Current Progress in the Development of Zika Virus Vaccines
Abstract
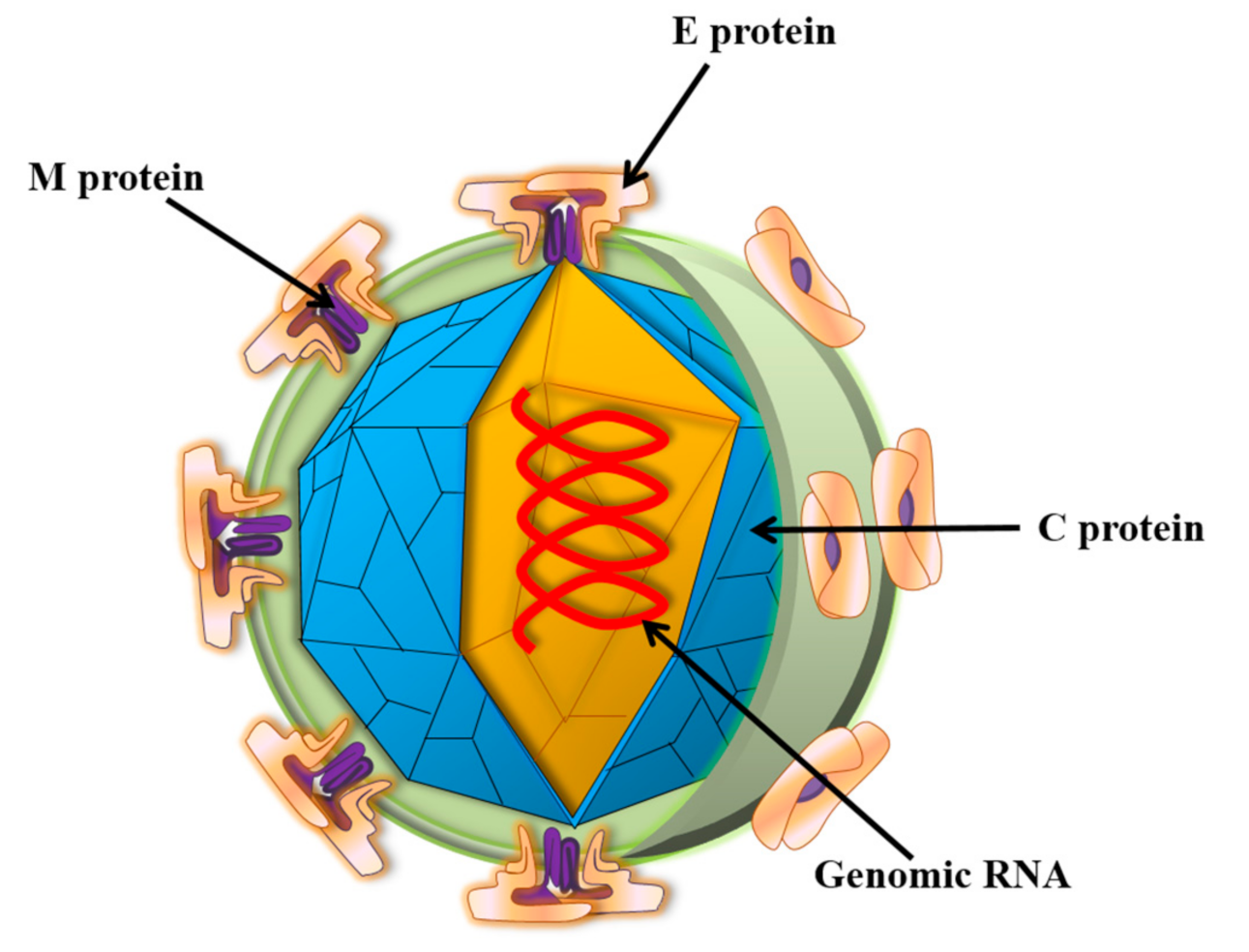
:1. Introduction
2. Development of Vaccines against ZIKV Infection
2.1. DNA Vaccines against ZIKV Infection
2.2. Subunit Vaccines against ZIKV Infection
2.3. Live-Attenuated Vaccines against ZIKV Infection
2.4. Virus-Vector-Based Vaccines against ZIKV Infection
2.5. Purified Inactivated Zika Vaccines (PIZV)
2.6. Virus-Like Particle (VLP)-Based Vaccines against ZIKV Infection
2.7. mRNA-Based Vaccines against ZIKV Infection
| Vaccine’s Name or Component | Immunogenicity in the Induction of Immune Responses | Animal Model | Vaccine Doses | Administration Route | Virus Challenged | Ref. |
|---|---|---|---|---|---|---|
| prM and E (HEK293 expression system) | Induced a protective antibody response | AG129 mice | Two doses at day 0 and 32 | i.m. | Prototype Zika Nica 2-16 strain | [69] |
| prM and E (Baculovirus expression system) | Stimulated ZIKV-specific IgG and neutralizing antibodies, as well as T-cell responses | BALB/c mice | Three doses at two-week intervals | i.m. | ZIKV strain SZ-WIV01 | [70] |
| EDIII (Nicotiana benthamiana plant expression system) | Elicited potent humoral and cellular immune responses correlated with protective immunity against multiple strains | C57BL/6 mice | Three doses at three-week intervals | s.c. | Puerto Rico strain PRVABC59 | [71] |
| prM and E | Intradermal electroporation of as little as 1 µg of this vaccine elicited potent humoral and cellular immune responses in BALB/c and IFNAR−/− C57BL/6 mice, resulting in complete protection of the latter mice against ZIKV infection. | BALB/c and IFNAR−/− C57BL/6 mice | Two doses at four-week interval | i.d. | ZIKV strain MR-766 | [72] |
| Conferred protection and sterilizing immunity in immunocompetent mice against ZIKV infection and diminished ADE in vitro, as well as in vivo | AG129, BALB/c and C57BL/6 mice | Single dose and two doses at three-week interval | i.m. | African ZIKV strain (Dakar 41519) | [73] | |
| Induced potent and durable protective responses in mice and non-human primates | BALB/c and C57BL/6 mice; rhesus macaques (Macaca mulatta) | Single dose | i.d. | Puerto Rico strain PRVABC59 | [74] |
2.8. Other Types of Vaccines against ZIKV Infection
3. Animal Models as Tools to Assist in the Development of Zika Vaccines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, H.H.; Yip, B.S.; Huang, L.M.; Wu, S.C. Zika virus structural biology and progress in vaccine development. Biotechnol. Adv. 2018, 36, 47–53. [Google Scholar] [CrossRef]
- Fontes-Garfias, C.R.; Shan, C.; Luo, H.; Muruato, A.E.; Medeiros, D.B.A.; Mays, E.; Xie, X.; Zou, J.; Roundy, C.M.; Wakamiya, M.; et al. Functional analysis of glycosylation of Zika virus envelope protein. Cell Rep. 2017, 21, 1180–1190. [Google Scholar] [CrossRef] [Green Version]
- Song, B.H.; Yun, S.I.; Woolley, M.; Lee, Y.M. Zika virus: History, epidemiology, transmission, and clinical presentation. J. Neuroimmunol. 2017, 308, 50–64. [Google Scholar] [CrossRef] [Green Version]
- Pang, W.; Lin, Y.L.; Xin, R.; Chen, X.X.; Lu, Y.; Zheng, C.B.; Yang, L.M.; Zheng, Y.T. Zika virus transmission via breast milk in suckling mice. Clin. Microbiol. Infect. 2021, 27, 469. [Google Scholar] [CrossRef]
- Regla-Nava, J.A.; Viramontes, K.M.; Vozdolska, T.; Huynh, A.T.; Villani, T.; Gardner, G.; Johnson, M.; Ferro, P.J.; Shresta, S.; Kim, K. Detection of Zika virus in mouse mammary gland and breast milk. PLoS Negl. Trop. Dis. 2019, 13, e0007080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driggers, R.W.; Ho, C.Y.; Korhonen, E.M.; Kuivanen, S.; Jääskeläinen, A.J.; Smura, T.; Rosenberg, A.; Hill, D.A.; DeBiasi, R.L.; Vezina, G.; et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 2016, 374, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Noronha, L.D.; Zanluca, C.; Azevedo, M.L.; Luz, K.G.; Santos, C.N. