Establishment of a Suspension MDBK Cell Line in Serum-Free Medium for Production of Bovine Alphaherpesvirus-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Culture Media and Animals
2.2. Cell Counting
2.3. Selection of Suspension Medium
2.4. Adaptation of Adherent MDBK Cells to Grow in Suspension and Serum-Free Culture
2.5. Establishment of Growth Curve of Suspension-Adapted MDBK Cells
2.6. Examination of Tumorigenicity for Suspension-Adapted MDBK Cells
2.7. Virus Strain and Virus Titration
2.8. Optimization of BoHV-1 Production in Suspension-Adapted MDBK Cells
2.9. Production of BoHV-1 in Adherent MDBK Cells
2.10. Immunogenicity of BoHV-1
2.11. Statistical Analysis
3. Results
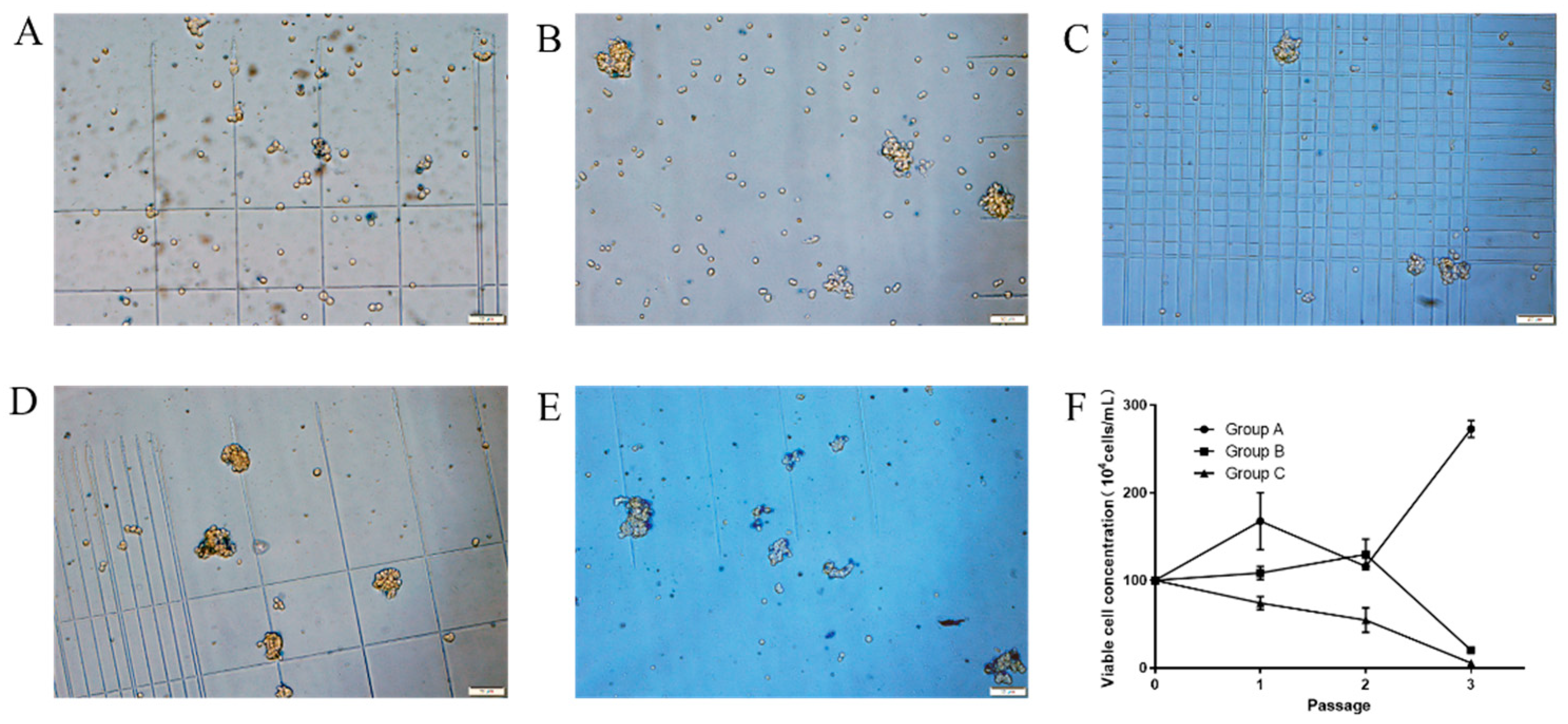
3.1. Screening of Commercially Available Medium for Suspended Growth of MDBK Cells
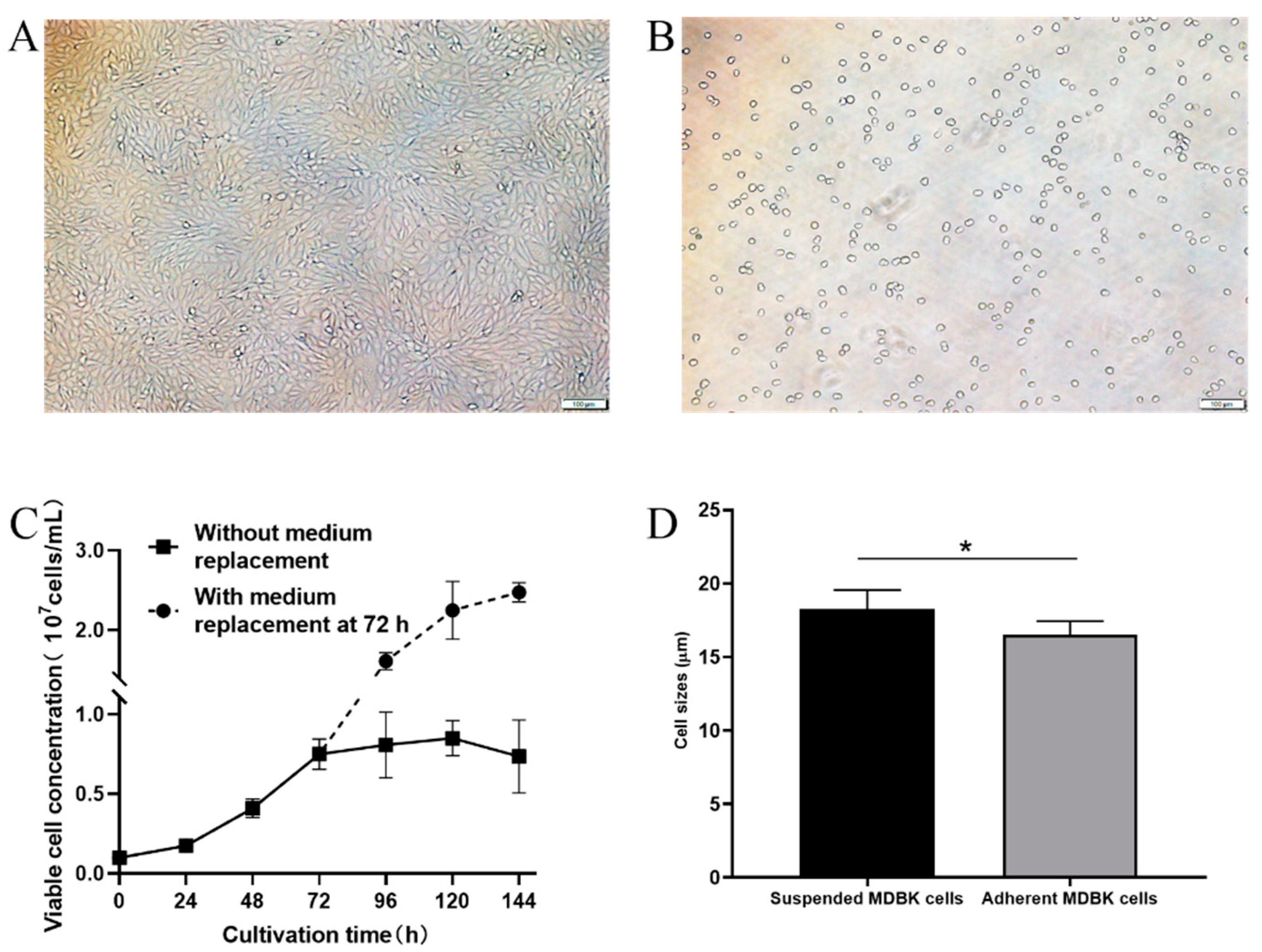
3.2. Adaptation of MDBK Cells to Suspension Culture in Serum-Free Medium
3.3. Characterization of Suspension-Adapted MDBK Cells
3.4. Production of BoHV-1 in Suspension-Adapted MDBK Cells
3.5. Immunogenicity of Inactivated BoHV-1 Produced in Suspended MDBK Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, L.; Silva, E.; Santos, M.H.; Heluy, T.R.; Biaggio, R.T.; Ferreira, A.R.; Milhomens, J.; Kashima, S.; el Nemer, W.; el Hoss, S.; et al. Serum-free suspension cultured human cells can produce a high-level of recombinant human erythropoietin. Eng. Rep. 2020, 2, e12172. [Google Scholar] [CrossRef]
- Gélinas, J.F.; Azizi, H.; Kiesslich, S.; Lanthier, S.; Perdersen, J.; Chahal, P.S.; Ansorge, S.; Kobinger, G.; Gilbert, R.; Kamen, A.A. Production of rVSV-ZEBOV in serum-free suspension culture of HEK 293SF cells. Vaccine 2019, 37, 6624–6632. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Fan, W.; Chen, Q.; Shi, B. An Efficient Transient Expression System for Enhancing the Generation of Monoclonal Antibodies in 293 Suspension Cells. Curr. Pharm. Biotechnol. 2017, 18, 351. [Google Scholar] [CrossRef]
- Qin, Z.; Ju, H.; Liu, Y.; Gao, T.; Wang, W.; Aurelian, L.; Zhao, P.; Qi, Z. Fetal bovine serum inhibits hepatitis C virus attachment to host cells. J. Virol. Methods 2013, 193, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Iki, S.; Yokota, S.; Okabayashi, T.; Yokosawa, N.; Nagata, K.; Fujii, N. Serum-dependent expression of promyelocytic leukemia protein suppresses propagation of influenza virus. Virology 2005, 343, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmori, K.; Masuda, K.; DeBoer, D.J.; Sakaguchi, M.; Tsujimoto, H. Immunoblot analysis for IgE-reactive components of fetal calf serum in dogs that developed allergic reactions after non-rabies vaccination. Vet. Immunol. Immunop. 