Prospective Cohort Study of the Kinetics of Specific Antibodies to SARS-CoV-2 Infection and to Four SARS-CoV-2 Vaccines Available in Serbia, and Vaccine Effectiveness: A 3-Month Interim Report
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Data Collection
2.4. Procedures
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
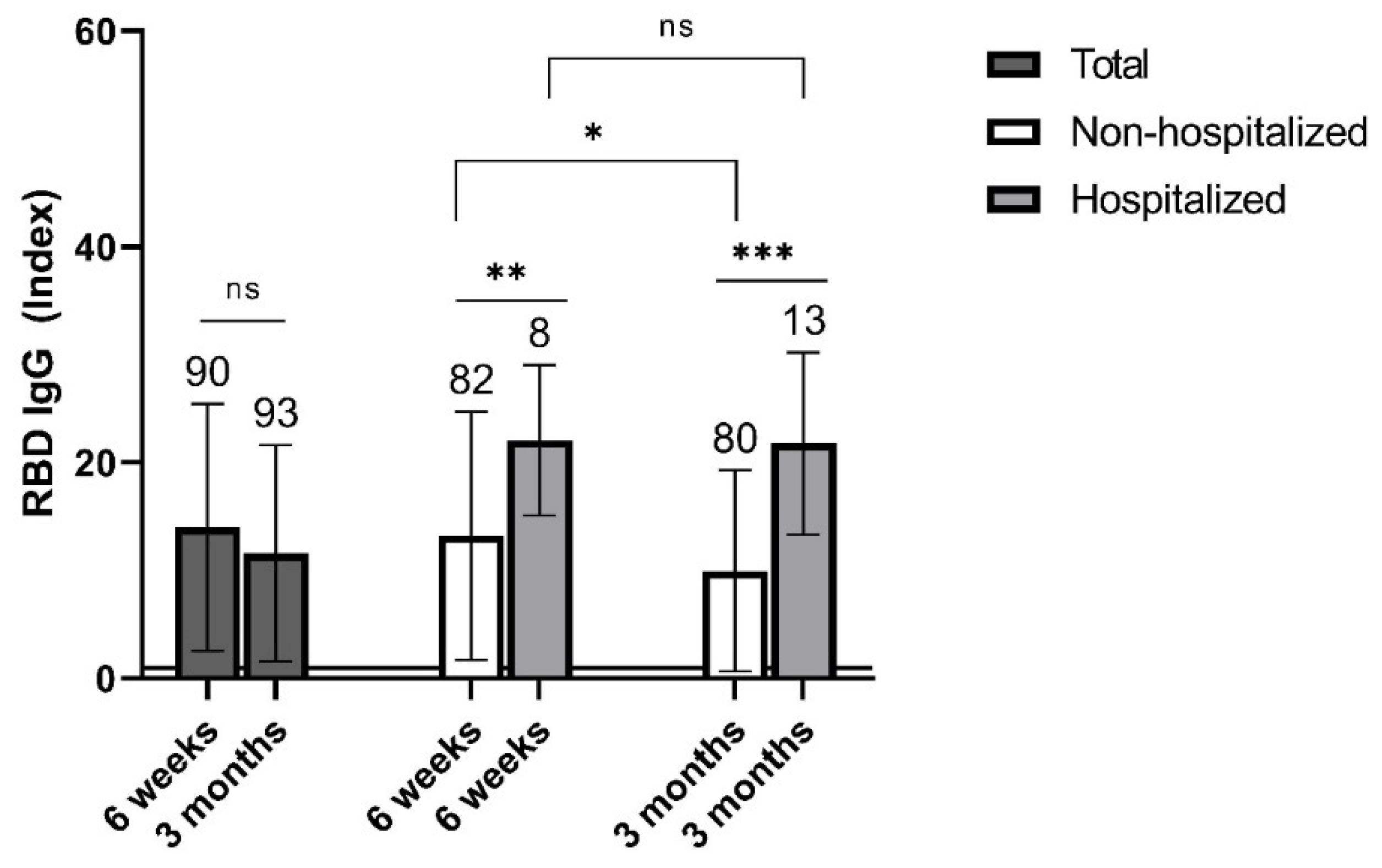