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem. Inst. Oswaldo Cruz 2016, 111, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, M.G.; Schwartz, D.A. Zika virus infection in pregnancy, microcephaly, and maternal and fetal health: What we think, what we know, and what we think we know. Arch. Pathol. Lab. Med. 2017, 141, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbiani, D.F.; Bozzacco, L.; Keeffe, J.R.; Khouri, R.; Olsen, P.C.; Gazumyan, A.; Schaefer-Babajiw, D.; Avila-Rios, S.; Nogueira, L.; Patel, R.; et al. Recurrent potent human neutralizing antibodies to Zika virus in Brazil and Mexico. Cell 2017, 169, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Tang, W.W.; Sheets, N.; Ellison, J.; Sette, A.; Kim, K.; Shresta, S. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8+ T cells. Nat. Microbiol. 2017, 2, 17036. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.H.; McGowan, E.; Jadi, R.; Young, E.; Lopez, C.A.; Baric, R.S.; Lazear, H.M.; de Silva, A.M. Lack of durable cross-neutralizing antibodies against Zika virus from Dengue virus infection. Emerg. Infect. Dis. 2017, 23, 773–781. [Google Scholar] [CrossRef]
- Danko, J.R.; Beckett, C.G.; Porter, K.R. Development of dengue DNA vaccines. Vaccine 2011, 29, 7261–7266. [Google Scholar] [CrossRef] [Green Version]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A mouse model of Zika virus pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a novel murine model to study Zika virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Li, S.; Ma, S.; Jia, L.; Zhang, F.; Zhang, Y.; Zhang, J.; Wong, G.; Zhang, S.; Lu, X.; et al. Zika virus causes testis damage and leads to male infertility in mice. Cell 2017, 168, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Griffin, B.D.; Muthumani, K.; Warner, B.M.; Majer, A.; Hagan, M.; Audet, J.; Stein, D.R.; Ranadheera, C.; Racine, T.; De La Vega, M.A.; et al. DNA vaccination protects mice against Zika virus-induced damage to the testes. Nat. Commun. 2017, 8, 15743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Fan, D.; Wang, L.; Song, J.; Feng, K.; Li, M.; Wang, P.; Chen, H.; An, J. Maternal immunization with a DNA vaccine candidate elicits specific passive protection against post-natal Zika virus infection in immunocompetent BALB/c mice. Vaccine 2018, 36, 3522–3532. [Google Scholar] [CrossRef] [PubMed]
- De La Vega, M.A.; Piret, J.; Griffin, B.D.; Rhéaume, C.; Venable, M.C.; Carbonneau, J.; Couture, C.; das Neves Almeida, R.; Tremblay, R.R.; Magalhães, K.G.; et al. Zika-induced male infertility in mice is potentially reversible and preventable by deoxyribonucleic acid immunization. J. Infect. Dis. 2019, 219, 365–374. [Google Scholar] [CrossRef]
- Gaudinski, M.R.; Houser, K.V.; Morabito, K.M.; Hu, Z.; Yamshchikov, G.; Rothwell, R.S.; Berkowitz, N.; Mendoza, F.; Saunders, J.G.; Novik, L.; et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 2018, 391, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Hraber, P.; Bradfute, S.; Clarke, E.; Ye, C.; Pitard, B. Amphiphilic block copolymer delivery of a DNA vaccine against Zika virus. Vaccine 2018, 36, 6911–6917. [Google Scholar] [CrossRef]
- Grubor-Bauk, B.; Wijesundara, D.K.; Masavuli, M.; Abbink, P.; Peterson, R.L.; Prow, N.A.; Larocca, R.A.; Mekonnen, Z.A.; Shrestha, A.; Eyre, N.S.; et al. NS1 DNA vaccination protects against Zika infection through T cell-mediated immunity in immunocompetent mice. Sci. Adv. 2019, 5, eaax2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowd, K.A.; Ko, S.Y.; Morabito, K.M.; Yang, E.S.; Pelc, R.S.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid development of a DNA vaccine for Zika virus. Science 2016, 354, 237–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.; Xie, X.; Luo, H.; Shan, C.; Muruato, A.E.; Weaver, S.C.; Wang, T.; Shi, P.Y. A single-dose plasmid-launched live-attenuated Zika vaccine induces protective immunity. EBioMedicine 2018, 36, 92–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Zheng, B.J.; Lu, L.; Zhou, Y.; Jiang, S.; Du, L. Advancements in the development of subunit influenza vaccines. Microbes. Infect. 2015, 17, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- To, A.; Medina, L.O.; Mfuh, K.O.; Lieberman, M.M.; Wong, T.A.S.; Namekar, M.; Nakano, E.; Lai, C.Y.; Kumar, M.; Nerurkar, V.R.; et al. Recombinant Zika virus subunits are immunogenic and efficacious in mice. MSphere 2018, 3, e00576-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, L.O.; To, A.; Lieberman, M.M.; Wong, T.A.S.; Namekar, M.; Nakano, E.; Andersen, H.; Yalley-Ogunro, J.; Greenhouse, J.; Higgs, S.; et al. A recombinant subunit based Zika virus vaccine is efficacious in non-human primates. Front. Immunol. 2018, 9, 2464. [Google Scholar] [CrossRef]