2007, 115, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.V.T.; Ho, K.W.; Yap, M.G.S. Evaluation of insulin-mimetic trace metals as insulin replacements in mammalian cell cultures. Cytotechnology 2004, 45, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Robinson, D.; Salmon, P. A novel function for selenium in biological system: Selenite as a highly effective iron carrier for Chinese hamster ovary cell growth and monoclonal antibody production. Biotechnol. Bioeng. 2006, 95, 1188–1197. [Google Scholar] [CrossRef]
- Madin, S.H.; Darby, N.J. Established kidney cell lines of normal adult bovine and ovine origin. Proc. Soc. Exp. Biol. Med. 1958, 98, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Conceição, M.M.; Tonso, A.; Freitas, C.B.; Pereira, C.A. Viral antigen production in cell cultures on microcarriers Bovine parainfluenza 3 virus and MDBK cells. Vaccine 2007, 25, 7785–7795. [Google Scholar] [CrossRef]
- Lesko, J.; Veber, P.; Hrda, M.; Feketeova, M. Large-scale production of infectious bovine rhinotracheitis virus in cell culture on microcarriers. Acta Virol. 1993, 37, 73–78. [Google Scholar]
- Elazhary, M.A.S.Y.; Derbyshire, J.B. The cultivation of bovine adenovirus type 3 in cultures of suspended MDBK cells. Vet. Microbiol. 1977, 2, 283–288. [Google Scholar] [CrossRef]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.L.; Turkington, H.L.; Cosby, S.L. The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments. Vaccines 2021, 9, 337. [Google Scholar] [CrossRef]
- Miles, D.G. Overview of the North American beef cattle industry and the incidence of bovine respiratory disease (BRD). Anim. Health Res. Rev. 2009, 10, 101–103. [Google Scholar] [CrossRef]
- Madin, S.H.; Mckercher, D.G.; York, C.J. Isolation of the infectious bovine rhinotracheitis virus. Science 1956, 124, 721–722. [Google Scholar] [CrossRef] [PubMed]
- Cowley, D.J.; Clegg, T.A.; Doherty, M.L.; More, S.J. Aspects of bovine herpesvirus-1 infection in dairy and beef herds in the Republic of Ireland. Acta Vet. Scand. 2011, 53, 40. [Google Scholar] [CrossRef]
