Interests of the Non-Human Primate Models for HIV Cure Research
Abstract
:1. Introduction
2. Could Studies in Humans and In Vitro Models Be Sufficient for HIV Cure Research?
3. Animal Models Used for Research on HIV Cure
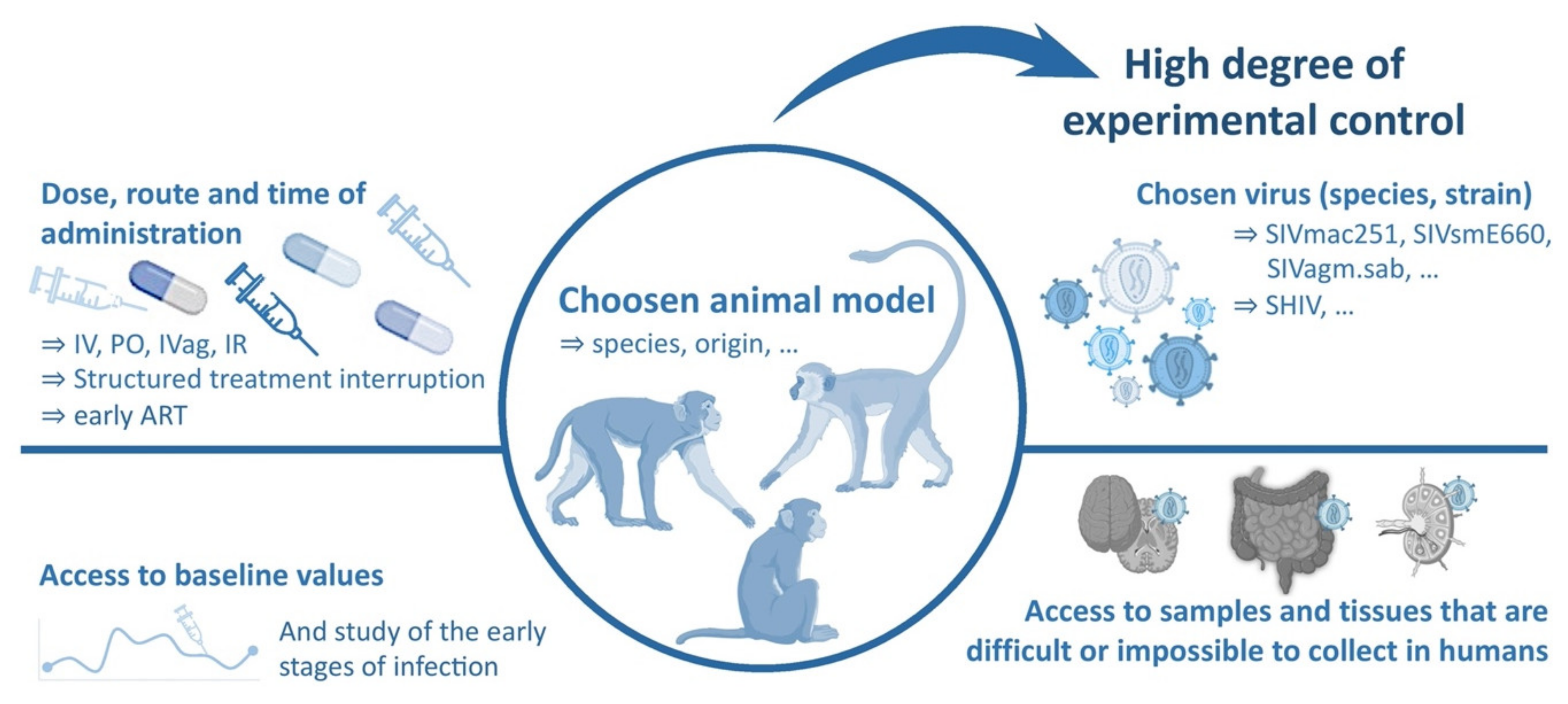
3.1. General Considerations
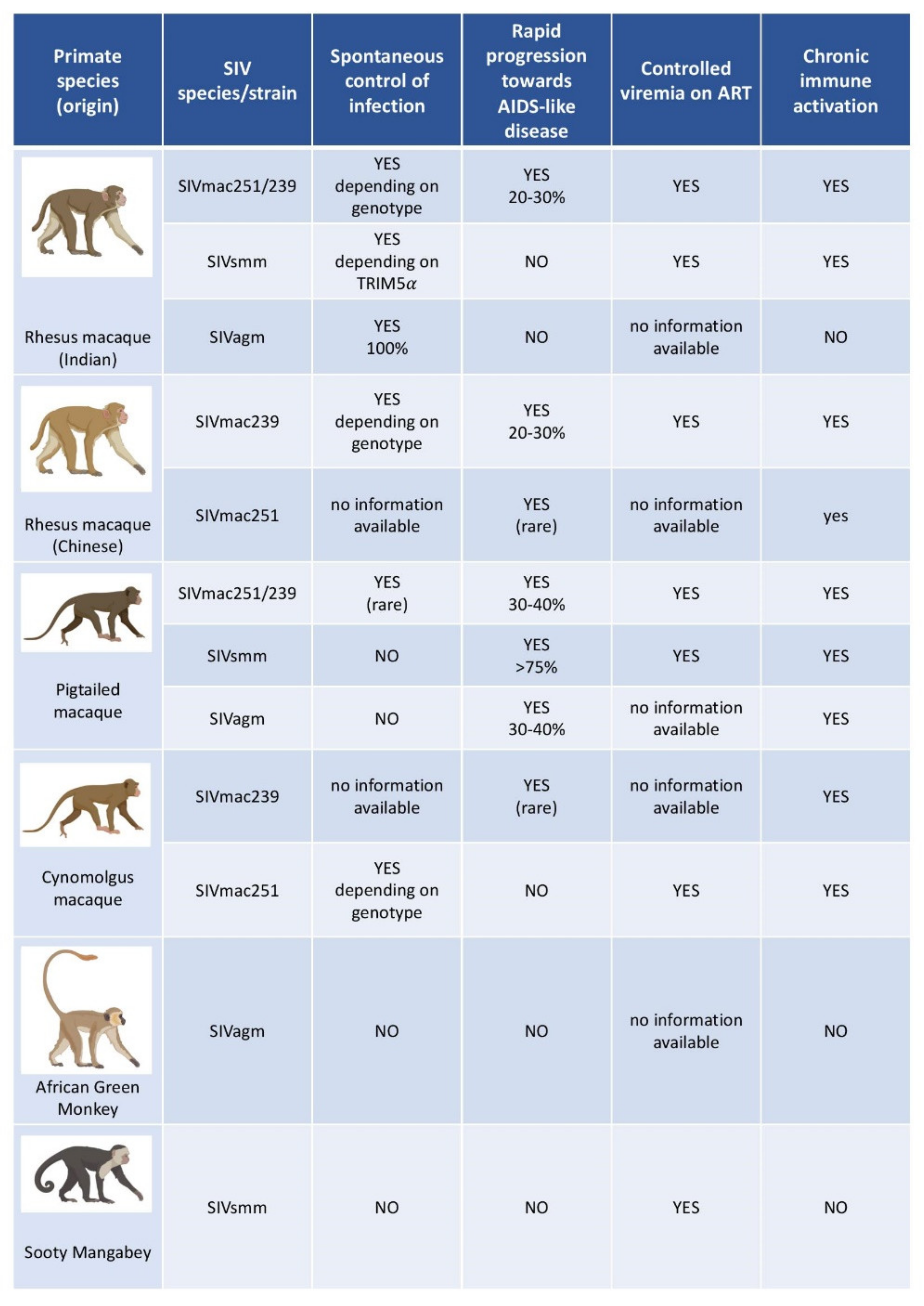
3.2. The Use of Macaques as a Model of HIV Infection
3.3. Viruses Used to Infect NHP Models of HIV Infection
3.4. Essential Insights on HIV Infection Obtained with the Help of NHP Models
4. The Case of Natural SIV Hosts and Their Potential Use for HIV Cure Research
- (i)
- First, natural hosts exhibit high levels of viremia, similar to those observed in untreated pathogenic SIV infections of RMs and HIV infections of humans both in primary and chronic infection [82,83,84,85,86,87]. During chronic infection, the virus thus continues to replicate at high levels, in most cases to about 104–107 SIV RNA copies/mL of plasma, without immune damage [85]. These characteristics are similar to the “viremic non-progressors”, very rare human individuals who show high viremia but maintain CD4 T cell counts and avoid disease progression for years [88].
- (ii)
- Natural hosts avoid chronic immune activation, which is the driving force of CD4+ T cell depletion and progression to AIDS in humans [84,89,90,91,92,93]. SIVagm and SIVsmm infections trigger a potent type I-interferon (IFN) production in acute infection, but this inflammatory response is rapidly controlled [94]. After the acute phase of infection, immune activation is controlled and returns to near pre-infection levels [89,94]. This could be of relevance for the lack of intestinal tissue damage (see below).