- Yang, M.; Dent, M.; Lai, H.; Sun, H.; Chen, Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vaccine 2017, 35, 4287–4294. [Google Scholar] [CrossRef]
- Zhu, X.; Li, C.; Afridi, S.K.; Zu, S.; Xu, J.W.; Quanquin, N.; Yang, H.; Cheng, G.; Xu, Z. E90 subunit vaccine protects mice from Zika virus infection and microcephaly. Acta. Neuropathol. Commun. 2018, 6, 77. [Google Scholar] [CrossRef]
- Han, J.F.; Qiu, Y.; Yu, J.Y.; Wang, H.J.; Deng, Y.Q.; Li, X.F.; Zhao, H.; Sun, H.X.; Qin, C.F. Immunization with truncated envelope protein of Zika virus induces protective immune response in mice. Sci. Rep. 2017, 7, 10047. [Google Scholar] [CrossRef] [Green Version]
- Tai, W.; He, L.; Wang, Y.; Sun, S.; Zhao, G.; Luo, C.; Li, P.; Zhao, H.; Fremont, D.H.; Li, F.; et al. Critical neutralizing fragment of Zika virus EDIII elicits cross-neutralization and protection against divergent Zika viruses. Emerg. Microbes Infect. 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Durbin, A.; Wvilder-Smith, A. An update on Zika vaccine developments. Expert Rev. Vaccines 2017, 16, 781–787. [Google Scholar] [CrossRef]
- Collins, N.D.; Shan, C.; Nunes, B.T.D.; Widen, S.G.; Shi, P.Y.; Barrett, A.D.T.; Sarathy, V.V. Using next generation sequencing to study the genetic diversity of candidate live attenuated Zika vaccines. Vaccines 2020, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Shan, C.; Muruato, A.E.; Nunes, B.T.D.; Luo, H.; Xie, X.; Medeiros, D.B.A.; Wakamiya, M.; Tesh, R.B.; Barrett, A.D.; Wang, T.; et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat. Med. 2017, 23, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Muruato, A.E.; Jagger, B.W.; Richner, J.; Nunes, B.T.D.; Medeiros, D.B.A.; Xie, X.; Nunes, J.G.C.; Morabito, K.M.; Kong, W.P.; et al. A single-dose live-attenuated vaccine prevents Zika virus pregnancy transmission and testis damage. Nat. Commun. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Jagger, B.W.; Shan, C.; Fontes, C.R.; Dowd, K.A.; Cao, B.; Himansu, S.; Caine, E.A.; Nunes, B.T.D.; Medeiros, D.B.A.; et al. Vaccine mediated protection against Zika virus-induced congenital disease. Cell 2017, 170, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Kum, D.B.; Xia, H.; Luo, H.; Shan, C.; Zou, J.; Muruato, A.E.; Medeiros, D.B.A.; Nunes, B.T.D.; Dallmeier, K.; et al. A single-dose live-attenuated Zika virus vaccine with controlled infection rounds that protects against vertical transmission. Cell Host Microbe 2018, 24, 487–499. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhou, D. Adenoviral vector-based strategies against infectious disease and cancer. Hum. Vaccines Immunother. 2016, 12, 2064–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullard, B.L.; Corder, B.N.; Gorman, M.J.; Diamond, M.S.; Weaver, E.A. Efficacy of a T cell-biased adenovirus vector as a Zika virus vaccine. Sci. Rep. 2018, 8, 18017. [Google Scholar] [CrossRef]
- Bullard, B.L.; Corder, B.N.; Gordon, D.N.; Pierson, T.C.; Weaver, E.A. Characterization of a species E adenovirus vector as a Zika virus vaccine. Sci. Rep. 2020, 10, 3613. [Google Scholar] [CrossRef]