- Han, Z.; Gao, J.; Li, K.; Shahzad, M.; Nabi, F.; Zhang, D.; Li, J.; Liu, Z. Prevalence of Circulating Antibodies to Bovine Herpesvirus 1 in Yaks (Bos grunniens) on the Qinghai-Tibetan Plateau, China. J. Wildl. Dis. 2016, 52, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.G.; Denwood, M.J.; de Sousa, A.B.S.C.; Alves, C.J.; Pituco, E.M.; de Campos, N.R.A.; de Stefano, E.; Nielsen, S.S.; de Azevedo, S.S. Bayesian estimation of herd-level prevalence and risk factors associated with BoHV-1 infection in cattle herds in the State of Paraiba, Brazil. Prev. Vet. Med. 2019, 169, 104705. [Google Scholar] [CrossRef] [PubMed]
- Sayers, R.G. Associations between exposure to bovine herpesvirus 1 (BoHV-1) and milk production, reproductive performance, and mortality in Irish dairy herds. J. Dairy Sci. 2017, 100, 1340–1352. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, Y.K.; Yin, B.; Liang, J.B.; Jiang, F.; Wu, W.X. Toll-Like Receptor 2 (TLR2) and TLR4 Mediate the IgA Immune Response Induced by Mycoplasma hyopneumoniae. Infect. Immun. 2019, 88, e619–e697. [Google Scholar] [CrossRef] [Green Version]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Quattrocchi, V.; Soria, I.; Langellotti, C.A.; Gnazzo, V.; Gammella, M.; Moore, D.P.; Zamorano, P.I. A DNA Vaccine Formulated with Chemical Adjuvant Provides Partial Protection against Bovine Herpes Virus Infection in Cattle. Front. Immunol. 2017, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Rescorla, F.J. Cell surface adhesion molecules and adhesion-initiated signaling: Understanding of anoikis resistance mechanisms and therapeutic opportunities. Cell Signal 2012, 24, 393–401. [Google Scholar] [CrossRef]
- Sinacore, M.S.; Drapeau, D.; Adamson, S.R. Adaptation of mammalian cells to growth in serum-free media. Mol. Biotechnol. 2000, 15, 249–257. [Google Scholar] [CrossRef]
- Huang, D.; Peng, W.; Ye, Q.; Liu, X.; Zhao, L.; Fan, L.; Xia-Hou, K.; Jia, H.; Luo, J.; Zhou, L.; et al. Serum-Free Suspension Culture of MDCK Cells for Production of Influenza H1N1 Vaccines. PLoS ONE 2015, 10, e141686. [Google Scholar] [CrossRef] [PubMed]
- Nyon, M.P.; Du, L.; Tseng, C.K.; Seid, C.A.; Pollet, J.; Naceanceno, K.S.; Agrawal, A.; Algaissi, A.; Peng, B.; Tai, W.; et al. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine 2018, 36, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Perrin, P.; Madhusudana, S.; Gontier-Jallet, C.; Petres, S.; Tordo, N.; Merten, O.W. An experimental rabies vaccine produced with a new BHK-21 suspension cell culture process: Use of serum-free medium and perfusion-reactor system. Vaccine 1995, 13, 1244–1250. [Google Scholar] [CrossRef]
