Simultaneous Immunization with Multiple Diverse Immunogens Alters Development of Antigen-Specific Antibody-Mediated Immunity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antigen Expression and Purification
2.2. Vaccination
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
3. Results
3.1. Simultaneous Immunization with Multiple Diverse Immunogens
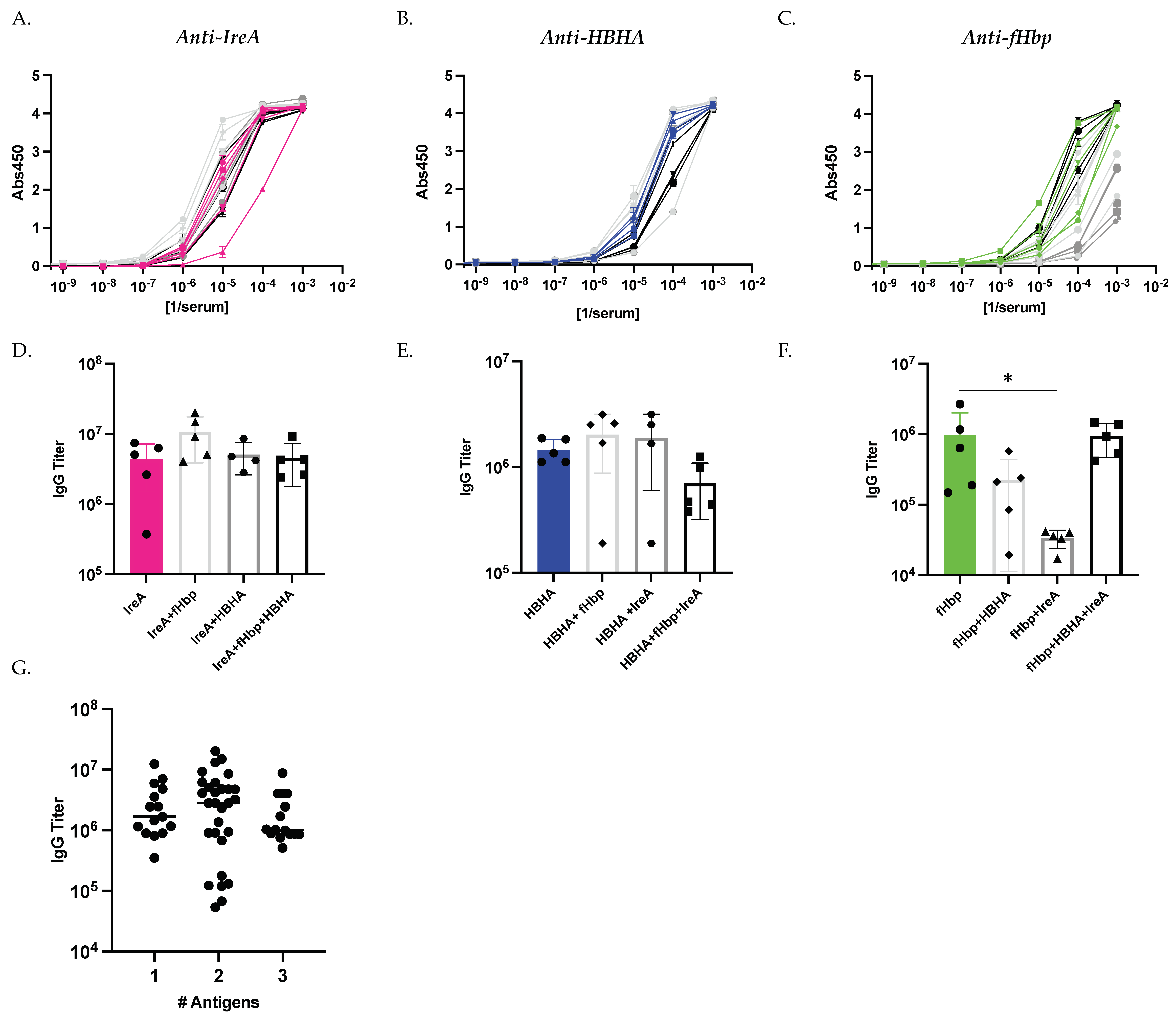
3.2. Antigen Immunization Combinations Elicit Comparable Antibody Titers by Study Conclusion
3.3. Vaccination Type Affects Development of Antigen-Specific Antibody Titers
3.4. Cocktail Immunization Alters Development of Antigen-Specific Antibody-Mediated Immunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [Green Version]
- Hajj Hussein, I.; Chams, N.; Chams, S.; El Sayegh, S.; Badran, R.; Raad, M.; Gerges-Geagea, A.; Leone, A.; Jurjus, A. Vaccines Through Centuries: Major Cornerstones of Global Health. Front. Public Health 2015, 3, 269. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Helmreich, S. A history of herd immunity. Lancet 2020, 396, 810–811. [Google Scholar] [CrossRef]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Nandi, A.; Shet, A. Why vaccines matter: Understanding the broader health, economic, and child development benefits of routine vaccination. Hum. Vaccin. Immunother. 2020, 16, 1900–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Piot, P.; Larson, H.J.; O’Brien, K.L.; N’Kengasong, J.; Ng, E.; Sow, S.; Kampmann, B. Immunization: Vital progress, unfinished agenda. Nature 2019, 575, 119–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreano, E.; D’Oro, U.; Rappuoli, R.; Finco, O. Vaccine Evolution and Its Application to Fight Modern Threats. Front. Immunol. 2019, 10, 1722. [Google Scholar] [CrossRef] [Green Version]
- Mascola, J.R.; Fauci, A.S. Novel vaccine technologies for the 21st century. Nat. Rev. Immunol. 2020, 20, 87–88. [Google Scholar] [CrossRef]