3.2. Levels of Specific IgG Antibodies in Non-Vaccinated Individuals with Past COVID-19
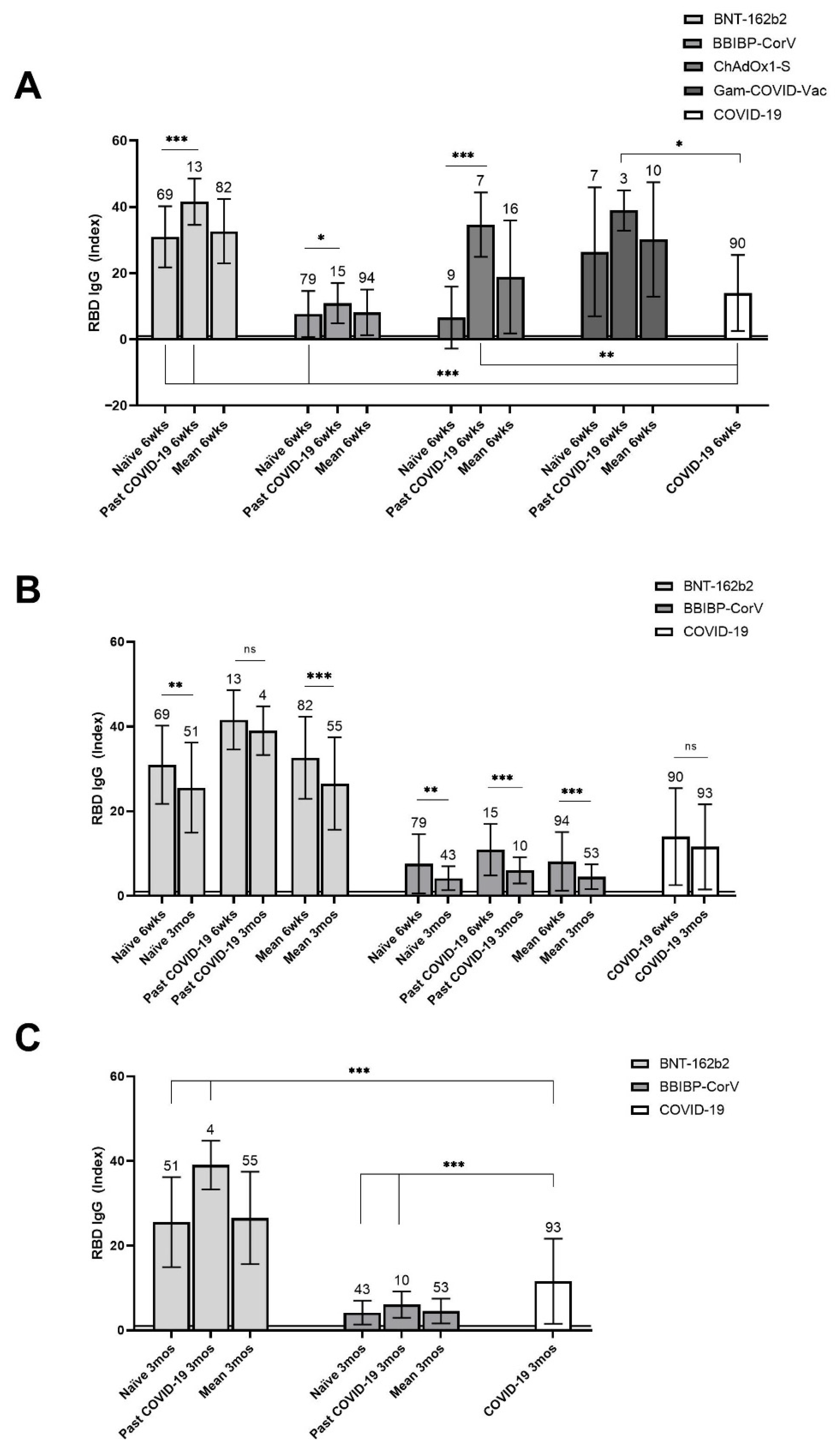
3.3. Rate and Levels of Specific IgG Antibodies at 6 Weeks Post First Vaccine Dose
3.4. Rate of Detection and Levels of Specific Antibodies at 3 Months Post First Vaccine Dose
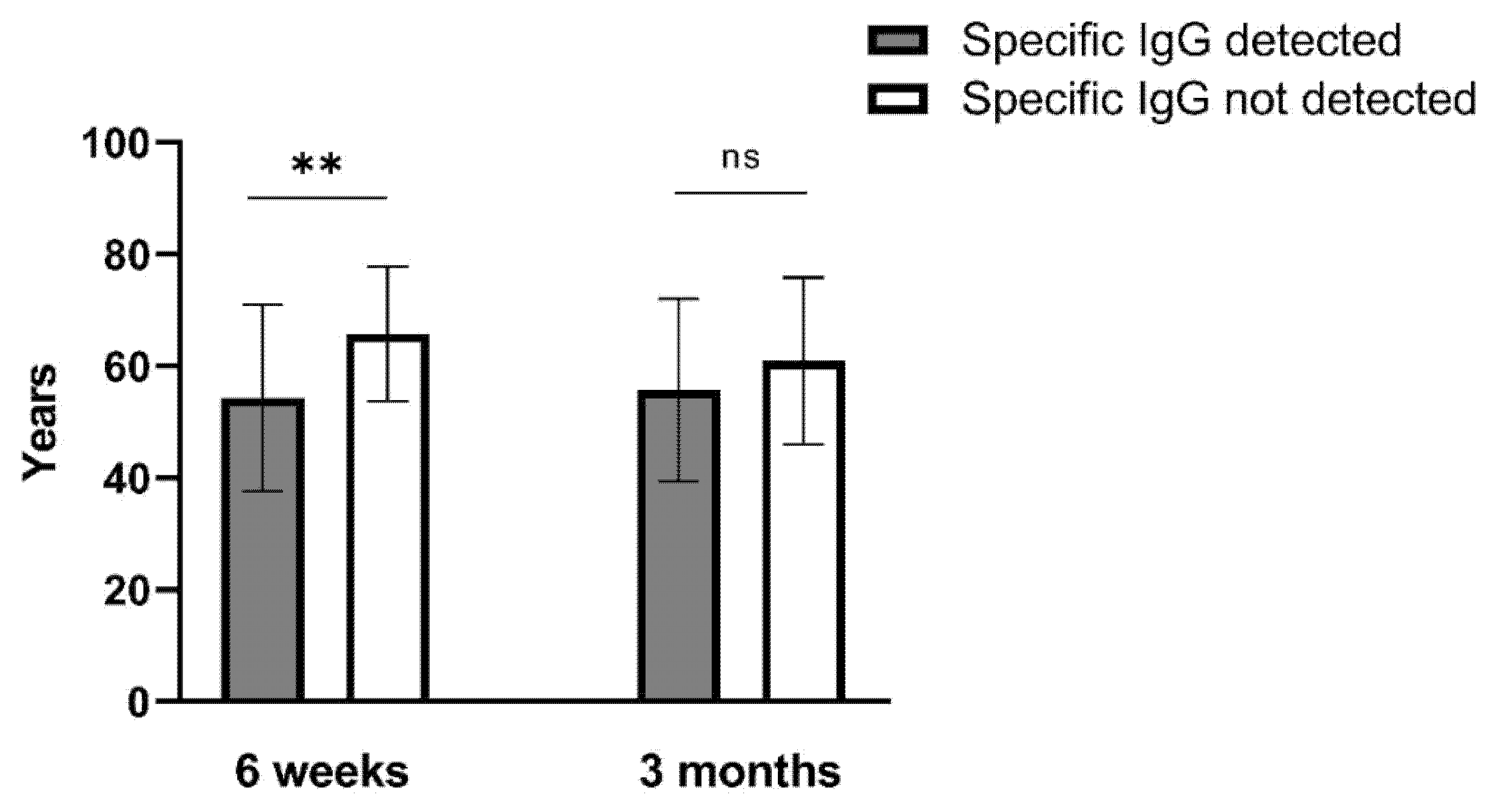
3.5. Analysis of the Vaccinal-Specific Antibody Levels According to Age
3.6. Detection of Specific IgM Antibodies
3.7. Vaccine Safety and Effectiveness
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process, Guidance Document. 16 June 2021. Available online: https://extranet.who.int/pqweb/sites/default/files/documents/Status_of_COVID-19_Vaccines_within_WHO_EUL-PQ_evaluation_process-16June2021_Final.pdf (accessed on 23 June 2021).