- Shen, C.F.; Guilbault, C.; Li, X.; Elahi, S.M.; Ansorge, S.; Kamen, A.; Gilbert, R. Development of suspension adapted Vero cell culture process technology for production of viral vaccines. Vaccine 2019, 37, 6996–7002. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Hou, R. MDBK Acclimation Suspension Method and Two-Stage Virus Production. Process. Patent Application No. 201711098901.9, 25 September 2018. [Google Scholar]
- Hao, P.; Liu, G.; Wen, X.; Kong, C.; Ji, Y.; Cang, F.; Shang, X. Bovine Kidney Cell Capable of Being Subjected to Suspension Culture, Suspension Culture Method for Bovine Kidney Cell and Application. Thereof. Patent Application No. 201710154847.9, 26 September 2017. [Google Scholar]
- Lee, S.S. Lateral inhibition-induced pattern formation controlled by the size and geometry of the cell. J. Theor. Biol. 2016, 404, 51–65. [Google Scholar] [CrossRef]
- Tekin, S.; Padua, M.B.; Brad, A.M.; Hansen, P.J. Antiproliferative actions of ovine uterine serpin. Am. J. Reprod. Immunol. 2005, 53, 136–143. [Google Scholar] [CrossRef]
- Welter, M.W. Adaptation and serial passage of bovine coronavirus in an established diploid swine testicular cell line and subsequent development of a modified live vaccine. Adv. Exp. Med. Biol. 1998, 440, 707–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Cui, N.; Zheng, P.S.; Yang, W.T. BMX/Etk promotes cell proliferation and tumorigenicity of cervical cancer cells through PI3K/AKT/mTOR and STAT3 pathways. Oncotarget 2017, 8, 49238–49252. [Google Scholar] [CrossRef] [PubMed]
- Petiot, E.; Jacob, D.; Lanthier, S.; Lohr, V.; Ansorge, S.; Kamen, A.A. Metabolic and kinetic analyses of influenza production in perfusion HEK293 cell culture. BMC Biotechnol. 2011, 11, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dill, V.; Ehret, J.; Zimmer, A.; Beer, M.; Eschbaumer, M. Cell Density Effects in Different Cell Culture Media and Their Impact on the Propagation of Foot-And-Mouth Disease Virus. Viruses 2019, 11, 511. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ze’Ev, A. Differential control of cytokeratins and vimentin synthesis by cell-cell contact and cell spreading in cultured epithelial cells. J. Cell Biol. 1984, 99, 1424–1433. [Google Scholar] [CrossRef] [Green Version]