- Steffen, T.; Hassert, M.; Hoft, S.G.; Stone, E.T.; Zhang, J.; Geerling, E.; Grimberg, B.T.; Roberts, M.S.; Pinto, A.K.; Brien, J.D. Immunogenicity and efficacy of a recombinant human adenovirus type 5 vaccine against Zika virus. Vaccines 2020, 8, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Chan, J.F.W.; Poon, V.K.M.; Wu, S.; Chan, C.C.S.; Hou, L.; Yip, C.C.Y.; Ren, C.; Cai, J.P.; Zhao, M.; et al. Immunization with a novel human type 5 adenovirus-vectored vaccine expressing the premembrane and envelope proteins of Zika virus provides consistent and sterilizing protection in multiple immunocompetent and immunocompromised animal models. J. Infect. Dis. 2018, 218, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.; et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Larocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng’ang’a, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Yu, J.; Lu, M.; Ma, Y.; Attia, Z.; Shan, C.; Xue, M.; Liang, X.; Craig, K.; Makadiya, N.; et al. A Zika virus vaccine expressing premembrane-envelope-NS1 polyprotein. Nat. Commun. 2018, 9, 3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Hu, J.; Guo, J.; Wu, C.; Xiong, S.; Dong, C. A vesicular stomatitis virus-based vaccine carrying Zika virus capsid protein protects mice from viral infection. Virol. Sin. 2019, 34, 106–110. [Google Scholar] [CrossRef]
- Abbink, P.; Maxfield, L.F.; Ng’ang’a, D.; Borducchi, E.N.; Iampietro, M.J.; Bricault, C.A.; Teigler, J.E.; Blackmore, S.; Parenteau, L.; Wagh, K.; et al. Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J. Virol. 2015, 89, 1512–1522. [Google Scholar] [CrossRef] [Green Version]
- Boutin, S.; Monteilhet, V.; Veron, P.; Leborgne, C.; Benveniste, O.; Montus, M.F.; Masurier, C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010, 21, 704–712. [Google Scholar] [CrossRef]
- Li, C.; Narkbunnam, N.; Samulski, R.J.; Asokan, A.; Hu, G.; Jacobson, L.J.; Manco-Johnson, M.J.; Monahan, P.E. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012, 19, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Mingozzi, F.; High, K.A. Overcoming the host immune response to adeno-associated virus gene delivery vectors: The race between clearance, tolerance, neutralization, and escape. Annu. Rev. Virol. 2017, 4, 511–534. [Google Scholar] [CrossRef]
- Meliani, A.; Boisgerault, F.; Hardet, R.; Marmier, S.; Collaud, F.; Ronzitti, G.; Leborgne, C.; Verdera, H.C.; Sola, M.S.; Charles, S.; et al. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat. Commun. 2018, 9, 4098. [Google Scholar] [CrossRef] [Green Version]
- von Pawel-Rammingen, U.; Johansson, B.P.; Björck, L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002, 21, 1607–1615. [Google Scholar] [CrossRef] [Green Version]
- Wenig, K.; Chatwell, L.; von Pawel-Rammingen, U.; Björck, L.; Huber, R.; Sondermann, P. Structure of the streptococcal endopeptidase IdeS, a cysteine proteinase with strict specificity for IgG. Proc. Natl. Acad. Sci. USA 2004, 101, 17371–17376. [Google Scholar] [CrossRef] [Green Version]
- Kizlik-Masson, C.; Deveuve, Q.; Zhou, Y.; Vayne, C.; Thibault, G.; McKenzie, S.E.; Pouplard, C.; Loyau, S.; Gruel, Y.; Rollin, J. Cleavage of anti-PF4/heparin IgG by a bacterial protease and potential benefit in heparin-induced thrombocytopenia. Blood 2019, 133, 2427–2435. [Google Scholar] [CrossRef]
- Lorant, T.; Bengtsson, M.; Eich, T.; Eriksson, B.M.; Winstedt, L.; Järnum, S.; Stenberg, Y.; Robertson, A.K.; Mosén, K.; Björck, L.; et al. Safety, immunogenicity, pharmacokinetics, and efficacy of degradation of anti-HLA antibodies by IdeS (imlifidase) in chronic kidney disease patients. Am. J. Transplant. 2018, 18, 2752–2762. [Google Scholar] [CrossRef]
- Nandakumar, K.S.; Holmdahl, R. Therapeutic cleavage of IgG: New avenues for treating inflammation. Trends Immunol. 2008, 29, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Leborgne, C.; Barbon, E.; Alexander, J.M.; Hanby, H.; Delignat, S.; Cohen, D.M.; Collaud, F.; Muraleetharan, S.; Lupo, D.; Silverberg, J.; et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat. Med. 2020, 26, 1096–1101. [Google Scholar] [CrossRef]
- Austin, A.L.; Galasso, B.; Nickens, C.; Knollmann-Ritschel, B.; Sharma, A. Inactivation of Zika virus by photoactive iodonaphthyl azide preserves immunogenic potential of the virus. Pathogens 2019, 8, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, W.R.; Livengood, J.A.; Giebler, H.A.; Stovall, J.L.; Boroughs, K.L.; Sonnberg, S.; Bohning, K.J.; Dietrich, E.A.; Ong, Y.T.; Danh, H.K.; et al. Purified inactivated Zika vaccine candidates afford protection against lethal challenge in mice. Sci. Rep. 2018, 8, 16509. [Google Scholar] [CrossRef]