- (iii)
- Natural hosts show strong viral control in secondary lymphoid tissue (SLT) [95,96]. Indeed, AGM and SM exhibit a strong control of viral replication in lymph nodes (LN) (in both T cell zone and B cell follicles) shortly after the peak of viremia, which persists throughout infection, despite high viremia levels. Viral control in SLT of SIVagm-infected AGM is mediated by NK cells [97]. The latter express C-X-C chemokine receptor type 5 (CXCR5) in SLT during SIVagm infection and are able to migrate into B cell follicles [95,96,97]. This represents a striking difference with pathogenic infections, where the virus persists in LN and where B cell follicles represent “sanctuary sites” for the virus. Immune activation, including expression of IFN-stimulated gene (ISGs), is particularly rapidly controlled in SLT. The rapid and strong viral control most likely contributes to this rapid resolution of inflammation. The LNs of natural hosts also show neither lymphadenopathy nor fibrosis. Importantly, the network of follicular dendritic cells (FDC) remains intact, unlike in HIV-1 and SIVmac infections [95]. During SIVagm infection, these FDC produce high levels of IL-15. NK cells accumulate preferentially in these IL-15+ follicles during SIVagm infection [97].
- (iv)
- Central memory CD4+ T-cells (TCM) in SMs have been reported to be infected at a lower frequency than in non-natural hosts. Based on this observation, it has been suggested that long-lived TCM cells are relatively resistant to SIV infection. Indeed, it has been shown that in sooty mangabeys, TCM exhibit low levels of SIV co-receptor CCR5 expression and are less likely to be infected in vivo and in vitro (compared with sooty mangabeys effector memory CD4+ T cells and RM central memory CD4+ T cells) [98]. However, SIVsmm and SIVagm do not require CCR5 to infect CD4+ T cells but can efficiently use other co-receptors, such as CXCR6. Thus, the underlying mechanism of the lower infection rate of Tcm could be another one. For instance, the fact that SIVsmm infection is strongly controlled in SLT but not in the intestine might play a role since the frequency of TCM is higher in SLT than in the intestine. Whatever the mechanism, the preservation of long-lived cells in lymphoid tissues in natural hosts can contribute to the reduced pathogenicity [83,98,99,100].
- (v)
- Natural hosts of SIV preserve their intestinal mucosal immune system and the integrity of the intestinal barrier. Thus, they efficiently prevent the translocation of microbial products from the intestinal lumen into the systemic circulation [101,102]. In addition, no early preferential depletion of Th17 cells is observed during SIV infection of natural hosts [71,103,104]. Th17 maintenance in the gut could positively contribute to preserve the intestinal barrier integrity [71]. A recent study [105] by Raehtz et al. documenting early SIV infection of AGMs showed that despite a strong but transient interferon-based inflammatory response, the levels of plasma markers associated with enteropathy did not increase. They did not document a significant increase in apoptosis of mucosal enterocytes or lymphocytes, nor damage to the mucosal epithelium [105]. Also, unconventional CD8+ T cells expressing regulatory molecules expand in the intestine of SIVagm-infected AGM and the increase of these cells was associated with lower levels of intestinal inflammation as measured by IL-23 [106]. It is also possible that stronger or more efficient tissue repair mechanisms operate in natural hosts of SIV. Barrenas et al. [107] demonstrated that monocytes from AGMs rapidly activate and maintain evolutionarily conserved regenerative wound healing mechanisms in mucosal tissues, possibly via fibronectin production and TGF-beta signature.
5. Evaluation of HIV Cure Strategies with the Help of NHP Models
5.1. Latency Reversing Agents for “Shock and Kill Strategies”
5.1.1. Epigenetic and Signal Agonist LRAs
5.1.2. Immunomodulatory LRAs
5.2. Block and Lock Strategies
5.3. Immunotherapies to Elicit and Strengthen Potent Immune Responses
5.3.1. Broadly Neutralizing Antibodies and Beyond
5.3.2. Therapeutic Vaccines
5.3.3. Immune Checkpoint Blockers
5.3.4. Therapies Harnessing Natural Killer Cells
5.3.5. Targeting Tissue Damage and Mucosal Immunity
5.4. Gene Editing and Gene Therapies
5.5. Approaches Targeting the Cells to the Right Place
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Barre-Sinoussi, F.; Chermann, J.; Rey, F.; Nugeyre, M.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vezinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [Green Version]
- UNAIDS. Fact Sheet 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 23 July 2021).
- Ndung’u, T.; McCune, J.M.; Deeks, S.G. Why and where an HIV cure is needed and how it might be achieved. Nature 2019, 576, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tellez, T.; Huot, N.; Ploquin, M.J.; Rascle, P.; Jacquelin, B.; Müller-Trutwin, M. Non-human primates in HIV research: Achievements, limits and alternatives. Infect. Genet. Evol. 2016, 46, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Pandrea, I. Animal Models for HIV Cure Research. Front. Immunol. 2016, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Del Prete, G.Q.; Lifson, J.D. Nonhuman Primate Models for Studies of AIDS Virus Persistence during Suppressive Combination Antiretroviral Therapy. In Current Topics in Microbiology and Immunology; Silvestri, G., Lichterfeld, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 417, pp. 69–109. ISBN 978-3-030-02815-2. [Google Scholar]
- Marsden, M.D. Benefits and limitations of humanized mice in HIV persistence studies. Retrovirology 2020, 17, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, Y.; Beatty, C.; Biradar, S.; Castronova, I.; Ho, S.; Melody, K.; Bility, M.T. Moving beyond the mousetrap: Current and emerging humanized mouse and rat models for investigating prevention and cure strategies against HIV infection and associated pathologies. Retrovirology 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosur, V.; Skelly, D.A.; Francis, C.; Low, B.E.; Kohar, V.; Burzenski, L.M.; Amiji, M.A.; Shultz, L.D.; Wiles, M.V. Improved mouse models and advanced genetic and genomic technologies for the study of neutrophils. Drug Discov. Today 2020, 25, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Weichseldorfer, M.; Heredia, A.; Reitz, M.; Bryant, J.L.; Latinovic, O.S. Use of Humanized Mouse Models for Studying HIV-1 Infection, Pathogenesis and Persistence. J. AIDS HIV Treat. 2020, 2. [Google Scholar] [CrossRef]
- Del Prete, G.Q.; Lifson, J.D.; Keele, B.F. Nonhuman primate models for the evaluation of HIV-1 preventive vaccine strategies: Model Parameter Considerations and Consequences. Curr. Opin. HIV AIDS 2016, 11, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Gardner, M.B.; Luciw, P.A. Macaque Models of Human Infectious Disease. ILAR J. 2008, 49, 220–255. [Google Scholar] [CrossRef] [Green Version]
- Colby, D.J.; Sarnecki, M.; Barouch, D.H.; Tipsuk, S.; Stieh, D.J.; Kroon, E.; Schuetz, A.; Intasan, J.; Sacdalan, C.; Pinyakorn, S.; et al. Safety and immunogenicity of Ad26 and MVA vaccines in acutely treated HIV and effect on viral rebound after antiretroviral therapy interruption. Nat. Med. 2020, 26, 498–501. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Futur. Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Understanding Animal Research. Nobel Prize. Available online: http://www.Animalresearch.Info/En/Medical-Advances/Nobel-Prizes/ (accessed on 23 July 2021).
