COVID-19 Vaccines in Cancer Patients. Seropositivity and Safety. Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Statistical Analysis
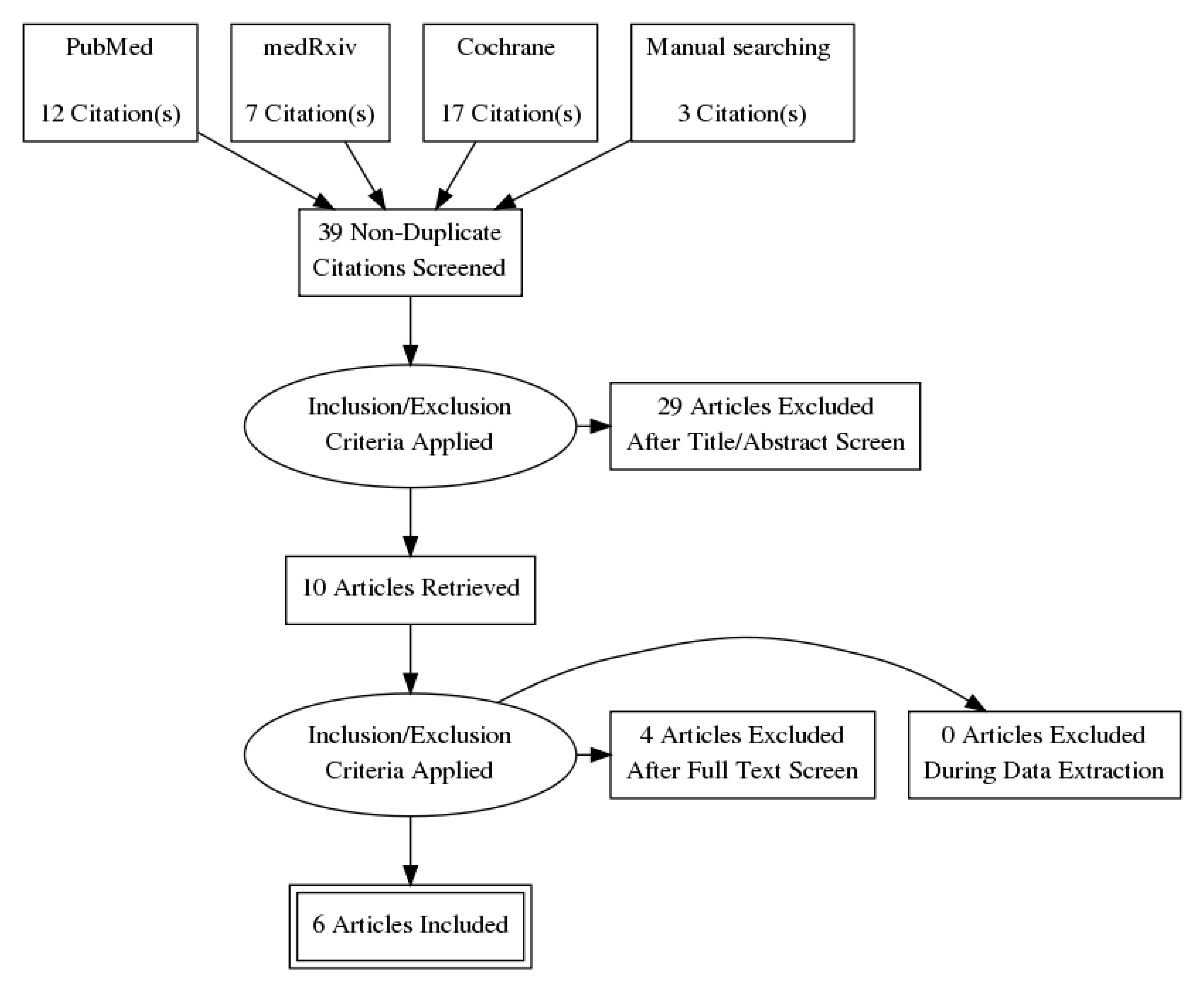
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chamilos, G.; Lionakis, M.S.; Kontoyiannis, D.P. Are all patients with cancer at heightened risk for severe Coronavirus Disease 2019 (COVID-19)? Clin. Infect. Dis. 2021, 72, 351–356. [Google Scholar] [CrossRef]
- Stroppa, E.M.; Toscani, I.; Citterio, C.; Anselmi, E.; Zaffignani, E.; Codeluppi, M.; Cavanna, L. Coronavirus Disease-2019 in cancer patients. A report of the first 25 cancer patients in a Western country (Italy). Futur. Oncol. 2020, 16, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, L.; Citterio, C.; Toscani, I.; Franco, C.; Magnacavallo, A.; Caprioli, S.; Cattadori, E.; Di Nunzio, C.; Pane, R.; Schiavo, R.; et al. Cancer patients with COVID-19: A retrospective study of 51 patients in the district of Piacenza, Northern Italy. Futur. Sci. OA 2020, 7, FSO645. [Google Scholar] [CrossRef] [PubMed]
- Bitar, N.; Kattan, J.; Kourie, H.R.; Mukherji, D.; El Saghir, N. Caring for Cancer Patients during the COVID-19 Pandemic. Available online: http://www.lsmo-lb.org/news/caring-for-cancer-patients-during-the-covid-19-pandemic (accessed on 2 July 2021).