- Lecouturier, V.; Bernard, M.C.; Berry, C.; Carayol, S.; Richier, E.; Boudet, F.; Heinrichs, J. Immunogenicity and protection conferred by an optimized purified inactivated Zika vaccine in mice. Vaccine 2019, 37, 2679–2686. [Google Scholar] [CrossRef]
- Lecouturier, V.; Pavot, V.; Berry, C.; Donadieu, A.; de Montfort, A.; Boudet, F.; Rokbi, B.; Jackson, N.; Heinrichs, J. An optimized purified inactivated Zika vaccine provides sustained immunogenicity and protection in cynomolgus macaques. NPJ Vaccines 2020, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Young, G.; Bohning, K.J.; Zahralban-Steele, M.; Hather, G.; Tadepalli, S.; Mickey, K.; Godin, C.S.; Sanisetty, S.; Sonnberg, S.; Patel, H.K.; et al. Complete protection in macaques conferred by purified inactivated Zika vaccine: Defining a correlate of protection. Sci. Rep. 2020, 10, 3488. [Google Scholar] [CrossRef]
- Abbink, P.; Larocca, R.A.; Visitsunthorn, K.; Boyd, M.; De La Barrera, R.A.; Gromowski, G.D.; Kirilova, M.; Peterson, R.; Li, Z.; Nanayakkara, O.; et al. Durability and correlates of vaccine protection against Zika virus in rhesus monkeys. Sci. Transl. Med. 2017, 9, eaao4163. [Google Scholar] [CrossRef] [Green Version]
- Stephenson, K.E.; Tan, C.S.; Walsh, S.R.; Hale, A.; Ansel, J.L.; Kanjilal, D.G.; Jaegle, K.; Peter, L.; Borducchi, E.N.; Nkolola, J.P.; et al. Safety and immunogenicity of a Zika purified inactivated virus vaccine given via standard, accelerated, or shortened schedules: A single-centre, double-blind, sequential-group, randomised, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 2020, 20, 1061–1070. [Google Scholar] [CrossRef]
- Dussupt, V.; Sankhala, R.S.; Gromowski, G.D.; Donofrio, G.; De La Barrera, R.A.; Larocca, R.A.; Zaky, W.; Mendez-Rivera, L.; Choe, M.; Davidson, E.; et al. Potent Zika and dengue cross-neutralizing antibodies induced by Zika vaccination in a dengue-experienced donor. Nat. Med. 2020, 26, 228–235. [Google Scholar] [CrossRef]
- Yang, Y.; Shan, C.; Zou, J.; Muruato, A.E.; Bruno, D.N.; de Almeida Medeiros Daniele, B.; Vasconcelos, P.F.C.; Rossi, S.L.; Weaver, S.C.; Xie, X.; et al. A cDNA clone-launched platform for high-yield production of inactivated Zika vaccine. EBioMedicine 2017, 17, 145–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boigard, H.; Alimova, A.; Martin, G.R.; Katz, A.; Gottlieb, P.; Galarza, J.M. Zika virus-like particle (VLP) based vaccine. PLoS Negl. Trop. Dis. 2017, 11, e0005608. [Google Scholar] [CrossRef] [Green Version]
- Garg, H.; Sedano, M.; Plata, G.; Punke, E.B.; Joshi, A. Development of virus-like-particle vaccine and reporter assay for Zika virus. J. Virol. 2017, 91, e00834-17. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, D.; Mendy, J.; Manayani, D.; Vang, L.; Wang, C.; Richard, T.; Guenther, B.; Aruri, J.; Avanzini, J.; Garduno, F.; et al. Passive transfer of immune sera induced by a Zika virus-like particle vaccine protects AG129 mice against lethal Zika virus challenge. EBioMedicine 2018, 27, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Zhang, T.; Zhang, Y.; Wang, H.; Deng, F. Zika virus baculovirus-expressed virus-like particles induce neutralizing antibodies in mice. Virol. Sin. 2018, 33, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Sci. Rep. 2017, 7, 7679. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Portela Catani, J.P.; Mc Cafferty, S.; Couck, L.; Van Den Broeck, W.; Gorlé, N.; Vandenbroucke, R.E.; Devriendt, B.; Ulbert, S.; Cnops, L.; et al. Immunogenicity and protection efficacy of a naked self-replicating mRNA-based Zika virus vaccine. Vaccines 2019, 7, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richner, J.M.; Himansu, S.; Dowd, K.A.; Butler, S.L.; Salazar, V.; Fox, J.M.; Julander, J.G.; Tang, W.W.; Shresta, S.; Pierson, T.C.; et al. Modified mRNA vaccines protect against Zika virus infection. Cell 2017, 169, 176. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Xie, X.; Yang, Y.; Muruato, A.E.; Zou, J.; Shan, C.; Nunes, B.T.; Medeiros, D.B.; Vasconcelos, P.F.; Weaver, S.C.; Rossi, S.L.; et al. Understanding Zika virus stability and developing a chimeric vaccine through functional analysis. MBio 2017, 8, e02134-16. [Google Scholar] [CrossRef] [Green Version]