- Balls, M. It’s Time to Reconsider the Principles of Humane Experimental Technique. Altern. Lab. Anim. 2020, 48, 40–46. [Google Scholar] [CrossRef]
- Huot, N.; Rascle, P.; Müller-Trutwin, M. Apport Des Modèles Animaux Dans La Recherche Sur Le VIH. Virologie 2019, 23, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Hatziioannou, T.; Evans, D.T. Animal models for HIV/AIDS research. Nat. Rev. Microbiol. 2012, 10, 852–867. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Cirión, A.; Jacquelin, B.; Barré-Sinoussi, F.; Müller-Trutwin, M. Immune responses during spontaneous control of HIV and AIDS: What is the hope for a cure? Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130436. [Google Scholar] [CrossRef] [Green Version]
- Compton, A.; Malik, H.S.; Emerman, M. Host gene evolution traces the evolutionary history of ancient primate lentiviruses. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etienne, L.; Nerrienet, E.; Lebreton, M.; Bibila, G.T.; Foupouapouognigni, Y.; Rousset, D.; Nana, A.; Djoko, C.F.; Tamoufe, U.; Aghokeng, A.F.; et al. Characterization of a new simian immunodeficiency virus strain in a naturally infected Pan troglodytes troglodyteschimpanzee with AIDS related symptoms. Retrovirology 2011, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudicell, R.S.; Jones, J.H.; Wroblewski, E.E.; Learn, G.H.; Li, Y.; Robertson, J.D.; Greengrass, E.; Grossmann, F.; Kamenya, S.; Pintea, L.; et al. Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics. PLoS Pathog. 2010, 6, e1001116. [Google Scholar] [CrossRef]
- Keele, B.F.; Jones, J.; Terio, K.A.; Estes, J.D.; Rudicell, R.S.; Wilson, M.; Li, Y.; Learn, G.; Beasley, T.M.; Schumacher-Stankey, J.; et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 2009, 460, 515–519. [Google Scholar] [CrossRef] [Green Version]
- DeGrazia, D.; Beauchamp, T.L. Beyond the 3 Rs to a More Comprehensive Framework of Principles for Animal Research Ethics. ILAR J. 2019, ilz011. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Symonowicz, C.; Medina, L.V.; Bratcher, N.A.; Buckmaster, C.A.; Klein, H.; Anderson, L.C. Culture of Care: Organizational Responsibilities. In Management of Animal Care and Use Programs in Research, Education, and Testing; Weichbrod, R.H., Thompson, G.A., Norton, J.N., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: London, UK, 2018; ISBN 978-1-4987-4844-5. [Google Scholar]
- Hampshire, V.A.; Gilbert, S.H. Refinement, Reduction, and Replacement (3R) Strategies in Preclinical Testing of Medical Devices. Toxicol. Pathol. 2018, 47, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauci, A.S.; Desrosiers, R.C. Pathogenesis of HIV and SIV. In Retroviruses; Coffin, J.M., Hughes, S.H., Varmus, H.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997; ISBN 978-0-87969-571-2. [Google Scholar]
- Nath, B.M.; Schumann, K.E.; Boyer, J.D. The chimpanzee and other non-human-primate models in HIV-1 vaccine research. Trends Microbiol. 2000, 8, 426–431. [Google Scholar] [CrossRef]
- Mohammadi, H.; Bienzle, D. Pharmacological Inhibition of Feline Immunodeficiency Virus (FIV). Viruses 2012, 4, 708–724. [Google Scholar] [CrossRef]
- Krakoff, E.; Gagne, R.B.; VandeWoude, S.; Carver, S. Variation in Intra-individual Lentiviral Evolution Rates: A Systematic Review of Human, Nonhuman Primate, and Felid Species. J. Virol. 2019, 93, e00538-19. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.V. Humanized mice for HIV and AIDS research. Curr. Opin. Virol. 2016, 19, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.V. In vivo platforms for analysis of HIV persistence and eradication. J. Clin. Investig. 2016, 126, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Fukazawa, Y.; Lum, R.; Okoye, A.A.; Park, H.; Matsuda, K.; Bae, J.Y.; Hagen, S.I.; Shoemaker, R.; Deleage, C.; Lucero, C.; et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med. 2015, 21, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loffredo, J.T.; Maxwell, J.; Qi, Y.; Glidden, C.E.; Borchardt, G.J.; Soma, T.; Bean, A.T.; Beal, D.R.; Wilson, N.A.; Rehrauer, W.M.; et al. Mamu-B * 08 -Positive Macaques Control Simian Immunodeficiency Virus Replication. J. Virol. 2007, 81, 8827–8832. [Google Scholar] [CrossRef] [Green Version]
- Cumont, M.-C.; Diop, O.; Vaslin, B.; Elbim, C.; Viollet, L.; Monceaux, V.; Lay, S.; Silvestri, G.; Le Grand, R.; Müller-Trutwin, M.; et al. Early Divergence in Lymphoid Tissue Apoptosis between Pathogenic and Nonpathogenic Simian Immunodeficiency Virus Infections of Nonhuman Primates. J. Virol. 2008, 82, 1175–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruel, T.; Hamimi, C.; Dereuddre-Bosquet, N.; Cosma, A.; Shin, S.Y.; Corneau, A.; Versmisse, P.; Karlsson, I.; Malleret, B.; Targat, B.; et al. Long-Term Control of Simian Immunodeficiency Virus (SIV) in Cynomolgus Macaques Not Associated with Efficient SIV-Specific CD8 + T-Cell Responses. J. Virol. 2015, 89, 3542–3556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, K.C.; Jin, Z.; Rudersdorf, R.; Hughes, A.L.; O’Connor, D.H. Unusually High Frequency MHC Class I Alleles in Mauritian Origin Cynomolgus Macaques. J. Immunol. 2005, 175, 5230–5239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, S.L.; Blasky, A.J.; Pendley, C.J.; Becker, E.A.; Wiseman, R.W.; Karl, J.A.; Hughes, A.L.; O’Connor, D. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics 2007, 59, 449–462. [Google Scholar] [CrossRef] [Green Version]
- Mee, E.T.; Badhan, A.; Karl, J.A.; Wiseman, R.W.; Cutler, K.; Knapp, L.A.; Almond, N.; O’Connor, D.H.; Rose, N.J. MHC haplotype frequencies in a UK breeding colony of Mauritian cynomolgus macaques mirror those found in a distinct population from the same geographic origin. J. Med. Primatol. 2009, 38, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohns, M.S.; Greene, J.M.; Cain, B.T.; Pham, N.H.; Gostick, E.; Price, D.A.; O’Connor, D.H. Expansion of Simian Immunodeficiency Virus (SIV)-Specific CD8 T Cell Lines from SIV-Naive Mauritian Cynomolgus Macaques for Adoptive Transfer. J. Virol. 2015, 89, 9748–9757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatt, N.R.; Harris, L.D.; Vinton, C.L.; Sung, H.; Briant, J.A.; Tabb, B.; Morcock, D.; McGinty, J.W.; Lifson, J.D.; Lafont, B.; et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010, 3, 387–398. [Google Scholar] [CrossRef]
- Mandell, D.T.; Kristoff, J.; Gaufin, T.; Gautam, R.; Ma, D.; Sandler, N.; Haret-Richter, G.; Xu, C.; Aamer, H.; Dufour, J.; et al. Pathogenic Features Associated with Increased Virulence upon Simian Immunodeficiency Virus Cross-Species Transmission from Natural Hosts. J. Virol. 2014, 88, 6778–6792. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Jasinska, A.; Kristoff, J.; Grobler, J.P.; Turner, T.; Jung, Y.; Schmitt, C.; Raehtz, K.; Feyertag, F.; Sosa, N.M.; et al. SIVagm Infection in Wild African Green Monkeys from South Africa: Epidemiology, Natural History, and Evolutionary Considerations. PLoS Pathog. 2013, 9, e1003011. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, G.; Paiardini, M.; Pandrea, I.; Lederman, M.M.; Sodora, D.L. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Investig. 2007, 117, 3148–3154. [Google Scholar] [CrossRef]
- Sauter, D.; Kirchhoff, F. Properties of Human and Simian Immunodeficiency Viruses. In Natural Hosts of SIV; Elsevier: Amsterdam, The Netherlands, 2014; pp. 69–84. [Google Scholar] [CrossRef]
- Simon, F.; Mauclère, P.; Roques, P.; Loussert-Ajaka, I.; Müller-Trutwin, M.; Saragosti, S.; Georges-Courbot, M.C.; Barré-Sinoussi, F.; Brun-Vézinet, F. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat. Med. 1998, 4, 1032–1037. [Google Scholar] [CrossRef]