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Uhrlaub, J.L.; Ripperger, T.J.; Jergović, M.; Dalgai, S.; et al. Immune responses to COVID-19 mRNA vaccines in patients with solid tumors on active, immunosuppressive cancer therapy. medRxiv 2021. [Google Scholar] [CrossRef]
- Desai, A.; Gainor, J.F.; Hegde, A.; Schram, A.M.; Curigliano, G.; Pal, S.; Liu, S.V.; Halmos, B.; Groisberg, R.; Grande, E.; et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat. Rev. Clin. Oncol. 2021, 18, 313–319. [Google Scholar] [CrossRef]
- Ribas, A.; Sengupta, R.; Locke, T.; Zaidi, S.K.; Campbell, K.M.; Carethers, J.M.; Jaffee, E.M.; Wherry, E.J.; Soria, J.C.; D’Souza, G.; et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2020, 11, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.B.H. Cancer Groups Urge CDC to Prioritize Cancer Patients for COVID-19 Vaccination. Available online: https://cancerletter.com/articles/20210108_2/ (accessed on 17 June 2021).
- SITC Statement on SARS-CoV-2 Vaccination and Cancer Immunotherapy. Available online: https://www.sitcancer.org/aboutsitc/press-releases/2020/sitc-statement-sars-cov-2-vaccination-cancer-immunotherapy (accessed on 11 June 2021).
- National Comprehensive Cancer Network. Preliminary Recommendations of the NCCN COVID-19 Vaccination Advisory Committee* Version 1.0 1/22/2021. Available online: https://www.nccn.org/covid-19/pdf/COVID-19_Vaccination_Guidance_V1.0.pdf (accessed on 10 June 2021).
- Sociedad Española de Oncología Médica (SEOM). Posicionamiento y Recomendaciones de Seom en Relación con la Campaña de Vacunación Frente al COVID-19 en Pacientes Con Cáncer. Available online: https://seom.org/images/Posicionamiento_SEOM_vacunacion_COVID19_pacientes_con_cancer.pdf (accessed on 10 June 2021).
- Garassino, M.C.; Vyas, M.; de Vries, E.G.E.; Kanesvaran, R.; Giuliani, R.; Peters, S. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann. Oncol. 2021, 32, 579–581. [Google Scholar] [CrossRef] [PubMed]
- AIOM CIPOMO COMU. Documento AIOM CIPOMO COMU Vaccinazione COVID-19 per i Pazienti Oncologici ver 1.0. Available online: https://www.aiom.it/wp-content/uploads/2020/12/20201231_Vaccino_COVID_19_AIOM_CIPOMO_COMU_1.0.pdf (accessed on 25 June 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021, 39, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Iacono, D.; Cerbone, L.; Palombi, L.; Cavalieri, E.; Sperduti, I.; Cocchiara, R.A.; Mariani, B.; Parisi, G.; Garfuli, C. Serological response to COVID-19 vaccination in patients with cancer older than 80 years. J. Geriatr. Oncol. 2021, 21, 1879–4068. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; del Molino del Barrio, I.; Alaguthurai, T.; Domingo-vila, C.; Hayday, T.S.; Graham, C.; Seow, J.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B.; et al. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140. [Google Scholar] [CrossRef]
- Pimpinelli, F.; Marchesi, F.; Piaggio, G.; Giannarelli, D.; Papa, E.; Falcucci, P.; Pontone, M.; Di Martino, S.; Laquintana, V.; La Malfa, A.; et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: Preliminary data from a single institution. J. Hematol. Oncol. 2021, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Arbel, Y.S.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Loannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Ueda, M.; Martins, R.; Hendrie, P.C.; McDonnell, T.; Crews, J.R.; Wong, T.L.; McCreery, B.; Jagels, B.; Crane, A.; Byrd, D.R.; et al. Managing Cancer Care During the COVID-19 Pandemic: Agility and Collaboration Toward a Common Goal. J. Natl. Compr. Canc. Netw. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Oncology. COVID-19 and cancer: 1 year on. Lancet Oncol. 2021, 22, 411. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html (accessed on 21 June 2021).