- Nandy, A.; Basak, S.C. A brief review of computer-assisted approaches to rational design of peptide vaccines. Int. J. Mol. Sci. 2016, 17, 666. [Google Scholar] [CrossRef] [Green Version]
- Rayner, J.O.; Kalkeri, R.; Goebel, S.; Cai, Z.; Green, B.; Lin, S.; Snyder, B.; Hageli, K.; Walters, K.B.; Koide, F. Comparative pathogenesis of Asian and African-lineage Zika virus in Indian rhesus macaque’s and development of a non-human primate model suitable for the evaluation of new drugs and vaccines. Viruses 2018, 10, 229. [Google Scholar] [CrossRef] [Green Version]
- Sumathy, K.; Kulkarni, B.; Gondu, R.K.; Ponnuru, S.K.; Bonguram, N.; Eligeti, R.; Gadiyaram, S.; Praturi, U.; Chougule, B.; Karunakaran, L.; et al. Protective efficacy of Zika vaccine in AG129 mouse model. Sci. Rep. 2017, 7, 46375. [Google Scholar] [CrossRef] [Green Version]
- Zompi, S.; Santich, B.H.; Beatty, P.R.; Harris, E. Protection from secondary dengue virus infection in a mouse model reveals the role of serotype cross-reactive B and T cells. J. Immunol. 2012, 188, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of lethal Zika virus infection in AG129 Mice. PLoS. Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Huang, X.; Yang, Y. Type I IFN signaling on both B and CD4 T cells is required for protective antibody response to adenovirus. J. Immunol. 2007, 178, 3505–3510. [Google Scholar] [CrossRef] [PubMed]

| Vaccine’s Name or Component | Immunogenicity in the Induction of Immune Responses | Animal Model | Vaccine Doses | Administration Route | Virus Challenged | Ref. |
|---|---|---|---|---|---|---|
| prM and E | Completely protected mice against ZIKV-associated damage to the testes and sperm and prevented viral persistence in the testes | Type-I interferon knockout mice | Two doses at two-week interval | i.m. | Puerto Rico Strain PRVABC59 | [17] |
| pVAX1-ZME (prM and E) | Induced robust ZIKV-specific cellular and long-term humoral immune responses with high and sustained neutralizing activity, which provided passive protection against ZIKV infection in neonatal mice | BALB/c mice | Three doses at three-week intervals | i.m. | (SMGC-1 strain, GenBank accession number: KX266255 | [18] |
| GLS-5700 (prM and E) | Prevented fertility loss in male IFNAR−/− mice | C57BL/6J mice and IFNAR−/− mice | Two doses at two-week interval | i.m. | Puerto Rico Strain PRVABC59 | [19] |
| VRC5288 and VRC5283 | Induced detectable T-cell response and antibody response with neutralization activity. The immunogenicity of VRC5283 was better than that of VRC5288. | Humans | Single dose, two and three doses | i.m | No | [20] |
| prM and E | Elicited protective responses against multiple diverse ZIKV isolates | C57BL/6c mice | Four doses at days 0, 24, 42, and 199 | i.m | Puerto Rico Strain PRVABC59 | [21] |
| pVAX-NS1, pVAX-tpaNS1, pVAX-tpaNS1-IMX313P (NS1) | pVAX-tpaNS1 vaccination induced significantly higher NS1-specific antibody titers and CD4+, as well as CD8+, T-cell responses compared to pVAX-NS1 and pVAX-tpaNS1- IMX313P | BALB/c and IFNAR−/− mice | Three doses at two-week intervals | i.d. | ZIKVzkv2015 | [22] |
| Vaccine’s Name or Component | Immunogenicity in the Induction of Immune Responses | Animal Model | Vaccine Doses | Administration Route | Virus Challenged | Ref. |
|---|---|---|---|---|---|---|
| E | Induced robust antigen binding IgG titers and high levels of neutralizing antibodies in the mice, which protected against viremia after ZIKV infection | Swiss Webster, BALB/c, and C57BL/6 mice | Three doses at three-week intervals | i.m. | Puerto Rico Strain PRVABC59 | [26] |
| Induced high neutralizing antibody titers | Cynomolgus macaques and BALB/c mice | Three doses at three-week intervals | i.m. | Puerto Rico Strain PRVABC59 | [27] | |