- Santiago, M.L.; Range, F.; Keele, B.F.; Li, Y.; Bailes, E.; Bibollet-Ruche, F.; Fruteau, C.; Noë, R.; Peeters, M.; Brookfield, J.F.Y.; et al. Simian Immunodeficiency Virus Infection in Free-Ranging Sooty Mangabeys (Cercocebus atys atys) from the Taï Forest, Côte d’Ivoire: Implications for the Origin of Epidemic Human Immunodeficiency Virus Type 2. J. Virol. 2005, 79, 12515–12527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, V.M.; Lifson, J.D. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and Prevention. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2000; Volume 49, pp. 437–477. [Google Scholar] [CrossRef]
- Van Rompay, K.K.; Blackwood, E.J.; Landucci, G.; Forthal, D.; Marthas, M.L. Role of CD8+ cells in controlling replication of nonpathogenic Simian Immunodeficiency Virus SIVmac1A11. Virol. J. 2006, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatissian, E.; Monceaux, V.; Cumont, M.-C.; Kieny, M.-P.; Aubertin, A.-M.; Hurtrel, B. Persistence of Pathogenic Challenge Virus in Macaques Protected by Simian Immunodeficiency Virus SIVmacΔ nef. J. Virol. 2001, 75, 1507–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandrea, I.; Gaufin, T.; Gautam, R.; Kristoff, J.; Mandell, D.; Montefiori, D.; Keele, B.F.; Ribeiro, R.; Veazey, R.S.; Apetrei, C. Functional Cure of SIVagm Infection in Rhesus Macaques Results in Complete Recovery of CD4+ T Cells and Is Reverted by CD8+ Cell Depletion. PLoS Pathog. 2011, 7, e1002170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandrea, I.; Cornell, E.; Wilson, C.; Ribeiro, R.M.; Ma, N.; Kristoff, J.; Xu, C.; Haret-Richter, G.S.; Trichel, A.; Apetrei, C.; et al. Coagulation biomarkers predict disease progression in SIV-infected nonhuman primates. Blood 2012, 120, 1357–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courgnaud, V.; Saurin, W.; Villinger, F.; Sonigo, P. Different Evolution of Simian Immunodeficiency Virus in a Natural Host and a New Host. Virology 1998, 247, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Courgnaud, V.; Müller-Trutwin, M.; Sonigo, P. Évolution et virulence des lentivirus de primates. Med. Sci. 2004, 20, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Donahoe, R.M.; O’Neil, S.P.; Marsteller, F.A.; Novembre, F.J.; Anderson, D.C.; Lankford-Turner, P.; McClure, H.H. Probable deceleration of progression of Simian AIDS affected by opiate dependency: Studies with a rhesus macaque/SIVsmm9 model. JAIDS J. Acquir. Immune Defic. Syndr. 2009, 50, 241–249. [Google Scholar] [CrossRef]
- Wiederin, J.L.; Donahoe, R.M.; Anderson, J.R.; Yu, F.; Fox, H.S.; Gendelman, H.E.; Ciborowski, P.S. Plasma Proteomic Analysis of Simian Immunodeficiency Virus Infection of Rhesus Macaques. J. Proteome Res. 2010, 9, 4721–4731. [Google Scholar] [CrossRef] [Green Version]
- Van Rompay, K.K. Tackling HIV and AIDS: Contributions by non-human primate models. Lab Anim. 2017, 46, 259–270. [Google Scholar] [CrossRef]
- Barouch, D.H.; Whitney, J.B.; Moldt, B.; Klein, F.; Oliveira, T.; Liu, J.; Stephenson, K.; Chang, H.-W.; Shekhar, K.; Gupta, S.; et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 2013, 503, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.G.; Ford, J.C.; Lewis, M.S.; Ventura, A.B.; Hughes, C.M.; Coyne-Johnson, L.; Whizin, N.; Oswald, K.; Shoemaker, R.; Swanson, T.; et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011, 473, 523–527. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.G.; Jr, M.P.; Ventura, A.B.; Hughes, C.M.; Gilbride, R.M.; Ford, J.C.; Oswald, K.; Shoemaker, R.; Li, Y.; Lewis, M.S.; et al. Immune clearance of highly pathogenic SIV infection. Nature 2013, 502, 100–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, S.G.; Sacha, J.B.; Hughes, C.M.; Ford, J.C.; Burwitz, B.J.; Scholz, I.; Gilbride, R.M.; Lewis, M.S.; Gilliam, A.N.; Ventura, A.B.; et al. Cytomegalovirus Vectors Violate CD8+ T Cell Epitope Recognition Paradigms. Science 2013, 340, 1237874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, S.G.; Wu, H.L.; Burwitz, B.J.; Hughes, C.M.; Hammond, K.B.; Ventura, A.B.; Reed, J.S.; Gilbride, R.M.; Ainslie, E.; Morrow, D.W.; et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science 2016, 351, 714–720. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.G.; Marshall, E.E.; Malouli, D.; Ventura, A.B.; Hughes, C.M.; Ainslie, E.; Ford, J.C.; Morrow, D.; Gilbride, R.M.; Bae, J.Y.; et al. A live-attenuated RhCMV/SIV vaccine shows long-term efficacy against heterologous SIV challenge. Sci. Transl. Med. 2019, 11, eaaw2607. [Google Scholar] [CrossRef]
- Abad-Fernandez, M.; Goonetilleke, N. Human cytomegalovirus-vectored vaccines against HIV. Curr. Opin. HIV AIDS 2019, 14, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Mattapallil, J.; Douek, D.C.; Hill, B.J.; Nishimura, Y.; Martin, M.A.; Roederer, M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005, 434, 1093–1097. [Google Scholar] [CrossRef]
- Li, Q.; Duan, L.; Estes, J.D.; Ma, Z.-M.; Rourke, T.; Wang, Y.; Reilly, C.; Carlis, J.V.; Miller, C.J.; Haase, A.T. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 2005, 434, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Picker, L.J.; Hagen, S.I.; Lum, R.; Reed-Inderbitzin, E.F.; Daly, L.M.; Sylwester, A.W.; Walker, J.; Siess, D.C.; Piatak, M.; Wang, C.; et al. Insufficient Production and Tissue Delivery of CD4+Memory T Cells in Rapidly Progressive Simian Immunodeficiency Virus Infection. J. Exp. Med. 2004, 200, 1299–1314. [Google Scholar] [CrossRef]
- Reeves, R.K.; Evans, T.I.; Gillis, J.; Johnson, R.P. Simian Immunodeficiency Virus Infection Induces Expansion of α4β7 + and Cytotoxic CD56 + NK Cells. J. Virol. 2010, 84, 8959–8963. [Google Scholar] [CrossRef] [Green Version]
- Reeves, R.K.; Gillis, J.; Wong, F.E.; Yu, Y.; Connole, M.; Johnson, R.P. CD16− natural killer cells: Enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood 2010, 115, 4439–4446. [Google Scholar] [CrossRef]
- Reeves, R.K.; Evans, T.I.; Gillis, J.; Wong, F.E.; Kang, G.; Li, Q.; Johnson, R.P. SIV Infection Induces Accumulation of Plasmacytoid Dendritic Cells in the Gut Mucosa. J. Infect. Dis. 2012, 206, 1462–1468. [Google Scholar] [CrossRef] [Green Version]
- Brenchley, J.M.; Paiardini, M.; Knox, K.S.; Asher, A.I.; Cervasi, B.; Asher, T.E.; Scheinberg, P.; Price, D.; Hage, C.A.; Kholi, L.M.; et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 2008, 112, 2826–2835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veazey, R.S.; DeMaria, M.; Chalifoux, L.V.; Shvetz, D.E.; Pauley, D.R.; Knight, H.L.; Rosenzweig, M.; Johnson, R.P.; Desrosiers, R.C.; Lackner, A.A. Gastrointestinal Tract as a Major Site of CD4+ T Cell Depletion and Viral Replication in SIV Infection. Science 1998, 280, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, J.B.; Wahl, A.; Baker, C.; Spagnuolo, R.A.; Foster, J.; Zakharova, O.; Wietgrefe, S.; Caro-Vegas, C.; Madden, V.; Sharpe, G.; et al. Macrophages sustain HIV replication in vivo independently of T cells. J. Clin. Investig. 2016, 126, 1353–1366. [Google Scholar] [CrossRef]
- Araínga, M.; Edagwa, B.; Mosley, R.L.; Poluektova, L.Y.; Gorantla, S.; Gendelman, H.E. A mature macrophage is a principal HIV-1 cellular reservoir in humanized mice after treatment with long acting antiretroviral therapy. Retrovirology 2017, 14, 17. [Google Scholar] [CrossRef] [Green Version]
- Honeycutt, J.B.; Thayer, W.O.; Baker, C.E.; Ribeiro, R.; Lada, S.M.; Cao, Y.; Cleary, R.A.; Hudgens, M.G.; Richman, D.D.; Garcia, J.V. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med. 2017, 23, 638–643. [Google Scholar] [CrossRef]
