapy of HPV-associated cancer: DNA/plant-derived vaccines and new orthotopic mouse models. Cancer Immunol. Immunother. 2015, 64, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- IGN. iBio Reports Successful COVID-19 Vaccine Toxicology Study Results and Announces Next-Gen COVID-19 Vaccine Program. Available online: Globenewswire.com/news-release/2021/05/06/2225071/0/en/iBio-Reports-Successful-COVID-19-Vaccine-Toxicology-Study-Results-and-Announces-Next-Gen-COVID-19-Vaccine-Program.html (accessed on 11 May 2021).
- IGN. iBio and Planet Biotechnology Enter into Exclusive Worldwide License Agreement FOR THE Development of a COVID-19 Therapeutic. Available online: https://www.globenewswire.com/fr/news-release/2020/08/28/2085354/0/en/iBio-and-Planet-Biotechnology-Enter-into-Exclusive-Worldwide-License-Agreement-for-the-Development-of-a-COVID-19-Therapeutic.html (accessed on 11 May 2021).
- Bian, L.; Gao, F.; Zhang, J.; He, Q.; Mao, Q.; Xu, M.; Liang, Z. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 365–373. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Nie, J.; Zhang, L.; Hao, H.; Liu, S.; Zhao, C.; Zhang, Q.; Liu, H.; Nie, L. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell 2020, 182, 1284–1294. [Google Scholar] [CrossRef]
- Faria, N.R.; Mellan, T.A.; Whittaker, C.; Claro, I.M.; Candido, D.D.S.; Mishra, S.; Crispim, M.A.E.; Sales, F.C.S.; Hawryluk, I.; McCrone, J.T.; et al. Genomics and epidemiology of the P.1 SARS-CoV-2 lineage in Manaus, Brazil. Science 2021, 372, 815–821. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv 2020. [Google Scholar] [CrossRef]
- Rambaut, A.; Holmes, E.C.; O’Toole, Á.; Hill, V.; McCrone, J.T.; Ruis, C.; du Plessis, L.; Pybus, O.G. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat. Microbiol. 2020, 5, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.K.; Mazumdar, K.; Gordy, J.T. The nucleocapsid protein of SARS-CoV-2: A target for vaccine development. J. Virol. 2020, 94, e00647-20. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.C.; de Magalhães, M.T.Q.; Homan, E.J. Immunoinformatic analysis of SARS-CoV-2 nucleocapsid protein and identification of COVID-19 vaccine targets. Front. Immunol. 2020, 11, 587615. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yang, M.; Hong, Z.; Zhang, L.; Huang, Z.; Chen, X.; He, S.; Zhou, Z.; Zhou, Z.; Chen, Q.; et al. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B 2020, 10, 1228–1238. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Kou, G.; Zheng, Y.; Ding, Y.; Ni, W.; Wang, Q.; Tan, L.; Wu, W.; Tang, S.; et al. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e00461-20. [Google Scholar] [CrossRef] [Green Version]
- NYT. Tracking Coronavirus Vaccinations around the World. Available online: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html (accessed on 19 June 2021).
- CNN. Tracking Covid-19 Vaccinations Worldwide. Available online: https://edition.cnn.com/interactive/2021/health/global-covid-vaccinations (accessed on 19 June 2021).