| EDIII | Induced high titer of IgG and ZIKV-neutralizing antibodies and showed no evidence of ADE induction in mouse serum | C57BL/6 mice | Four doses at three-week intervals | s.c. | Puerto Rico Strain PRVABC59 | [28] |
| E90 (Consisting of the first 450 amino acids at the N-terminal region of E protein) | Immunization of pregnant mice with E90 protected the developing brains of offspring, both in utero and in the neonatal period, from subsequent ZIKV infection and microcephaly. E90 induced robust ZIKV-specific humoral responses in adult BALB/c mice. | ICR (CD-1 immunocompetent) mice; BALB/c mice | Two doses at two-week interval | i.p. | GZ01 and FSS13025 strains | [29,30] |
| EDIII fragments (E296–406; E298–409; E301–404) | Induced sustained broad-spectrum neutralization antibodies and passive transfer of the E298–409-specific antibodies prevented ZIKV infection in newborns and immunocompromised adults. | BALB/c mice and A129 mice | Five doses at days 0, 21, 42, 210, and 300 | i.m. | R103451 and FLR strains | [31] |
| Vaccine’s Name or Component | Immunogenicity in the Induction of Immune Responses | Animal Model | Vaccine Doses | Administration Route | Virus Challenged | Ref. |
|---|---|---|---|---|---|---|
| ZIKV-3′ UTR-10-LAV | Showed complete protection from viremia and induced a saturated neutralizing antibody response | A129 mice | Single dose | s.c. | Cambodian strain FSS13025 and Puerto Rico strain PRVABC59 | [34] |
| ZIKV-3′ UTR-20-LAV | Induced strong immune responses and protected ZIKV-induced damage to testes in mice; induced sterilizing immunity in NHPs | A129 mice and rhesus macaques | Single dose | s.c. | Cambodian strain FSS13025 and Puerto Rico strain PRVABC59 | [35] |
| ZIKV-NS1-LAV (NS1) | Markedly diminished viral RNA levels in maternal, placental, and fetal tissues, which resulted in protection against placental damage and fetal death | A129 mice andrhesus macaques | Single dose | s.c. | Puerto Rico strain PRVABC59 | [36] |
| LAV (with 9-amino-acid deletion in the C protein) | Not only elicited protective immunity that completely prevented viremia, morbidity and mortality, but also fully prevented infection of pregnant mice and maternal-to-fetal transmission | A129 mice | Single dose | s.c. | Puerto Rico strain PRVABC59 | [37] |
| Vaccine’s Name or Component | Immunogenicity in the Induction of Immune Responses | Animal Model | Vaccine Doses | Administration Route | Virus Challenged | Ref. |
|---|---|---|---|---|---|---|
| Ad4-prM-E and Ad5-prM-E | Ad5-prM-E vaccination induced both humoral and T-cell responses, while Ad4-prM-E induced only a T-cell response. | C57BL/6 mice | Two doses at three-week interval | i.m. | Puerto Rico strain PRVABC59 | [39] |
| hAd5-prM-E | Induced both cell-mediated and humoral immune responses, which conferred protection against a ZIKV challenge | C57BL/6 mice and Ifnar1−/− mice | Single dose | i.n. | Puerto Rico strain PRVABC59 | [41] |
| Ad5-Sig-prM-Env (prM-E) and Ad5-Env (E) | Both vaccines elicited robust humoral and cellular immune responses in immunocompetent BALB/c mice, as well as in A129 mice, but Ad5-Sig-prM-Env-vaccinated mice resulted in significantly higher ZIKV-specific neutralizing antibody titers and lower viral loads than Ad5-Env-vaccinated mice. | BALB/c mice and A129 mice | Single dose | i.m. | Puerto Rico strain PRVABC59 | [42] |
| ChAdOx1 | Induced high levels of protective responses in challenged mice | BALB/c mice | Single dose | i.m. | Brazilian ZIKV | [43] |
| RhAd52-prMEnv | Induced ZIKV-specific neutralizing antibodies in rhesus monkeys; antibodies sufficient for protection against ZIKV challenge in mice | Rhesus monkeys and BALB/c mice | Single dose | i.m. | Brazilian ZIKV and Puerto Rico strain PRVABC59 | [44] |
| rVSV-prM-E-NS1 | Induced ZIKV-specific antibody and T-cell immune responses that conferred partial protection against ZIKV infection | A129 mice and BALB/c mice | Single dose | i.n. | Cambodian strain FSS13025 | [45] |