- Haase, A.T. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010, 464, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Haase, A.T. Early Events in Sexual Transmission of HIV and SIV and Opportunities for Interventions. Annu. Rev. Med. 2011, 62, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.R.; Rakasz, E.; Skinner, P.; White, C.; Abel, K.; Ma, Z.-M.; Compton, L.; Napoé, G.; Wilson, N.; Miller, C.J.; et al. CD8 + T-Lymphocyte Response to Major Immunodominant Epitopes after Vaginal Exposure to Simian Immunodeficiency Virus: Too Late and Too Little. J. Virol. 2005, 79, 9228–9235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodora, D.L.; Gettie, A.; Miller, C.J.; Marx, P.A. Vaginal transmission of SIV: Assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res. Hum. Retrovir. 1998, 14 (Suppl. 1), 119–123. [Google Scholar]
- Nomura, T.; Matano, T. Association of MHC-I genotypes with disease progression in HIV/SIV infections. Front. Microbiol. 2012, 3, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodora, D.L.; Allan, J.S.; Apetrei, C.; Brenchley, J.M.; Douek, D.C.; Else, J.G.; Estes, J.D.; Hahn, B.H.; Hirsch, V.M.; Kaur, A.; et al. Toward an AIDS vaccine: Lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat. Med. 2009, 15, 861–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apetrei, C.; Sumpter, B.; Souquiere, S.; Chahroudi, A.; Makuwa, M.; Reed, P.; Ribeiro, R.M.; Pandrea, I.; Roques, P.; Silvestri, G. Immunovirological Analyses of Chronically Simian Immunodeficiency Virus SIVmnd-1- and SIVmnd-2-Infected Mandrills (Mandrillus sphinx). J. Virol. 2011, 85, 13077–13087. [Google Scholar] [CrossRef] [Green Version]
- Chahroudi, A.; Bosinger, S.E.; Vanderford, T.H.; Paiardini, M.; Silvestri, G. Natural SIV Hosts: Showing AIDS the Door. Science 2012, 335, 1188–1193. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, G.; Sodora, D.L.; Koup, R.A.; Paiardini, M.; O’Neil, S.P.; McClure, H.M.; Staprans, S.I.; Feinberg, M.B. Nonpathogenic SIV Infection of Sooty Mangabeys Is Characterized by Limited Bystander Immunopathology Despite Chronic High-Level Viremia. Immunity 2003, 18, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Pandrea, I.; Silvestri, G.; Onanga, R.; Veazey, R.S.; Marx, P.A.; Hirsch, V.; Apetrei, C. Simian immunodeficiency viruses replication dynamics in African non-human primate hosts: Common patterns and species-specific differences. J. Med. Primatol. 2006, 35, 194–201. [Google Scholar] [CrossRef]
- Goldstein, S.; Brown, C.R.; Ourmanov, I.; Pandrea, I.; Buckler-White, A.; Erb, C.; Nandi, J.S.; Foster, G.J.; Autissier, P.; Schmitz, J.E.; et al. Comparison of Simian Immunodeficiency Virus SIVagmVer Replication and CD4 + T-Cell Dynamics in Vervet and Sabaeus African Green Monkeys. J. Virol. 2006, 80, 4868–4877. [Google Scholar] [CrossRef] [Green Version]
- Rey-Cuillé, M.-A.; Berthier, J.-L.; Bomsel-Demontoy, M.-C.; Chaduc, Y.; Montagnier, L.; Hovanessian, A.G.; Chakrabarti, L. Simian Immunodeficiency Virus Replicates to High Levels in Sooty Mangabeys without Inducing Disease. J. Virol. 1998, 72, 3872–3886. [Google Scholar] [CrossRef] [Green Version]
- Rotger, M.; Dalmau, J.; Rauch, A.; McLaren, P.; Bosinger, S.E.; Martinez, R.; Sandler, N.G.; Roque, A.; Liebner, J.; Battegay, M.; et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J. Clin. Investig. 2011, 121, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Bosinger, S.E.; Li, Q.; Gordon, S.N.; Klatt, N.R.; Duan, L.; Xu, L.; Francella, N.; Sidahmed, A.; Smith, A.J.; Cramer, E.M.; et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J. Clin. Investig. 2009, 119, 3556–3572. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.D.; Gordon, S.N.; Zeng, M.; Chahroudi, A.M.; Dunham, R.M.; Staprans, S.I.; Reilly, C.S.; Silvestri, G.; Haase, A.T. Early Resolution of Acute Immune Activation and Induction of PD-1 in SIV-Infected Sooty Mangabeys Distinguishes Nonpathogenic from Pathogenic Infection in Rhesus Macaques. J. Immunol. 2008, 180, 6798–6807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquelin, B.; Mayau, V.; Targat, B.; Liovat, A.-S.; Kunkel, D.; Petitjean, G.; Dillies, M.-A.; Roques, P.; Butor, C.; Silvestri, G.; et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Investig. 2009, 119, 3544–3555. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.D.; Tabb, B.; Sodora, D.L.; Paiardini, M.; Klatt, N.R.; Douek, D.C.; Silvestri, G.; Müller-Trutwin, M.; Vasile-Pandrea, I.; Apetrei, C.; et al. Downregulation of Robust Acute Type I Interferon Responses Distinguishes Nonpathogenic Simian Immunodeficiency Virus (SIV) Infection of Natural Hosts from Pathogenic SIV Infection of Rhesus Macaques. J. Virol. 2010, 84, 7886–7891. [Google Scholar] [CrossRef] [Green Version]
- Lederer, S.; Favre, D.; Walters, K.-A.; Proll, S.; Kanwar, B.; Kasakow, Z.; Baskin, C.R.; Palermo, R.; McCune, J.M.; Katze, M.G. Transcriptional Profiling in Pathogenic and Non-Pathogenic SIV Infections Reveals Significant Distinctions in Kinetics and Tissue Compartmentalization. PLoS Pathog. 2009, 5, e1000296. [Google Scholar] [CrossRef] [Green Version]
- Jacquelin, B.; Petitjean, G.; Kunkel, D.; Liovat, A.-S.; Jochems, S.; Rogers, K.A.; Ploquin, M.J.; Madec, Y.; Barré-Sinoussi, F.; Dereuddre-Bosquet, N.; et al. Innate Immune Responses and Rapid Control of Inflammation in African Green Monkeys Treated or Not with Interferon-Alpha during Primary SIVagm Infection. PLoS Pathog. 2014, 10, e1004241. [Google Scholar] [CrossRef]
- Huot, N.; Bosinger, S.E.; Paiardini, M.; Reeves, R.K.; Müller-Trutwin, M. Lymph Node Cellular and Viral Dynamics in Natural Hosts and Impact for HIV Cure Strategies. Front. Immunol. 2018, 9, 780. [Google Scholar] [CrossRef] [Green Version]
- Huot, N.; Rascle, P.; Garcia-Tellez, T.; Jacquelin, B.; Müller-Trutwin, M. Innate immune cell responses in non pathogenic versus pathogenic SIV infections. Curr. Opin. Virol. 2016, 19, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Huot, N.; Jacquelin, B.; Garcia-Tellez, T.; Rascle, P.; Ploquin, M.J.; Madec, Y.; Reeves, R.K.; Derreudre-Bosquet, N.; Müller-Trutwin, M. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat. Med. 2017, 23, 1277–1286. [Google Scholar] [CrossRef]
- Paiardini, M.; Cervasi, B.; Reyes-Aviles, E.; Micci, L.; Ortiz, A.M.; Chahroudi, A.; Vinton, C.; Gordon, S.N.; Bosinger, S.E.; Francella, N.; et al. Low levels of SIV infection in sooty mangabey central memory CD4+ T cells are associated with limited CCR5 expression. Nat. Med. 2011, 17, 830–836. [Google Scholar] [CrossRef]
- Meythaler, M.; Wang, Z.; Martinot, A.; Pryputniewicz, S.; Kasheta, M.; McClure, H.M.; O’Neil, S.P.; Kaur, A. Early Induction of Polyfunctional Simian Immunodeficiency Virus (SIV)-Specific T Lymphocytes and Rapid Disappearance of SIV from Lymph Nodes of Sooty Mangabeys during Primary Infection. J. Immunol. 2011, 186, 5151–5161. [Google Scholar] [CrossRef]
- Heeney, J. AIDS: A disease of impaired Th-cell renewal? Immunol. Today 1995, 16, 515–520. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Price, D.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [Green Version]
- Pantaleo, G.; Graziosi, C.; Demarest, J.F.; Butini, L.; Montroni, M.; Fox, C.H.; Orenstein, J.M.; Kotler, D.P.; Fauci, A.S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 1993, 362, 355–358. [Google Scholar] [CrossRef]
- Favre, D.; Lederer, S.; Kanwar, B.; Ma, Z.-M.; Proll, S.; Kasakow, Z.; Mold, J.; Swainson, L.; Barbour, J.D.; Baskin, C.R.; et al. Critical Loss of the Balance between Th17 and T Regulatory Cell Populations in Pathogenic SIV Infection. PLoS Pathog. 2009, 5, e1000295. [Google Scholar] [CrossRef] [PubMed]
- Paiardini, M. Th17 cells in natural SIV hosts. Curr. Opin. HIV AIDS 2010, 5, 166–172. [Google Scholar] [CrossRef]