- Delany, I.; Rappuoli, R.; Seib, K.L. Vaccines, reverse vaccinology, and bacterial pathogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a012476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sette, A.; Rappuoli, R. Reverse vaccinology: Developing vaccines in the era of genomics. Immunity 2010, 33, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines (Basel) 2019, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef]
- Scarselli, M.; Aricò, B.; Brunelli, B.; Savino, S.; Di Marcello, F.; Palumbo, E.; Veggi, D.; Ciucchi, L.; Cartocci, E.; Bottomley, M.J.; et al. Rational Design of a Meningococcal Antigen Inducing Broad Protective Immunity. Sci. Transl. Med. 2011, 3, 91ra62. [Google Scholar] [CrossRef]
- Alteri, C.J.; Hagan, E.C.; Sivick, K.E.; Smith, S.N.; Mobley, H.L. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009, 5, e1000586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagan, E.C.; Mobley, H.L. Uropathogenic Escherichia coli outer membrane antigens expressed during urinary tract infection. Infect. Immun. 2007, 75, 3941–3949. [Google Scholar] [CrossRef] [Green Version]
- Russo, T.A.; Carlino, U.B.; Johnson, J.R. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 2001, 69, 6209–6216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, P.; Copland, A.; Diogo, G.R.; Harris, S.; Spallek, R.; Oehlmann, W.; Singh, M.; Basile, J.; Rottenberg, M.; Paul, M.J.; et al. Nanoparticle-Fusion Protein Complexes Protect against Mycobacterium tuberculosis Infection. Mol. Ther. 2018, 26, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Pickett, T.; Delogu, G.; Dheenadhayalan, V.; Debrie, A.S.; Locht, C.; Brennan, M.J. The mycobacterial heparin-binding hemagglutinin is a protective antigen in the mouse aerosol challenge model of tuberculosis. Infect. Immun. 