| VSV-Capsid and VSV-ZikaE260-425 | Both vaccines induced strong ZIKV-specific humoral responses in immunized BALB/c mice, but VSV-Capsid immunization elicited significantly higher levels of IFN-γ+ CD8+ and CD4+ T-cells than that of VSV-ZikaE260-425 vaccine. | BALB/c mice | Single dose | i.n. | Puerto Rico strain PRVABC59 | [46] |
| Vaccine’s Name or Component | Immunogenicity in the Induction of Immune responses | Animal Model | Vaccine Doses | Administration Route | Virus Challenged | Ref. |
|---|---|---|---|---|---|---|
| Alum-adjuvant mixed purified inactivated ZIKV vaccine (PIZV) | Two-dose vaccination of the candidates was highly immunogenic in the mouse models, which protected AG129 mice against lethal ZIKV challenge. Passive transfer of naïve mice with ZIKV-immune serum also showed full protection against lethal ZIKV challenge. | CD-1 and AG129 mice | Three doses at four-week intervals | i.m. | Puerto Rico strain PRVABC59 | [59] |
| Induced robust neutralizing antibody responses and provided complete protection from homologous ZIKV strain challenge | BALB/c mice and cynomolgus macaques | Two doses at three/four-week interval | i.m. | Puerto Rico strain PRVABC59 | [60,61] | |
| PIZV | Elicited a dose-dependent and long-lasting neutralizing antibody responses | Indian rhesus macaques | Two doses at four-week interval | i.m. | Puerto Rico strain PRVABC59 | [62] |
| Two dose-vaccination of the Type of vaccine gave a robust protection against ZIKV challenge. | rhesus macaques | Two doses at four-week interval | s.c., i.m. | Brazil ZKV2015 | [63] | |
| Safe and well tolerated in humans up to 52 weeks of follow-up; but two doses not durable for immunogenicity required | Phase I clinical trial | Single dose and two doses at two/four-week interval | i.m. | No | [64] |
| Vaccine Types | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| DNA vaccines | Chemically stable and cost effective; easy and safe to scale up; can induce both humoral and cellular immune responses and are capable of mediating long-term protection | Have the potential of integrating the exogenous gene into the host genome, leading to induction of host autoimmunity | [17,18,19,20,21,22,25] |
| Subunit vaccines | Rapid, stable, and consistent production | Normally need multiple doses with appropriate adjuvants | [26,27,28,29,30,31] |
| Live-attenuated vaccines | Single dose could induce high immune responses, rapid induction of durable immunity | Safety problems and need cold-chain storage facilities | [24,34,35,36,37] |
| Virus-vector-based vaccines | Single dose could induce higher and faster immune responses with lasting protection | Pre-existing immunity problem | [39,41,42,43,44,45,48,49] |
| Inactivated vaccines | Easy production and storage; convenient to make multivalent vaccines | Safety problems; need multiple injections; unable to deal with mutant viruses | [59,60,61,62,63,64] |
| VLP-based vaccines | Noninfectious and could induce robust antibodies; multiple choices of expression systems | Application for clinical use needs further studies | [69,70,71] |
| mRNA-based vaccines | Rapid and flexible production; could induce potent humoral and cellular immune responses | Need cold-chain storage facilities; new technology, lack of historical accumulation | [72,73,74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Li, C.; Shi, W.; Hu, X.; Nandakumar, K.S.; Jiang, S.; Zhang, N. Current Progress in the Development of Zika Virus Vaccines. Vaccines 2021, 9, 1004. https://doi.org/10.3390/vaccines9091004
Zhou K, Li C, Shi W, Hu X, Nandakumar KS, Jiang S, Zhang N. Current Progress in the Development of Zika Virus Vaccines. Vaccines. 2021; 9(9):1004. https://doi.org/10.3390/vaccines9091004
Chicago/Turabian StyleZhou, Kehui, Chaoqun Li, Wen Shi, Xiaodan Hu, Kutty Selva Nandakumar, Shibo Jiang, and Naru Zhang. 2021. "Current Progress in the Development of Zika Virus Vaccines" Vaccines 9, no. 9: 1004. https://doi.org/10.3390/vaccines9091004
APA StyleZhou, K., Li, C., Shi, W., Hu, X., Nandakumar, K. S., Jiang, S., & Zhang, N. (2021). Current Progress in the Development of Zika Virus Vaccines. Vaccines, 9(9), 1004. https://doi.org/10.3390/vaccines9091004