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: A randomized clinical trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Serbia: What Share of the Population has been Fully Vaccinated Against COVID-19? Our World in Data. Available online: https://ourworldindata.org/coronavirus/country/serbia#what-share-of-the-population-has-been-fully-vaccinated-against-covid-19 (accessed on 23 June 2021).
- Nathalie, R.; Soizic, D.; Nadège, C.; Matthieu, P.; Frédérique, R.; Sylvie, P.; Julien, L.; Carole, T.; Catherine, P.; Guillaume, G.; et al. Performance characteristics of the Vidas SARS-CoV-2 IgM and IgG serological assays. J. Clin. Microbiol. 2021, 59, e02292-20. [Google Scholar] [CrossRef]
- U Beogradu Najviše Ljudi Primilo Sinofarm Vakcinu, Najmanje Astrazeneku, Daily Danas. Available online: https://www.danas.rs/drustvo/u-beogradu-najvise-ljudi-primilo-sinofarm-vakcinu-najmanje-astrazeneku/ (accessed on 23 June 2021).
- Tok Vakcinacije: Pogledajte Koliko se Ljudi Vakcinisalo u Vašem Mestu, Centar za Istraživačko Novinarstvo Srbije. Available online: https://www.cins.rs/tok-vakcinacije-pogledajte-koliko-se-ljudi-vakcinisalo-u-vasem-mestu/ (accessed on 23 June 2021).
- Bruni, M.; Cecatiello, V.; Diaz-Basabe, A.; Lattanzi, G.; Mileti, E.; Monzani, S.; Pirovano, L.; Rizzelli, F.; Visintin, C.; Bonizzi, G.; et al. Persistence of anti-SARS-CoV-2 antibodies in non-hospitalized COVID-19 convalescent health care workers. J. Clin. Med. 2020, 9, 3188. [Google Scholar] [CrossRef] [PubMed]
- Duysburgh, E.; Mortgat, L.; Barbezange, C.; Dierick, K.; Fischer, N.; Heyndrickx, L.; Hutse, V.; Thomas, I.; Van Gucht, S.; Vuylsteke, B.; et al. Persistence of IgG response to SARS-CoV-2. Lancet Infect. Dis. 2021, 21, 163–164. [Google Scholar] [CrossRef]
- Miljanović, D.; Milićević, O.; Lončar, A.; Abazović, D.; Despot, D.; Banko, A. The first molecular characterization of Serbian SARS-CoV-2 isolates from a unique early second wave in Europe. Front Microbiol. 2021, 12, 691154. [Google Scholar] [CrossRef] [PubMed]
- Interim Guidelines for COVID-19 Antibody Testing in Clinical and Public Health settings, Center for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html (accessed on 23 June 2021).
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2021, 15, e0244126. [Google Scholar]
- Peluso, M.J.; Takahashi, S.; Hakim, J.; Kelly, J.D.; Torres, L.; Iyer, N.S.; Turcios, K.; Janson, O.; Munter, S.E.; Thanh, C.; et al. SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. Sci. Adv. 2021, 7, eabh3409. [Google Scholar] [CrossRef] [PubMed]
- Assis, R.; Jain, A.; Nakajima, R.; Jasinskas, A.; Kahn, S.; Palma, A.; Parker, D.M.; Chau, A.; Leung, A.; Grabar, C.; et al. Substantial differences in SARS-CoV-2 antibody responses elicited by natural infection and mRNA vaccination. bioRxiv 2021. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2020, 384, 80–82. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef]
- Eligibility Criteria for Booster Shot in Bahrain Outlined, Zawya, Refinitive. Available online: https://www.zawya.com/mena/en/life/story/Eligibility_criteria_for_booster_shot_in_Bahrain_outlined-SNG_216259172/ (accessed on 23 June 2021).