2004, 72, 6799–6805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pethe, K.; Alonso, S.; Biet, F.; Delogu, G.; Brennan, M.J.; Locht, C.; Menozzi, F.D. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 2001, 412, 190–194. [Google Scholar] [CrossRef]
- Donald, R.G.; Hawkins, J.C.; Hao, L.; Liberator, P.; Jones, T.R.; Harris, S.L.; Perez, J.L.; Eiden, J.J.; Jansen, K.U.; Anderson, A.S. Meningococcal serogroup B vaccines: Estimating breadth of coverage. Hum. Vaccin. Immunother. 2017, 13, 255–265. [Google Scholar] [CrossRef] [Green Version]
- McNeil, L.K.; Zagursky, R.J.; Lin, S.L.; Murphy, E.; Zlotnick, G.W.; Hoiseth, S.K.; Jansen, K.U.; Anderson, A.S. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol. Mol. Biol. Rev. 2013, 77, 234–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulendran, B.; Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 2011, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Tai, W.; Yang, Y.; Zhao, G.; Zhu, Q.; Sun, S.; Liu, C.; Tao, X.; Tseng, C.K.; Perlman, S.; et al. Introduction of neutralizing immunogenicity index to the rational design of MERS coronavirus subunit vaccines. Nat. Commun. 2016, 7, 13473. [Google Scholar] [CrossRef] [PubMed]
- Sanders, R.W.; Derking, R.; Cupo, A.; Julien, J.P.; Yasmeen, A.; de Val, N.; Kim, H.J.; Blattner, C.; de la Peña, A.T.; Korzun, J.; et al. A Next-Generation Cleaved, Soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, Expresses Multiple Epitopes for Broadly Neutralizing but Not Non-Neutralizing Antibodies. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.A.; Rainho-Tomko, J.N.; Williams, Z.P.; Lanza, L.; Peredelchuk, M.; Kishko, M.; Pavot, V.; Alamares-Sapuay, J.; Adhikarla, H.; Gupta, S.; et al. A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain. Sci. Immunol. 2020, 5, eaba6466. [Google Scholar] [CrossRef]