- Raehtz, K.D.; Barrenäs, F.; Xu, C.; Busman-Sahay, K.; Valentine, A.; Law, L.; Ma, D.; Policicchio, B.B.; Wijewardana, V.; Brocca-Cofano, E.; et al. African green monkeys avoid SIV disease progression by preventing intestinal dysfunction and maintaining mucosal barrier integrity. PLoS Pathog. 2020, 16, e1008333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huot, N.; Rascle, P.; Tchitchek, N.; Wimmer, B.; Passaes, C.; Contreras, V.; Desjardins, D.; Stahl-Hennig, C.; Le Grand, R.; Saez-Cirion, A.; et al. Role of NKG2a/c+CD8+ T cells in pathogenic versus non-pathogenic SIV infections. iScience 2021, 24, 102314. [Google Scholar] [CrossRef] [PubMed]
- Barrenas, F.; Raehtz, K.; Xu, C.; Law, L.; Green, R.R.; Silvestri, G.; Bosinger, S.E.; Nishida, A.; Li, Q.; Lu, W.; et al. Macrophage-associated wound healing contributes to African green monkey SIV pathogenesis control. Nat. Commun. 2019, 10, 5101. [Google Scholar] [CrossRef]
- Deeks, S.G. Shock and Kill. Nature 2012, 487, 439–440. [Google Scholar] [CrossRef]
- Jiang, G.; Nguyen, D.; Archin, N.M.; Yukl, S.A.; Méndez-Lagares, G.; Tang, Y.; Elsheikh, M.M.; Thompson, G.R.; Hartigan-O’Connor, D.J.; Margolis, D.M.; et al. HIV latency is reversed by ACSS2-driven histone crotonylation. J. Clin. Investig. 2018, 128, 1190–1198. [Google Scholar] [CrossRef] [Green Version]
- Fulda, S. Molecular Pathways: Targeting Death Receptors and Smac Mimetics. Clin. Cancer Res. 2014, 20, 3915–3920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, C.C.; Mavigner, M.; Sampey, G.C.; Brooks, A.D.; Spagnuolo, R.A.; Irlbeck, D.M.; Mattingly, C.; Ho, P.T.; Schoof, N.; Cammon, C.G.; et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 2020, 578, 160–165. [Google Scholar] [CrossRef]
- Pache, L.; Dutra, M.S.; Spivak, A.; Marlett, J.M.; Murry, J.; Hwang, Y.; Maestre, A.M.; Manganaro, L.; Vamos, M.; Teriete, P.; et al. BIRC2/cIAP1 Is a Negative Regulator of HIV-1 Transcription and Can Be Targeted by Smac Mimetics to Promote Reversal of Viral Latency. Cell Host Microbe 2015, 18, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Dashti, A.; Waller, C.; Mavigner, M.; Schoof, N.; Bar, K.J.; Shaw, G.M.; Vanderford, T.H.; Liang, S.; Lifson, J.D.; Dunham, R.M.; et al. SMAC mimetic plus triple combination bispecific HIVxCD3 DART® molecules in SHIV.C.CH505-infected, ART-suppressed rhesus macaques. J. Virol. 2020, 94, e00793-20. [Google Scholar] [CrossRef] [PubMed]
- Mavigner, M.; Liao, L.E.; Brooks, A.D.; Ke, R.; Mattingly, C.; Schoof, N.; McBrien, J.; Carnathan, D.; Liang, S.; Vanderford, T.H.; et al. CD8 Lymphocyte Depletion Enhances the Latency Reversal Activity of the SMAC Mimetic AZD5582 in ART-Suppressed SIV-Infected Rhesus Macaques. J. Virol. 2021, 95, e01429-20. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, J.T.; Gunst, J.D.; Højen, J.F.; Tolstrup, M.; Søgaard, O.S. The Use of Toll-Like Receptor Agonists in HIV-1 Cure Strategies. Front. Immunol. 2020, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.B.; Novis, C.L.; Bosque, A. Targeting Cellular and Tissue HIV Reservoirs with Toll-Like Receptor Agonists. Front. Immunol. 2019, 10, 2450. [Google Scholar] [CrossRef] [Green Version]
- Borducchi, E.N.; Cabral, C.; Stephenson, K.; Liu, J.; Abbink, P.; Ng’Ang’A, D.; Nkolola, J.P.; Brinkman, A.L.; Peter, L.; Lee, B.C.; et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 2016, 540, 284–287. [Google Scholar] [CrossRef]
- Borducchi, E.N.; Liu, J.; Nkolola, J.P.; Cadena, A.M.; Yu, W.-H.; Fischinger, S.; Broge, T.; Abbink, P.; Mercado, N.B.; Chandrashekar, A.; et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 2018, 563, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Borducchi, E.N.; Liu, J.; Nkolola, J.P.; Cadena, A.M.; Yu, W.-H.; Fischinger, S.; Broge, T.; Abbink, P.; Mercado, N.B.; Chandrashekar, A.; et al. Publisher Correction: Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 2018, 564, E8. [Google Scholar] [CrossRef] [PubMed]
- Zerbato, J.; Purves, H.V.; Lewin, S.R.; Rasmussen, T.A. Between a shock and a hard place: Challenges and developments in HIV latency reversal. Curr. Opin. Virol. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Lim, S.-Y.; Osuna, C.E.; Hraber, P.T.; Hesselgesser, J.; Gerold, J.M.; Barnes, T.L.; Sanisetty, S.; Seaman, M.S.; Lewis, M.G.; Geleziunas, R.; et al. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci. Transl. Med. 2018, 10, eaao4521. [Google Scholar] [CrossRef] [Green Version]
- McGary, C.S.; Deleage, C.; Harper, J.; Micci, L.; Ribeiro, S.P.; Paganini, S.; Kuri-Cervantes, L.; Benne, C.; Ryan, E.S.; Balderas, R.; et al. CTLA-4+PD-1− Memory CD4+ T Cells Critically Contribute to Viral Persistence in Antiretroviral Therapy-Suppressed, SIV-Infected Rhesus Macaques. Immunity 2017, 47, 776–788.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, J.; Gordon, S.; Chan, C.N.; Wang, H.; Lindemuth, E.; Galardi, C.; Falcinelli, S.D.; Raines, S.L.M.; Read, J.L.; Nguyen, K.; et al. CTLA-4 and PD-1 dual blockade induces SIV reactivation without control of rebound after antiretroviral therapy interruption. Nat. Med. 2020, 26, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Wykes, M.; Lewin, S.R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2017, 18, 91–104. [Google Scholar] [CrossRef]
- Mediouni, S.; Kessing, C.F.; Jablonski, J.A.; Thenin-Houssier, S.; Clementz, M.; Kovach, M.D.; Mousseau, G.; De Vera, I.M.S.; Li, C.; Kojetin, D.J.; et al. The Tat inhibitor didehydro-cortistatin A suppresses SIV replication and reactivation. FASEB J. 2019, 33, 8280–8293. [Google Scholar] [CrossRef] [PubMed]
- VanSant, G.; Vranckx, L.S.; Zurnic, I.; Van Looveren, D.; Van De Velde, P.; Nobles, C.; Gijsbers, R.; Christ, F.; Debyser, Z. Impact of LEDGIN treatment during virus production on residual HIV-1 transcription. Retrovirology 2019, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Naranbhai, V.; Stern, J.; Roche, M.; Dantanarayana, A.; Ke, R.; Tennakoon, S.; Solomon, A.; Hoh, R.; Hartogensis, W.; et al. Variation in cell-associated unspliced HIV RNA on antiretroviral therapy is associated with the circadian regulator brain-and-muscle-ARNT-like-1. AIDS 2018, 32, 2119–2128. [Google Scholar] [CrossRef]
- Ngassaki-Yoka, C.-D.; Chatterjee, D.; Zhang, Y.; Wiche Salinas, T.; Raymond Marchand, L.; Cermakian, N.; Routy, J.-P.; Solt, L.; Ancuta, P. The Circadian Clock Machinery Regulates HIV Transcription in CD4+ T Cells; Universite de Montreal: Montreal, QC, USA, 2021. [Google Scholar]
- Scheid, J.F.; Mouquet, H.; Feldhahn, N.; Seaman, M.S.; Velinzon, K.; Pietzsch, J.; Ott, R.G.; Anthony, R.M.; Zebroski, H.; Hurley, A.; et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 2009, 458, 636–640. [Google Scholar] [CrossRef]
- Eroshkin, A.M.; Leblanc, A.; Weekes, D.; Post, K.; Li, Z.; Rajput, A.; Butera, S.T.; Burton, D.R.; Godzik, A. bNAber: Database of broadly neutralizing HIV antibodies. Nucleic Acids Res. 2013, 42, D1133–D1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, Y.; Gautam, R.; Chun, T.-W.; Sadjadpour, R.; Foulds, K.E.; Shingai, M.; Klein, F.; Gazumyan, A.; Golijanin, J.; Donaldson, M.; et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 2017, 543, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, P.; Grüll, H.; Nogueira, L.; Pai, J.A.; Butler, A.L.; Millard, K.; Lehmann, C.; Suárez, I.; Oliveira, T.; Lorenzi, J.C.C.; et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 2018, 561, 479–484. [Google Scholar] [CrossRef]