| Vaccine | Vaccine Platform | Target Antigen | Development Phase | Developer | Country | References |
|---|---|---|---|---|---|---|
| CoVLP | VLP | S protein | Phase 2/3 | Medicago | Canada | [50,58] |
| KBP-201 | cVLP | RBD | Phase 1/2 | Kentucky Bioprocessing | United State | [59] |
| IBIO-200 | VLP | S protein | preclinical | iBio | United State | [51] |
| IBIO-201 | Conjugated protein subunit | S protein | preclinical | iBio | United State | [51] |
| IBIO-202 | Protein subunit | N protein | preclinical | iBio | United State | [51] |
| RBD | Protein subunit | RBD | preclinical | G+FLAS Life Sciences | South Korea | [57] |
| Baiya SARS-CoV Vax 1 | Protein subunit | NM * | preclinical | Baiya phytopharm | Thailand | [60] |
| S1 protein | Protein subunit | S1 protein | preclinical | Akdeniz University | Turkey | [61] |
| RBD | Protein subunit | RBD | preclinical | Akdeniz University | Turkey | [61] |
| N protein | Protein subunit | N protein | preclinical | Akdeniz University | Turkey | [61] |
| Disease | Pathogen | Antigen | Host Plant | Expression System | Route of Administration | Clinical Phase | References |
|---|---|---|---|---|---|---|---|
| Seasonal Influenza | A/H1N1, A/H3N2, B/Brisbane, B/Phuket | HA Quadrivalent | Nicotiana benthamiana | Transient VLP | Intramuscular | Phase 3 completed | [48,66] |
| COVID-19 | SARS-CoV-2 | Spike protein | Nicotiana benthamiana | Transient VLP | Intramuscular | Phase 3 | [50,58] |
| COVID-19 | SARS-CoV-2 | RBD | Nicotiana benthamiana | Transient cVLP | Intramuscular | Phase 1/2 | [59] |
| Influenza | H5N1 | HA (H5) | Nicotiana benthamiana | Transient | Intramuscular | Phase 2 completed | [79] |
| Influenza | H5N1 | HA | Nicotiana benthamiana | Transient | Intramuscular | Phase 1 completed | [73] |
| Influenza | H1N1 virus | HA | Nicotiana benthamiana | Transient | Intramuscular | Phase 1 completed | [70] |
| Malaria | Plasmodium falciparum | Pfs25 VLP | Nicotiana benthamiana | Transient cVLP | Intramuscular | Phase 1 completed | [71,72] |
| Influenza | H7N9 | HA (H7) | Nicotiana benthamiana | Transient | Intramuscular | Phase 1 | [27,74] |
| Cholera | Vibrio Cholera | CTB | Rice | Transgenic | Edible | Phase 1 | [75] |
| Hepatitis B | HBV | HBsAg | Potato | Transgenic | Edible | Phase 1 | [76] |
| Hepatitis B | HBV | HBsAg | Lettuce | Transgenic | Edible | Phase 1 | [35] |
| Rabies | Rabies virus | G protein | Spinach | Transient | Oral | Phase 1 | [36] |
| Gastroenteritis | Norwalk virus | Capsid protein | Potato | Transgenic | Oral | Phase 1 | [77] |
| Gastroenteritis | Norwalk virus | NM * | Nicotiana benthamiana | Transient VLP | NA | Phase 1 | [78] |
| Anthrax | Bacillus anthracis | Protective antigen | Nicotiana benthamiana | Transient | N | Phase I | [64] |
| Gastroenteritis | Rotavirus | NM * | Nicotiana benthamiana | Transient VLP | NA | Phase 1 | [65] |
| Disease | Antigen | Host Plant | Expression System | Efficacy in Preclinical Study | References |
|---|---|---|---|---|---|
| Plague | F1 and V | Tomato | Transgenic | 50% protection in mice | [80] |
| Plague | F1 and V | Nicotiana benthamiana | Transient | 88% protection in monkeys | [69] |
| HIV/AIDS Pandemic | HIV multi proteins | Nicotiana benthamiana | Transplantomic | Induced humoral and cellular immune response in mice. | [82] |
| HIV/AIDS Pandemic | p24 | Arabidopsis thaliana | Transgenic | Induced humoral immune response in mice. | [86] |
| Dengue fever | SP and NSPs | Nicotiana benthamiana | Transient VLP | Induced humoral immune response in mice. | [89] |
| Dengue fever | EDIII-1-4 tetravalent antigen | Lettuce | Transplantomic | Induced specific antibodies in rabbits. | [88] |
| Yellow fever | Envelop protein | Nicotiana benthamiana | Transient | 77% protection in mice and induced cellular and humoral immune response in monkey. | [83] |
| Yellow fever | Envelop protein | Nicotiana benthamiana | Transient | 100% protection in mice and induced cellular and humoral immune response in monkey. | [84] |
| Ebola | Glycoprotein (GP1) | Nicotiana benthamiana | Transient | Induced anti-Ebola antibodies in mice. | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharjan, P.M.; Choe, S. Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines. Vaccines 2021, 9, 992. https://doi.org/10.3390/vaccines9090992
Maharjan PM, Choe S. Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines. Vaccines. 2021; 9(9):992. https://doi.org/10.3390/vaccines9090992
Chicago/Turabian StyleMaharjan, Puna Maya, and Sunghwa Choe. 2021. "Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines" Vaccines 9, no. 9: 992. https://doi.org/10.3390/vaccines9090992
APA StyleMaharjan, P. M., & Choe, S. (2021). Plant-Based COVID-19 Vaccines: Current Status, Design, and Development Strategies of Candidate Vaccines. Vaccines, 9(9), 992. https://doi.org/10.3390/vaccines9090992