- Kwong, P.D.; Mascola, J.R.; Nabel, G.J. Rational design of vaccines to elicit broadly neutralizing antibodies to HIV-1. Cold Spring Harbor Perspect. Med. 2011, 1, a007278. [Google Scholar] [CrossRef]
- Galli, V.; Simionatto, S.; Marchioro, S.B.; Klabunde, G.H.; Conceicao, F.R.; Dellagostin, O.A. Recombinant secreted antigens from Mycoplasma hyopneumoniae delivered as a cocktail vaccine enhance the immune response of mice. Clin. Vaccine Immunol. 2013, 20, 1370–1376. [Google Scholar] [CrossRef] [Green Version]
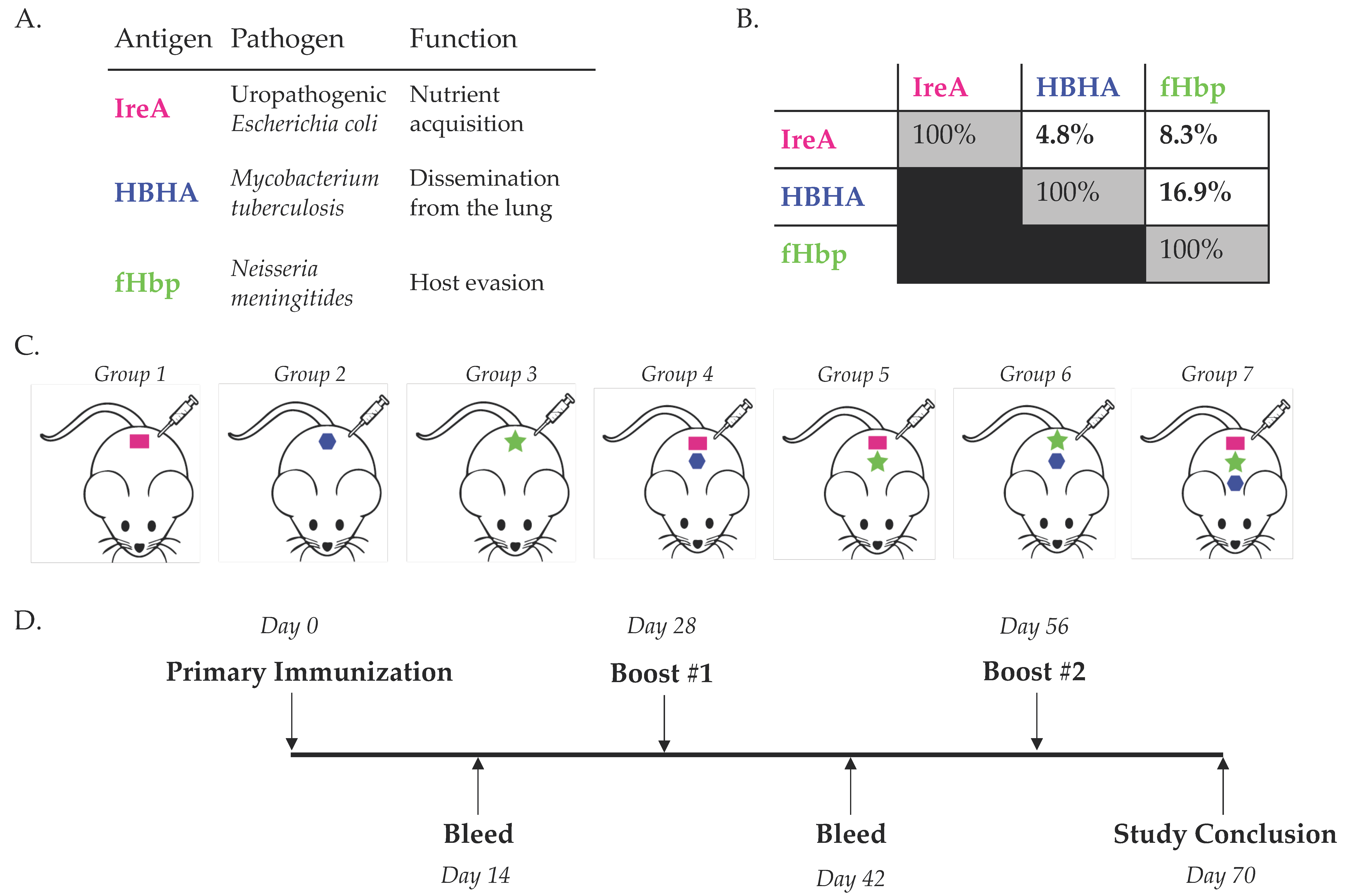


| Antigen | Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| IreA | 20 | 20 | 20 | 20 | ||||
| HBHA | 5.9 | 5.9 | 5.9 | 5.9 | ||||
| fHbp | 7.4 | 7.4 | 7.4 | 7.4 | ||||
| Total (μg) | 20 | 5.9 | 7.4 | 25.9 | 27.4 | 13.3 | 33.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilewski, K.A.; Kramer, K.J.; Georgiev, I.S. Simultaneous Immunization with Multiple Diverse Immunogens Alters Development of Antigen-Specific Antibody-Mediated Immunity. Vaccines 2021, 9, 964. https://doi.org/10.3390/vaccines9090964
Pilewski KA, Kramer KJ, Georgiev IS. Simultaneous Immunization with Multiple Diverse Immunogens Alters Development of Antigen-Specific Antibody-Mediated Immunity. Vaccines. 2021; 9(9):964. https://doi.org/10.3390/vaccines9090964
Chicago/Turabian StylePilewski, Kelsey A., Kevin J. Kramer, and Ivelin S. Georgiev. 2021. "Simultaneous Immunization with Multiple Diverse Immunogens Alters Development of Antigen-Specific Antibody-Mediated Immunity" Vaccines 9, no. 9: 964. https://doi.org/10.3390/vaccines9090964
APA StylePilewski, K. A., Kramer, K. J., & Georgiev, I. S. (2021). Simultaneous Immunization with Multiple Diverse Immunogens Alters Development of Antigen-Specific Antibody-Mediated Immunity. Vaccines, 9(9), 964. https://doi.org/10.3390/vaccines9090964