- Gardner, M.R. Promise and Progress of an HIV-1 Cure by Adeno-Associated Virus Vector Delivery of Anti-HIV-1 Biologics. Front. Cell. Infect. Microbiol. 2020, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Byrareddy, S.N.; Arthos, J.; Cicala, C.; Villinger, F.; Ortiz, K.T.; Little, D.; Sidell, N.; Kane, M.A.; Yu, J.; Jones, J.; et al. Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy. Science 2016, 354, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, N.; Mason, R.D.; Song, K.; Gorman, J.; Welles, H.C.; Arthos, J.; Cicala, C.; Min, S.; King, H.A.D.; Belli, A.J.; et al. Blocking α4β7 integrin binding to SIV does not improve virologic control. Science 2019, 365, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Calenda, G.; Keawvichit, R.; Arrode-Brusés, G.; Pattanapanyasat, K.; Frank, I.; Byrareddy, S.N.; Arthos, J.; Cicala, C.; Grasperge, B.; Blanchard, J.L.; et al. Integrin α4β7 Blockade Preferentially Impacts CCR6+ Lymphocyte Subsets in Blood and Mucosal Tissues of Naive Rhesus Macaques. J. Immunol. 2017, 200, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, P.J.; Cicala, C.; Byrareddy, S.N.; Ortiz, K.; Little, D.; Lindsay, K.E.; Gumber, S.; Hong, J.J.; Jelicic, K.; Rogers, K.A.; et al. Early treatment of SIV+ macaques with an α4β7 mAb alters virus distribution and preserves CD4+ T cells in later stages of infection. Mucosal Immunol. 2017, 11, 932–946. [Google Scholar] [CrossRef]
- Valle-Casuso, J.C.; Angin, M.; Volant, S.; Passaes, C.; Monceaux, V.; Mikhailova, A.; Bourdic, K.; Avettand-Fenoel, V.; Boufassa, F.; Sitbon, M.; et al. Cellular Metabolism Is a Major Determinant of HIV-1 Reservoir Seeding in CD4+ T Cells and Offers an Opportunity to Tackle Infection. Cell Metab. 2018, 29, 611–626.e5. [Google Scholar] [CrossRef] [Green Version]
- Huot, N.; Rascle, P.; Planchais, C.; Contreras, V.; Passaes, C.; Le Grand, R.; Beignon, A.-S.; Kornobis, E.; Legendre, R.; Varet, H.; et al. CD32+CD4+ T Cells Sharing B Cell Properties Increase With Simian Immunodeficiency Virus Replication in Lymphoid Tissues. Front. Immunol. 2021, 12, 2238. [Google Scholar] [CrossRef]
- Letvin, N.L.; Mascola, J.R.; Sun, Y.; Gorgone, D.A.; Buzby, A.P.; Xu, L.; Yang, Z.-Y.; Chakrabarti, B.; Rao, S.S.; Schmitz, J.E.; et al. Preserved CD4+ Central Memory T Cells and Survival in Vaccinated SIV-Challenged Monkeys. Science 2006, 312, 1530–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattapallil, J.J.; Douek, D.C.; Buckler-White, A.; Montefiori, D.; Letvin, N.L.; Nabel, G.J.; Roederer, M. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 2006, 203, 1533–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, N.A.; Reed, J.; Napoe, G.S.; Piaskowski, S.; Szymanski, A.; Furlott, J.; Gonzalez, E.J.; Yant, L.J.; Maness, N.J.; May, G.E.; et al. Vaccine-Induced Cellular Immune Responses Reduce Plasma Viral Concentrations after Repeated Low-Dose Challenge with Pathogenic Simian Immunodeficiency Virus SIVmac239. J. Virol. 2006, 80, 5875–5885. [Google Scholar] [CrossRef] [Green Version]
- Nakamura-Hoshi, M.; Takahara, Y.; Matsuoka, S.; Ishii, H.; Seki, S.; Nomura, T.; Yamamoto, H.; Sakawaki, H.; Miura, T.; Tokusumi, T.; et al. Therapeutic vaccine-mediated Gag-specific CD8+ T-cell induction under anti-retroviral therapy augments anti-virus efficacy of CD8+ cells in simian immunodeficiency virus-infected macaques. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mothe, B.; Brander, C. Small steps forward for HIV vaccine development. Nat. Med. 2020, 26, 466–467. [Google Scholar] [CrossRef]
- Mylvaganam, G.H.; Chea, L.S.; Tharp, G.K.; Hicks, S.; Velu, V.; Iyer, S.S.; Deleage, C.; Estes, J.D.; Bosinger, S.E.; Freeman, G.J.; et al. Combination anti–PD-1 and antiretroviral therapy provides therapeutic benefit against SIV. JCI Insight 2018, 3, e122940. [Google Scholar] [CrossRef]
- Velu, V.; Titanji, K.; Zhu, B.; Husain, S.; Pladevega, A.; Lai, L.; Vanderford, T.H.; Chennareddi, L.; Silvestri, G.; Freeman, G.J.; et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 2008, 458, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Bugide, S.; Janostiak, R.; Wajapeyee, N. Epigenetic Mechanisms Dictating Eradication of Cancer by Natural Killer Cells. Trends Cancer 2018, 4, 553–566. [Google Scholar] [CrossRef]
- Navarro, A.G.; Björklund, A.T.; Chekenya, M. Therapeutic Potential and Challenges of Natural Killer Cells in Treatment of Solid Tumors. Front. Immunol. 2015, 6, 202. [Google Scholar] [CrossRef] [Green Version]
- Minetto, P.; Guolo, F.; Pesce, S.; Greppi, M.; Obino, V.; Ferretti, E.; Sivori, S.; Genova, C.; Lemoli, R.M.; Marcenaro, E. Harnessing NK Cells for Cancer Treatment. Front. Immunol. 2019, 10, 2836. [Google Scholar] [CrossRef] [Green Version]
- Veluchamy, J.P.; Kok, N.; van der Vliet, H.; Verheul, H.; De Gruijl, T.D.; Spanholtz, J. The Rise of Allogeneic Natural Killer Cells As a Platform for Cancer Immunotherapy: Recent Innovations and Future Developments. Front. Immunol. 2017, 8, 631. [Google Scholar] [CrossRef] [PubMed]
- Petitdemange, C.; Maucourant, C.; Tarantino, N.; Rey, J.; Vieillard, V. Glycogen synthetase kinase 3 inhibition drives MIC-A/B to promote cytokine production by human natural killer cells in Dengue virus type 2 infection. Eur. J. Immunol. 2019, 50, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Guma, M.; Angulo, A.; Vilches, C.; Gómez-Lozano, N.; Malats, N.; López-Botet, M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okoye, A.A.; DeGottardi, M.Q.; Fukazawa, Y.; Vaidya, M.; Abana, C.O.; Konfe, A.L.; Fachko, D.N.; Duell, D.M.; Li, H.; Lum, R.; et al. Role of IL-15 Signaling in the Pathogenesis of Simian Immunodeficiency Virus Infection in Rhesus Macaques. J. Immunol. 2019, 203, 2928–2943. [Google Scholar] [CrossRef]
- Ramsuran, V.; Naranbhai, V.; Horowitz, A.; Qi, Y.; Martin, M.P.; Yuki, Y.; Gao, X.; Walker-Sperling, V.; Del Prete, G.Q.; Schneider, D.K.; et al. Elevated HLA-A expression impairs HIV control through inhibition of NKG2A-expressing cells. Science 2018, 359, 86–90. [Google Scholar] [CrossRef] [Green Version]
- Ferlazzo, G.; Pack, M.; Thomas, D.; Paludan, C.; Schmid, D.; Strowig, T.; Bougras, G.; Muller, W.A.; Moretta, L.; Münz, C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. USA 2004, 101, 16606–16611. [Google Scholar] [CrossRef] [Green Version]
- Imamura, M.; Shook, D.; Kamiya, T.; Shimasaki, N.; Chai, S.M.H.; Coustan-Smith, E.; Imai, C.; Campana, D. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood 2014, 124, 1081–1088. [Google Scholar] [CrossRef] [Green Version]
- Garrido, C.; Abad-Fernandez, M.; Tuyishime, M.; Pollara, J.J.; Ferrari, G.; Soriano-Sarabia, N.; Margolis, D.M. Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo. J. Virol. 2018, 92, e00235-18. [Google Scholar] [CrossRef] [Green Version]
- Webb, G.M.; Molden, J.; Busman-Sahay, K.; Abdulhaqq, S.; Wu, H.L.; Weber, W.C.; Bateman, K.B.; Reed, J.S.; Northrup, M.; Maier, N.; et al. The human IL-15 superagonist N-803 promotes migration of virus-specific CD8+ T and NK cells to B cell follicles but does not reverse latency in ART-suppressed, SHIV-infected macaques. PLoS Pathog. 2020, 16, e1008339. [Google Scholar] [CrossRef]
- McBrien, J.B.; Mavigner, M.; Franchitti, L.; Smith, S.A.; White, E.; Tharp, G.K.; Walum, H.; Busman-Sahay, K.; Aguilera-Sandoval, C.R.; Thayer, W.O.; et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature 2020, 578, 154–159. [Google Scholar] [CrossRef]