- Ramos, I.; Stamatakis, K.; Oeste, C.L.; Pérez-Sala, D. Vimentin as a Multifaceted Player and Potential Therapeutic Target in Viral Infections. Int. J. Mol. Sci. 2020, 21, 4675. [Google Scholar] [CrossRef]
- le Tu, G. Study on Immunogenicity of Infectious Bovine Rhinotracheitis Inactivated Vaccine Seed and Establishment of Mycoplasma Bovis Challenge Model. Master’s Thesis, China Agricultural University, Beijing, China, 2020. [Google Scholar]
- Bastian, M.; Holsteg, M.; Hanke-Robinson, H.; Duchow, K.; Cussler, K. Bovine Neonatal Pancytopenia: Is this alloimmune syndrome caused by vaccine-induced alloreactive antibodies? Vaccine 2011, 29, 5267–5275. [Google Scholar] [CrossRef]
- Benedictus, L.; Bell, C.R. The risks of using allogeneic cell lines for vaccine production: The example of Bovine Neonatal Pancytopenia. Expert Rev. Vaccines 2017, 16, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Euler, K.N.; Hauck, S.M.; Ueffing, M.; Deeg, C.A. Bovine neonatal pancytopenia--comparative proteomic characterization of two BVD vaccines and the producer cell surface proteome (MDBK). BMC Vet. Res. 2013, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Mastelic, B.; Garcon, N.; Del, G.G.; Golding, H.; Gruber, M.; Neels, P.; Fritzell, B. Predictive markers of safety and immunogenicity of adjuvanted vaccines. Biologicals 2013, 41, 458–468. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Huang, S.; Hao, C.; Wang, Z.; Zhao, H.; Liu, M.; Tian, X.; Ge, L.; Wu, W.; Peng, C. Establishment of a Suspension MDBK Cell Line in Serum-Free Medium for Production of Bovine Alphaherpesvirus-1. Vaccines 2021, 9, 1006. https://doi.org/10.3390/vaccines9091006
Wang P, Huang S, Hao C, Wang Z, Zhao H, Liu M, Tian X, Ge L, Wu W, Peng C. Establishment of a Suspension MDBK Cell Line in Serum-Free Medium for Production of Bovine Alphaherpesvirus-1. Vaccines. 2021; 9(9):1006. https://doi.org/10.3390/vaccines9091006
Chicago/Turabian StyleWang, Pengpeng, Shulin Huang, Chengwu Hao, Zhanhui Wang, Haoran Zhao, Mengyao Liu, Xinrui Tian, Letu Ge, Wenxue Wu, and Chen Peng. 2021. "Establishment of a Suspension MDBK Cell Line in Serum-Free Medium for Production of Bovine Alphaherpesvirus-1" Vaccines 9, no. 9: 1006. https://doi.org/10.3390/vaccines9091006
APA StyleWang, P., Huang, S., Hao, C., Wang, Z., Zhao, H., Liu, M., Tian, X., Ge, L., Wu, W., & Peng, C. (2021). Establishment of a Suspension MDBK Cell Line in Serum-Free Medium for Production of Bovine Alphaherpesvirus-1. Vaccines, 9(9), 1006. https://doi.org/10.3390/vaccines9091006