- Salazar, E.; Kuchipudi, S.V.; Christensen, P.A.; Eagar, T.; Yi, X.; Zhao, P.; Jin, Z.; Long, S.W.; Olsen, R.J.; Chen, J.; et al. Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization. J. Clin. Investig. 2020, 130, 6728–6738. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.-J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 2020, 183, 1024–1042.e21. [Google Scholar] [CrossRef] [PubMed]

| A. Unvaccinated participants with past COVID-19. | |||||||||
| Participants N (%) | Sex | Age | Hospitalization | ||||||
| F (%) | M (%) | ≤65 (%) | >65 (%) | Yes (%) | No (%) | ||||
| Past COVID-19 Unvaccinated | 110 | 59 (53.6) | 51 (46.4) | 81 (73.6) | 29 (26.4) | 17 (15.5) | 93 (84.5) | ||
| B. Vaccinated participants. | |||||||||
| Vaccinated Per Vaccine | Participants N (%) | Sex | Age | COVID-19 Naïve Tested Prior to Vaccination | Past COVID-19 | ||||
| F (%) | M (%) | ≤65 (%) | >65 (%) | Negative/Tested (N/N) | Baseline IgG Levels (mean ± SD) | N (%) | Days from COVID-19 to Vaccination (mean ± SD) | ||
| Total | 285 | 157 (55.1) | 128 (44.9) | 218 (76.5) | 68 (23.8) | 67/67 | 0.045 ± 0.064 | 47 (16.4) | 157.5 ± 94.7 |
| BNT-162b2 | 100 (35) | 64 (64) | 36 (36) | 91 (91) | 9 (9) | 43/43 | 0.043 ± 0.044 | 17 (17) | 153.3 ± 93.3 |
| BBIBP-CorV | 148 (51.9) | 75 (50.6) | 73 (49) | 91 (61.5) | 57 (38.5) | 18/18 | 0.06 ± 0.01 | 20 (13.5) | 131.4 ± 87.2 |
| ChAdOx1-S | 25 (8.7) | 12 (48) | 13 (52) | 23 (92) | 2 (8) | 4/4 | 0.015 ± 0.015 | 7 (28) | 242.4 ± 94.3 |
| Gam-COVID-Vac | 12 (4.2) | 6 (50) | 6 (50) | 12 (100) | 0 | 2/2 | 0.023 ± 0.016 | 3 (25) | 156.7 ± 73.9 |
| BNT-162b2 | BBIBP-CorV | ChAdOx1-S | Gam-COVID-Vac | NI | p | |
|---|---|---|---|---|---|---|
| 6 weeks N (%) | 34/82 (41.4) | 19/95 (20) | 3/16 (18.7) | 0/10 | 66/90 (73.3) | <0.001 * |
| 3 months N (%) | 3/55 (5.4) | 3/53 (5.7) | NA | NA | 41/93 (44.1) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lijeskić, O.; Klun, I.; Stamenov Djaković, M.; Gligorić, N.; Štajner, T.; Srbljanović, J.; Djurković-Djaković, O. Prospective Cohort Study of the Kinetics of Specific Antibodies to SARS-CoV-2 Infection and to Four SARS-CoV-2 Vaccines Available in Serbia, and Vaccine Effectiveness: A 3-Month Interim Report. Vaccines 2021, 9, 1031. https://doi.org/10.3390/vaccines9091031
Lijeskić O, Klun I, Stamenov Djaković M, Gligorić N, Štajner T, Srbljanović J, Djurković-Djaković O. Prospective Cohort Study of the Kinetics of Specific Antibodies to SARS-CoV-2 Infection and to Four SARS-CoV-2 Vaccines Available in Serbia, and Vaccine Effectiveness: A 3-Month Interim Report. Vaccines. 2021; 9(9):1031. https://doi.org/10.3390/vaccines9091031
Chicago/Turabian StyleLijeskić, Olivera, Ivana Klun, Marija Stamenov Djaković, Nenad Gligorić, Tijana Štajner, Jelena Srbljanović, and Olgica Djurković-Djaković. 2021. "Prospective Cohort Study of the Kinetics of Specific Antibodies to SARS-CoV-2 Infection and to Four SARS-CoV-2 Vaccines Available in Serbia, and Vaccine Effectiveness: A 3-Month Interim Report" Vaccines 9, no. 9: 1031. https://doi.org/10.3390/vaccines9091031
APA StyleLijeskić, O., Klun, I., Stamenov Djaković, M., Gligorić, N., Štajner, T., Srbljanović, J., & Djurković-Djaković, O. (2021). Prospective Cohort Study of the Kinetics of Specific Antibodies to SARS-CoV-2 Infection and to Four SARS-CoV-2 Vaccines Available in Serbia, and Vaccine Effectiveness: A 3-Month Interim Report. Vaccines, 9(9), 1031. https://doi.org/10.3390/vaccines9091031