- McBrien, J.B.; Mavigner, M.; Franchitti, L.; Smith, S.A.; White, E.; Tharp, G.K.; Walum, H.; Busman-Sahay, K.; Aguilera-Sandoval, C.R.; Thayer, W.O.; et al. Author Correction: Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature 2020, 578, E21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBrien, J.B.; Wong, A.K.H.; White, E.; Carnathan, D.G.; Lee, J.H.; Safrit, J.T.; Vanderford, T.H.; Paiardini, M.; Chahroudi, A.; Silvestri, G. Combination of CD8β Depletion and Interleukin-15 Superagonist N-803 Induces Virus Reactivation in Simian-Human Immunodeficiency Virus-Infected, Long-Term ART-Treated Rhesus Macaques. J. Virol. 2020, 94, e00755-20. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.; Zinter, M.; Stanfield-Oakley, S.; Carpp, L.N.; Edwards, R.W.; Denny, T.; Moodie, Z.; Laher, F.; Bekker, L.-G.; McElrath, M.J.; et al. Vaccine-Induced Antibodies Mediate Higher Antibody-Dependent Cellular Cytotoxicity After Interleukin-15 Pretreatment of Natural Killer Effector Cells. Front. Immunol. 2019, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Papasavvas, E.; Azzoni, L.; Kossenkov, A.V.; Dawany, N.; Morales, K.H.; Fair, M.; Ross, B.N.; Lynn, K.; Mackiewicz, A.; Mounzer, K.; et al. NK Response Correlates with HIV Decrease in Pegylated IFN-α2a-Treated Antiretroviral Therapy-Suppressed Subjects. J. Immunol. 2019, 203, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Micci, L.; Ryan, E.S.; Fromentin, R.; Bosinger, S.E.; Harper, J.; He, T.; Paganini, S.; Easley, K.; Chahroudi, A.; Benne, C.; et al. Interleukin-21 combined with ART reduces inflammation and viral reservoir in SIV-infected macaques. J. Clin. Investig. 2015, 125, 4497–4513. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.; Huot, N.; Micci, L.; Tharp, G.; King, C.; Rascle, P.; Shenvi, N.; Wang, H.; Galardi, C.; Upadhyay, A.A.; et al. IL-21 and IFNα therapy rescues terminally differentiated NK cells and limits SIV reservoir in ART-treated macaques. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Martins, R.; Carlos, A.R.; Braza, F.; Thompson, J.A.; Bastos-Amador, P.; Ramos, S.; Soares, M.P. Disease Tolerance as an Inherent Component of Immunity. Annu. Rev. Immunol. 2019, 37, 405–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiardini, M.; Müller-Trutwin, M. HIV-associated chronic immune activation. Immunol. Rev. 2013, 254, 78–101. [Google Scholar] [CrossRef] [Green Version]
- Barker, B.; Gladstone, M.N.; Gillard, G.O.; Panas, M.W.; Letvin, N.L. Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur. J. Immunol. 2010, 40, 3085–3096. [Google Scholar] [CrossRef] [PubMed]
- Parrish-Novak, J.; Dillon, S.R.; Nelson, A.; Hammond, A.; Sprecher, C.A.; Gross, J.A.; Johnston, J.A.; Madden, K.; Xu, W.; West, J.; et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000, 408, 57–63. [Google Scholar] [CrossRef]
- Korn, T.; Bettelli, E.; Gao, W.; Awasthi, A.; Jäger, A.; Strom, T.B.; Oukka, M.; Kuchroo, V.K. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 2007, 448, 484–487. [Google Scholar] [CrossRef]
- Pallikkuth, S.; Micci, L.; Ende, Z.S.; Iriele, R.I.; Cervasi, B.; Lawson, B.; McGary, C.S.; Rogers, K.A.; Else, J.G.; Silvestri, G.; et al. Maintenance of Intestinal Th17 Cells and Reduced Microbial Translocation in SIV-infected Rhesus Macaques Treated with Interleukin (IL)-21. PLoS Pathog. 2013, 9, e1003471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, A.M.; Klase, Z.A.; DiNapoli, S.; Vujkovic-Cvijin, I.; Carmack, K.; Perkins, M.R.; Calantone, N.; Vinton, C.L.; Riddick, N.E.; Gallagher, J.; et al. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal Immunol. 2015, 9, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.; Uppada, S.B.; Pandey, K.; King, C.; Nguyen, K.; Shim, I.; Rogers, K.; Villinger, F.; Paiardini, M.; Byrareddy, S.N. Safety and Immunological Evaluation of Interleukin-21 Plus Anti-α4β7 mAb Combination Therapy in Rhesus Macaques. Front. Immunol. 2020, 11, 1275. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Jadlowsky, J.K.; Shaw, P.A.; Tian, L.; Esparza, E.; Brennan, A.L.; Kim, S.; Naing, S.Y.; Richardson, M.W.; Vogel, A.N.; et al. CCR5-edited CD4+ T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. J. Clin. Investig. 2021, 131, e144486. [Google Scholar] [CrossRef] [PubMed]
- Younan, P.; Kowalski, J.; Kiem, H.-P. Genetic Modification of Hematopoietic Stem Cells as a Therapy for HIV/AIDS. Viruses 2013, 5, 2946–2962. [Google Scholar] [CrossRef] [Green Version]
- Leibman, R.S.; Riley, J.L. Engineering T Cells to Functionally Cure HIV-1 Infection. Mol. Ther. 2015, 23, 1149–1159. [Google Scholar] [CrossRef] [Green Version]
- Rust, B.J.; Kean, L.S.; Colonna, L.; Brandenstein, K.E.; Poole, N.H.; Obenza, W.; Enstrom, M.R.; Maldini, C.R.; Ellis, G.I.; Fennessey, C.M.; et al. Robust expansion of HIV CAR T cells following antigen boosting in ART-suppressed nonhuman primates. Blood 2020, 136, 1722–1734. [Google Scholar] [CrossRef]
- Barber-Axthelm, I.M.; Barber-Axthelm, V.; Sze, K.Y.; Zhen, A.; Suryawanshi, G.W.; Chen, I.S.; Zack, J.A.; Kitchen, S.G.; Kiem, H.-P.; Peterson, C.W. Stem cell–derived CAR T cells traffic to HIV reservoirs in macaques. JCI Insight 2021, 6, e141502. [Google Scholar] [CrossRef]
- Ayala, V.I.; Deleage, C.; Trivett, M.T.; Jain, S.; Coren, L.V.; Breed, M.W.; Kramer, J.A.; Thomas, J.; Estes, J.D.; Lifson, J.D.; et al. CXCR5-Dependent Entry of CD8 T Cells into Rhesus Macaque B-Cell Follicles Achieved through T-Cell Engineering. J. Virol. 2017, 91, e02507-16. [Google Scholar] [CrossRef] [Green Version]
- Thalhauser, S.; Peterhoff, D.; Wagner, R.; Breunig, M. Critical design criteria for engineering a nanoparticulate HIV-1 vaccine. J. Control. Release 2019, 317, 322–335. [Google Scholar] [CrossRef]
- Surve, D.H.; Jirwankar, Y.B.; Dighe, V.D.; Jindal, A.B. Long-Acting Efavirenz and HIV-1 Fusion Inhibitor Peptide Co-loaded Polymer–Lipid Hybrid Nanoparticles: Statistical Optimization, Cellular Uptake, and In Vivo Biodistribution. Mol. Pharm. 2020, 17, 3990–4003. [Google Scholar] [CrossRef] [PubMed]
- Surve, D.H.; Jindal, A.B. Recent advances in long-acting nanoformulations for delivery of antiretroviral drugs. J. Control. Release 2020, 324, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.T.; Cottrell, C.A.; Antanasijevic, A.; Carnathan, D.G.; Cossette, B.J.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Viviano, F.; Fischinger, S.; et al. Targeting HIV Env immunogens to B cell follicles in nonhuman primates through immune complex or protein nanoparticle formulations. Npj Vaccines 2020, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Francica, J.R.; Laga, R.; Lynn, G.M.; Mužíková, G.; Androvič, L.; Aussedat, B.; Walkowicz, W.E.; Padhan, K.; Ramirez-Valdez, R.A.; Parks, R.; et al. Star nanoparticles delivering HIV-1 peptide minimal immunogens elicit near-native envelope antibody responses in nonhuman primates. PLoS Biol. 2019, 17, e3000328. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.; Sweeney, E.E.; Fernandes, R. Nanoparticle-Based Immunoengineered Approaches for Combating HIV. Front. Immunol. 2020, 11, 789. [Google Scholar] [CrossRef]
- Pino, M.; Paganini, S.; Deleage, C.; Padhan, K.; Harper, J.L.; King, C.T.; Micci, L.; Cervasi, B.; Mudd, J.C.; Gill, K.P.; et al. Fingolimod retains cytolytic T cells and limits T follicular helper cell infection in lymphoid sites of SIV persistence. PLoS Pathog. 2019, 15, e1008081. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrade, G.; Huot, N.; Petitdemange, C.; Lazzerini, M.; Orta Resendiz, A.; Jacquelin, B.; Müller-Trutwin, M. Interests of the Non-Human Primate Models for HIV Cure Research. Vaccines 2021, 9, 958. https://doi.org/10.3390/vaccines9090958
Terrade G, Huot N, Petitdemange C, Lazzerini M, Orta Resendiz A, Jacquelin B, Müller-Trutwin M. Interests of the Non-Human Primate Models for HIV Cure Research. Vaccines. 2021; 9(9):958. https://doi.org/10.3390/vaccines9090958
Chicago/Turabian StyleTerrade, Gauthier, Nicolas Huot, Caroline Petitdemange, Marie Lazzerini, Aurelio Orta Resendiz, Beatrice Jacquelin, and Michaela Müller-Trutwin. 2021. "Interests of the Non-Human Primate Models for HIV Cure Research" Vaccines 9, no. 9: 958. https://doi.org/10.3390/vaccines9090958
APA StyleTerrade, G., Huot, N., Petitdemange, C., Lazzerini, M., Orta Resendiz, A., Jacquelin, B., & Müller-Trutwin, M. (2021). Interests of the Non-Human Primate Models for HIV Cure Research. Vaccines, 9(9), 958. https://doi.org/10.3390/vaccines9090958