| Author Reference /Country | Vaccine/ n. Inoculations | Study Design | Tot. Patients | Sex (f%) of Patients/ Controls | Age Median (Range) Patients/ Controls | Patients on Active Treatment n(%) | Solid Tumors | Hematologic Malignances | Controls | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Sc n(%) | Tot | Sc n(%) | Tot | Sc n(%) | |||||||
| Thakkar et al. [17]/USA | BNT/2, mRNA/2, Ad */1 | CS | 200 | 58/62 | 67 (27–90)/ 64 (37–82) | 135 (67.5) | 134 | 131 (97.76) | 66 | 56 (84.85) | 26 | 26 (100) |
| Iacono et al. [18]/Italy | BNT/2 | CS | 36 | 58.4/nr | 82/nr § | 31 (86) | 26 | 25 (96.15) | 10 | 4 (40) | 72 | 52 (100) |
| Monin et al. [19]/UK | BNT/2 | PR | 24 | 48/48 # | 73 (64.5–79.5)/ 40.5 (31.3–50) # | 13 (45.83) | 19 | 18 (94.74) | 5 | 3 (60) | 12 | 12 (100) |
| Massarweh et al. [20]/ Israel | BNT/2 | PR | 102 | 43/68 | 66 (56–72)/ 62 (49–70) | 102 (100) | 102 | 92 (90.2) | 0 | 0 | 78 | 78 (100) |
| Pimpinelli et al. [21]/Italy | BNT/2 | PR | 92 | MM 45.23, MPM 48/50 | MM 73 (47–78) MPM 70 (28–80)/ 81 (79–87) | 92 (100) | 0 | 0 | MM 42 MPM 50 | 33 (78.6) 44 (88) | 36 | 36 (100) |
| Herishanuet al. [22]/Israel | BNT/2 | PR | 167 | 32.9 | 71 (63–76)/ 68 (64–74) | 75 (44.9) | 0 | 0 | 167 | 66 (39.52) | 52 | 52 (100) |
| Author Reference | Positive Cutoff/Unit | Median Serum IgG Level (Pre-Vaccinations) | Patients’ Median Serum IgG Level (Post-Vaccinations) | Controls’ Median Serum IgG Level (Post-Vaccinations) |
|---|---|---|---|---|
| Thakkar et al. [17] | ≥50 AU/mL | np | Solid tumors 7858 Hematologic malignancies 2528 | Higher than 15,000 |
| Iacono et al. [18] | ≥50 AU/mL | np | 2396.10 (range 0–32,763) | 8737.49 (range 398.90–976,280) |
| Monin et al. [19] | 70 EC50 dilution units | np | nr | nr |
| Massarweh et al. [20] | ≥50 AU/mL | np | 1931 (IQR, 509–4386) | 7160 (IQR, 3129–11,241) |
| Pimpinelli et al. [21] | ≥15 AU/mL | GMC (CI): MM 4.2 (3.9–4.6) MPM 4.6 (4.2–5.2) Controls 3.8 (3.8–3.8) | GMC (CI): MM (42 patients) 106.7 (62.3–179.7). MPM (50 patients) 172.9 (106.5–257.0) | GMC (CI): 353.3 (255.6–470.0) |
| Herishanu et al. [22] | ≥0.80 U/mL | np | 0.824 (IQR, 0.4–167.3) | 1084 (IQR, 128.9–1879) |
| Author Reference | AE G1–2 n(%) | AE G3–4 n(%) |
|---|---|---|
| Thakkar et al. [17] | BNT152b2 37% mRNA1273 34% Ad26.COV2 26% | 0 |
| Iacono et al. [18] | Not reported | 0 |
| Monin et al. [19] | 29% patients, 69% controls | 0 |
| Massarweh et al. [20] | Not reported | Not reported |
| Pimpinelli et al. [21] | Most common side effect 16% pain | 0 |
| Herishanu et al. [22] | 39 (23.4) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavanna, L.; Citterio, C.; Toscani, I. COVID-19 Vaccines in Cancer Patients. Seropositivity and Safety. Systematic Review and Meta-Analysis. Vaccines 2021, 9, 1048. https://doi.org/10.3390/vaccines9091048
Cavanna L, Citterio C, Toscani I. COVID-19 Vaccines in Cancer Patients. Seropositivity and Safety. Systematic Review and Meta-Analysis. Vaccines. 2021; 9(9):1048. https://doi.org/10.3390/vaccines9091048
Chicago/Turabian StyleCavanna, Luigi, Chiara Citterio, and Ilaria Toscani. 2021. "COVID-19 Vaccines in Cancer Patients. Seropositivity and Safety. Systematic Review and Meta-Analysis" Vaccines 9, no. 9: 1048. https://doi.org/10.3390/vaccines9091048
APA StyleCavanna, L., Citterio, C., & Toscani, I. (2021). COVID-19 Vaccines in Cancer Patients. Seropositivity and Safety. Systematic Review and Meta-Analysis. Vaccines, 9(9), 1048. https://doi.org/10.3390/vaccines9091048







